Effects of Different Types of LAB on Dynamic Fermentation Quality and Microbial Community of Native Grass Silage during Anaerobic Fermentation and Aerobic Exposure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites and Silage Preparation
2.2. Chemical Composition
2.3. Fermentation Composition
2.4. Aerobic Stability
2.5. Microbial Counting and Sequencing
2.6. Statistical Analyses
3. Results
3.1. Chemical Composition and Microbial Community Structure before Ensiling
3.2. Effect of Additives and Days of Anaerobic Fermentation and Aerobic Exposure on Chemical Parameters of Native Grass Silage
3.3. Effect of Additives and Days of Anaerobic Fermentation and Aerobic Exposure on Fermentation Quality of Native Grass Silage
3.4. Effect of Additives and Days of Anaerobic Fermentation and Aerobic Exposure on Microorganism Counts of Native Grass Silage
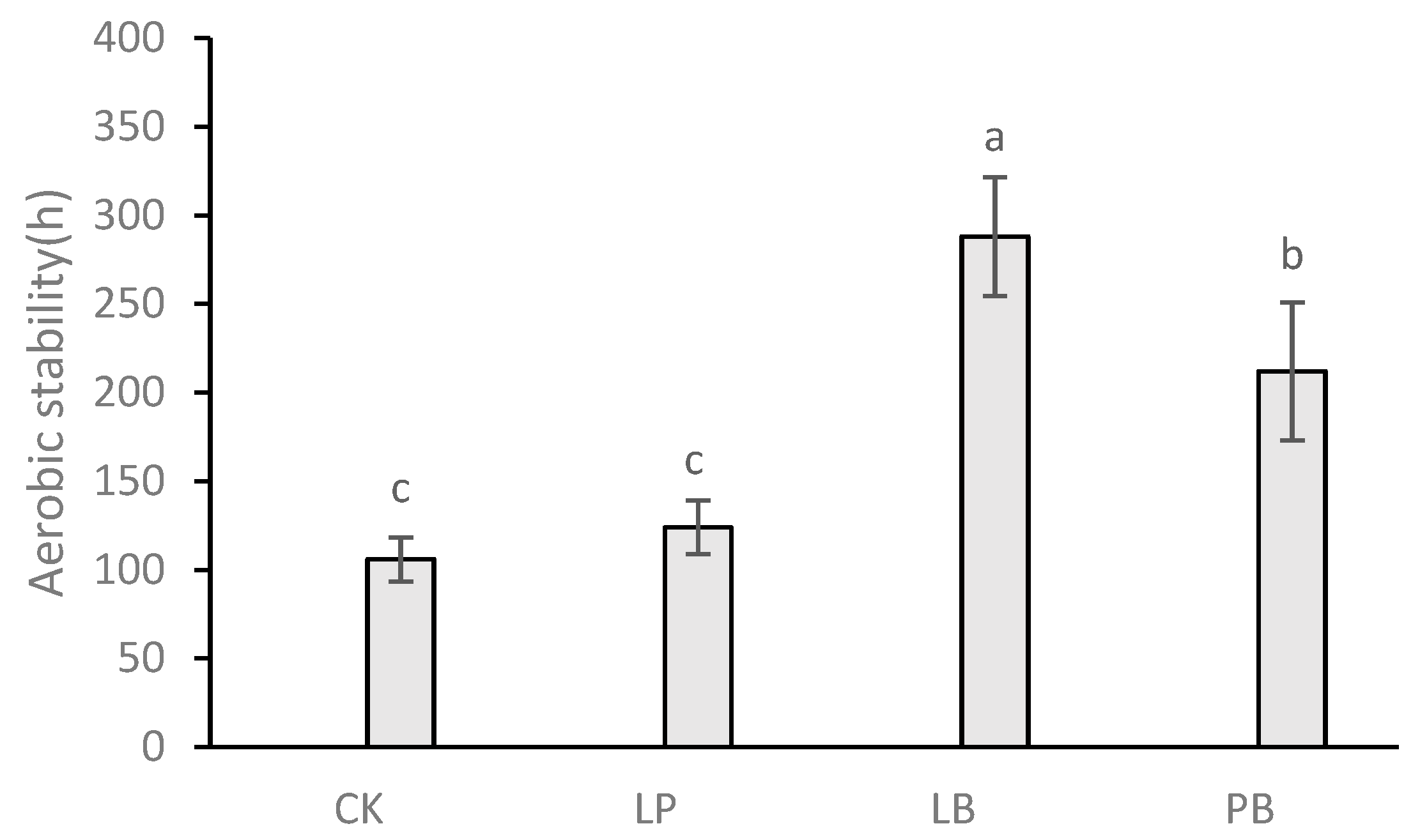
3.5. Effect of Lactic Acid Bacteria Additives on Aerobic Stability during Aerobic Exposure
3.6. Effect of Additives and Days of Anaerobic Fermentation and Aerobic Exposure on Microbial Alpha Diversity of Native Grass Silage
3.7. Effect of Additives and Days of Anaerobic Fermentation and Aerobic Exposure on Microbial Community Dynamics of Native Grass Silage
3.8. Correlation of Microbial Genera Level with Silage Quality in Native Grass Silage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- TäLle, M.; Deák, B.; Poschlod, P.; Valkó, O.; Westerberg, L.; Milberg, P. Grazing vs. mowing: A meta-analysis of biodiversity benefits for grassland management. Agric. Ecosyst. Environ. 2016, 222, 200–212. [Google Scholar] [CrossRef]
- Kebede, G.; Assefa, G.; Feyissa, F.; Mengistu, A. A review on some management and improvement practices of natural pasture in the mid and high altitude areas of ethiopia. Int. J. Livest. Res. 2016, 6, 1–14. [Google Scholar] [CrossRef]
- Long, R.J.; Zhang, D.G.; Wang, X.; Hu, Z.Z.; Dong, S.K. Effect of strategic feed supplementation on productive and reproductive performance in yak cows. Prev. Vet. Med. 1999, 38, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Schnbach, P.; Wan, H.; Gierus, M.; Bai, Y.; Müller, K.; Lin, L.; Susenbeth, A.; Taube, F. Grassland responses to grazing: Effects of grazing intensity and management system in an Inner Mongolian steppe ecosystem. Plant Soil 2011, 340, 103–115. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, B.; Nishino, N.; Wang, X.; Yu, Z. Fermentation and microbial population dynamics during the ensiling of native grass and subsequent exposure to air. Anim. Sci. J. 2015, 87, 389–397. [Google Scholar] [CrossRef]
- Cai, Y. Identification and characterization of Enterococcus species isolated from forage crops and their influence on silage fermentation. J. Dairy Sci. 1999, 82, 2466–2471. [Google Scholar] [CrossRef]
- Du, Z.; Risu, N.; Gentu, G.; Jia, Y.; Cai, Y. Dynamic changes and characterization of the protein and carbohydrate fractions of native grass grown in Inner Mongolia during ensiling and the aerobic stage. Asian-Australas. J. Anim. Sci. 2020, 33, 556. [Google Scholar] [CrossRef] [Green Version]
- Cao, C.; Bao, W.; Li, W.; Zhao, F.; Kwok, L.Y.; Zhang, W.; Zhang, H. Changes in physico-chemical characteristics and viable bacterial communities during fermentation of alfalfa silages inoculated with Lactobacillus plantarum. World J. Microbiol. Biotechnol. 2021, 37, 127. [Google Scholar] [CrossRef]
- Huo, W.; Wang, X.; Wei, Z.; Zhang, H.; Guo, G. Effect of lactic acid bacteria on the ensiling characteristics and in vitro ruminal fermentation parameters of alfalfa silage. Ital. J. Anim. Sci. 2021, 20, 623–631. [Google Scholar] [CrossRef]
- Zi, X.; Li, M.; Chen, Y.; Lv, R.; Tang, J. Effects of Citric Acid and Lactobacillus plantarum on Silage Quality and Bacterial Diversity of King Grass Silage. Front. Microbiol. 2021, 12, 631096. [Google Scholar] [CrossRef]
- Tahir, M.; Li, J.; Xin, Y.; Wang, T.; Chen, C.; Zhong, Y.; Zhang, L.; Liu, H.; He, Y.; Wen, X.; et al. Response of fermentation quality and microbial community of oat silage to homofermentative lactic acid bacteria inoculation. Front. Microbiol. 2023, 13, 5452. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; da Silva, E.; Li, J.; Kung, L., Jr. Effect of homo-fermentative lactic acid bacteria inoculants on fermentation characteristics and bacterial and fungal communities in alfalfa silage. Fermentation 2022, 8, 621. [Google Scholar] [CrossRef]
- Yan, J.; Sun, Y.; Kang, Y.; Meng, X.; Zhang, H.; Cai, Y.; Zhu, W.; Yuan, X.; Cui, Z. An innovative strategy to enhance the ensiling quality and methane production of excessively wilted wheat straw: Using acetic acid or hetero-fermentative lactic acid bacterial community as additives. Waste Manag. 2022, 149, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, L.; Wu, G.; Wang, X.; Tan, Z. Effects of Lactobacillus plantarum on the fermentation profile and microbiological composition of wheat fermented silage under the freezing and thawing low temperatures. Front. Microbiol. 2021, 12, 671287. [Google Scholar] [CrossRef]
- Zhao, S.S.; Wang, Y.P.; Yang, F.Y.; Wang, Y.; Zhang, H. Screening a Lactobacillus plantarum strain for good adaption in alfalfa ensiling and demonstrating its improvement of alfalfa silage quality. J. Appl. Microbiol. 2020, 129, 233–242. [Google Scholar] [CrossRef]
- Danner, H.; Holzer, M.; Mayrhuber, E.; Braun, R. Acetic acid increases stability of silage under aerobic conditions. Appl. Environ. Microbiol. 2003, 69, 562. [Google Scholar] [CrossRef] [Green Version]
- Mcdonald, P.; Henderson, A.R.; Heron, S. The Biochemistry of Silage; Chalcombe Publications: Marlow, UK, 1991. [Google Scholar]
- Zhang, F.; Miao, F.; Wang, X.; Lu, W.; Ma, C. Effects of homo- and hetero-fermentative lactic acid bacteria on the quality and aerobic stability of corn silage. Can. J. Anim. Sci. 2021, 101, 761–770. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Zhao, J.; Dong, Z.; Dong, D.; Shao, T. Silage fermentation characteristics and microbial diversity of alfalfa (Medicago sativa L.) in response to exogenous microbiota from temperate grasses. World J. Microbiol. Biotechnol. 2021, 37, 1–14. [Google Scholar] [CrossRef]
- Li, Y.; Nishino, N. Effects of inoculation of Lactobacillus rhamnosus and Lactobacillus buchneri on fermentation, aerobic stability and microbial communities in whole crop corn silage. Grassl. Sci. 2011, 57, 184–191. [Google Scholar] [CrossRef]
- Silva, E.; Smith, M.; Savage, R.; Polukis, S.; Kung, L. Effects of Lactobacillus hilgardii 4785 and Lactobacillus buchneri 40788 on the bacterial community, fermentation, and aerobic stability of high-moisture corn silage. J. Appl. Microbiol. 2020, 130, 1481–1493. [Google Scholar] [CrossRef]
- Li, Y.; Du, S.; Sun, L.; Cheng, Q.; Hao, J.; Lu, Q.; Ge, G.; Wang, Z.; Jia, Y. Effects of Lactic acid bacteria and molasses additives on dynamic fermentation quality and microbial community of native grass silage. Front. Microbiol. 2022, 13, 830121. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R. A method for the extraction of plant samples and the determination of total soluble carbohydrates. J. Sci. Food Agric. 1958, 9, 714–717. [Google Scholar] [CrossRef]
- Sun, L.; Na, N.; Li, X.; Li, Z.; Wang, C.; Wu, X.; Xiao, Y.; Yin, G.; Liu, S.; Liu, Z. Impact of packing density on the bacterial community, fermentation, and in vitro digestibility of whole-crop barley silage. Agriculture 2021, 11, 672. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Broderick, G.A.; Kang, J.H. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Reich, L.J.; Kung, L. Effects of combining Lactobacillus buchneri 40788 with various lactic acid bacteria on the fermentation and aerobic stability of corn silage. Anim. Feed Sci. Technol. 2010, 159, 105–109. [Google Scholar] [CrossRef]
- Cai, Y.; Benno, Y.; Ogawa, M.; Kumai, S. Effect of applying lactic acid bacteria isolated from forage crops on fermentation characteristics and aerobic deterioration of silage. J. Dairy Sci. 1999, 82, 520–526. [Google Scholar] [CrossRef]
- Guan, H.; Yan, Y.; Li, X.; Li, X.; Shuai, Y.; Feng, G.; Ran, Q.; Cai, Y.; Li, Y.; Zhang, X. Microbial communities and natural fermentation of corn silages prepared with farm bunker-silo in Southwest China. Bioresour. Technol. 2018, 265, 282–290. [Google Scholar] [CrossRef]
- Cai, Y.M.; Benno, Y.; Ogawa, M.; Ohmomo, S.; Nakase, T. Influence of Lactobacillus spp. from an Inoculant and of Weissella and Leuconostoc spp. from forage crops on silage fermentation. Appl. Env. Microbiol. 1998, 64, 2982–2987. [Google Scholar] [CrossRef] [Green Version]
- Ávila, C.L.S.; Pinto, J.C.; Figueiredo, H.; Schwan, R.F. Effects of an indigenous and a commercial Lactobacillus buchneri strain on quality of sugar cane silage. Grass Forage Sci. 2010, 64, 384–394. [Google Scholar] [CrossRef]
- Si, H.; Liu, H.; Li, Z.; Nan, W.; Jin, C.; Sui, Y.; Li, G. Effect of Lactobacillus plantarum and Lactobacillus buchneri addition on fermentation, bacterial community and aerobic stability in lucerne silage. Anim. Prod. Sci. 2018, 59, 1528–1536. [Google Scholar] [CrossRef]
- Ge, G.; Hou, M.; Liu, T.; Jia, Y.; Cai, Y. Microbial population, chemical composition and silage fermentation of native grasses growing on the Inner Mongolian Plateau. Grassl. Sci. 2018, 64, 226–233. [Google Scholar] [CrossRef]
- Tabacco, E.; Righi, F.; Quarantelli, A.; Borreani, G. Dry matter and nutritional losses during aerobic deterioration of corn and sorghum silages as influenced by different lactic acid bacteria inocula. J. Dairy Sci. 2011, 94, 1409–1419. [Google Scholar] [CrossRef]
- Ren, H.; Feng, Y.; Pei, J.; Li, J.; Wang, Z.; Fu, S.; Zheng, Y.; Li, Z.; Peng, Z. Effects of Lactobacillus plantarum additive and temperature on the ensiling quality and microbial community dynamics of cauliflower leaf silages. Bioresour. Technol. 2020, 307, 123238. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wang, M.; Ke, W.; Guo, X. Screening of high 1, 2-propanediol production by Lactobacillus buchneri strains and their effects on fermentation characteristics and aerobic stability of whole-plant corn silage. Agriculture 2021, 11, 590. [Google Scholar] [CrossRef]
- Carvalho, B.F.; Ávila, C.L.S.; Miguel, M.G.C.P.; Pinto, J.C.; Santos, M.C.; Schwan, R.F. Aerobic stability of sugar-cane silage inoculated with tropical strains of lactic acid bacteria. Grass Forage Sci. 2015, 70, 308–323. [Google Scholar] [CrossRef]
- Nazar, M.; Wang, S.; Zhao, J.; Dong, Z.; Shao, T. The feasibility and effects of exogenous epiphytic microbiota on the fermentation quality and microbial community dynamics of whole crop corn. Bioresour. Technol. 2020, 306, 123106. [Google Scholar] [CrossRef]
- Bartkiene, E.; Gruzauskas, R.; Ruzauskas, M.; Zokaityte, E.; Starkute, V.; Klupsaite, D.; Vadopalas, L.; Badaras, S.; Özogul, F. Changes in the microbial community and biogenic amine content in rapeseed meal during fermentation with an antimicrobial combination of Lactic acid bacteria strains. Fermentation 2022, 8, 136. [Google Scholar] [CrossRef]
- Li, J.; Meng, Q.; Wang, C.; Song, C.; Lyu, Y.; Li, J.; Shan, A. The interaction between temperature and citric acid treatment in the anaerobic fermentation of Chinese cabbage waste. J. Clean. Prod. 2023, 383, 135502. [Google Scholar] [CrossRef]
- Soundharrajan, I.; Park, H.S.; Rengasamy, S.; Sivanesan, R.; Choi, K.C. Application and future prospective of lactic acid bacteria as natural additives for silage production—A review. Appl. Sci. 2021, 11, 8127. [Google Scholar] [CrossRef]
- Nascimento Agarussi, M.; Pereira, O.; da Silva, L.; da Silva, V.; de Paula, R.; Fonseca e Silva, F.; Guimarães Ribeiro, K. Effect of Various Strains of Lactobacillus buchneri on the Fermentation Quality and Aerobic Stability of Corn Silage. Agriculture 2022, 12, 95. [Google Scholar] [CrossRef]
- Da Silva, N.; Nascimento, C.; Nascimento, F.; De Resende, F.; Daniel, J.; Siqueira, G. Fermentation and aerobic stability of rehydrated corn grain silage treated with different doses of Lactobacillus buchneri or a combination of Lactobacillus plantarum and Pediococcus acidilactici. J. Dairy Sci. 2018, 101, 4158–4167. [Google Scholar] [CrossRef] [PubMed]
- Mugabe, W.; Shao, T.; Li, J.; Dong, Z.; Yuan, X. Effect of hexanoic acid, Lactobacillus plantarum and their combination on the aerobic stability of napier grass silage. J. Appl. Microbiol. 2020, 129, 823–831. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Zhou, W.; Yang, F.-y.; Chen, X.-y.; Zhang, Q. Effects of wilting and Lactobacillus plantarum addition on the fermentation quality and microbial community of Moringa oleifera leaf silage. Front. Microbiol. 2018, 9, 1817. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Li, X.; Guan, H.; Huang, L.; Ma, X.; Peng, Y.; Li, Z.; Nie, G.; Zhou, J.; Yang, W. Microbial community and fermentation characteristic of Italian ryegrass silage prepared with corn stover and lactic acid bacteria. Bioresour. Technol. 2019, 279, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Xie, Z.; Hu, L.; Chen, G.; Zhang, Z. Lactobacillus plantarum and molasses alter dynamic chemical composition, microbial community, and aerobic stability of mixed (amaranth and rice straw) silage. J. Sci. Food Agric. 2021, 101, 5225–5235. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Xu, J.; Guo, L.; Chen, F.; Jiang, D.; Lin, Y.; Guo, C.; Li, X.; Chen, Y.; Ni, K. Exploring the effects of different bacteria additives on fermentation quality, microbial community and in vitro gas production of forage oat silage. Animals 2022, 12, 1122. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Lu, Q.; Sun, L.; Du, S.; Liu, T.; Hou, M.; Ge, G.; Wang, Z.; Jia, Y. Effects of lactic acid bacteria additives on the quality, volatile chemicals and microbial community of leymus chinensis silage during aerobic exposure. Front. Microbiol. 2022, 13, 938153. [Google Scholar] [CrossRef]
- Du, Z.; Sun, L.; Chen, C.; Lin, J.; Yang, F.; Cai, Y. Exploring microbial community structure and metabolic gene clusters during silage fermentation of paper mulberry, a high-protein woody plant. Anim. Feed Sci. Technol. 2021, 275, 114766. [Google Scholar] [CrossRef]
- Li, M.; Zi, X.; Zhou, H.; Lv, R.; Tang, J.; Cai, Y. Effect of lactic acid bacteria, molasses, and their combination on the fermentation quality and bacterial community of cassava foliage silage. Anim. Sci. J. 2021, 92, e13635. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Wang, W.-K.; Wu, Q.-C.; Zhang, F.; Li, W.-J.; Yang, Z.-M.; Bo, Y.-K.; Yang, H.-J. The Effect of different lactic acid bacteria inoculants on silage quality, phenolic acid profiles, bacterial community and in vitro rumen fermentation characteristic of whole corn silage. Fermentation 2022, 8, 285. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, R.; Wang, C.; Dong, W.; Zhang, Z.; Zhao, L.; Zhang, X. Effects of Cellulase and Lactobacillus plantarum on Fermentation Quality, Chemical Composition, and Microbial Community of Mixed Silage of Whole-Plant Corn and Peanut Vines. Appl. Biochem. Biotechnol. 2022, 194, 2465–2480. [Google Scholar] [CrossRef] [PubMed]
- Kung, L., Jr.; Tung, R.; Maciorowski, K.; Buffum, K.; Knutsen, K.; Aimutis, W. Effects of plant cell-wall-degrading enzymes and lactic acid bacteria on silage fermentation and composition. J. Dairy Sci. 1991, 74, 4284–4296. [Google Scholar] [CrossRef]
- Carvalho, B.; Sales, G.; Schwan, R.; Ávila, C. Criteria for lactic acid bacteria screening to enhance silage quality. J. Appl. Microbiol. 2021, 130, 341–355. [Google Scholar] [CrossRef]
- Gang, G.; Chen, S.; Qiang, L.; Zhang, S.; Tao, S.; Cong, W.; Wang, Y.; Xu, Q.; Huo, W. The effect of lactic acid bacteria inoculums on in vitro rumen fermentation, methane production, ruminal cellulolytic bacteria populations and cellulase activities of corn stover silage. J. Integr. Agric. 2020, 19, 838–847. [Google Scholar] [CrossRef]
- Sun, L.; Bai, C.; Xu, H.; Na, N.; Jiang, Y.; Yin, G.; Liu, S.; Xue, Y. Succession of bacterial community during the initial aerobic, intense fermentation, and stable phases of whole-plant corn silages treated with lactic acid bacteria suspensions prepared from other silages. Front. Microbiol. 2021, 12, 655095. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Sun, L.; Lin, Y.; Yang, F.; Cai, Y. The use of PacBio SMRT technology to explore the microbial network and fermentation characteristics of woody silage prepared with exogenous carbohydrate additives. J. Appl. Microbiol. 2021, 131, 2193–2211. [Google Scholar] [CrossRef] [PubMed]
- Xian, Z.; Wu, J.; Deng, M.; Wang, M.; Tian, H.; Liu, D.; Li, Y.; Liu, G.; Sun, B.; Guo, Y. Effects of cellulase and Lactiplantibacillus plantarum on the fermentation parameters, nutrients, and bacterial community in Cassia alata silage. Front. Microbiol. 2022, 13, 926065. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Bu, C.; Zou, L.; Hu, Y.; Zheng, Z.; Ouyang, J. A comprehensive review on microbial production of 1, 2-propanediol: Micro-organisms, metabolic pathways, and metabolic engineering. Biotechnol. Biofuels 2021, 14, 216. [Google Scholar] [CrossRef]
- Lv, H.; Pian, R.; Xing, Y.; Zhou, W.; Yang, F.; Chen, X.; Zhang, Q. Effects of citric acid on fermentation characteristics and bacterial diversity of Amomum villosum silage. Bioresour. Technol. 2020, 307, 123290. [Google Scholar] [CrossRef]
- Keshri, J.; Chen, Y.; Pinto, R.; Kroupitski, Y.; Weinberg, Z.G. Microbiome dynamics during ensiling of corn with and without Lactobacillus plantarum inoculant. Appl. Microbiol. Biotechnol. 2018, 102, 4025–4037. [Google Scholar] [CrossRef]
- Liu, B.; Huan, H.; Gu, H.; Xu, N.; Shen, Q.; Ding, C. Dynamics of a microbial community during ensiling and upon aerobic exposure in lactic acid bacteria inoculation-treated and untreated barley silages. Bioresour. Technol. 2019, 273, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yuan, Z.; Sun, Y.; Kong, X.; Dong, P.; Zhang, J. A reused method for molasses-processed wastewater: Effect on silage quality and anaerobic digestion performance of Pennisetum purpereum. Bioresour. Technol. 2017, 241, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Sa, D.W.; Lu, Q.; Wang, Z.; Ge, G.; Sun, L.; Jia, Y. The potential and effects of saline-alkali alfalfa microbiota under salt stress on the fermentation quality and microbial. BMC Microbiol. 2021, 21, 149. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Jiang, D.; Zheng, M.; Tian, P.; Zheng, M.; Xu, C. Microbial community dynamics during alfalfa silage with or without clostridial fermentation. Sci. Rep. 2020, 10, 17782. [Google Scholar] [CrossRef] [PubMed]
- Pitiwittayakul, N.; Bureenok, S.; Schonewille, J.T. Selective thermotolerant lactic acid bacteria isolated from fermented juice of epiphytic lactic acid bacteria and their effects on fermentation quality of stylo silages. Front. Microbiol. 2021, 12, 673946. [Google Scholar] [CrossRef]
- Chen, L.; Bai, S.; You, M.; Xiao, B.; Li, P.; Cai, Y. Effect of a low temperature tolerant lactic acid bacteria inoculant on the fermentation quality and bacterial community of oat round bale silage. Anim. Feed Sci. Technol. 2020, 269, 114669. [Google Scholar] [CrossRef]
- Drouin, P.; Mari, L.J.; Schmidt, R.J. Lactic acid bacteria as microbial silage additives: Current status and future outlook. New Adv. Ferment. Process. 2019, 226, 89326. [Google Scholar] [CrossRef] [Green Version]
- Ferrero, F.; Tabacco, E.; Borreani, G. Lentilactobacillus hilgardii inoculum, dry matter contents at harvest and length of conservation affect fermentation characteristics and aerobic stability of corn silage. Front. Microbiol. 2021, 12, 675563. [Google Scholar] [CrossRef]
- Yang, H.; Wang, B.; Zhang, Q.; Cheng, H.; Yu, Z. Improvement of fermentation quality in the fermented total mixed ration with oat silage. Microorganisms 2021, 9, 420. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, X.-y.; Qin, C.-q.; Li, T.-t.; Liu, W.-h.; Ren, D.-F. Fermentation of rose residue by Lactiplantibacillus plantarum B7 and Bacillus subtilis natto promotes polyphenol content and beneficial bioactivity. J. Biosci. Bioeng. 2022, 134, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Chang, J.; Yu, J.; Li, S.; Niu, H. Diversity of bacterial community during ensiling and subsequent exposure to air in whole-plant maize silage. Asian-Australas. J. Anim. Sci. 2018, 31, 1464. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Sheng, F.; Hu, X.; Huang, Z.; Tian, X.; Wu, Z. Nutrition promotion of brewer’s spent grain by symbiotic fermentation adding Bacillus velezensis and Levilactobacillus brevis. Food Biosci. 2022, 49, 101941. [Google Scholar] [CrossRef]
- Kim, I.S.; Hur, Y.K.; Kim, E.J.; Ahn, Y.-T.; Kim, J.G.; Choi, Y.-J.; Huh, C.S. Comparative analysis of the microbial communities in raw milk produced in different regions of Korea. Asian-Australas. J. Anim. Sci. 2017, 30, 1643. [Google Scholar] [CrossRef] [Green Version]
- Vongkamjan, K.; Switt, A.M.; den Bakker, H.C.; Fortes, E.D.; Wiedmann, M. Silage collected from dairy farms harbors an abundance of listeriaphages with considerable host range and genome size diversity. Appl. Environ. Microbiol. 2012, 78, 8666–8675. [Google Scholar] [CrossRef] [Green Version]



| Items | Sample | SEM | |
|---|---|---|---|
| Chemical composition | Dry matter (% FM) | 51.12 | 0.12 |
| Acid detergent fiber (% DM) | 33.92 | 0.33 | |
| Neutral detergent fiber (% DM) | 67.95 | 0.25 | |
| Crude protein (% DM) | 8.31 | 0.16 | |
| Crude fat (% DM) | 2.74 | 0.07 | |
| Coarse ash (% DM) | 5.32 | 0.09 | |
| Water soluble carbohydrate (% DM) | 6.38 | 0.33 | |
| Microbial counts | Lactic acid bacteria (log10 cfu/g FM) | 2.81 | 0.23 |
| Aerobic bacteria (log10 cfu/g FM) | 7.32 | 0.12 | |
| Coliform bacteria (log10 cfu/g FM) | ND | ND | |
| Yeasts (log10 cfu/g FM) | 3.84 | 0.20 | |
| Molds (log10 cfu/g FM) | 3.62 | 0.26 |
| Items | Treatment | Days | Significance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 7 | 15 | 60 | B4 | B8 | SEM | T | D | T × D | ||
| DM (% FM) | CK | 47.68Aa | 44.66Ab | 45.65Ab | 46.01Bb | 42.02Bc | 40.34Ad | 0.28 | ** | ** | NS |
| LP | 46.95Aa | 44.26Ab | 45.71Aab | 46.54ABa | 42.25Bc | 41.36Ac | |||||
| LB | 47.42Aa | 45.13Abc | 46.91Aab | 47.11Aab | 44.63Ac | 42.53Ad | |||||
| PB | 47.88Aa | 44.62Abc | 46.33Aab | 46.93ABa | 43.40ABcd | 41.73Ad | |||||
| ADF (% DM) | CK | 32.87Bc | 34.65ABb | 35.05ABb | 34.28Abc | 34.93Cb | 37.83Aa | 0.20 | ** | ** | ** |
| LP | 34.33Ab | 34.52ABb | 35.16aABb | 34.25Ab | 35.89BCa | 35.39Bab | |||||
| LB | 33.43ABc | 34.89Abc | 35.92Ab | 35.46Ab | 37.93Aa | 37.92Aa | |||||
| PB | 32.27Bd | 32.79Bd | 34.51Bc | 35.74Ab | 36.94ABa | 37.33Aa | |||||
| NDF (% DM) | CK | 63.56ABc | 64.02Abc | 63.46Ac | 62.89Ac | 65.17Bb | 67.17Aa | 0.24 | ** | ** | ** |
| LP | 64.80Aab | 63.64Ab | 64.62Aab | 64.78Aab | 66.67ABa | 65.05Bab | |||||
| LB | 64.06ABbc | 64.32Abc | 66.00Ab | 63.32Ac | 68.62Aa | 68.22Aa | |||||
| PB | 62.95Bc | 62.23Bc | 64.65Abc | 62.77Ac | 66.60ABab | 68.51Aa | |||||
| CP (% DM) | CK | 8.87ABa | 8.28Ba | 8.25Aa | 7.94Ba | 7.67Ba | 7.90Aa | 0.07 | ** | ** | NS |
| LP | 8.38ABab | 8.64ABa | 8.49Aa | 7.97Bab | 7.98Bab | 7.73Ab | |||||
| LB | 7.90Bb | 9.15ABa | 7.64Ab | 8.09ABb | 7.88Bb | 7.93Ab | |||||
| PB | 9.02Aab | 9.54Aa | 8.05Ac | 8.34Abc | 8.46Abc | 8.17Abc | |||||
| EE (% DM) | CK | 3.99Aa | 3.25Aab | 2.87Aab | 2.62Ab | 3.34Aab | 2.95Aab | 0.07 | NS | NS | NS |
| LP | 2.81Aa | 2.73ABa | 2.22Aa | 3.12Aa | 2.79Aa | 3.26Aa | |||||
| LB | 2.38Ab | 2.35Bb | 2.63Aab | 2.72Aab | 2.55Aab | 3.23Aa | |||||
| PB | 2.89Aa | 2.54ABa | 2.71Aa | 3.01Aa | 2.97Aa | 2.71Aa | |||||
| Ash (% DM) | CK | 4.45Aa | 5.19Aa | 4.95Aa | 4.68Ba | 4.70Ba | 4.65Ba | 0.04 | NS | NS | NS |
| LP | 4.83Aa | 4.81Aa | 4.75Aab | 4.48Bbc | 4.72Bab | 4.38Cc | |||||
| LB | 4.58Ab | 4.92Aab | 5.04Aa | 5.12Aa | 5.28Aa | 5.00Aa | |||||
| PB | 5.15Aa | 5.13Aa | 4.81Ab | 4.60Bb | 4.89Bab | 4.85Aab | |||||
| WSC (% DM) | CK | 2.98Aa | 3.21Aa | 2.79Aa | 2.90Aa | 3.20Aa | 1.95Bb | 0.11 | ** | ** | ** |
| LP | 2.96Aab | 3.35Aa | 2.60ABab | 2.44Ab | 2.27Bb | 2.86Aab | |||||
| LB | 2.96Aa | 2.92Aa | 1.51Cb | 0.57Bc | 0.77Cc | 0.89Dc | |||||
| PB | 2.79Aa | 3.07Aa | 2.06BCb | 1.28Bc | 1.01Cc | 1.30Cc | |||||
| Items | Treatment | Days | Significance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 7 | 15 | 60 | B4 | B8 | SEM | T | D | T × D | ||
| pH value | CK | 5.57ABb | 5.58Ab | 5.55Ab | 5.47Ab | 5.62Aab | 6.04Aa | 0.07 | ** | ** | ** |
| LP | 5.67Aa | 4.31Ccd | 4.17Cd | 4.24Cd | 4.53Bc | 5.17Bb | |||||
| LB | 5.10Ba | 4.68Bbc | 4.59Bc | 4.72Bbc | 4.74Bbc | 4.92Bab | |||||
| PB | 5.32ABa | 4.30Ccd | 4.20Cd | 4.56BCbcd | 4.64Bbc | 4.98Bab | |||||
| Lactic acid (g/kg DM) | CK | 0.91ABb | 1.18Cb | 1.33Bb | 3.15Ca | 1.15Cb | 0.74Cb | 0.71 | ** | ** | ** |
| LP | 3.38Ad | 7.00Bcd | 9.98ABbc | 16.73Aa | 12.53Aab | 11.31Abc | |||||
| LB | 0.17Bb | 4.00BCab | 8.39Ba | 8.16Ba | 6.33Ba | 5.97Ba | |||||
| PB | 1.92ABb | 12.23Aa | 18.20Aa | 15.27Aa | 14.10Aa | 13.63Aa | |||||
| Acetic acid (g/kg DM) | CK | 7.20Aa | 7.43Aa | 8.24ABa | 6.28Ca | 3.37Cb | 1.33Bb | 0.38 | ** | ** | ** |
| LP | 6.43Abc | 4.81Bc | 6.60BCbc | 9.03Ba | 7.53Bab | 6.19Abc | |||||
| LB | 3.81Ac | 1.94Cc | 4.40Cc | 12.77Aa | 11.03Aab | 8.85Ab | |||||
| PB | 3.89Ac | 8.30Aab | 10.77Aab | 11.54Aa | 9.20ABab | 7.61Ab | |||||
| NH3-N (g/kg DM) | CK | 0.24Ad | 0.33Acd | 0.47Ac | 0.71Ab | 0.84Aab | 0.93ABa | 0.03 | ** | ** | NS |
| LP | 0.23Ac | 0.17Ac | 0.33Ac | 0.65Ab | 0.67Bb | 1.04Aa | |||||
| LB | 0.29Ade | 0.17Ae | 0.43Acd | 0.61Abc | 0.68Bb | 0.89ABa | |||||
| PB | 0.14Ad | 0.16Ad | 0.36Ac | 0.59Ab | 0.65Bb | 0.78Ba | |||||
| Items | Treatment | Days | Significance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 7 | 15 | 60 | B4 | B8 | SEM | T | D | T × D | ||
| Lactic acid bacteria (log10 cfu/g FM) | CK | 6.43Aa | 5.3Bb | 4.44Bc | 2.73Cd | 5.11Abc | 6.87Aa | 0.17 | ** | ** | ** |
| LP | 6.73Aa | 7.09Aa | 7.24Aa | 5.18Bb | 2.32Bd | 3.56Cc | |||||
| LB | 6.71Aa | 6.93Aa | 7.01Aa | 5.71Ab | 4.98Ac | 4.47BCd | |||||
| PB | 6.73Aa | 7.08Aa | 7.07Aa | 4.86Bb | 4.41Ab | 5.11Bb | |||||
| Aerobic bacteria (log10 cfu/g FM) | CK | 5.77Aab | 6.88Aa | 5.82Cab | 2.58Bc | 4.99Ab | 6.83Aa | 0.18 | * | ** | ** |
| LP | 6.74Aa | 7.08Aa | 6.54ABa | 4.60Ab | 2.62Cd | 3.50Bc | |||||
| LB | 5.93Ab | 7.09Aa | 6.24Bab | 4.37Ac | 4.09Bc | 4.05Bc | |||||
| PB | 6.62Aa | 7.15Aa | 6.9Aa | 4.42Ab | 4.45ABb | 4.30Bb | |||||
| Coliform bacteria (log10 cfu/g FM) | CK | 1.31Ab | 3.59Aa | 3.39a | ND | 1.90ab | 3.90Aa | 0.19 | ** | ** | * |
| LP | 1.10Aa | ND | ND | ND | ND | 1.39Ba | |||||
| LB | 1.63Aab | 2.98ABa | ND | ND | ND | 2.06ABab | |||||
| PB | 1.19Ab | 1.20ABb | ND | ND | ND | 3.31ABa | |||||
| Yeast (log10 cfu/g FM) | CK | 6.62Aa | 6.92Ba | 6.84Aa | 3.18Bc | 5.43Ab | 6.95Aa | 0.16 | * | ** | ** |
| LP | 6.73Aa | 6.91Ba | 6.80Aa | 4.47Ab | 3.10Bc | 5.11Bb | |||||
| LB | 5.76Aab | 6.92Ba | 6.89Aa | 4.38Ac | 4.84Abc | 5.82ABab | |||||
| PB | 6.91Aa | 7.39Aa | 6.95Aa | 4.42Ab | 4.74Ab | 5.14Bb | |||||
| Molds (log10 cfu/g FM) | CK | 1.74bc | 1.12bc | ND | ND | 2.90b | 7.01a | 0.19 | ** | ** | ** |
| LP | ND | ND | ND | ND | ND | ND | |||||
| LB | ND | ND | ND | ND | ND | ND | |||||
| PB | ND | ND | ND | ND | ND | ND | |||||
| Items | Treatment | Days | Significance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 7 | 15 | 60 | B4 | B8 | SEM | T | D | T × D | ||
| OTU | CK | 21.67Ac | 76Aa | 57.33Aab | 39.67Abc | 37.67ABbc | 23.00Bc | 2.34 | ** | ** | NS |
| LP | 18.33Ab | 17Bb | 16.33Bb | 54.33Aa | 53.67Aa | 63.33Aa | |||||
| LB | 26.00Ab | 20.67Bb | 17.33Bb | 41.00Aab | 38.67ABab | 51.67Aa | |||||
| PB | 19.00Aab | 12.00Bb | 16.67Bab | 36.67Aa | 25.00Bab | 29.33Bab | |||||
| Chao1 | CK | 36.44Ab | 101.33Aa | 70.00Aab | 52.76Ab | 48.70Ab | 30.71Bb | 3.34 | NS | * | ** |
| LP | 26.11Ab | 20.33Bb | 33.00Ab | 86.60Aa | 68.82Aa | 76.83Aa | |||||
| LB | 42.23Aa | 30.67Ba | 45.50Aa | 74.00Aa | 63.11Aa | 62.76Aa | |||||
| PB | 28.90Aab | 18.42Bb | 29.11Aab | 51.46Aab | 42.73Aab | 54.29ABa | |||||
| Shannon | CK | 0.90Ab | 3.07Aa | 1.14Ab | 1.31Ab | 1.48Ab | 0.85Bb | 0.14 | ** | ** | ** |
| LP | 0.33Ac | 0.55Bbc | 0.29Bc | 1.93Ab | 0.97ABbc | 3.37Aa | |||||
| LB | 1.34Aa | 0.54Bbc | 0.24Bc | 0.78Ab | 0.79BCb | 1.06Bab | |||||
| PB | 0.63Aab | 1.01Ba | 0.89Aab | 0.93Aab | 0.32Cab | 0.29Bb | |||||
| Simpson | CK | 0.29ABb | 0.77Aa | 0.27Ab | 0.36Ab | 0.48Aab | 0.27Bb | 0.03 | * | ** | ** |
| LP | 0.10Bc | 0.17Cc | 0.07Bc | 0.54Aab | 0.25Bbc | 0.77Aa | |||||
| LB | 0.56Aa | 0.15Cbc | 0.06Bc | 0.24Ab | 0.24Bb | 0.27Bb | |||||
| PB | 0.22ABbc | 0.48Ba | 0.38Aab | 0.22Abc | 0.09Bc | 0.06Bc | |||||
| ACE | CK | 79.40Aa | 96.98Aa | 74.76Aa | 67.29Aa | 67.62Aa | 40.45Ba | 4.39 | NS | * | NS |
| LP | 32.83Ab | 24.64Bb | 42.03Ab | 94.75Aa | 89.28Aa | 93.54Aa | |||||
| LB | 53.05Aab | 43.60Bb | 44.07Ab | 122.99Aa | 77.94Aab | 72.6ABab | |||||
| PB | 34.58Aa | 31.91Ba | 45.79Aa | 66.23Aa | 53.19Aa | 75.22ABa | |||||
| Coverage | CK | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.00 | NS | NS | NS |
| LP | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | |||||
| LB | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | |||||
| PB | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Liu, Y.; Wang, Z.; Bao, J.; Zhao, M.; Si, Q.; Sun, P.; Ge, G.; Jia, Y. Effects of Different Types of LAB on Dynamic Fermentation Quality and Microbial Community of Native Grass Silage during Anaerobic Fermentation and Aerobic Exposure. Microorganisms 2023, 11, 513. https://doi.org/10.3390/microorganisms11020513
Zhang J, Liu Y, Wang Z, Bao J, Zhao M, Si Q, Sun P, Ge G, Jia Y. Effects of Different Types of LAB on Dynamic Fermentation Quality and Microbial Community of Native Grass Silage during Anaerobic Fermentation and Aerobic Exposure. Microorganisms. 2023; 11(2):513. https://doi.org/10.3390/microorganisms11020513
Chicago/Turabian StyleZhang, Jiawei, Yichao Liu, Zhijun Wang, Jian Bao, Muqier Zhao, Qiang Si, Pengbo Sun, Gentu Ge, and Yushan Jia. 2023. "Effects of Different Types of LAB on Dynamic Fermentation Quality and Microbial Community of Native Grass Silage during Anaerobic Fermentation and Aerobic Exposure" Microorganisms 11, no. 2: 513. https://doi.org/10.3390/microorganisms11020513
APA StyleZhang, J., Liu, Y., Wang, Z., Bao, J., Zhao, M., Si, Q., Sun, P., Ge, G., & Jia, Y. (2023). Effects of Different Types of LAB on Dynamic Fermentation Quality and Microbial Community of Native Grass Silage during Anaerobic Fermentation and Aerobic Exposure. Microorganisms, 11(2), 513. https://doi.org/10.3390/microorganisms11020513




