Developmentally Programmed Switches in DNA Replication: Gene Amplification and Genome-Wide Endoreplication in Tetrahymena
Abstract
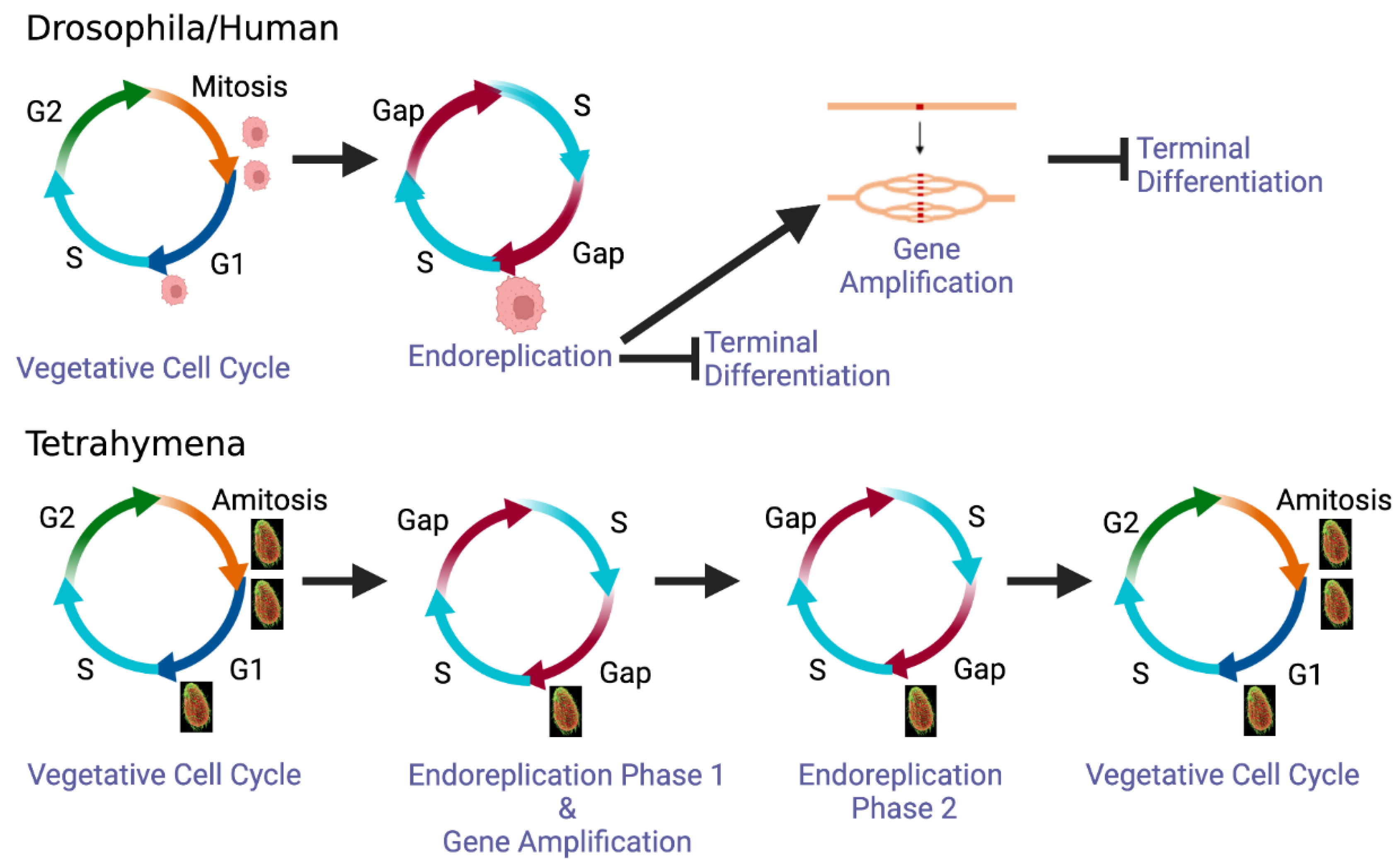
1. Introduction
2. Materials and Methods
2.1. Tetrahymena Culture and Strains
2.2. 5-Ethynyl-2′-deoxyuridine (EdU) Labeling
2.3. Quantitative PCR (qPCR)
2.4. Flow Cytometry
2.5. DNA Fiber Analysis
2.6. Two-Dimensional (2D) Gel Electrophoresis of DNA Replication Intermediates (RIs)
3. Results
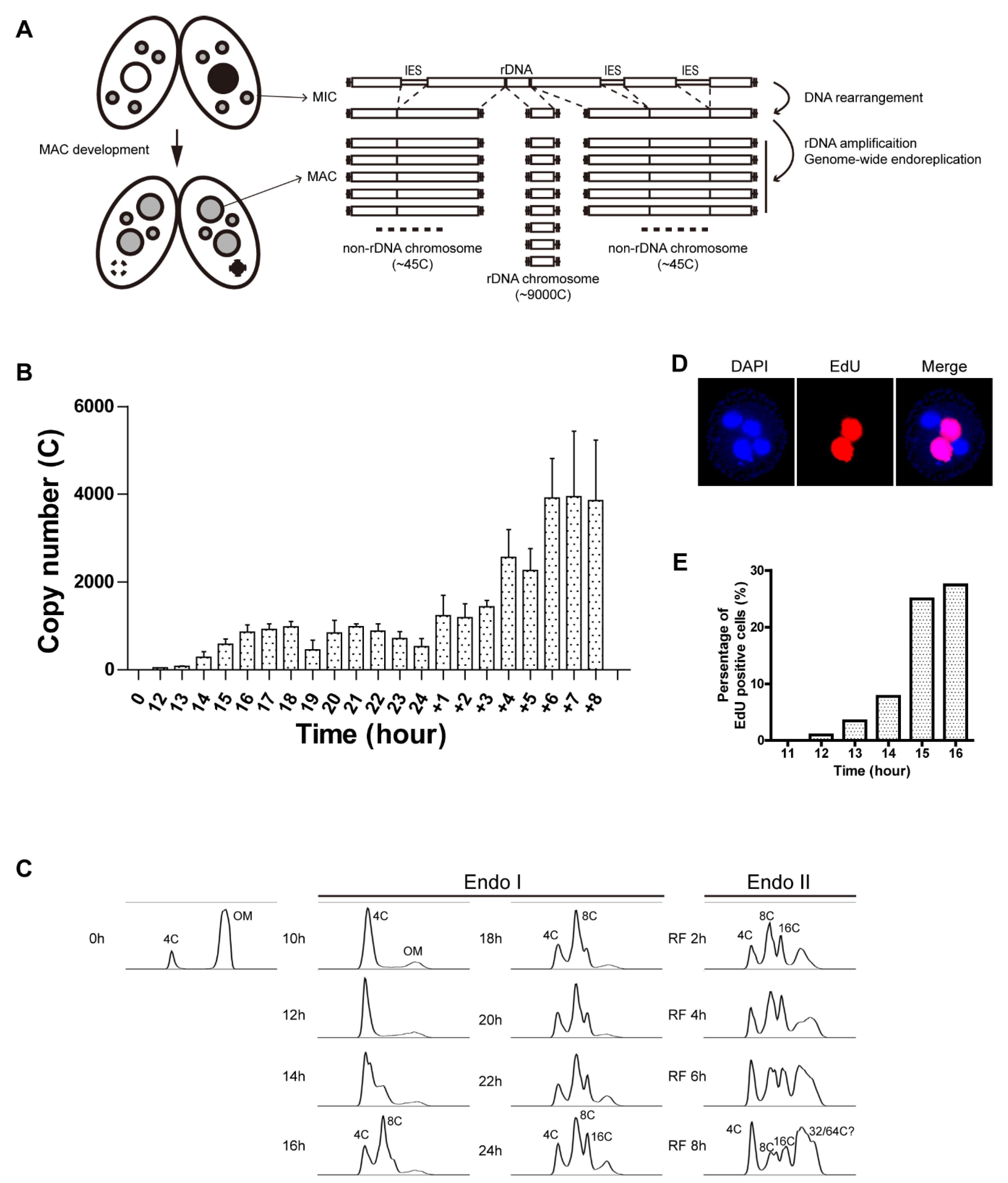
3.1. Quantitative Analysis of rDNA Gene Amplification in the Developing Macronucleus
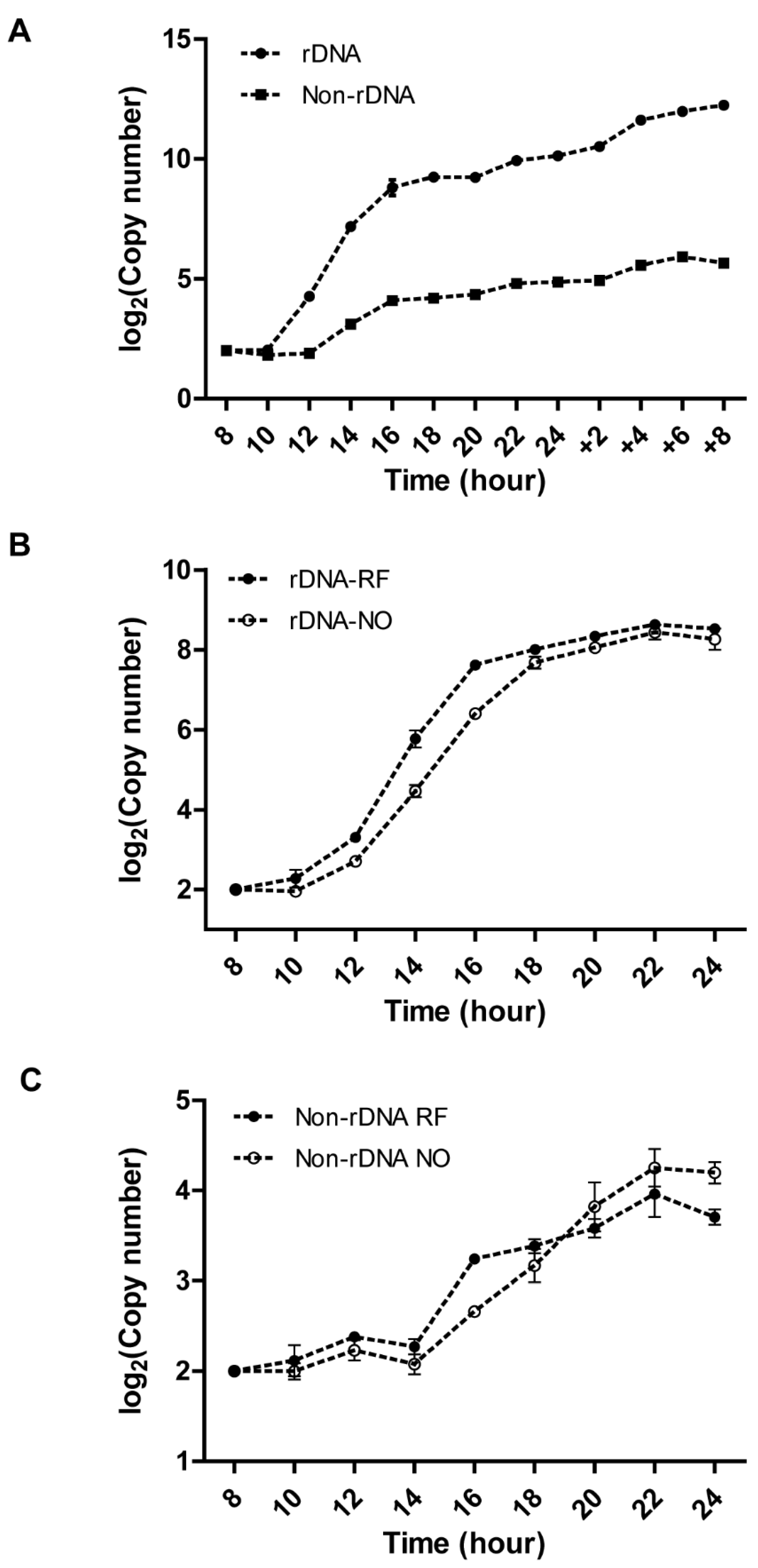
3.2. Kinetics of rDNA and Non-rDNA Copy Number Increases during Development
3.3. Effect of Re-Feeding on Endoreplication Phases 1 and 2
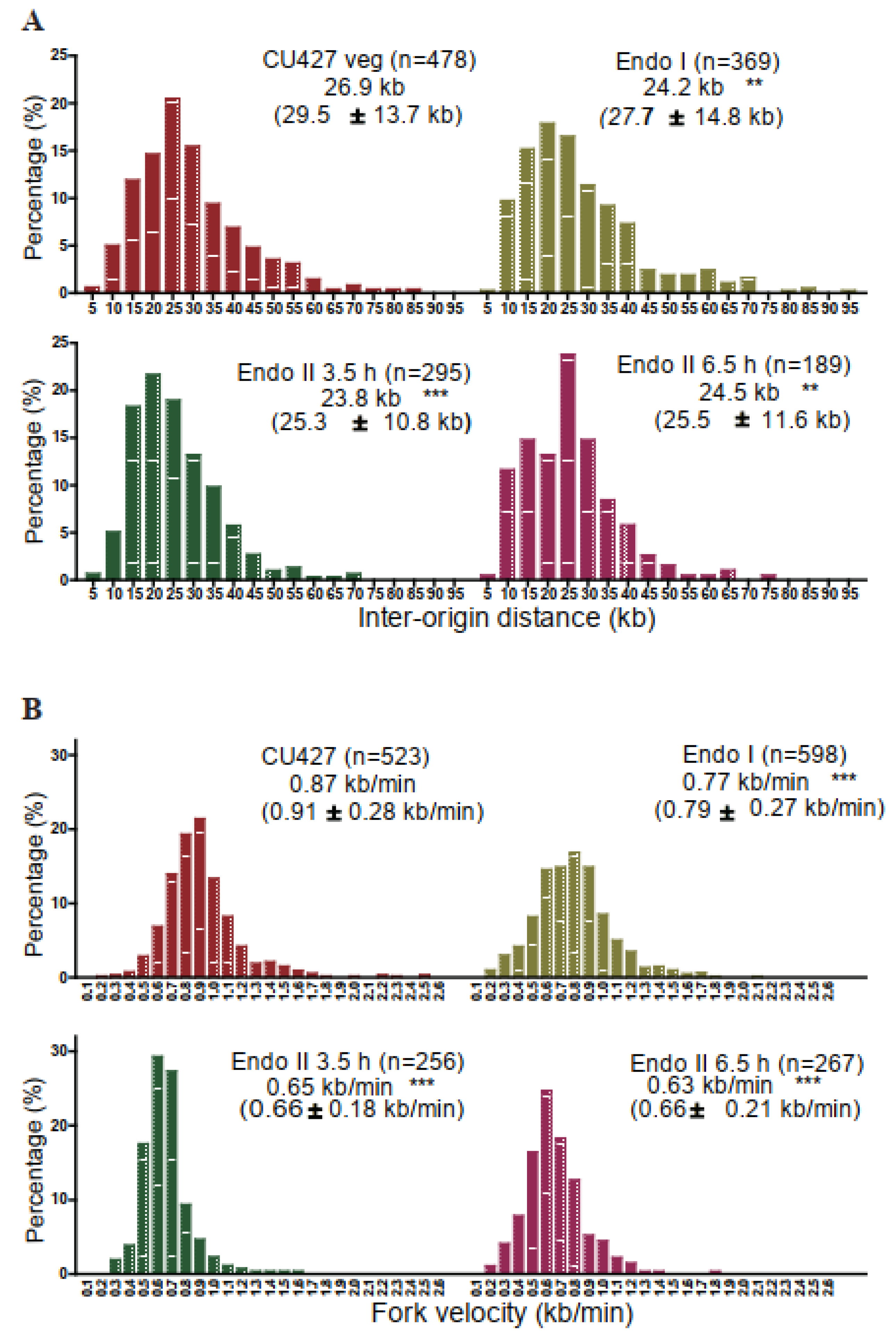
3.4. Replication Initiation and Elongation in Endoreplicating Non-rDNA Chromosomes
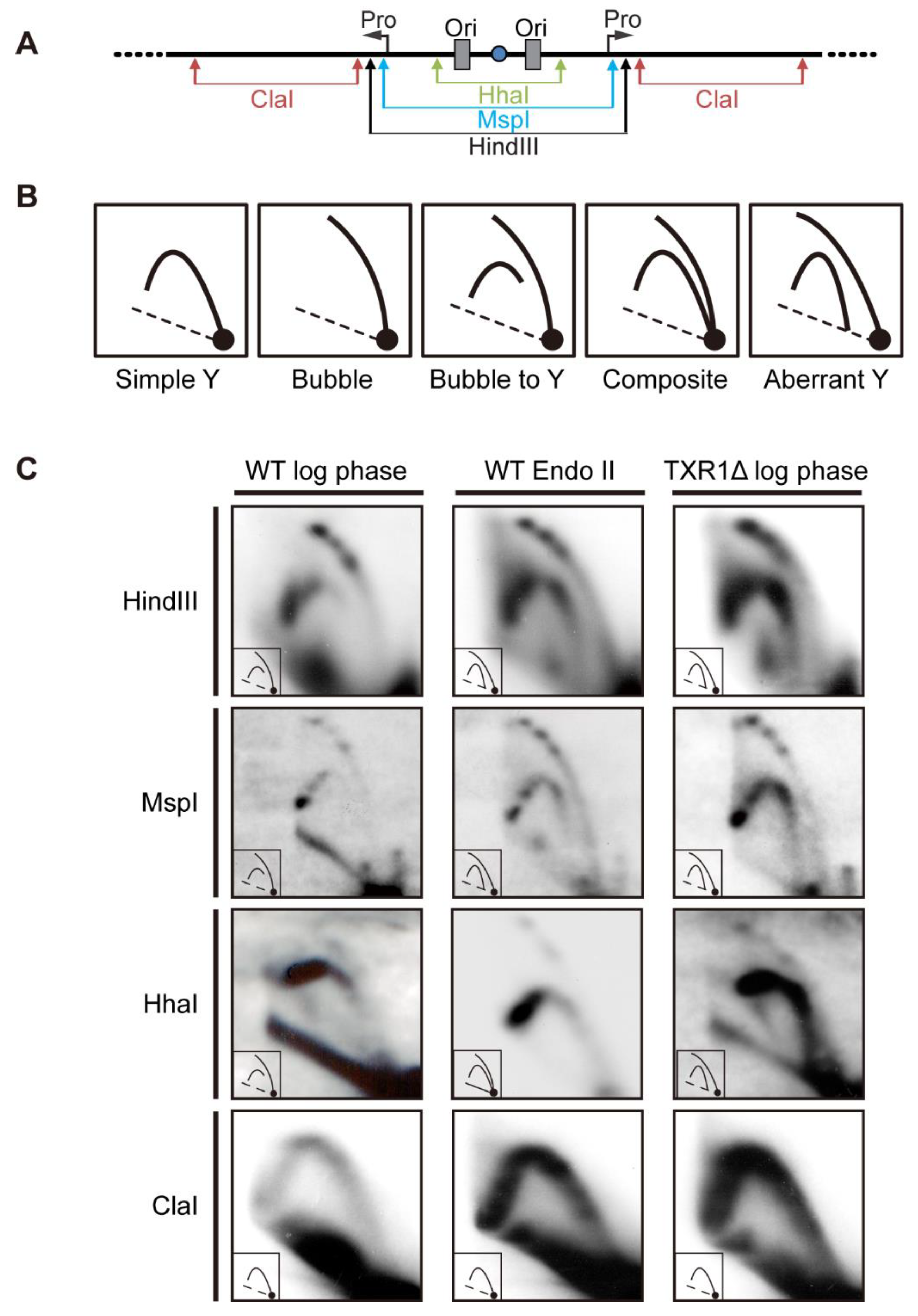
3.5. Localization of Aberrant Replication Intermediates in Endoreplicating rDNA Minichromosomes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morgan, D.O. Cyclin-Dependent kinases: Engines, Clocks, and Microprocessors. Annu. Rev. Cell Dev. Biol. 1997, 13, 261–291. [Google Scholar] [CrossRef] [PubMed]
- Dolson, A.; Sauty, S.M.; Shaban, K.; Yankulov, K. Dbf4-Dependent Kinase: DDK-ated to post-initiation events in DNA replication. Cell Cycle 2021, 20, 2348–2360. [Google Scholar] [CrossRef] [PubMed]
- Orr-Weaver, T.L. When bigger is better: The role of polyploidy in organogenesis. Trends Genet. 2015, 31, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Shu, Z.; Row, S.; Deng, W.-M. Endoreplication: The Good, the Bad, and the Ugly. Trends Cell Biol. 2018, 28, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Nordman, J.T.; Kozhevnikova, E.N.; Verrijzer, C.P.; Pindyurin, A.V.; Andreyeva, E.N.; Shloma, V.V.; Zhimulev, I.F.; Orr-Weaver, T.L. DNA Copy-Number Control through Inhibition of Replication Fork Progression. Cell Rep. 2014, 9, 841–849. [Google Scholar] [CrossRef]
- Austin, R.J.; Orr-Weaver, T.L.; Bell, S. Drosophila ORC specifically binds to ACE3, an origin of DNA replication control element. Genes Dev. 1999, 13, 2639–2649. [Google Scholar] [CrossRef]
- Claycomb, J.; MacAlpine, D.M.; Evans, J.G.; Bell, S.; Orr-Weaver, T.L. Visualization of replication initiation and elongation in Drosophila. J. Cell Biol. 2002, 159, 225–236. [Google Scholar] [CrossRef]
- Spradling, A.C.; Mahowald, A.P. Amplification of genes for chorion proteins during oogenesis in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 1980, 77, 1096–1100. [Google Scholar] [CrossRef]
- Calvi, B.R.; Lilly, M.A. Spradling AC Cell cycle control of chorion gene amplification. Genes Dev. 1998, 12, 734–744. [Google Scholar] [CrossRef]
- Albertson, D.G. Gene amplification in cancer. Trends Genet. 2006, 22, 447–455. [Google Scholar] [CrossRef]
- Karrer, K.M. Nuclear dualism. Methods Cell Biol. 2012, 109, 29–52. [Google Scholar] [PubMed]
- Cole, E.; Sugai, T. Developmental progression of Tetrahymena through the cell cycle and conjugation. Methods Cell Biol. 2012, 109, 177–236. [Google Scholar] [PubMed]
- Zhang, L.; Cervantes, M.D.; Pan, S.; Lindsley, J.; Dabney, A.; Kapler, G.M. Transcriptome analysis of the binucleate ciliate Tetrahymena thermophila with asynchronous nuclear cell cycles. Mol Biol Cell. 2023. [Google Scholar] [CrossRef] [PubMed]
- Cole, E.S.; Soelter, T.A. A Mutational Analysis of Conjugation in Tetrahymena thermophila 2. Phenotypes affecting middle and late development: Third prezygotic nuclear division, pronuclear exchange, pronuclear fusion, and postzygotic development. Dev. Biol. 1997, 189, 233–245. [Google Scholar] [CrossRef]
- Yakisich, J.S.; Kapler, G.M. Role of the PI3-kinase pathway in programmed nuclear death and the fate of pharmacologically-induced survival nuclei in Tetrahymena thermophila. Cell Death Differ. 2004, 10, 1146–1149. [Google Scholar] [CrossRef]
- Yao, M.C.; Fuller, P.; Xi, X. Programmed DNA deletion as an RNA-guided system of genome defense. Science 2003, 300, 1581–1584. [Google Scholar] [CrossRef]
- Eisen, J.A.; Coyne, R.S.; Wu, M.; Wu, D.; Thiagarajan, M.; Wortman, J.R.; Badger, J.H.; Ren, Q.; Amedeo, P.; Jones, K.M.; et al. Macronuclear Genome Sequence of the Ciliate Tetrahymena thermophila, a Model Eukaryote. PLoS Biol. 2006, 4, e286. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, L.; Mochizuki, K.; Xiong, J.; Miao, W.; Wang, G. Absolute quantification of chromosome copy numbers in the polyploid macronucleus of Tetrahymena thermophila at the single-cell level. J. Eukaryot. Microbiol. 2022, 69, 12907. [Google Scholar] [CrossRef]
- Lee, P.-H.; Meng, X.; Kapler, G.M. Developmental Regulation of the Tetrahymena thermophila Origin Recognition Complex. PLoS Genet. 2015, 11, e1004875. [Google Scholar] [CrossRef]
- Sandoval, P.Y.; Lee, P.-H.; Meng, X.; Kapler, G.M. Checkpoint Activation of an Unconventional DNA Replication Program in Tetrahymena. PLoS Genet. 2015, 11, e1005405. [Google Scholar] [CrossRef]
- Zhang, Z.; Macalpine, D.M.; Kapler, G.M. Developmental regulation of DNA replication: Replication fork barriers and programmed gene amplification in Tetrahymena thermophila. Mol. Cell Biol. 1997, 17, 6147–6156. [Google Scholar] [CrossRef] [PubMed]
- Larson, D.D.; Blackburn, E.H.; Yaeger, P.C.; Orias, E. Control of rDNA replication in Tetrahymena involves a cis-acting upstream repeat of a promoter element. Cell 1986, 47, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Chastain, P.D.; Nakamura, J.; Swenberg, J.; Kaufman, D. Nonrandom AP site distribution in highly proliferative cells. FASEB J. 2006, 20, 2612–2614. [Google Scholar] [CrossRef] [PubMed]
- Schwab, R.A.; Niedzwiedz, W. Visualization of DNA Replication in the Vertebrate Model System DT40 using the DNA Fiber Technique. J. Vis. Exp. 2011, 1, e3255. [Google Scholar] [CrossRef]
- Stewart, J.A.; Wang, F.; Chaiken, M.F.; Kasbek, C.; Chastain, P.D., 2nd; Wright, W.E.; Price, C.M. Human CST promotes telomere duplex replication and general replication restart after fork stalling. EMBO J. 2012, 31, 3537–3549. [Google Scholar] [CrossRef]
- Iida, T.; Kobayashi, T. How do cells count multi-copy genes?: “Musical Chair” model for preserving the number of rDNA copies. Curr. Genet. 2019, 65, 883–885. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Li, S.; Yin, L.; Cole, E.S.; Udani, R.A.; Karrer, K.M. Progeny of germ line knockouts of ASI2, a gene encoding a putative signal transduction receptor in Tetrahymena thermophila, fail to make the transition from sexual reproduction to vegetative growth. Dev. Biol. 2006, 295, 633–646. [Google Scholar] [CrossRef]
- MacAlpine, D.M.; Zhang, Z.; Kapler, G.M. Type I elements mediate replication fork pausing at conserved upstream sequences in the Tetrahymena thermophila rDNA minichromosome. Mol. Cell. Biol. 1997, 17, 4517–4525. [Google Scholar] [CrossRef]
- Mohammad, M.M.; Smith, A.G.; Donti, T.R.; Kapler, G.M. Tetrahymena ORC contains a ribosomal RNA fragment that participates in rDNA origin recognition. EMBO J. 2007, 26, 5048–5060. [Google Scholar] [CrossRef]
- Donti, T.R.; Datta, S.; Sandoval, P.Y.; Kapler, G.M. Differential targeting of Tetrahymena ORC to ribosomal DNA and non-rDNA replication origins. EMBO J. 2009, 28, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Alzu, A.; Bermejo, R.; Begnis, M.; Lucca, C.; Piccini, D.; Carotenuto, W.; Saponaro, M.; Brambati, A.; Cocito, A.; Foiani, M.; et al. Senataxin Associates with Replication Forks to Protect Fork Integrity across RNA-Polymerase-II-Transcribed Genes. Cell 2012, 151, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Xiong, J.; Zhang, C.; Berquist, B.R.; Yang, R.; Zhao, M.; Molascon, A.J.; Kwiatkowski, S.Y.; Yuan, D.; Qin, Z.; et al. Impaired replication elongation in Tetrahymena mutants deficient in histone H3 Lys 27 monomethylation. Genes Dev. 2013, 27, 1662–1679. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.J.; Gallagher, R.C.; Blackburn, E.H. Replication of an rRNA gene origin plasmid in the Tetrahymena thermophila macronucleus is prevented by transcription through the origin from an RNA polymerase I promoter. Mol. Cell. Biol. 1995, 15, 3372–3381. [Google Scholar] [CrossRef]
- Berendes, H.D. Salivary gland function and chromosomal puffing patterns in Drosophila hydei. Chromosoma 1965, 17, 35–77. [Google Scholar] [CrossRef]
- Cross, J. How to make a placenta: Mechanisms of trophoblast cell differentiation in mice—A Review. Placenta 2005, 26, S3–S9. [Google Scholar] [CrossRef]
- Slabodnick, M.M.; Ruby, J.G.; Reiff, S.B.; Swart, E.C.; Gosai, S.; Prabakaran, S.; Witkowska, E.; Larue, G.E.; Fisher, S.; Freeman, R.M.; et al. The Macronuclear Genome of Stentor coeruleus Reveals Tiny Introns in a Giant Cell. Curr. Biol. 2017, 27, 569–575. [Google Scholar] [CrossRef]
- Zeng, J.; Huynh, N.; Phelps, B.; King-Jones, K. Snail synchronizes endocycling in a TOR-dependent manner to coordinate entry and escape from endoreplication pausing during the Drosophila critical weight checkpoint. PLoS Biol. 2000, 18, e3000609. [Google Scholar] [CrossRef]
- Noto, T.; Mochizuki, K. Whats, hows and whys of programmed DNA elimination in Tetrahymena. Open Biol. 2017, 7, 170–172. [Google Scholar] [CrossRef]
- Chalker, D.L. Dynamic nuclear reorganization during genome remodeling of Tetrahymena. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2008, 1783, 2130–2136. [Google Scholar] [CrossRef]
- Yin, L.; Gater, S.T.; Karrer, K.M. A Developmentally Regulated Gene, ASI2, Is Required for Endocycling in the Macronuclear Anlagen of Tetrahymena. Eukaryot. Cell 2010, 9, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Akematsu, T.; Pearlman, R.E.; Endoh, H. Gigantic macroautophagy in programmed nuclear death ofTetrahymena thermophila. Autophagy 2010, 6, 901–911. [Google Scholar] [CrossRef]
- Fox, D.T.; Gall, J.G.; Spradling, A.C. Error-prone polyploid mitosis during normal Drosophila development. Genes Dev. 2010, 24, 2294–2302. [Google Scholar] [CrossRef] [PubMed]
- Hassel, C.; Zhang, B.; Dixon, M.; Calvi, B.R. Induction of endocycles represses apoptosis independently of differentiation and predisposes cells to genome instability. Development 2014, 141, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, S.; Maqbool, S.B.; Kolpakas, A.; Murnen, K.; Calvi, B.R. Endocycling cells do not apoptose in response to DNA rereplication genotoxic stress. Genes Dev. 2008, 22, 3158–3171. [Google Scholar] [CrossRef] [PubMed]
- Yarosh, W.; Spradling, A.C. Incomplete replication generates somatic DNA alterations within Drosophila polytene salivary gland cells. Genes Dev. 2014, 28, 1840–1855. [Google Scholar] [CrossRef] [PubMed]
- Osheim, Y.N.; Miller, O.L., Jr.; Beyer, A.L. Visualization of Drosophila melanogaster chorion genes undergoing amplification. Mol. Cell Biol. 1998, 8, 2811–2821. [Google Scholar]
- Sun, J.; Smith, L.; Armento, A.; Deng, W.-M. Regulation of the endocycle/gene amplification switch by Notch and ecdysone signaling. J. Cell Biol. 2008, 182, 885–896. [Google Scholar] [CrossRef]
- Gomes, C.J.; Centuori, S.M.; Harman, M.W.; Putnam, C.W.; Wolgemuth, C.W.; Martinez, J.D. The induction of endoreduplication and polyploidy by elevated expression of 14-3-3γ. Genes Cancer 2017, 8, 771–783. [Google Scholar] [CrossRef]
- Tan, Z.; Chu, D.Z.V.; Chan, Y.J.A.; Lu, Y.E.; Rancati, G. Mammalian Cells Undergo Endoreduplication in Response to Lactic Acidosis. Sci. Rep. 2018, 8, 2890. [Google Scholar] [CrossRef]
- Adachi, S.; Minamisawa, K.; Okushima, Y.; Inagaki, S.; Yoshiyama, K.; Kondou, Y.; Kaminuma, E.; Kawashima, M.; Toyoda, T.; Matsui, M.; et al. Programmed induction of endoreduplication by DNA double-strand breaks in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 10004–10009. [Google Scholar] [CrossRef] [PubMed]
- Coyne, R.S.; A Nikiforov, M.; Smothers, J.F.; Allis, C.; Yao, M.-C. Parental Expression of the Chromodomain Protein Pdd1p Is Required for Completion of Programmed DNA Elimination and Nuclear Differentiation. Mol. Cell 1999, 4, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yuan, Y.; Liang, A.; Wang, W. Chromodomain protein Tcd1 is required for macronuclear genome rearrangement and repair in Tetrahymena. Sci. Rep. 2015, 5, 10243. [Google Scholar] [CrossRef] [PubMed]
- Yakisich, J.S.; Sandoval, P.Y.; Morrison, T.L.; Kapler, G.M. TIF1 activates the intra-S phase checkpoint response in the diploid micronucleus and amitotic polyploid macronucleus of Tetrahymena. Mol. Biol. Cell 2006, 17, 5185–5197. [Google Scholar] [CrossRef]
- Feng, L.; Wang, G.; Hamilton, E.P.; Xiong, J.; Yan, G.; Chen, K.; Chen, X.; Dui, W.; Plemens, A.; Khadr, L.; et al. A germline-limited piggyBac transposase gene is required for precise excision in Tetrahymena genome rearrangement. Nucleic Acids Res. 2017, 45, 9481–9502. [Google Scholar] [CrossRef]
- Liang, C.; Spitzer, J.D.; Smith, H.S.; Gerbi, S.A. Replication initiates at a confined region during DNA amplification in Sciara DNA puff II/9A. Genes Dev. 1993, 7, 1072–1084. [Google Scholar] [CrossRef]
- Doerder, F.P.; Ditaranto, J.; DeBault, L.E. Evolutionary constraints on quantitative variation and regulation of macronuclear DNA content in the genus Tetrahymena. J. Cell Sci. 1981, 49, 177–193. [Google Scholar] [CrossRef]
- Park, S.Y.; Asano, M. The origin recognition complex is dispensable for endoreplication in Drosophila. Proc. Natl. Acad. Sci. USA 2008, 105, 12343–12348. [Google Scholar] [CrossRef]
- Okano-Uchida, T.; Kent, L.N.; Ouseph, M.M.; McCarty, B.; Frank, J.J.; Kladney, R.; Cuitino, M.C.; Thompson, J.C.; Coppola, V.; Asano, M.; et al. Endoreduplication of the mouse genome in the absence of ORC1. Genes Dev. 2018, 32, 978–990. [Google Scholar] [CrossRef]
- Shibata, E.; Kiran, M.; Shibata, Y.; Singh, S.; Kiran, S.; Dutta, A. Two subunits of human ORC are dispensable for DNA replication and proliferation. Elife 2016, 5, 19084. [Google Scholar] [CrossRef]
- Shibata, E.; Dutta, A. A human cancer cell line initiates DNA replication normally in the absence of ORC5 and ORC2 proteins. J. Biol. Chem. 2020, 295, 16949–16959. [Google Scholar] [CrossRef] [PubMed]
- Challoner, P.B.; Amin, A.A.; Pearlman, R.E.; Blackburn, E.H. Conserved arrangements of repeated DNA sequences in nontranscribed spacers of ciliate ribosomal RNA genes: Evidence for molecular coevolution. Nucleic Acids Res. 1985, 11, 2661–2680. [Google Scholar] [CrossRef] [PubMed]
- Polleys, E.J.; House, N.C.M.; Freudenreich, C.H. Role of recombination and replication fork restart in repeat instability. DNA Repair 2017, 56, 156–165. [Google Scholar] [CrossRef] [PubMed]
| Strain | Micronuclear Genotype | Macronuclear Phenotype |
|---|---|---|
| CU427 | chx1-1/chx1-1 | paromomycin-sensitive cycloheximide-sensitive |
| CU428 | mpr1-1/mpr1-1 | paromomycin-sensitive 6-methylpurine-sensitive |
| SB1934 | rDNA [C3-1]/rDNA [C3-1]; mpr1-1/mpr1-1 | paromomycin-sensitive 6-methylpurine-sensitive rDNA [B] |
| SB4202 | pmr1[C3-1/pmr1[C3-1]; CHX1[C3]/CHX1[C3] | paromomycin-sensitive cycloheximide-resistant rDNA [B] |
| g-H3.2+GFP-3 | hht2[3′neo2, GFPc] hht2[3′neo2, GFPc] | paromomycin-sensitive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, X.; Dang, H.Q.; Kapler, G.M. Developmentally Programmed Switches in DNA Replication: Gene Amplification and Genome-Wide Endoreplication in Tetrahymena. Microorganisms 2023, 11, 491. https://doi.org/10.3390/microorganisms11020491
Meng X, Dang HQ, Kapler GM. Developmentally Programmed Switches in DNA Replication: Gene Amplification and Genome-Wide Endoreplication in Tetrahymena. Microorganisms. 2023; 11(2):491. https://doi.org/10.3390/microorganisms11020491
Chicago/Turabian StyleMeng, Xiangzhou, Hung Quang Dang, and Geoffrey M. Kapler. 2023. "Developmentally Programmed Switches in DNA Replication: Gene Amplification and Genome-Wide Endoreplication in Tetrahymena" Microorganisms 11, no. 2: 491. https://doi.org/10.3390/microorganisms11020491
APA StyleMeng, X., Dang, H. Q., & Kapler, G. M. (2023). Developmentally Programmed Switches in DNA Replication: Gene Amplification and Genome-Wide Endoreplication in Tetrahymena. Microorganisms, 11(2), 491. https://doi.org/10.3390/microorganisms11020491






