Changes in the Rhizosphere Prokaryotic Community Structure of Halodule wrightii Monospecific Stands Associated to Submarine Groundwater Discharges in a Karstic Costal Area
Abstract
1. Introduction
2. Materials and Methods
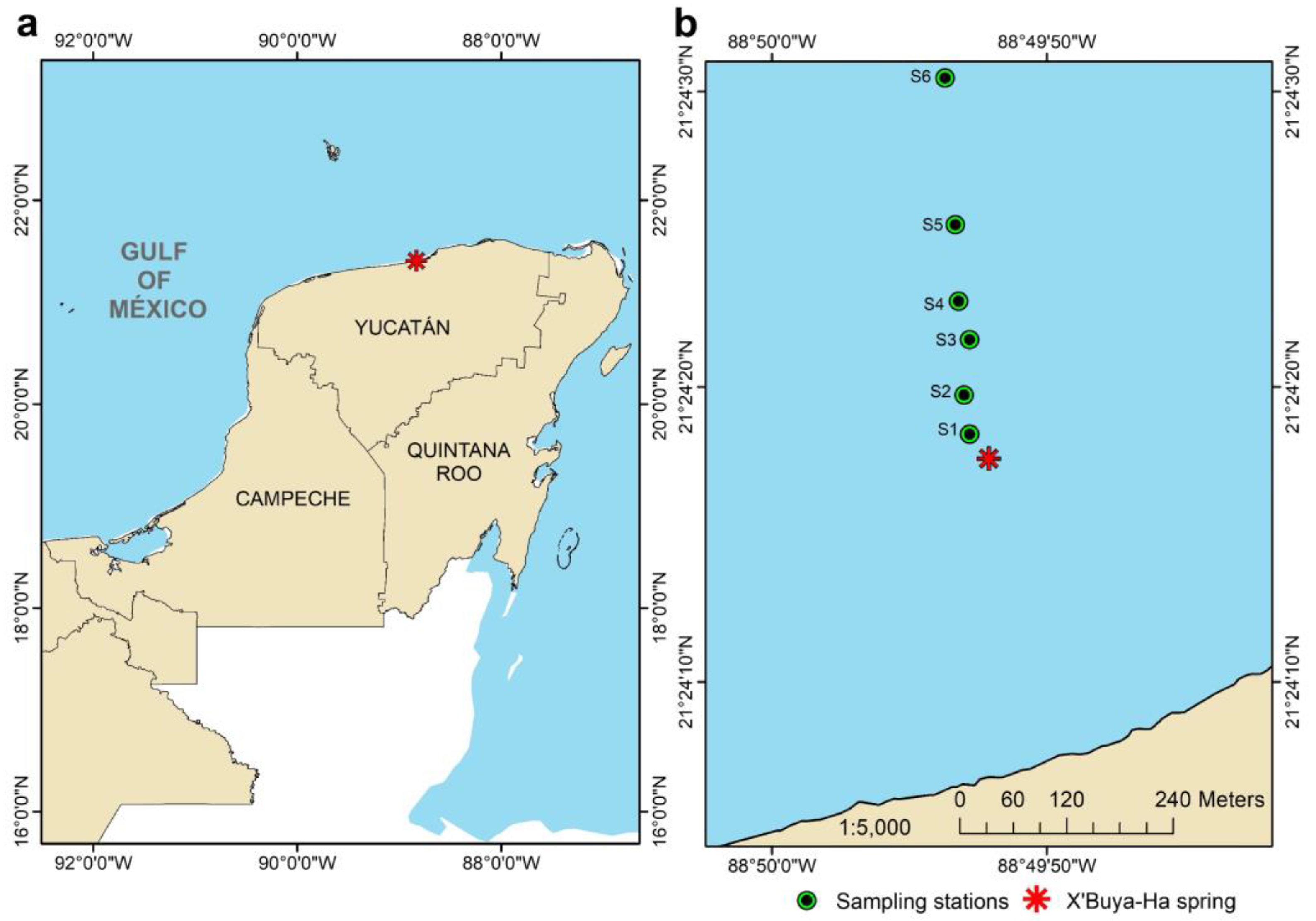
2.1. Site Description and Sampling
2.2. Environmental Characterization
2.3. DNA Extraction and 16S rRNA Gene Sequencing
2.4. Bioinformatic Analysis
3. Results
3.1. Environmental Characteristics
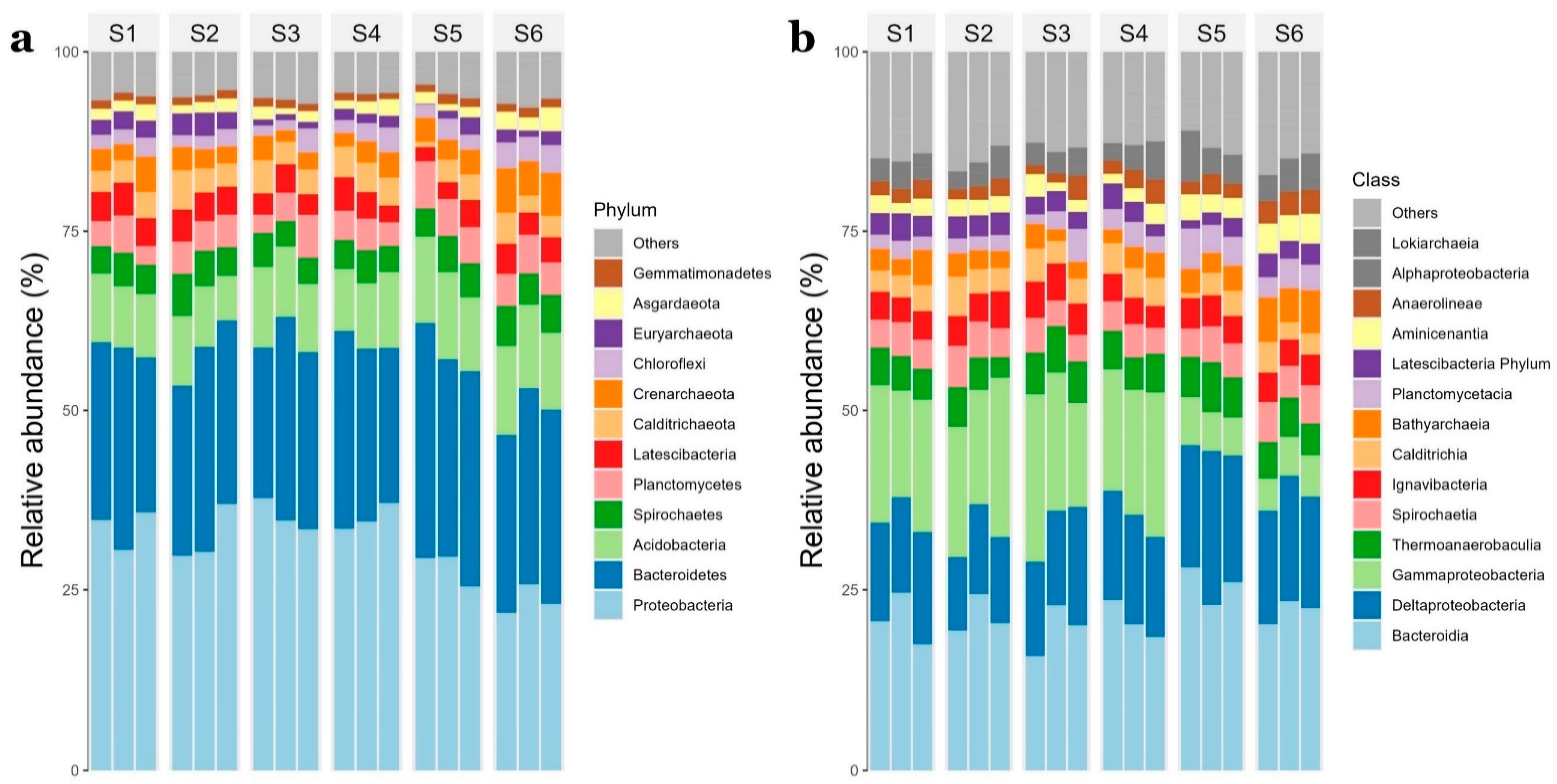
3.2. Summary of 16S rRNA Data, Microbial Diversity, and Microbial Community Composition
3.3. Comparison of Prokaryotic Community Structure
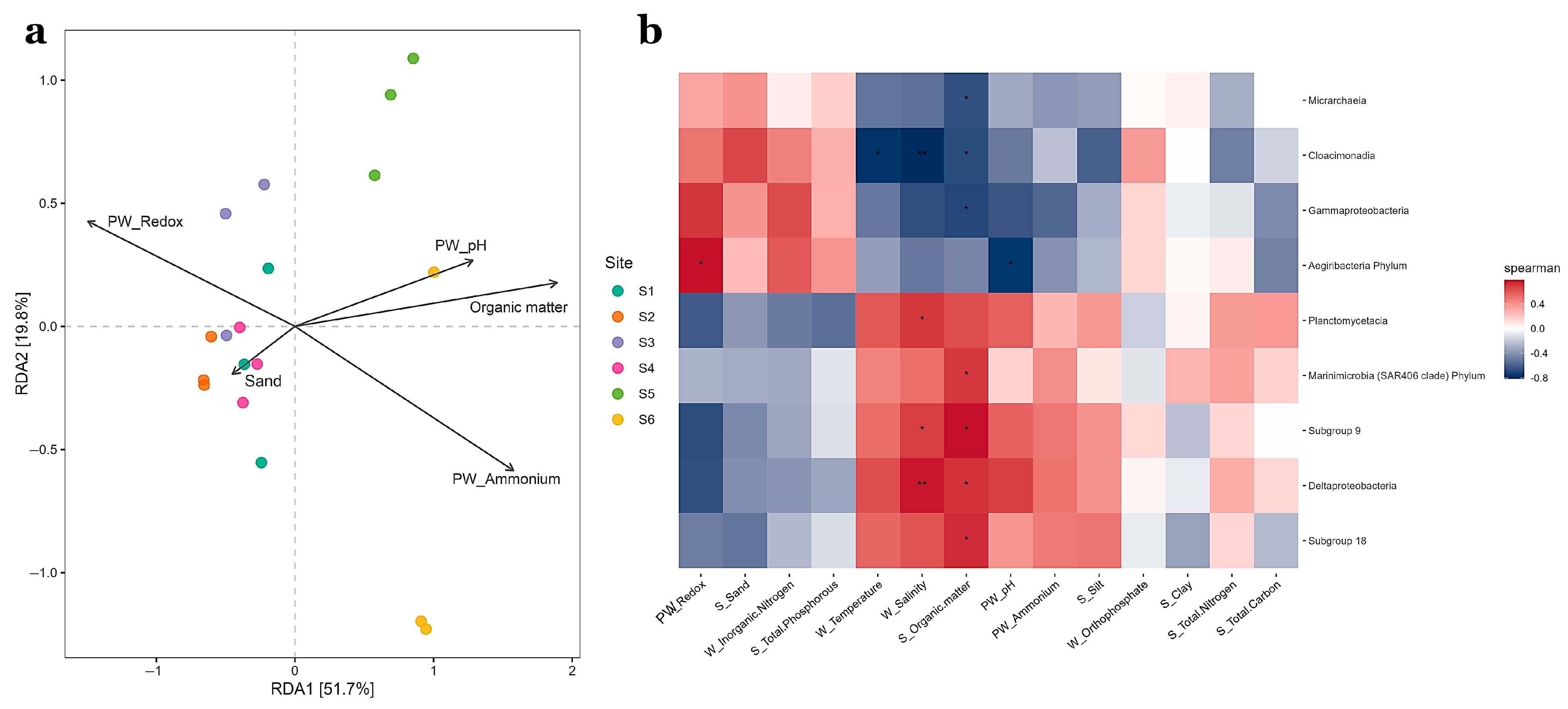
3.4. Effect of Abiotic Factors over Prokaryotic Community Structure
3.5. Predicted Functional Profiles
4. Discussion
4.1. Prokaryotic Core Community
4.2. Environmental Drivers and Comparison of Prokaryotic Community Structure
4.3. Functional Profiling
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reynolds, L.; Waycott, M.; McGlathery, K.; Orth, R. Ecosystem Services Returned through Seagrass Restoration: Restoration of Ecosystem Services. Restor. Ecol. 2016, 24, 583–588. [Google Scholar] [CrossRef]
- Nordlund, L.M.; Koch, E.W.; Barbier, E.B.; Creed, J.C. Seagrass Ecosystem Services and Their Variability across Genera and Geographical Regions. PLoS ONE 2016, 11, e0163091. [Google Scholar] [CrossRef] [PubMed]
- Cúcio, C.; Engelen, A.H.; Costa, R.; Muyzer, G. Rhizosphere Microbiomes of European + Seagrasses Are Selected by the Plant, But Are Not Species Specific. Front. Microbiol. 2016, 7, 440. [Google Scholar] [CrossRef]
- Fraser, M.W.; Gleeson, D.B.; Grierson, P.F.; Laverock, B.; Kendrick, G.A. Metagenomic Evidence of Microbial Community Responsiveness to Phosphorus and Salinity Gradients in Seagrass Sediments. Front. Microbiol. 2018, 9, 1703. [Google Scholar] [CrossRef]
- Seymour, J.R.; Laverock, B.; Nielsen, D.A.; Trevathan-Tackett, S.M.; Macreadie, P.I. The Microbiology of Seagrasses. In Seagrasses of Australia: Structure, Ecology and Conservation; Larkum, A.W.D., Kendrick, G.A., Ralph, P.J., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 343–392. [Google Scholar]
- Sogin, E.M.; Michellod, D.; Gruber-Vodicka, H.R.; Bourceau, P.; Geier, B.; Meier, D.V.; Seidel, M.; Ahmerkamp, S.; Schorn, S.; D’Angelo, G.; et al. Sugars Dominate the Seagrass Rhizosphere. Nat. Ecol. Evol. 2022, 6, 866–877. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, C.; Yu, S.; Jiang, Z.; Liu, S.; Wu, Y.; Huang, X. Rhizosphere Microbial Community Structure Is Selected by Habitat but Not Plant Species in Two Tropical Seagrass Beds. Front. Microbiol. 2020, 11, 161. [Google Scholar] [CrossRef]
- Apostolaki, E.; Holmer, M.; Santinelli, V.; Karakassis, I. Species-Specific Response to Sulfide Intrusion in Indigenous and Non-Indigenous Mediterranean Seagrasses under Stress. Mar. Environ. Res. 2017, 134, 85–95. [Google Scholar] [CrossRef]
- Christiaen, B.; McDonald, A.; Cebrian, J.; Ortmann, A.C. Response of the Microbial Community to Environmental Change during Seagrass Transplantation. Aquat. Bot. 2013, 109, 31–38. [Google Scholar] [CrossRef]
- Tarquinio, F.; Attlan, O.; Vanderklift, M.A.; Berry, O.; Bissett, A. Distinct Endophytic Bacterial Communities Inhabiting Seagrass Seeds. Front. Microbiol. 2021, 12, 703014. [Google Scholar] [CrossRef]
- Zou, D.; Liu, H.; Li, M. Community, Distribution, and Ecological Roles of Estuarine Archaea. Front. Microbiol. 2020, 11, 2060. [Google Scholar] [CrossRef]
- Cifuentes, A.; Antón, J.; Benlloch, S.; Donnelly, A.; Herbert, R.A.; Rodríguez-Valera, F. Prokaryotic Diversity in Zostera Noltii-Colonized Marine Sediments. Appl. Environ. Microbiol. 2000, 66, 1715–1719. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhang, H.; Song, Z.; Huang, Y.; Hu, X. Seasonal Dynamics of Bathyarchaeota-Dominated Benthic Archaeal Communities Associated with Seagrass (Zostera Japonica) Meadows. J. Mar. Sci. Eng. 2021, 9, 1304. [Google Scholar] [CrossRef]
- Evans, P.N.; Parks, D.H.; Chadwick, G.L.; Robbins, S.J.; Orphan, V.J.; Golding, S.D.; Tyson, G.W. Methane Metabolism in the Archaeal Phylum Bathyarchaeota Revealed by Genome-Centric Metagenomics. Science 2015, 350, 434–438. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, M.; Perumal, V.; Feng, X.; Fang, J.; Xie, J.; Sievert, S.M.; Wang, F. Genomic and Enzymatic Evidence for Acetogenesis among Multiple Lineages of the Archaeal Phylum Bathyarchaeota Widespread in Marine Sediments. Nat. Microbiol. 2016, 1, 1–9. [Google Scholar] [CrossRef]
- Conte, C.; Rotini, A.; Manfra, L.; D’Andrea, M.M.; Winters, G.; Migliore, L. The Seagrass Holobiont: What We Know and What We Still Need to Disclose for Its Possible Use as an Ecological Indicator. Water 2021, 13, 406. [Google Scholar] [CrossRef]
- Jiang, Z.; Huang, X.; Zhang, J. Effect of Nitrate Enrichment and Salinity Reduction on the Seagrass Thalassia Hemprichii Previously Grown in Low Light. J. Exp. Mar. Biol. Ecol. 2013, 443, 114–122. [Google Scholar] [CrossRef]
- Leoni, V.; Vela, A.; Pasqualini, V.; Pergent-Martini, C.; Pergent, G. Effects of Experimental Reduction of Light and Nutrient Enrichments (N and P) on Seagrasses: A Review. Aquat. Conserv. Mar. Freshw. Ecosyst. 2008, 18, 202–220. [Google Scholar] [CrossRef]
- Vogel, M.A.; Mason, O.U.; Miller, T.E. Composition of Seagrass Phyllosphere Microbial Communities Suggests Rapid Environmental Regulation of Community Structure. FEMS Microbiol. Ecol. 2021, 97, 3. [Google Scholar] [CrossRef]
- Björk, M.; Short, F.; Mcleod, E.; Beer, S. Managing Seagrasses for Resilience to Climate Change; IUCN: Gland, Switzerland, 2008. [Google Scholar]
- Stockbridge, J.; Jones, A.R.; Gillanders, B.M. A Meta-Analysis of Multiple Stressors on Seagrasses in the Context of Marine Spatial Cumulative Impacts Assessment. Sci. Rep. 2020, 10, 11934. [Google Scholar] [CrossRef]
- Waycott, M.; Collier, C.; Mcmahon, K.; Ralph, P.; McKenzie, L.; Udy, J.; Grech, A. Vulnerability of Seagrasses in the Great Barrier Reef to Climate Change; ECU Publications: Queensland, Australia, 2007; pp. 193–235. [Google Scholar]
- Jiang, Z.; Li, L.; Fang, Y.; Lin, J.; Liu, S.; Wu, Y.; Huang, X. Eutrophication Reduced the Release of Dissolved Organic Carbon from Tropical Seagrass Roots through Exudation and Decomposition. Mar. Environ. Res. 2022, 179, 105–703. [Google Scholar] [CrossRef]
- Martin, B.C.; Gleeson, D.; Statton, J.; Siebers, A.R.; Grierson, P.; Ryan, M.H.; Kendrick, G.A. Low Light Availability Alters Root Exudation and Reduces Putative Beneficial Microorganisms in Seagrass Roots. Front. Microbiol. 2018, 8, 2667. [Google Scholar] [CrossRef] [PubMed]
- Vogel, M.A.; Mason, O.U.; Miller, T.E. Host and Environmental Determinants of Microbial Community Structure in the Marine Phyllosphere. PLoS ONE 2020, 15, e0235441. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, C.L.; Voerman, S.E.; Lang, J.M.; Stachowicz, J.J.; Eisen, J.A. Microbial Communities in Sediment from Zostera Marina Patches, but Not the Z. Marina Leaf or Root Microbiomes, Vary in Relation to Distance from Patch Edge. PeerJ 2017, 5, e3246. [Google Scholar] [CrossRef] [PubMed]
- Ugarelli, K.; Laas, P.; Stingl, U. The Microbial Communities of Leaves and Roots Associated with Turtle Grass (Thalassia Testudinum) and Manatee Grass (Syringodium Filliforme) Are Distinct from Seawater and Sediment Communities, but Are Similar between Species and Sampling Sites. Microorganisms 2019, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.; Simioni, C.; Schmidt, É.C.; Ramlov, F.; Maraschin, M.; Bouzon, Z.L. The Influence of Salinity on Growth, Morphology, Leaf Ultrastructure, and Cell Viability of the Seagrass Halodule Wrightii Ascherson. Protoplasma 2017, 254, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Darnell, K.M.; Furman, B.T.; Heck, K.L., Jr.; Byron, D.; Reynolds, L.; Dunton, K.H. Seed Reserve Hot Spots for the Sub-Tropical Seagrass Halodule Wrightii (Shoal Grass) in the Northern Gulf of Mexico. Estuaries Coasts 2021, 44, 339–351. [Google Scholar] [CrossRef]
- Kantún-Manzano, C.; Silveira, J.; Flor, A.-C. Influence of Coastal Submarine Groundwater Discharges on Seagrass Communities in a Subtropical Karstic Environment. Bull. Environ. Contam. Toxicol. 2018, 100, 176–183. [Google Scholar] [CrossRef]
- Kantun Manzano, C.; Arcega-Cabrera, F.; Derrien, M.; Noreña-Barroso, E.; Herrera-Silveira, J. Submerged Groundwater Discharges as Source of Fecal Material in Protected Karstic Coastal Areas. Geofluids 2018, 1, 11. [Google Scholar] [CrossRef]
- ArandaCirerol, N.; Herrera-Silveira, J.A.; Comín, F.A. Nutrient Water Quality in a Tropical Coastal Zone with Groundwater Discharge, Northwest Yucatán, Mexico. Estuar. Coast. Shelf Sci. 2006, 68, 445–454. [Google Scholar] [CrossRef]
- Pacheco-Castro, R.; Salles, P.; Canul-Macario, C.; Paladio-Hernandez, A. On the Understanding of the Hydrodynamics and the Causes of Saltwater Intrusion on Lagoon Tidal Springs. Water 2021, 13, 3431. [Google Scholar] [CrossRef]
- Valle-Levinson, A.; Mariño-Tapia, I.; Enriquez, C.; Waterhouse, A.F. Tidal Variability of Salinity and Velocity Fields Related to Intense Point-Source Submarine Groundwater Discharges into the Coastal Ocean. Limnol. Oceanogr. 2011, 56, 1213–1224. [Google Scholar] [CrossRef]
- Edwards, J.; Santos-Medellín, C.; Sundaresan, V. Extraction and 16S RRNA Sequence Analysis of Microbiomes Associated with Rice Roots. Bio-protocol 2018, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, D.S.; Lebeis, S.L.; Paredes, S.H.; Yourstone, S.; Gehring, J.; Malfatti, S.; Tremblay, J.; Engelbrektson, A.; Kunin, V.; del Rio, T.G.; et al. Defining the Core Arabidopsis Thaliana Root Microbiome. Nature 2012, 488, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Parsons, T.R.; Maita, Y.; Lalli, C.M. A Manual of Chemical and Biological Methods for Seawater Analysis; Elsevier Science & Technology Books: Amsterdam, The Netherlands, 1984. [Google Scholar]
- Bouyoucos, G.J. The hydrometer as a new method for the mechanical analysis of soils. Soil Sci. 1927, 23, 343–354. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall, Inc.: Englewood Cliffs, NJ, USA, 1964; pp. 39–40. [Google Scholar]
- Aspila, K.I.; Agemian, H.; Chau, A.S.Y. A Semi-Automated Method for the Determination of Inorganic, Organic and Total Phosphate in Sediments. Analyst 1976, 101, 187–197. [Google Scholar] [CrossRef]
- Parada, A.E.; Needham, D.M.; Fuhrman, J.A. Every Base Matters: Assessing Small Subunit RRNA Primers for Marine Microbiomes with Mock Communities, Time Series and Global Field Samples. Environ. Microbiol. 2016, 18, 1403–1414. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.; O’Hara, R.; Simpson, G.; Solymos, P.; et al. Package ‘Vegan’. Community Ecology Package, Version 2; R Core Team: Vienna, Austria, 2013; Volume 2, pp. 1–295. [Google Scholar]
- Barnett, D.J.; Arts, I.C.; Penders, J. MicroViz: An R Package for Microbiome Data Visualization and Statistics. J. Open Source Softw. 2021, 6, 3201. [Google Scholar] [CrossRef]
- Xu, S.; Yu, G. Microbiota Process: A Comprehensive R Package for Managing and Analyzing Microbiome and Other Ecological Data within the Tidy Framework. R Package Version 1.8.1. Available online: https://github.com/YuLab-SMU/MicrobiotaProcess (accessed on 27 July 2022).
- Liu, C.; Cui, Y.; Li, X.; Yao, M. Microeco: An R Package for Data Mining in Microbial Community Ecology. FEMS Microbiol. Ecol. 2021, 97, 2. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Andrew, D.R.; Fitak, R.R.; Munguia-Vega, A.; Racolta, A.; Martinson, V.G.; Dontsova, K. Abiotic Factors Shape Microbial Diversity in Sonoran Desert Soils. Appl. Environ. Microbiol. 2012, 78, 7527–7537. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic Biomarker Discovery and Explanation. Genome Biol. 2011, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Aßhauer, K.P.; Wemheuer, B.; Daniel, R.; Meinicke, P. Tax4Fun: Predicting Functional Profiles from Metagenomic 16S RRNA Data. Bioinformatics 2015, 31, 2882–2884. [Google Scholar] [CrossRef] [PubMed]
- Louca, S.; Parfrey, L.W.; Doebeli, M. Decoupling Function and Taxonomy in the Global Ocean Microbiome. Science 2016, 353, 1272–1277. [Google Scholar] [CrossRef]
- Sun, Y.; Song, Z.; Zhang, H.; Liu, P.; Hu, X. Seagrass Vegetation Affect the Vertical Organization of Microbial Communities in Sediment. Mar. Environ. Res. 2020, 162, 105–174. [Google Scholar] [CrossRef] [PubMed]
- Dyksma, S.; Lenk, S.; Sawicka, J.E.; Mußmann, M. Uncultured Gammaproteobacteria and Desulfobacteraceae Account for Major Acetate Assimilation in a Coastal Marine Sediment. Front. Microbiol. 2018, 9, 3124. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; English, M.K.; Tomas, F.; Mueller, R.S. Recovery and Community Succession of the Zostera Marina Rhizobiome after Transplantation. Appl. Environ. Microbiol. 2021, 87, 3. [Google Scholar] [CrossRef] [PubMed]
- Pelikan, C.; Jaussi, M.; Wasmund, K.; Seidenkrantz, M.-S.; Pearce, C.; Kuzyk, Z.Z.A.; Herbold, C.W.; Røy, H.; Kjeldsen, K.U.; Loy, A. Glacial Runoff Promotes Deep Burial of Sulfur Cycling-Associated Microorganisms in Marine Sediments. Front. Microbiol. 2019, 10, 2558. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Wang, R.; Wang, H.; Gong, L.; Man, B.; Xu, Y. Distribution of Bathyarchaeota Communities Across Different Terrestrial Settings and Their Potential Ecological Functions. Sci. Rep. 2017, 7, 45028. [Google Scholar] [CrossRef]
- Zou, D.; Pan, J.; Liu, Z.; Zhang, C.; Liu, H.; Li, M. The Distribution of Bathyarchaeota in Surface Sediments of the Pearl River Estuary along Salinity Gradient. Front. Microbiol. 2020, 11, 285. [Google Scholar] [CrossRef]
- Sun, F.; Zhang, X.; Zhang, Q.; Liu, F.; Zhang, J.; Gong, J. Seagrass (Zostera Marina) Colonization Promotes the Accumulation of Diazotrophic Bacteria and Alters the Relative Abundances of Specific Bacterial Lineages Involved in Benthic Carbon and Sulfur Cycling. Appl. Environ. Microbiol. 2015, 81, 6901–6914. [Google Scholar] [CrossRef]
- Zhou, W.; Ding, D.; Yang, Q.; Ling, J.; Ahmad, M.; Lin, X.; Lin, L.; Zhang, Y.; Dong, J. Diversity and Abundance of Diazotrophic Communities of Seagrass Halophila Ovalis Based on Genomic and Transcript Level in Daya Bay, South China Sea. Arch. Microbiol. 2021, 203, 5577–5589. [Google Scholar] [CrossRef] [PubMed]
- Caffrey, J.M.; Bonaglia, S.; Conley, D.J. Short Exposure to Oxygen and Sulfide Alter Nitrification, Denitrification, and DNRA Activity in Seasonally Hypoxic Estuarine Sediments. FEMS Microbiol. Lett. 2019, 366, 1. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, T.L.; Bovee, R.J.; Sattin, S.R.; Mohr, W.; Gilhooly, W.P.; Lyons, T.W.; Pearson, A.; Macalady, J.L. Carbon and Sulfur Cycling below the Chemocline in a Meromictic Lake and the Identification of a Novel Taxonomic Lineage in the FCB Superphylum, Candidatus Aegiribacteria. Front. Microbiol. 2016, 7, 598. [Google Scholar] [CrossRef] [PubMed]
- Danovaro, R.; Molari, M.; Corinaldesi, C.; Dell’Anno, A. Macroecological Drivers of Archaea and Bacteria in Benthic Deep-Sea Ecosystems. Sci. Adv. 2016, 2, 4. [Google Scholar] [CrossRef]
- Munn, C.B. Marine Microbiology: Ecology & Applications, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2020; pp. 373–394. [Google Scholar]
- Wiegand, S.; Rast, P.; Kallscheuer, N.; Jogler, M.; Heuer, A.; Boedeker, C.; Jeske, O.; Kohn, T.; Vollmers, J.; Kaster, A.-K.; et al. Analysis of Bacterial Communities on North Sea Macroalgae and Characterization of the Isolated Planctomycetes Adhaeretor Mobilis Gen. Nov., Sp. Nov., Roseimaritima Multifibrata Sp. Nov., Rosistilla Ulvae Sp. Nov. and Rubripirellula Lacrimiformis Sp. Nov. Microorganisms 2021, 9, 1494. [Google Scholar] [CrossRef]
- Zhou, W.; Dong, J.; Ding, D.; Long, L.; Suo, A.; Lin, X.; Yang, Q.; Lin, L.; Zhang, Y.; Ling, J. Rhizosphere Microbiome Dynamics in Tropical Seagrass under Short-Term Inorganic Nitrogen Fertilization. Environ. Sci. Pollut. Res. 2021, 28, 19021–19033. [Google Scholar] [CrossRef]
- Krause, J.R.; Hinojosa-Corona, A.; Gray, A.B.; Herguera, J.C.; McDonnell, J.; Schaefer, M.V.; Ying, S.C.; Watson, E.B. Beyond Habitat Boundaries: Organic Matter Cycling Requires a System-Wide Approach for Accurate Blue Carbon Accounting. Limnol. Oceanogr. 2022, 67, S6–S18. [Google Scholar] [CrossRef]
- Krause-Jensen, D.; Carstensen, J.; Nielsen, S.; Dalsgaard, T.; Christensen, P.; Fossing, H.; Rasmussen, M. Sea Bottom Characteristics Affect Depth Limits of Eelgrass Zostera Marina L. Mar. Ecol. Prog. Ser. 2011, 425, 91–102. [Google Scholar] [CrossRef]
- Karmakar, R. State of the Art of Bacterial Chemotaxis. J. Basic Microbiol. 2021, 61, 366–379. [Google Scholar] [CrossRef]
- Kavamura, V.N.; Hayat, R.; Clark, I.M.; Rossmann, M.; Mendes, R.; Hirsch, P.R.; Mauchline, T.H. Inorganic Nitrogen Application Affects Both Taxonomical and Predicted Functional Structure of Wheat Rhizosphere Bacterial Communities. Front. Microbiol. 2018, 9, 1074. [Google Scholar] [CrossRef]
- Igamberdiev, A.U.; Kleczkowski, L.A. The Glycerate and Phosphorylated Pathways of Serine Synthesis in Plants: The Branches of Plant Glycolysis Linking Carbon and Nitrogen Metabolism. Front. Plant Sci. 2018, 9, 318. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Sun, F.; Zhao, H.; Mi, H.; He, S.; Chen, Y.; Liu, Y.; Lan, H.; Zhang, M.; Wang, Z. Compositional Changes of Sedimentary Microbes in the Yangtze River Estuary and Their Roles in the Biochemical Cycle. Sci. Total Environ. 2021, 760, 143–383. [Google Scholar] [CrossRef] [PubMed]




| Water | Porewater | ||||||
|---|---|---|---|---|---|---|---|
| Site | Salinity (PSU) | Temperature (°C) | Ninorg (µM) | Orthophosphate (µM) | pH | ORP (mV) | Ammonium (µM) |
| S1 | 30.83 ± 0.13 | 26.32 ± 1.02 | 14.26 ± 0.47 | 0.5 ± 0.033 | 7.07 ± 0.20 | −54.03 ± 7.82 | 17.23 ± 0.61 |
| S2 | 29.2 ± 1.05 | 25.7 ± 0.94 | 7.02 ± 0.1 | 0.2 ± 0.031 | 7.17 ± 0.06 | −56.67 ± 2.5 | 11.92 ± 0.60 |
| S3 | 31.4 ± 0.40 | 27.23 ± 0.45 | 9.29 ± 0.11 | 0.22 ± 0.015 | 7.13 ± 0.07 | −55.13 ± 3.52 | 10.52 ± 1.27 |
| S4 | 33.57 ± 0.03 | 28.34 ± 0.04 | 11.19 ± 0.53 | 0.17 ± 0.17 | 7.14 ± 0.09 | −55.6 ± 4.75 | 12.71 ± 2.16 |
| S5 | 33.62 ± 0.34 | 27.93 ± 0.34 | 4.86 ± 0.44 | 0.26 ± 0.04 | 7.41 ± 0.04 | −69.53 ± 1.66 | 15.48 ± 0.85 |
| S6 | 34.46 ± 0.18 | 29.02 ± 0.02 | 2.63 ± 0.24 | 0.08 ± 0.01 | 7.34 ± 0.09 | −65.4 ± 6.09 | 27.5 ± 0.71 |
| Sediment | |||||||
|---|---|---|---|---|---|---|---|
| Site | O.M (%) | Sand (%) | Silt (%) | Clay (%) | TP (µmol g−1) | TN (%) | TC (%) |
| S1 | 1.75 ± 0.21 | 93.42 ± 1.2 | 3.95 ± 1.26 | 2.63 ± 0.08 | 7.27 ± 0.23 | 0.36 ± 0.075 | 12.03 ± 1.35 |
| S2 | 1.34 ± 0.15 | 94.93 ± 0.06 | 1.12 ± 0.11 | 3.95 ± 1.17 | 6.56 ± 0.92 | 0.28 ± 0.05 | 13.44 ± 1.71 |
| S3 | 1.84 ± 0.1 | 94.33 ± 1.03 | 2.15 ± 0.18 | 3.52 ± 1.15 | 6.77 ± 0.59 | 1.57 ± 0.37 | 12.15 ± 1.43 |
| S4 | 1.75 ± 0.09 | 89.1 ± 0.82 | 7.03 ± 0.85 | 3.87 ± ±0.03 | 6.88 ± 0.45 | 1.38 ± 0.39 | 12.56 ± 2.31 |
| S5 | 2.27 ± 0.06 | 92.15 ± 1.98 | 4.58 ± 0.31 | 3.27 ± 1.18 | 6.78 ± 0.12 | 1.03 ± 0.18 | 13.31 ± 1.77 |
| S6 | 2.33 ± 0.1 | 87.83 ± 1.66 | 8.48 ± 2.03 | 3.68 ± 1.42 | 6.41 ± 0.11 | 1.12 ± 0.12 | 14.16 ± 1.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de la Garza Varela, A.; Aguirre-Macedo, M.L.; García-Maldonado, J.Q. Changes in the Rhizosphere Prokaryotic Community Structure of Halodule wrightii Monospecific Stands Associated to Submarine Groundwater Discharges in a Karstic Costal Area. Microorganisms 2023, 11, 494. https://doi.org/10.3390/microorganisms11020494
de la Garza Varela A, Aguirre-Macedo ML, García-Maldonado JQ. Changes in the Rhizosphere Prokaryotic Community Structure of Halodule wrightii Monospecific Stands Associated to Submarine Groundwater Discharges in a Karstic Costal Area. Microorganisms. 2023; 11(2):494. https://doi.org/10.3390/microorganisms11020494
Chicago/Turabian Stylede la Garza Varela, Alonso, M. Leopoldina Aguirre-Macedo, and José Q. García-Maldonado. 2023. "Changes in the Rhizosphere Prokaryotic Community Structure of Halodule wrightii Monospecific Stands Associated to Submarine Groundwater Discharges in a Karstic Costal Area" Microorganisms 11, no. 2: 494. https://doi.org/10.3390/microorganisms11020494
APA Stylede la Garza Varela, A., Aguirre-Macedo, M. L., & García-Maldonado, J. Q. (2023). Changes in the Rhizosphere Prokaryotic Community Structure of Halodule wrightii Monospecific Stands Associated to Submarine Groundwater Discharges in a Karstic Costal Area. Microorganisms, 11(2), 494. https://doi.org/10.3390/microorganisms11020494







