Short Course of Antibiotic Therapy for Gram-Negative Bacilli Bacteremia in Patients with Cancer and Hematopoietic Stem Cell Transplantation: Less Is Possible
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting, Patients, and Study Design
2.2. Definitions
2.3. Microbiological Studies
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuderer, N.M.; Dale, D.C.; Crawford, J.; Cosler, L.E.; Lyman, G.H. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 2006, 106, 2258–2266. [Google Scholar] [CrossRef] [PubMed]
- Bos, M.M.; Smeets, L.S.; Dumay, I.; de Jonge, E. Bloodstream infections in patients with or without cancer in a large community hospital. Infection 2013, 41, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Dandoy, C.E.; Ardura, M.I.; Papanicolaou, G.A.; Auletta, J.J. Bacterial bloodstream infections in the allogeneic hematopoietic cell transplant patient: New considerations for a persistent nemesis. Bone Marrow Transpl. 2017, 52, 1091–1106. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, A.; Malfuson, J.V.; Sanmartin, N.; Konopacki, J.; MacNab, C.; Souleau, B.; de Revel, T.; Elouennass, M.; Samson, T.; Soler, C.; et al. An 8-year survey of strains identified in blood cultures in a clinical haematology unit. Clin. Microbiol. Infect. 2014, 20, O7–O12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trecarichi, E.M.; Pagano, L.; Candoni, A.; Pastore, D.; Cattaneo, C.; Fanci, R.; Nosari, A.; Caira, M.; Spadea, A.; Busca, A.; et al. HeMABIS Registry—SEIFEM Group, Italy. Current epidemiology and antimicrobial resistance data for bacterial bloodstream infections in patients with hematologic malignancies: An Italian multicentre prospective survey. Clin. Microbiol. Infect. 2015, 21, 337–343. [Google Scholar] [CrossRef] [Green Version]
- Averbuch, D.; Tridello, G.; Hoek, J.; Mikulska, M.; Akan, H.; Yanez San Segundo, L.; Pabst, T.; Özçelik, T.; Klyasova, G.; Donnini, I.; et al. Antimicrobial Resistance in Gram-Negative Rods Causing Bacteremia in Hematopoietic Stem Cell Transplant Recipients: Intercontinental Prospective Study of the Infectious Diseases Working Party of the European Bone Marrow Transplantation Group. Clin. Infect. Dis. 2017, 65, 1819–1828. [Google Scholar] [CrossRef] [PubMed]
- Trecarichi, E.M.; Pagano, L.; Martino, B.; Candoni, A.; Di Blasi, R.; Nadali, G.; Fianchi, L.; Delia, M.; Sica, S.; Perriello, V.; et al. Haematologic Malignancies Associated Bloodstream Infections Surveillance (HEMABIS) registry-Sorveglianza Epidemiologica Infezioni Funginein Emopatie Maligne (SEIFEM) group, Italy. Bloodstream infections caused by Klebsiella pneumoniae in onco-hematological patients: Clinical impact of carbapenem resistance in a multicentre prospective survey. Am. J. Hematol. 2016, 91, 1076–1081. [Google Scholar] [CrossRef]
- Bush, K.; Bradford, P.A. Epidemiology of β-Lactamase-Producing Pathogens. Clin. Microbiol. Rev. 2020, 33, e00047-19. [Google Scholar] [CrossRef]
- Gao, H.; Liu, Y.; Wang, R.; Wang, Q.; Jin, L.; Wang, H. The transferability and evolution of NDM-1 and KPC-2 co-producing Klebsiella pneumoniae from clinical settings. EBioMedicine 2020, 51, 102599. [Google Scholar] [CrossRef] [Green Version]
- Han, R.; Shi, Q.; Wu, S.; Yin, D.; Peng, M.; Dong, D.; Zheng, Y.; Guo, Y.; Zhang, R.; Hu, F. China Antimicrobial Surveillance Network (CHINET) Study Group. Dissemination of Carbapenemases (KPC, NDM, OXA-48, IMP, and VIM) Among Carbapenem-Resistant Enterobacteriaceae Isolated From Adult and Children Patients in China. Front. Cell. Infect. Microbiol. 2020, 10, 314. [Google Scholar] [CrossRef]
- Paprocka, P.; Durnaś, B.; Mańkowska, A.; Król, G.; Wollny, T.; Bucki, R. Pseudomonas aeruginosa Infections in Cancer Patients. Pathogens 2022, 11, 679. [Google Scholar] [CrossRef] [PubMed]
- Royo-Cebrecos, C.; Laporte-Amargós, J.; Peña, M.; Ruiz-Camps, I.; Puerta-Alcalde, P.; Abdala, E.; Oltolini, C.; Akova, M.; Montejo, M.; Mikulska, M. Pseudomonas aeruginosa Bloodstream Infections in Patients with Cancer: Differences between Patients with Hematological Malignancies and Solid Tumors. Pathogens 2022, 11, 1132. [Google Scholar] [CrossRef] [PubMed]
- Herrera, F.; Laborde, A.; Jordán, R.; Berruezo, L.; Roccia Rossi, I.; Valledor, A.; Lambert, S.; Pereyra, M.; Nenna, A.; Dictar, M.; et al. Current Epidemiology of Bacteremia in Patients with Hematological Malignancies and Hematopoietic Stem Cell Transplantation and the Impact of Antibiotic Resistance on Survival. In Proceedings of the 31st of European Congress of Clinical Microbiology & Infectious Diseases (ECCMID), Vienna, Austria, 9–12 July 2021. [Google Scholar]
- Costantini, P.; Torres, D.; Dictar, M.; Nenna, A.; Valledor, A.; Jordán, R.; Laborde, A.; Lambert, S.; Benso, J.; Carena, A.; et al. Bacteremia in patients with solid tumors: Epidemiology, clinical features and risk factors for mortality. Results from a multicenter study in Argentina. Open Forum Infect. Dis. 2021, 8 (Suppl. S1), S565. [Google Scholar] [CrossRef]
- Yahav, D.; Shepshelovich, D.; Tau, N. Cost Analysis of New Antibiotics to Treat Multidrug-Resistant Bacterial Infections: Mind the Gap. Infect. Dis. Ther. 2021, 10, 621–630. [Google Scholar] [CrossRef]
- Tamma, P.D.; Avdic, E.; Li, D.X.; Dzintars, K.; Cosgrove, S.E. Association of Adverse Events with Antibiotic Use in Hospitalized Patients. JAMA Intern. Med. 2017, 177, 1308–1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schechner, V.; Fallach, N.; Braun, T.; Temkin, E.; Carmeli, Y. Antibiotic exposure and the risk of hospital-acquired diarrhoea and Clostridioides difficile infection: A cohort study. J. Antimicrob. Chemother. 2021, 76, 2182–2185. [Google Scholar] [CrossRef] [PubMed]
- Teshome, B.F.; Vouri, S.M.; Hampton, N.; Kollef, M.H.; Micek, S.T. Duration of Exposure to Antipseudomonal β-Lactam Antibiotics in the Critically Ill and Development of New Resistance. Pharmacotherapy 2019, 39, 261–270. [Google Scholar] [CrossRef]
- Yahav, D.; Paul, M.; Van Nieuwkoop, C.; Huttner, A. Is shorter always better? The pros and cons of treating Gram-negative bloodstream infections with 7 days of antibiotics. JAC Antimicrobal Resist. 2022, 4, dlac058. [Google Scholar] [CrossRef]
- Amjad, W.; Qureshi, W.; Malik, A.; Singh, R.; Jafri, S.M. The outcomes of Clostridioides difficile infection in inpatient liver transplant population. Transpl. Infect. Dis. 2022, 24, e13750. [Google Scholar] [CrossRef]
- Obeid, K.M.; Sapkota, S.; Cao, Q.; Richmond, S.; Watson, A.P.; Karadag, F.K.; Young, J.H.; Pruett, T.; Weisdorf, D.J.; Ustun, C.; et al. Early Clostridioides difficile infection characterizations, risks, and outcomes in allogeneic hematopoietic stem cell and solid organ transplant recipients. Transpl. Infect. Dis. 2022, 24, e13720. [Google Scholar] [CrossRef]
- Herrera, F.; Laborde, A.; Baldoni, N.; Jordán, R.; Roccia Rossi, I.; Valledor, A.; Costantini, P.; Dictar, M.; Nenna, A.; Caeiro, J.; et al. Risk factors for carbapenem-resistant Enterobacteriaceae Bacteremia in cancer patients: Results from ROCAS Study. In Proceedings of the 29th of European Congress of Clinical Microbiology & Infectious Diseases (ECCMID), Amsterdam, The Netherlands, 13–16 April 2019. [Google Scholar]
- Contejean, A.; Abbara, S.; Chentouh, R.; Alviset, S.; Grignano, E.; Gastli, N.Ç.; Casetta, A.; Willems, L.; Canouï, E.; Charlier, C.; et al. Antimicrobial stewardship in high-risk febrile neutropenia patients. Antimicrob. Resist. Infect. Control. 2022, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Yahav, D.; Franceschini, E.; Koppel, F.; Turjeman, A.; Babich, T.; Bitterman, R.; Neuberger, A.; Ghanem-Zoubi, N.; Santoro, A.; Eliakim-Raz, N.; et al. Bacteremia Duration Study Group. Seven Versus 14 Days of Antibiotic Therapy for Uncomplicated Gram-negative Bacteremia: A Noninferiority Randomized Controlled Trial. Clin. Infect. Dis. 2019, 69, 1091–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Dach, E.; Albrich, W.C.; Brunel, A.S.; Prendki, V.; Cuvelier, C.; Flury, D.; Gayet-Ageron, A.; Huttner, B.; Kohler, P.; Lemmenmeier, E.; et al. Effect of C-Reactive Protein-Guided Antibiotic Treatment Duration, 7-Day Treatment, or 14-Day Treatment on 30-Day Clinical Failure Rate in Patients With Uncomplicated Gram-Negative Bacteremia: A Randomized Clinical Trial. JAMA 2020, 323, 2160–2169. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.; Montero-Mateos, E.; Praena-Segovia, J.; León-Jiménez, E.; Natera, C.; López-Cortés, L.E.; Valiente, L.; Rosso-Fernández, C.M.; Herrero, M.; Aller-García, A.I.; et al. Seven-versus 14-day course of antibiotics for the treatment of bloodstream infections by Enterobacterales: A randomized, controlled trial. Clin. Microbiol. Infect. 2022, 28, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Imlay, H.; Laundy, N.C.; Forrest, G.N.; Slavin, M.A. Shorter antibiotic courses in the immunocompromised: The impossible dream? Clin. Microbiol. Infect. 2023, 29, 143–149. [Google Scholar] [CrossRef]
- Herrera, F.; Torres, D.; Laborde, A.; Berruezo, L.; Jordán, R.; Roccia Rossi, I.; Valledor, A.; Costantini, P.; Dictar, M.; Nenna, A.; et al. Development of a Clinical Score to Stratify the Risk for Carbapenem-resistant Enterobacterales Bacteremia in Patients with Cancer and Hematopoietic Stem Cell Transplantation. Antibiotics 2023, 12, 226. [Google Scholar] [CrossRef]
- Averbuch, D.; Cordonnier, C.; Livermore, D.M.; Mikulska, M.; Orasch, C.; Viscoli, C.; Gyssens, I.C.; Kern, W.; Klyasova, G.; Marchetti, O.; et al. ECIL4, a joint venture of EBMT, EORTC, ICHS, ESGICH/ESCMID and ELN. Targeted therapy against multi-resistant bacteria in leukemic and hematopoietic stem cell transplant recipients: Guidelines of the 4th European Conference on Infections in Leukemia (ECIL-4, 2011). Haematologica 2013, 98, 1836–1847. [Google Scholar] [CrossRef] [Green Version]
- Freifeld, A.G.; Bow, E.J.; Sepkowitz, K.A.; Boeckh, M.J.; Ito, J.I.; Mullen, C.A.; Raad, I.I.; Rolston, K.V.; Young, J.A.; Wingard, J.R. Infectious Diseases Society of America. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2011, 52, e56–e93. [Google Scholar] [CrossRef] [Green Version]
- Friedman, N.D.; Kaye, K.S.; Stout, J.E.; McGarry, S.A.; Trivette, S.L.; Briggs, J.P.; Lamm, W.; Clark, C.; MacFarquhar, J.; Walton, A.L.; et al. Health care-associated bloodstream infections in adults: A reason to change the accepted definition of community-acquired infections. Ann. Intern. Med. 2002, 137, 791–797. [Google Scholar] [CrossRef]
- Harris, P.N.A.; Tambyah, P.A.; Lye, D.C.; Mo, Y.; Lee, T.H.; Yilmaz, M.; Alenazi, T.H.; Arabi, Y.; Falcone, M.; Bassetti, M.; et al. MERINO Trial Investigators and the Australasian Society for Infectious Disease Clinical Research Network (ASID-CRN). Effect of Piperacillin-Tazobactam vs Meropenem on 30-Day Mortality for Patients With E. coli or Klebsiella pneumoniae Bloodstream Infection and Ceftriaxone Resistance: A Randomized Clinical Trial. JAMA 2018, 320, 984–994, Erratum in: JAMA 2019, 321, 2370. [Google Scholar] [CrossRef] [Green Version]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez, F.; Adachi, J.; Bonomo, R.A. Antibiotic-resistant gram-negative bacterial infections in patients with cancer. Clin. Infect. Dis. 2014, 59 (Suppl. S5), S335–S339. [Google Scholar] [CrossRef] [PubMed]
- Protocolo de PCR-Multiplex para la Detección de Carbapenemasas. Available online: http://antimicrobianos.com.ar/2019/10/protocolo-de-pcr-multiplex-para-la-deteccion-de-carbapenemasas/ (accessed on 20 January 2023).
- Leffler, D.A.; Lamont, J.T. Clostridium difficile infection. N. Engl. J. Med. 2015, 372, 1539–1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, L.C.; Gerding, D.N.; Johnson, S.; Bakken, J.S.; Carroll, K.C.; Coffin, S.E.; Dubberke, E.R.; Garey, K.W.; Gould, C.V.; Kelly, C.; et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. 2018, 66, 987–994. [Google Scholar] [CrossRef]
- Heinz, W.J.; Buchheidt, D.; Christopeit, M.; von Lilienfeld-Toal, M.; Cornely, O.A.; Einsele, H.; Karthaus, M.; Link, H.; Mahlberg, R.; Neumann, S.; et al. Diagnosis and empirical treatment of fever of unknown origin (FUO) in adult neutropenic patients: Guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Medical Oncology (DGHO). Ann. Hematol. 2017, 96, 1775–1792. [Google Scholar] [CrossRef] [Green Version]
- Averbuch, D.; Orasch, C.; Cordonnier, C.; Livermore, D.M.; Mikulska, M.; Viscoli, C.; Gyssens, I.C.; Kern, W.V.; Klyasova, G.; Marchetti, O.; et al. ECIL4, a joint venture of EBMT, EORTC, ICHS, ESGICH/ESCMID and ELN. European guidelines for empirical antibacterial therapy for febrile neutropenic patients in the era of growing resistance: Summary of the 2011 4th European Conference on Infections in Leukemia. Haematologica 2013, 98, 1826–1835, Erratum in: Haematologica 2014, 99, 400. [Google Scholar] [CrossRef] [Green Version]
- Schauwvlieghe, A.; Dunbar, A.; Storme, E.; Vlak, A.; Aerts, R.; Maertens, J.; Sciot, B.; Van Der Wel, T.; Papageorgiou, G.; Moors, I.; et al. Stopping antibiotic therapy after 72 h in patients with febrile neutropenia following intensive chemotherapy for AML/MDS (safe study): A retrospective comparative cohort study. eClinicalMedicine 2021, 35, 100855. [Google Scholar] [CrossRef]
- Le Clech, L.; Talarmin, J.P.; Couturier, M.A.; Ianotto, J.C.; Nicol, C.; Le Calloch, R.; Dos Santos, S.; Hutin, P.; Tandé, D.; Cogulet, V.; et al. Early discontinuation of empirical antibacterial therapy in febrile neutropenia: The ANTIBIOSTOP study. Infect. Dis. 2018, 50, 539–549. [Google Scholar] [CrossRef]
- Aguilar-Guisado, M.; Espigado, I.; Martín-Peña, A.; Gudiol, C.; Royo-Cebrecos, C.; Falantes, J.; Vázquez-López, L.; Montero, M.I.; Rosso-Fernández, C.; de la Luz Martino, M.; et al. Optimisation of empirical antimicrobial therapy in patients with haematological malignancies and febrile neutropenia (How Long study): An open-label, randomised, controlled phase 4 trial. Lancet Haematol. 2017, 4, e573–e583. [Google Scholar] [CrossRef]
- Gudiol, C.; Aguilar-Guisado, M.; Azanza, J.R.; Candel, F.J.; Cantón, R.; Carratalà, J.; Garcia-Vidal, C.; Jarque, I.; Lizasoain, M.; Gil-Bermejo, J.M.; et al. Executive summary of the consensus document of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC), the Spanish Network for Research in Infectious Diseases (REIPI) and the Spanish Society of Haematology and Haemotherapy (SEHH) on the management of febrile neutropenia in patients with hematological malignancies. Enferm. Infecc. Microbiol. Clin. (Engl. Ed.) 2020, 38, 174–181. [Google Scholar] [CrossRef]
- Verlinden, A.; Mikulska, M.; Knelange, N.S.; Averbuch, D.; Styczynski, J. Infectious Diseases Working Party (IDWP) of the European Group for Blood and Marrow Transplantation Group (EBMT). Current antimicrobial practice in febrile neutropenia across Europe and Asia: The EBMT Infectious Disease Working Party survey. Bone Marrow Transpl. 2020, 55, 1588–1594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turjeman, A.; von Dach, E.; Molina, J.; Franceschini, E.; Koppel, F.; Yelin, D.; Dishon-Benattar, Y.; Mussini, C.; Rodríguez-Baño, J.; Cisneros, J.M.; et al. Duration of antibiotic treatment for Gram-negative bacteremia-Systematic review and individual participant data (IPD) meta-analysis. eClinicalMedicine 2022, 55, 101750. [Google Scholar] [CrossRef] [PubMed]
- Metais, A.; Torregrosa Diaz, J.M.; Gallego Hernanz, M.P.; Pichon, M.; Desmier, D.; Roblot, F.; Rammaert, B. Efficacy of antibiotic short course for bloodstream infections in acute myeloid leukemia patients with febrile neutropenia: A retrospective comparative study. J. Infect. 2022, 84, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Marin, M.; Gudiol, C.; Ardanuy, C.; Garcia-Vidal, C.; Calvo, M.; Arnan, M.; Carratalà, J. Bloodstream infections in neutropenic patients with cancer: Differences between patients with haematological malignancies and solid tumours. J. Infect. 2014, 69, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Carena, A.; Laborde, A.; Roccia Rossi, I.; Guerrini, G.; Valledor, A.; Jordán, R.; Nenna, A.; Costantini, P.; Dictar, M.; Caeiro, J.; et al. Bacteremia in Cancer Patients. Comparison between Solid and Hematological Tumors and Impact on 30-day Mortality. Open Forum Infect. Dis. 2016, 3 (Suppl. S1), S599. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.Y.; Tien, F.M.; Sheng, W.H.; Huang, S.Y.; Yao, M.; Tang, J.L.; Tsay, W.; Tien, H.F.; Hsueh, P.R. Clinical and microbiological characteristics of bloodstream infections among patients with haematological malignancies with and without neutropenia at a medical centre in northern Taiwan, 2008–2013. Int. J. Antimicrob. Agents 2017, 49, 272–281. [Google Scholar] [CrossRef]
- Carena, A.; Laborde, A.; Roccia Rossi, I.; Guerrini, G.; Valledor, A.; Jordán, R.; Nenna, A.; Costantini, P.; Dictar, M.; Caeiro, J.; et al. Epidemiology and Clinical Impact of Bacteremia in Neutropenic and Nonneutropenic Patients with Cancer and Stem-Cell Transplant in the Era of Multiresistance. In Proceedings of the 19 Symposia on the Infections in the Immunocompromised Host, Santiago, Chile, 13–15 November 2016. [Google Scholar]
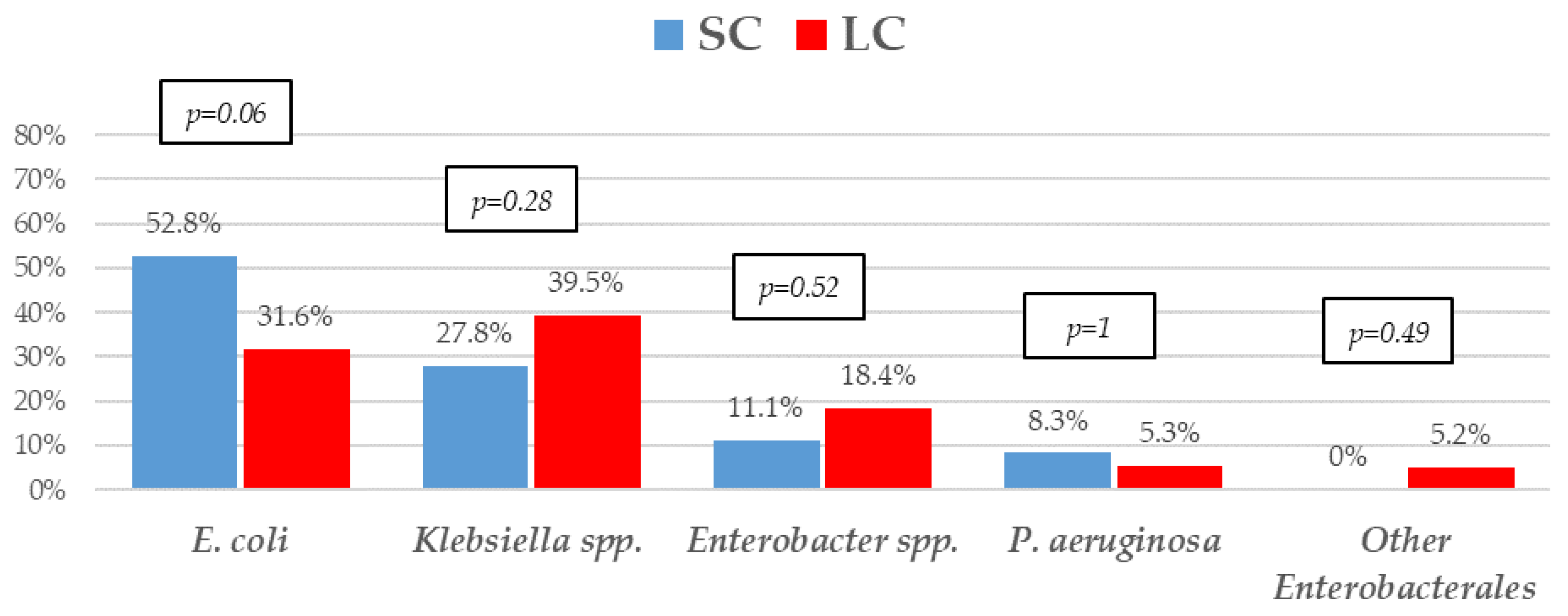


| Variables | SC n = 36 n (%) | LC n = 38 n (%) | * p-Value |
|---|---|---|---|
| Age (years) (median, IQR) | 57 (47–68) | 60 (47–66) | 0.71 |
| Male sex | 21 (58.3) | 18 (47.4) | 0.34 |
| Inclusion criteria | |||
| Hematologic malignancy | 19 (52.8) | 17 (44.7) | 0.49 |
| Solid malignancy | 9 (25) | 12 (31.6) | 0.53 |
| Hematopoietic stem cell transplant | 8 (22.2) | 9 (23.7) | 0.88 |
| Allogeneic HSCT | 4 (50) | 4 (44.4) | 1 |
| Type of hematologic malignancy | |||
| Acute leukemia | 7 (25.9) | 11 (42.3) | 0.21 |
| Lymphoma | 13 (48.2) | 9 (34.6) | 0.32 |
| Myelodysplastic syndrome | 3 (11.1) | 4 (15.4) | 1 |
| Multiple myeloma | 3 (11.1) | 2 (7.7) | 0.70 |
| Chronic myeloproliferative neoplasm | 1 (3.7) | 0 (0) | 1 |
| Stage of underlying diseases | |||
| Recently diagnosed | 8 (22.2) | 10 (26.3) | 0.68 |
| Complete remission | 9 (25) | 5 (13.1) | 0.24 |
| Partial remission | 5 (13.9) | 8 (21.1) | 0.54 |
| Refractory | 6 (16.7) | 8 (21.1) | 0.63 |
| Relapse | 8 (22.2) | 7 (18.4) | 0.68 |
| Recent chemotherapy (1 month prior to bacteremia) | 35 (97.2) | 34 (89.5) | 0.18 |
| Recent radiotherapy (1 month prior to bacteremia) | 0 (0) | 1 (2.6) | 1 |
| Current steroids use | 11 (30.5) | 10 (26.3) | 0.68 |
| Biological agents use | 13 (36.1) | 7 (18.4) | 0.09 |
| Charlson comorbidity index (median, IQR) | 2 (2–4) | 2 (2–3) | 0.69 |
| Neutropenia | 21 (58.3) | 23 (60.5) | 0.85 |
| High risk by their MASCC score | 20 (95.2) | 19 (82.6) | 0.19 |
| Neutropenia duration (days) (IQR) | 14 (6–41) | 12 (7–22) | 0.59 |
| Neutropenia > 10 days | 14 (51.9) | 14 (60.9) | 0.52 |
| Variables | SC n = 36 n (%) | LC n = 38 n (%) | * p-Value |
|---|---|---|---|
| Recent hospitalization (1 month prior to bacteremia) | 17 (47.2) | 16 (42.1) | 0.66 |
| Previous colonization with KPC-CPE | 5 (13.9) | 1 (2.6) | 0.10 |
| Previous colonization with ESBL | 7 (19.4) | 4 (10.5) | 0.34 |
| Previous colonization with MDR-PA | 1 (2.8) | 3 (7.9) | 0.61 |
| Previous infection with MDR-GNB | 3 (8.3) | 5 (13.2) | 0.71 |
| Recent antibiotic use | 20 (55.5) | 24 (63.2) | 0.51 |
| Recent piperacillin-tazobactam use | 13 (36.1) | 16 (42.1) | 0.56 |
| Recent 3rd or 4th generation cephalosporin use | 1 (2.8) | 1 (2.6) | 1 |
| Recent carbapenem use | 10 (27.8) | 9 (23.7) | 0.68 |
| >7 days of antibiotic use prior to bacteremia | 9 (25) | 14 (36.8) | 0.27 |
| Fluoroquinolone prophylaxis | 0 (0) | 0 (0) | |
| Recent colonization with KPC-CPE | 7 (19.4) | 2 (5.3) | 0.08 |
| Recent colonization with ESBL | 9 (25) | 6 (15.8) | 0.32 |
| Recent colonization with MDR-PA | 0 (0) | 2 (5.3) | 0.49 |
| Recent intensive care unit admission | 2 (5.6) | 2 (5.3) | 1 |
| Central venous catheter in place | 28 (77.8) | 26 (68.4) | 0.36 |
| Urinary catheter in place | 3 (8.3) | 1 (2.6) | 0.35 |
| Nosocomial infection | 19 (52.8) | 22 (57.8) | 0.66 |
| Healthcare-associated infection | 14 (38.9) | 8 (21.1) | 0.09 |
| Community-associated infection | 3 (8.3) | 8 (21.1) | 0.19 |
| Length of hospitalization prior to bacteremia | 0 (0–13) | 5 (0–15) | 0.27 |
| Variables | SC n = 36 n (%) | LC n = 38 n (%) | * p-Value |
|---|---|---|---|
| Bacteremia with clinical source | 26 (72.2) | 29 (76.3) | 0.69 |
| Abdominal | 14 (38.9) | 11 (28.9) | 0.36 |
| Central venous catheter | 6 (16.7) | 7 (18.4) | 0.84 |
| Urinary tract | 2 (5.6) | 4 (10.5) | 0.67 |
| Respiratory tract | 3 (8.3) | 2 (5.3) | 0.67 |
| Skin and soft tissue | 1 (2.8) | 1 (2.6) | 1 |
| Perianal | 1 (2.8) | 3 (7.9) | 0.61 |
| Other | 0 | 1 (2.6) | 1 |
| Fever | 35 (97.2) | 37 (97.4) | 0.97 |
| Hypotension | 10 (27.8) | 13 (34.2) | 0.55 |
| Empirical monotherapy antibiotic therapy | 22 (61.1) | 21 (55.3) | 0.61 |
| Piperacillin-tazobactam | 10 (45.5) | 12 (57.1) | 0.44 |
| Meropenem | 9 (40.9) | 7 (33.3) | 0.61 |
| Ceftriaxone | 0 (0) | 2 (9.5) | 0.23 |
| Ceftazidime-avibactam | 2 (9.1) | 0 (0) | 0.49 |
| Empirical combination antibiotic therapy | 14 (38.9) | 17 (44.7) | 0.61 |
| Meropenem + colistin | 4 (28.6) | 7 (41.2) | 0.71 |
| Meropenem + amikacin | 2 (14.13) | 6 (35.3) | 0.23 |
| Ceftazidime-avibactam + amikacin | 6 (42.8) | 0 (0) | 0.004 |
| Definitive monotherapy antibiotic therapy | 36 (100) | 33 (83.6) | 0.024 |
| Fluoroquinolones | 5 (13.9) | 11 (33.3) | 0.08 |
| Piperacillin-tazobactam | 8 (22.2) | 12 (36.4) | 0.19 |
| Meropenem | 9 (25) | 5 (15.2) | 0.37 |
| Ceftriaxone | 6 (16.7) | 5 (15.2) | 1 |
| Ceftazidime-avibactam | 5 (13.9) | 0 (0) | 0.05 |
| APACHE II score the day of bacteremia (median, IQR) | 21 (19–23) | 17 (14–20) | <0.001 |
| PITT score the day of bacteremia (median, IQR) | 1 (0–2) | 1 (0–2) | 0.22 |
| Shock at presentation | 1 (2.8) | 4 (10.5) | 0.36 |
| 30-day mortality | 1 (2.8) | 3 (7.9) | 0.61 |
| Infection-related mortality | 0 (0) | 0 (0) | |
| Recurrence of bacteremia | 1 (2.8) | 0 (0) | 0.49 |
| Length of hospitalization after bacteremia | 7 (7–12) | 12 (8–20) | 0.02 |
| Clostridioides difficile infection | 0 (0) | 3 (7.9) | 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera, F.; Torres, D.; Carena, A.; Nicola, F.; Rearte, A.; Temporiti, E.; Jorge, L.; Valentini, R.; Bues, F.; Relloso, S.; et al. Short Course of Antibiotic Therapy for Gram-Negative Bacilli Bacteremia in Patients with Cancer and Hematopoietic Stem Cell Transplantation: Less Is Possible. Microorganisms 2023, 11, 511. https://doi.org/10.3390/microorganisms11020511
Herrera F, Torres D, Carena A, Nicola F, Rearte A, Temporiti E, Jorge L, Valentini R, Bues F, Relloso S, et al. Short Course of Antibiotic Therapy for Gram-Negative Bacilli Bacteremia in Patients with Cancer and Hematopoietic Stem Cell Transplantation: Less Is Possible. Microorganisms. 2023; 11(2):511. https://doi.org/10.3390/microorganisms11020511
Chicago/Turabian StyleHerrera, Fabián, Diego Torres, Alberto Carena, Federico Nicola, Andrés Rearte, Elena Temporiti, Laura Jorge, Ricardo Valentini, Florencia Bues, Silvia Relloso, and et al. 2023. "Short Course of Antibiotic Therapy for Gram-Negative Bacilli Bacteremia in Patients with Cancer and Hematopoietic Stem Cell Transplantation: Less Is Possible" Microorganisms 11, no. 2: 511. https://doi.org/10.3390/microorganisms11020511
APA StyleHerrera, F., Torres, D., Carena, A., Nicola, F., Rearte, A., Temporiti, E., Jorge, L., Valentini, R., Bues, F., Relloso, S., & Bonvehí, P. (2023). Short Course of Antibiotic Therapy for Gram-Negative Bacilli Bacteremia in Patients with Cancer and Hematopoietic Stem Cell Transplantation: Less Is Possible. Microorganisms, 11(2), 511. https://doi.org/10.3390/microorganisms11020511






