Divalent Cation Signaling in Clostridium perfringens Spore Germination
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains Used in This Study
2.2. Spore Preparation and Purification
2.3. Spore Germination
2.4. Statistical Analysis
3. Results
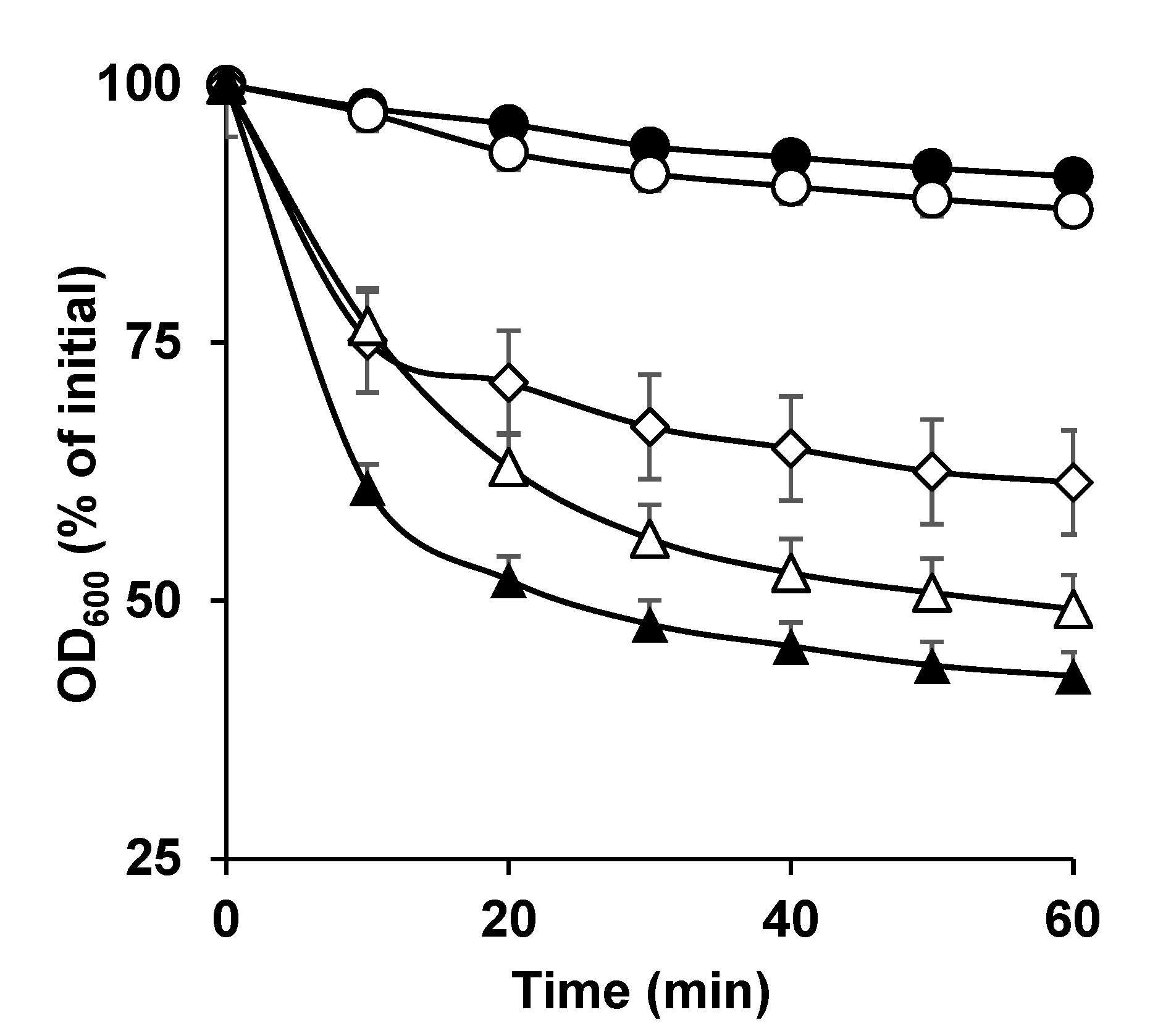
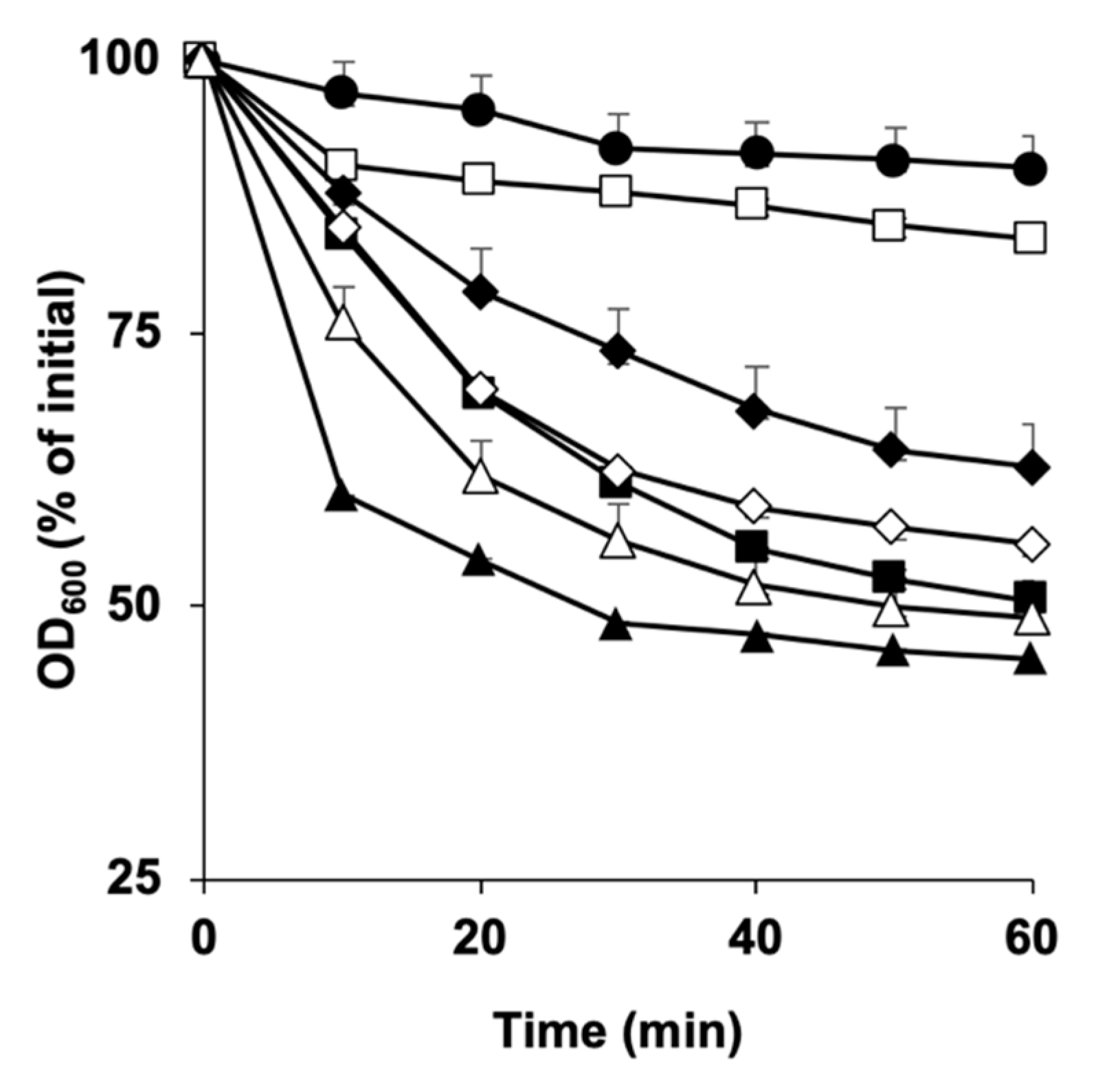
3.1. Germination of C. perfringens SM101 Spores in the Presence of Ca-DPA, DPA, and Ca2+
3.2. Germination of C. perfringens FP Isolate Spores with Different Calcium Salts
3.3. Germination of C. perfringens SM101 Spores with Other Spore Core-Specific Divalent Cations
3.4. Exogenous Ca2+ Is Essential for C. perfringens SM101 Spore Germination
3.5. Exogenous Mg2+ and Both Exogenous and Endogenous Mn2+ Are Essential for C. perfringens SM101 Spore Germination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grass, J.E.; Gould, L.H.; Mahon, B.E. Epidemiology of foodborne disease outbreaks caused by Clostridium perfringens, United States, 1998–2010. Foodborne Pathog. Dis. 2013, 10, 131–136. [Google Scholar] [CrossRef] [PubMed]
- McClane; Robertson, S.L.; Li, J. Clostridium Perfringens, 4th ed.; Doyle, M.P., Buchanan, R.L., Eds.; Food Microbiology: Fundamentals and Frontiers; ASM Press: Washington, DC, USA, 2013; pp. 465–489. [Google Scholar]
- Mehdizadeh Gohari, I.; Navarro, M.A.; Li, J.; Shrestha, A.; Uzal, F.; McClane, B.A. Pathogenicity and virulence of Clostridium perfringens. Virulence 2021, 12, 723–753. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, A.K. Clostridium botulinum, Clostridium perfringens, Clostridium difficile. In Foodborne Microbial Pathogens: Mechanisms and Pathogenesis; Bhunia, A.K., Ed.; Springer: New York, NY, USA, 2018; pp. 209–228. ISBN 978-1-4939-7349-1. [Google Scholar] [CrossRef]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States—Major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef]
- Uzal, F.A.; Freedman, J.C.; Shrestha, A.; Theoret, J.R.; Garcia, J.; Awad, M.M.; Adams, V.; Moore, R.J.; Rood, J.I.; McClane, B.A. Towards an understanding of the role of Clostridium perfringens toxins in human and animal disease. Future Microbiol. 2014, 9, 361–377. [Google Scholar] [CrossRef] [PubMed]
- Setlow, P. Summer meeting 201–When the sleepers wake: The germination of spores of Bacillus species. J. Appl. Microbiol. Summer Meet. 2013, 115, 1251–1268. [Google Scholar] [CrossRef] [PubMed]
- Paredes-Sabja, D.; Setlow, P.; Sarker, M.R. Germination of spores of Bacillales and Clostridiales species: Mechanisms and proteins involved. Trends Microbiol. 2011, 19, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Paredes-Sabja, D.; Torres, J.A.; Setlow, P.; Sarker, M.R. Clostridium perfringens Spore germination: Characterization of germinants and their receptors. J. Bacteriol. 2008, 190, 1190–1201. [Google Scholar] [CrossRef]
- Banawas, S.; Sarker, M.R. L-lysine (PH 6.0) induces germination of spores of Clostridium perfringens Type F isolates carrying chromosomal or plasmid-borne enterotoxin gene. Microb. Pathog. 2018, 123, 227–232. [Google Scholar] [CrossRef]
- Banawas, S.; Korza, G.; Paredes-Sabja, D.; Li, Y.; Hao, B.; Setlow, P.; Sarker, M.R. Location and stoichiometry of the protease CspB and the cortex-lytic enzyme SleC in Clostridium perfringens Spores. Food Microbiol. 2015, 50, 83–87. [Google Scholar] [CrossRef]
- Setlow, P.; Wang, S.; Li, Y.Q. Germination of spores of the orders Bacillales and Clostridiales. Annu. Rev. Microbiol. 2017, 71, 459–477. [Google Scholar] [CrossRef]
- Paredes-Sabja, D.; Setlow, P.; Sarker, M.R. SleC Is essential for cortex peptidoglycan hydrolysis during germination of spores of the pathogenic bacterium Clostridium perfringens. J. Bacteriol. 2009, 191, 2711–2720. [Google Scholar] [CrossRef] [PubMed]
- Foerster, H.F.; Foster, J.W. Response of bacillus spores to combinations of germinative compounds. J. Bacteriol. 1966, 91, 1168–1177. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.G.; Sidhu, R.; Yadav, N.K. Effect of monovalent and divalent ions upon the germination of Bacillus spores in the presence of nisin. J. Food Sci. 1971, 36, 896–898. [Google Scholar] [CrossRef]
- Jaye, M.; Ordal, Z.J. Germination of spores of Bacillus megaterium with divalent metal-dipicolinate chelates. J. Bacteriol. 1965, 89, 1617–1618. [Google Scholar] [CrossRef] [PubMed]
- Collie, R.E.; Kokai-Kun, J.F.; McClane, B.A. Phenotypic characterization of Enterotoxigenic Clostridium perfringens Isolates from non-foodborne human gastrointestinal diseases. Anaerobe 1998, 4, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Melville, S.B. Identification and characterization of sporulation-dependent promoters upstream of the enterotoxin gene (cpe) of Clostridium perfringens. J. Bacteriol. 1998, 180, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Paredes-Sabja, D.; Setlow, P.; Sarker, M.R. The protease CspB is essential for initiation of cortex hydrolysis and dipicolinic acid (DPA) release during germination of spores of Clostridium perfringens type A food poisoning isolates. Microbiology 2009, 155, 3464–3472. [Google Scholar] [CrossRef]
- Kokai-Kun, J.F.; Songer, J.G.; Czeczulin, J.R.; Chen, F.; McClane, B.A. Comparison of Western immunoblots and gene detection assays for identification of potentially enterotoxigenic isolates of Clostridium perfringens. J. Clin. Microbiol. 1994, 32, 2533–2539. [Google Scholar] [CrossRef]
- Duncan, C.L.; Strong, D.H. Improved medium for sporulation of Clostridium perfringens. Appl. Microbiol. 1968, 16, 82–89. [Google Scholar] [CrossRef]
- Kochan, T.J.; Somers, M.J.; Kaiser, A.M.; Shoshiev, M.S.; Hagan, A.K.; Hastie, J.L.; Giordano, N.P.; Smith, A.D.; Schubert, A.M.; Carlson, P.E.; et al. Intestinal calcium and bile salts facilitate germination of Clostridium difficile Spores. PLoS Pathog. 2017, 13, e1006443. [Google Scholar] [CrossRef]
- Setlow, P. Spore germination. Curr. Opin. Microbiol. 2003, 6, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Novak, J.S.; Juneja, V.K.; McClane, B.A. An ultrastructural comparison of spores from various strains of Clostridium perfringens and correlations with heat resistance parameters. Int. J. Food Microbiol. 2003, 86, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Paredes-Sabja, D.; Gonzalez, M.; Sarker, M.R.; Torres, J.A. Combined effects of hydrostatic pressure, temperature, and PH on the inactivation of spores of Clostridium perfringens Type A and Clostridium sporogenes in buffer solutions. J. Food Sci. 2007, 72, M202–M206. [Google Scholar] [CrossRef] [PubMed]
- Kiu, R.; Hall, L.J. An update on the human and animal enteric pathogen Clostridium perfringens. Emerg. Microbes Infect. 2018, 7, 141. [Google Scholar] [CrossRef] [PubMed]
- Fleming, H.P.; Ordal, Z.J. Responses of Bacillus subtilis spores to ionic environments during sporulation and germination. J. Bacteriol. 1964, 88, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Hudson, K.D.; Corfe, B.M.; Kemp, E.H.; Feavers, I.M.; Coote, P.J.; Moir, A. Localization of GerAA and GerAC germination proteins in the Bacillus subtilis Spore. J. Bacteriol. 2001, 183, 4317–4322. [Google Scholar] [CrossRef]
- Levinson, H.S.; Sevag, M.G. Stimulation of germination and respiration of the spores of Bacillus megaterium by manganese and monovalent anions. J. Gen. Physiol. 1953, 36, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Paredes-Sabja, D.; Udompijitkul, P.; Sarker, M.R. Inorganic phosphate and sodium ions are cogerminants for spores of Clostridium perfringens type A food poisoning-related isolates. Appl. Environ. Microbiol. 2009, 75, 6299–6305. [Google Scholar] [CrossRef]
- Ando, Y. Ionic germination of spores of Clostridium perfringens Type A. Jpn. J. Microbiol. 1974, 18, 433–439. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almatrafi, R.; Banawas, S.; Sarker, M.R. Divalent Cation Signaling in Clostridium perfringens Spore Germination. Microorganisms 2023, 11, 591. https://doi.org/10.3390/microorganisms11030591
Almatrafi R, Banawas S, Sarker MR. Divalent Cation Signaling in Clostridium perfringens Spore Germination. Microorganisms. 2023; 11(3):591. https://doi.org/10.3390/microorganisms11030591
Chicago/Turabian StyleAlmatrafi, Roua, Saeed Banawas, and Mahfuzur R. Sarker. 2023. "Divalent Cation Signaling in Clostridium perfringens Spore Germination" Microorganisms 11, no. 3: 591. https://doi.org/10.3390/microorganisms11030591
APA StyleAlmatrafi, R., Banawas, S., & Sarker, M. R. (2023). Divalent Cation Signaling in Clostridium perfringens Spore Germination. Microorganisms, 11(3), 591. https://doi.org/10.3390/microorganisms11030591





