Subsurface Bacterioplankton Structure and Diversity in the Strongly-Stratified Water Columns within the Equatorial Eastern Indian Ocean
Abstract
1. Introduction
2. Materials and Methods
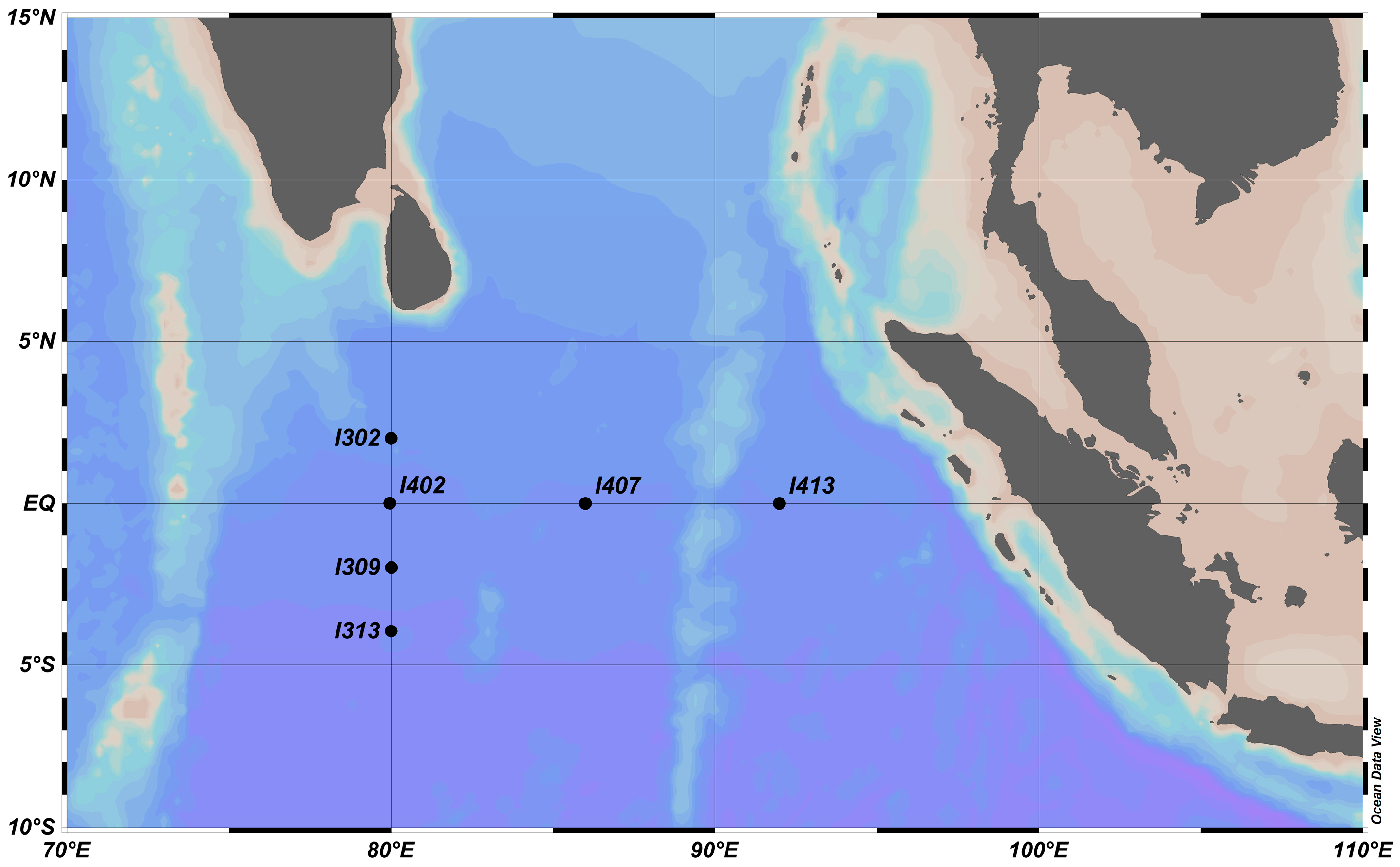
2.1. Sample Collection and Environmental Analysis
2.2. DNA Extraction, Library Preparation, and Sequencing
2.3. Downstream Analysis of Sequencing Reads
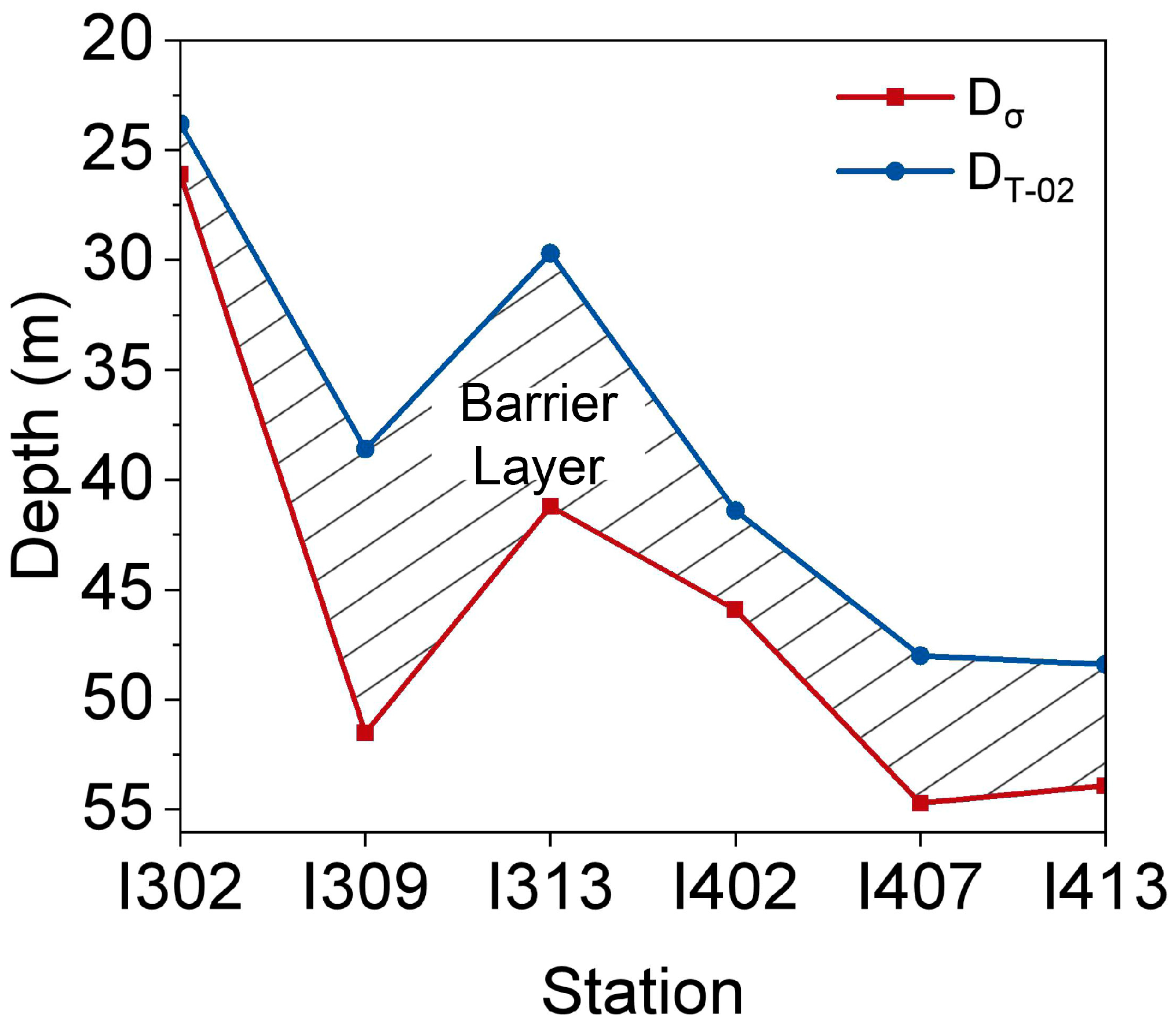
2.4. Determination of Stratification Strength and Mixed Layer Depth
2.5. Statistical Analyses
3. Results
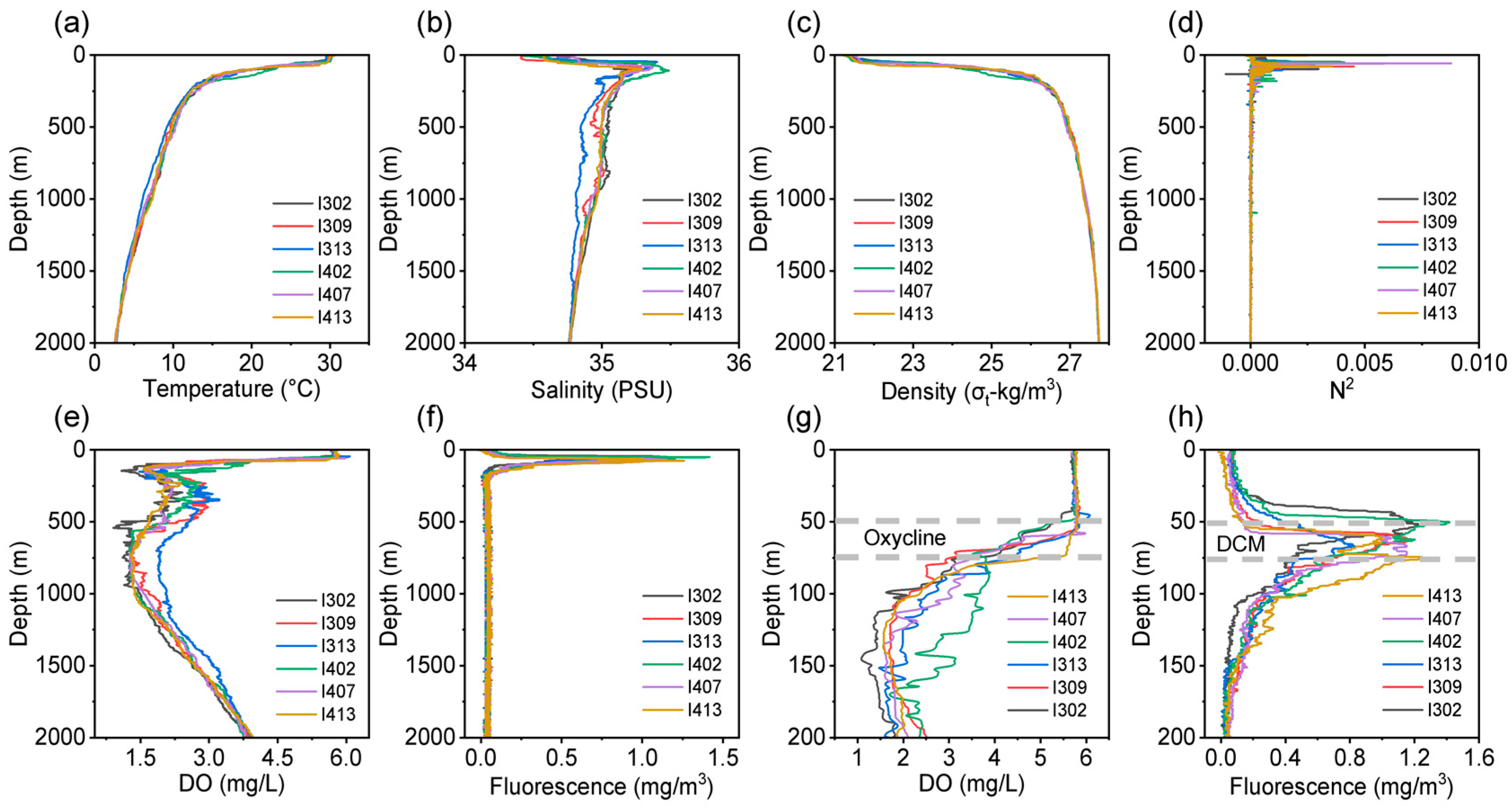
3.1. Physicochemical Gradients
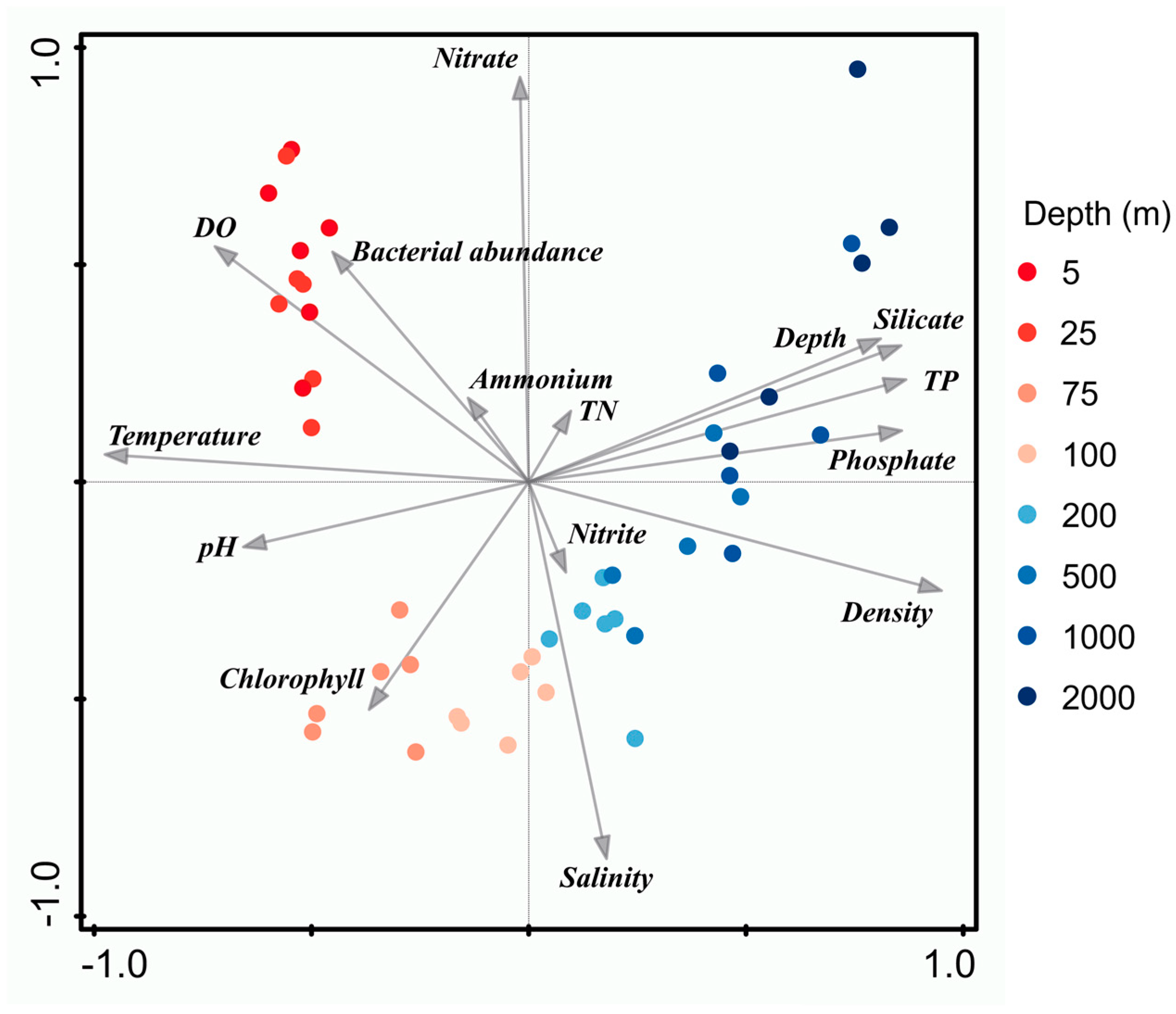
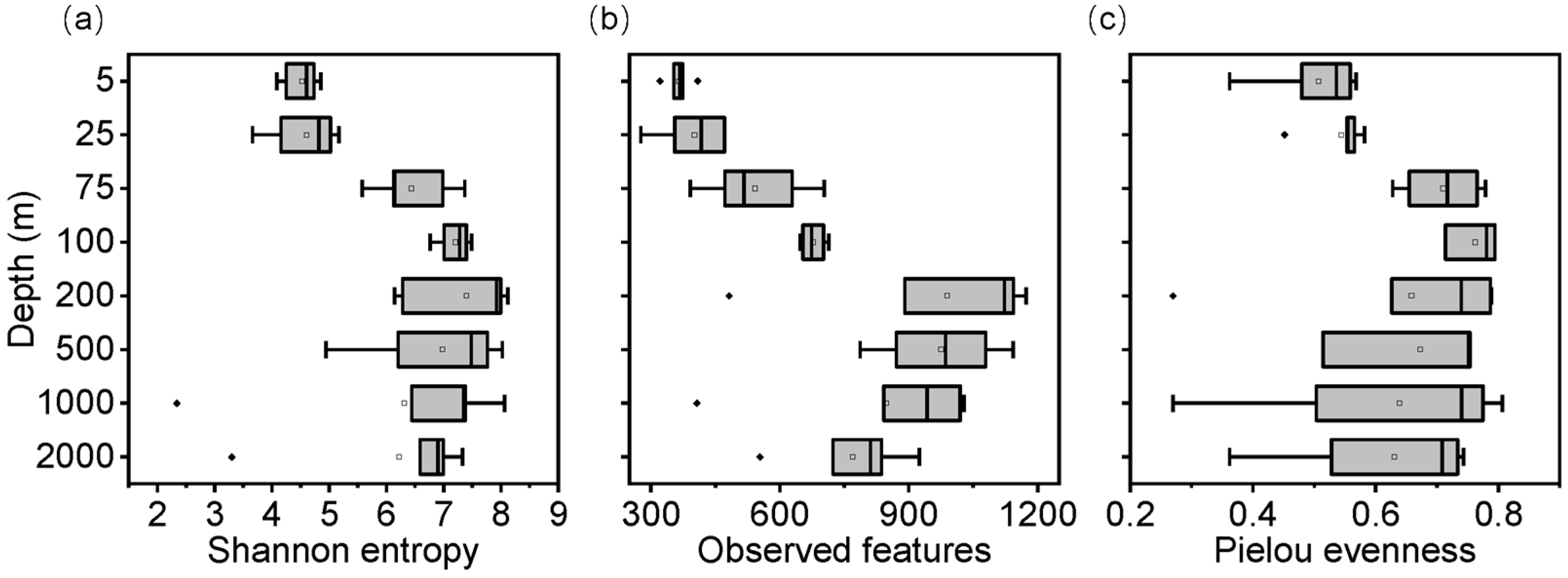
3.2. Vertical Alpha Diversity Patterns of Bacterioplankton
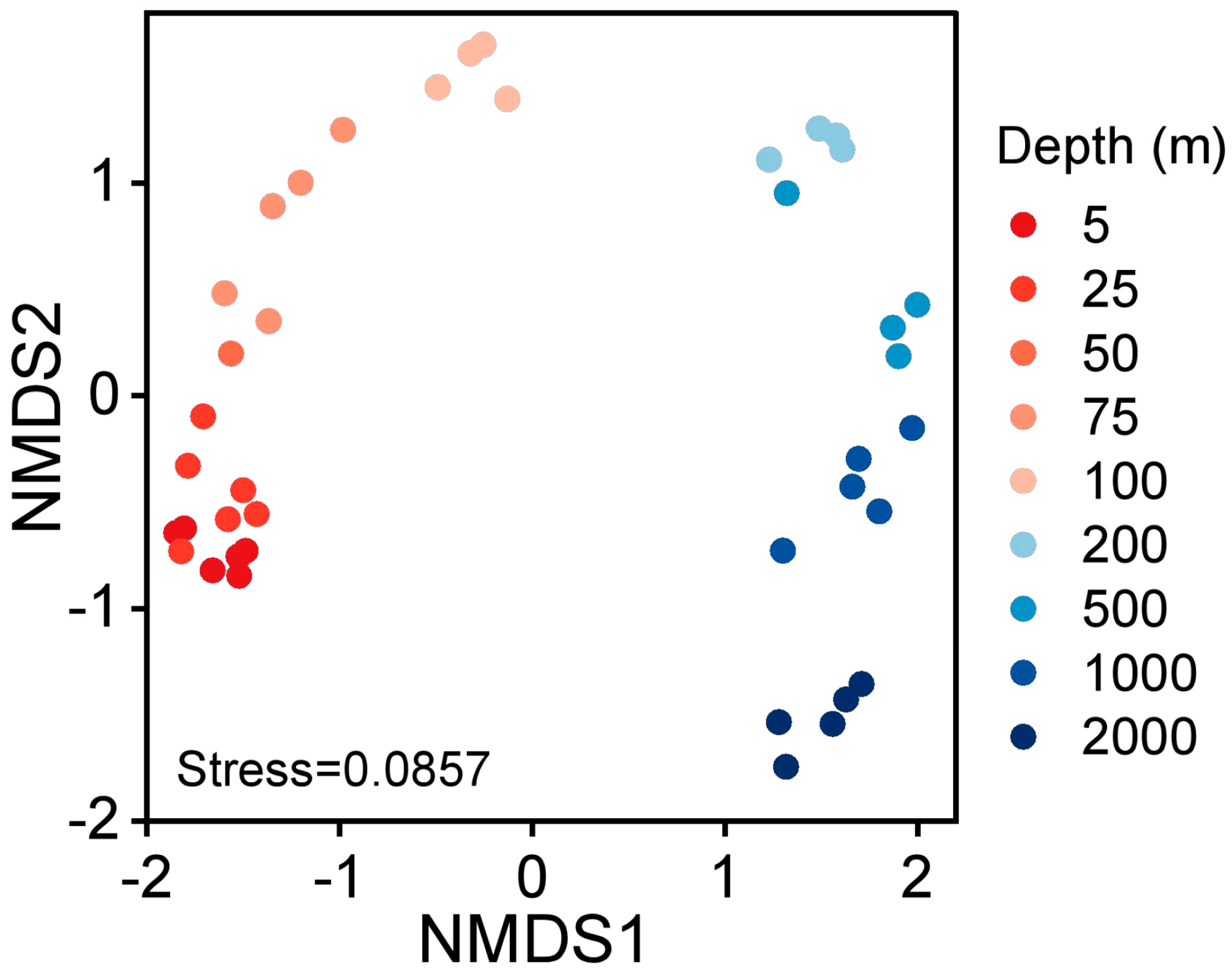
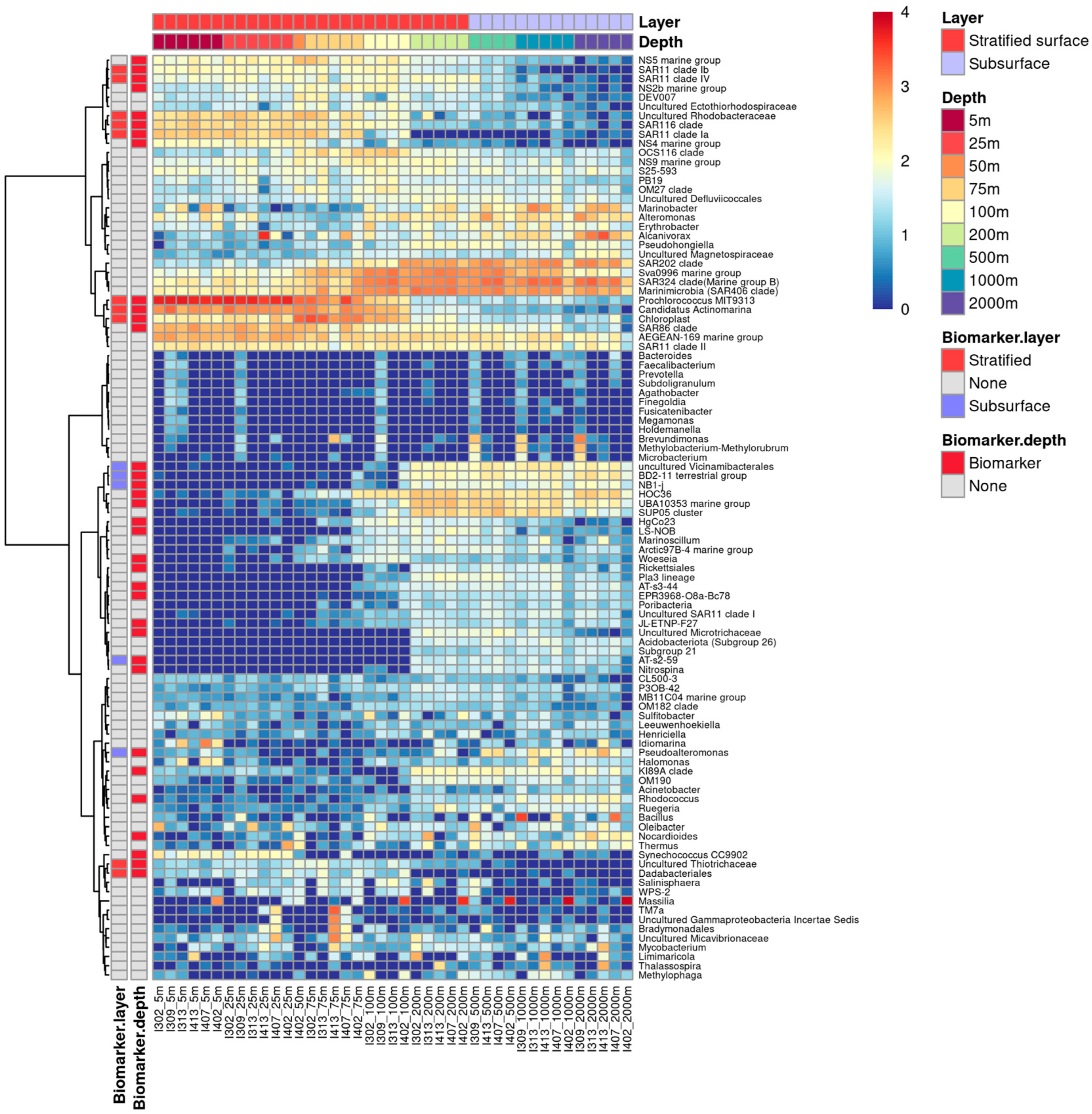
3.3. Depth-Related Bacterioplankton Community Compositions
4. Discussion
4.1. Vertical Environmental Patterns and Stratification
4.2. Impact of Stratification on the Vertical Structure of Bacterioplankton Communities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Laffoley, D.; Baxter, J.M. Explaining Ocean Warming: Causes, Scale, Effects and Consequences; IUCN: Gland, Switzerland, 2016. [Google Scholar]
- Boyd, P.W. Beyond ocean acidification. Nat. Geosci. 2011, 4, 273–274. [Google Scholar] [CrossRef]
- Boyd, P.W.; Strzepek, R.; Fu, F.; Hutchins, D.A. Environmental control of open-ocean phytoplankton groups: Now and in the future. Limnol. Oceanogr. 2010, 55, 1353–1376. [Google Scholar] [CrossRef]
- Doney, S.C. Oceanography -Plankton in a warmer world. Nature 2006, 444, 695–696. [Google Scholar] [CrossRef]
- Doney, S.C. The Growing Human Footprint on Coastal and Open-Ocean Biogeochemistry. Science 2010, 328, 1512–1516. [Google Scholar] [CrossRef]
- Li, G.; Cheng, L.; Zhu, J.; Trenberth, K.E.; Mann, M.E.; Abraham, J.P. Increasing ocean stratification over the past half-century. Nat. Clim. Chang. 2020, 10, 1116–1123. [Google Scholar] [CrossRef]
- Roxy, M.K.; Modi, A.; Murtugudde, R.; Valsala, V.; Panickal, S.; Kumar, S.P.; Ravichandran, M.; Vichi, M.; Lévy, M. A reduction in marine primary productivity driven by rapid warming over the tropical Indian Ocean. Geophys. Res. Lett. 2016, 43, 826–833. [Google Scholar] [CrossRef]
- Bertagnolli, A.D.; Stewart, F.J. Microbial niches in marine oxygen minimum zones. Nat. Rev. Genet. 2018, 16, 723–729. [Google Scholar] [CrossRef]
- Keeling, R.F.; Körtzinger, A.; Gruber, N. Ocean Deoxygenation in a Warming World. Annu. Rev. Mar. Sci. 2010, 2, 199–229. [Google Scholar] [CrossRef]
- Matear, R.J.; Hirst, A.C.; McNeil, B.I. Changes in dissolved oxygen in the Southern Ocean with climate change. Geochem. Geophys. Geosystems 2000, 1, 2000GC000086. [Google Scholar] [CrossRef]
- Sarmiento, J.L.; Hughes, T.M.C.; Stouffer, R.J.; Manabe, S. Simulated response of the ocean carbon cycle to anthropogenic climate warming. Nature 1998, 393, 245–249. [Google Scholar] [CrossRef]
- Cheng, W.-H.; Lu, H.-P.; Chen, C.-C.; Jan, S.; Hsieh, C.-H. Vertical Beta-Diversity of Bacterial Communities Depending on Water Stratification. Front. Microbiol. 2020, 11, 449. [Google Scholar] [CrossRef]
- Fu, W.; Randerson, J.T.; Moore, J.K. Climate change impacts on net primary production (NPP) and export production (EP) regulated by increasing stratification and phytoplankton community structure in the CMIP5 models. Biogeosciences 2016, 13, 5151–5170. [Google Scholar] [CrossRef]
- Bouman, H.A.; Ulloa, O.; Barlow, R.; Li, W.K.W.; Platt, T.; Zwirglmaier, K.; Scanlan, D.J.; Sathyendranath, S. Water-column stratification governs the community structure of subtropical marine picophytoplankton. Environ. Microbiol. Rep. 2011, 3, 473–482. [Google Scholar] [CrossRef]
- Campbell, L.; Landry, M.; Constantinou, J.; Nolla, H.; Brown, S.; Liu, H.; Caron, D. Response of microbial community structure to environmental forcing in the Arabian Sea. Deep Sea Res. Part I 1998, 45, 2301–2325. [Google Scholar] [CrossRef]
- Zinser, E.R.; Johnson, Z.I.; Coe, A.; Karaca, E.; Veneziano, D.; Chisholm, S.W. Influence of light and temperature on Prochlorococcus ecotype distributions in the Atlantic Ocean. Limnol. Oceanogr. 2007, 52, 2205–2220. [Google Scholar] [CrossRef]
- Irion, S.; Christaki, U.; Berthelot, H.; L’Helguen, S.; Jardillier, L. Small phytoplankton contribute greatly to CO2-fixation after the diatom bloom in the Southern Ocean. ISME J. 2021, 15, 2509–2522. [Google Scholar] [CrossRef] [PubMed]
- Frank, A.H.; Garcia, J.A.L.; Herndl, G.J.; Reinthaler, T. Connectivity between surface and deep waters determines prokaryotic diversity in the North Atlantic Deep Water. Environ. Microbiol. 2016, 18, 2052–2063. [Google Scholar] [CrossRef]
- Condie, S.A.; Bormans, M. The Influence of Density Stratification on Particle Settling, Dispersion and Population Growth. J. Theor. Biol. 1997, 187, 65–75. [Google Scholar] [CrossRef]
- Mestre, M.; Ruiz-González, C.; Logares, R.; Duarte, C.M.; Gasol, J.M.; Sala, M.M. Sinking particles promote vertical connectivity in the ocean microbiome. Proc. Natl. Acad. Sci. USA 2018, 115, E6799–E6807. [Google Scholar] [CrossRef] [PubMed]
- Cram, J.; Xia, L.C.; Needham, D.M.; Sachdeva, R.; Sun, F.; Fuhrman, J. Cross-depth analysis of marine bacterial networks suggests downward propagation of temporal changes. ISME J. 2015, 9, 2573–2586. [Google Scholar] [CrossRef]
- Baltar, F.; Arístegui, J.; Gasol, J.M.; Sintes, E.; Herndl, G.J. Evidence of prokaryotic metabolism on suspended particulate organic matter in the dark waters of the subtropical North Atlantic. Limnol. Oceanogr. 2009, 54, 182–193. [Google Scholar] [CrossRef]
- Miki, T.; Yokokawa, T.; Nagata, T.; Yamamura, N. Immigration of prokaryotes to local environments enhances remineralization efficiency of sinking particles: A metacommunity model. Mar. Ecol. Prog. Ser. 2008, 366, 1–14. [Google Scholar] [CrossRef]
- Deuser, W. Seasonal and interannual variations in deep-water particle fluxes in the Sargasso Sea and their relation to surface hydrography. Deep. Sea Res. Part A 1986, 33, 225–246. [Google Scholar] [CrossRef]
- Deuser, W.; Ross, E.; Anderson, R. Seasonality in the supply of sediment to the deep Sargasso Sea and implications for the rapid transfer of matter to the deep ocean. Deep. Sea Res. Part A 1981, 28, 495–505. [Google Scholar] [CrossRef]
- Boyd, P.W. Environmental factors controlling phytoplankton processes in the southern ocean. J. Phycol. 2002, 38, 844–861. [Google Scholar] [CrossRef]
- Cavender-Bares, K.K.; Karl, D.; Chisholm, S. Nutrient gradients in the western North Atlantic Ocean: Relationship to microbial community structure and comparison to patterns in the Pacific Ocean. Deep. Sea Res. Part I 2001, 48, 2373–2395. [Google Scholar] [CrossRef]
- Dhame, S.; Taschetto, A.S.; Santoso, A.; Meissner, K.J. Indian Ocean warming modulates global atmospheric circulation trends. Clim. Dyn. 2020, 55, 2053–2073. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, Y.; Feng, M.; Wang, T.; Zhang, N.; Wijffels, S. Decadal trends of the upper ocean salinity in the tropical Indo-Pacific since mid-1990s. Sci. Rep. 2015, 5, 16050. [Google Scholar] [CrossRef]
- Liu, X.; Xie, N.; Bai, M.; Li, J.; Wang, G. Composition change and decreased diversity of microbial eukaryotes in the coastal upwelling waters of South China Sea. Sci. Total. Environ. 2021, 795, 148892. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Sen, B.; Shang, J.; He, Y.; Xie, N.; Zhang, Y.; Zhang, J.; Johnson, Z.I.; Wang, G. Seasonal influence of scallop culture on nutrient flux, bacterial pathogens and bacterioplankton diversity across estuaries off the Bohai Sea Coast of Northern China. Mar. Pollut. Bull. 2017, 124, 411–420. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Holmes, S.P. Exact sequence variants should replace operational taxonomic units in marker-gene data analysis. ISME J. 2017, 11, 2639–2643. [Google Scholar] [CrossRef]
- Yilmaz, P.; Parfrey, L.W.; Yarza, P.; Gerken, J.; Pruesse, E.; Quast, C.; Schweer, T.; Peplies, J.; Ludwig, W.; Glöckner, F.O. The SILVA and “all-species living tree project (LTP)” taxonomic frameworks. Nucleic Acids Res. 2014, 42, D643–D648. [Google Scholar] [CrossRef]
- Sprintall, J.; Tomczak, M. Evidence of the barrier layer in the surface layer of the tropics. J. Geophys. Res. Atmos. 1992, 97, 7305–7316. [Google Scholar] [CrossRef]
- Lai, J. Canoco 5: A new version of an ecological multivariate data ordination program. Biodivers. Sci. 2013, 21, 765. [Google Scholar]
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Mandal, S.; Van Treuren, W.; White, R.A.; Eggesbø, M.; Knight, R.T.; Peddada, S.D. Analysis of composition of microbiomes: A novel method for studying microbial composition. Microb. Ecol. Health Dis. 2015, 26, 27663. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Wu, C.; Guo, C.; Gui, J.; Wei, Y.; Sun, J. The Composition and Primary Metabolic Potential of Microbial Communities Inhabiting the Surface Water in the Equatorial Eastern Indian Ocean. Biology 2021, 10, 248. [Google Scholar] [CrossRef]
- Qian, P.-Y.; Wang, Y.; Lee, O.O.; Lau, S.C.K.; Yang, J.; Lafi, F.F.; Al-Suwailem, A.; Wong, T.Y.H. Vertical stratification of microbial communities in the Red Sea revealed by 16S rDNA pyrosequencing. ISME J. 2011, 5, 568. [Google Scholar] [CrossRef]
- Zhao, F.; Filker, S.; Xu, K.; Huang, P.; Zheng, S. Patterns and Drivers of Vertical Distribution of the Ciliate Community from the Surface to the Abyssopelagic Zone in the Western Pacific Ocean. Front. Microbiol. 2017, 8, 2559. [Google Scholar] [CrossRef] [PubMed]
- Saji, N.H.; Goswami, B.N.; Vinayachandran, P.N.; Yamagata, T. A dipole mode in the tropical Indian Ocean. Nature 1999, 401, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Aparna, A.R.; Girishkumar, M.S. Mixed layer heat budget in the eastern equatorial Indian Ocean during the two consecutive positive Indian Ocean dipole events in 2018 and 2019. Clim. Dyn. 2022, 58, 3297–3315. [Google Scholar] [CrossRef]
- Shi, J.-R.; Xie, S.-P.; Talley, L.D. Evolving Relative Importance of the Southern Ocean and North Atlantic in Anthropogenic Ocean Heat Uptake. J. Clim. 2018, 31, 7459–7479. [Google Scholar] [CrossRef]
- Chu, P.C.; Fan, C. Objective determination of global ocean surface mixed layer depth. In Proceedings of the OCEANS 2010 MTS/IEEE SEATTLE, Seattle, WA, USA, 20–23 September 2010; pp. 1001–1007. [Google Scholar] [CrossRef]
- Fee, E.J.; Hecky, R.E.; Regehr, G.W.; Hendzel, L.L.; Wilkinson, P. Effects of Lake Size on Nutrient Availability in the Mixed Layer during Summer Stratification. Can. J. Fish. Aquat. Sci. 1994, 51, 2756–2768. [Google Scholar] [CrossRef]
- François, R.; Altabet, M.A.; Yu, E.-F.; Sigman, D.M.; Bacon, M.P.; Frank, M.; Bohrmann, G.; Bareille, G.; Labeyrie, L.D. Contribution of Southern Ocean surface-water stratification to low atmospheric CO2 concentrations during the last glacial period. Nature 1997, 389, 929–935. [Google Scholar] [CrossRef]
- Sigman, D.M.; Jaccard, S.L.; Haug, G.H. Polar ocean stratification in a cold climate. Nature 2004, 428, 59–63. [Google Scholar] [CrossRef]
- Behrenfeld, M.J.; O’Malley, R.T.; Siegel, D.A.; McClain, C.R.; Sarmiento, J.L.; Feldman, G.C.; Milligan, A.J.; Falkowski, P.G.; Letelier, R.M.; Boss, E.S. Climate-driven trends in contemporary ocean productivity. Nature 2006, 444, 752–755. [Google Scholar] [CrossRef]
- Goes, J.I.; Thoppil, P.G.; Gomes, H.D.R.; Fasullo, J.T. Warming of the Eurasian Landmass Is Making the Arabian Sea More Productive. Science 2005, 308, 545–547. [Google Scholar] [CrossRef]
- Gregg, W.W.; Casey, N.W.; McClain, C.R. Recent trends in global ocean chlorophyll. Geophys. Res. Lett. 2005, 32, L03606. [Google Scholar] [CrossRef]
- Jiang, C.F.; Qiao, F.L.; Wang, G.S.; Zhao, C. Verification of the Mixed Layer Depth of Indian Ocean Forecasting System. Adv. Mar. Sci. 2014, 437–449. [Google Scholar]
- Abirami, B.; Radhakrishnan, M.; Kumaran, S.; Wilson, A. Impacts of global warming on marine microbial communities. Sci. Total. Environ. 2021, 791, 147905. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, J.A.; Steele, J.A.; Hewson, I.; Schwalbach, M.S.; Brown, M.V.; Green, J.L.; Brown, J.H. A latitudinal diversity gradient in planktonic marine bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 7774–7778. [Google Scholar] [CrossRef] [PubMed]
- Xiao-Peng, Q.; Ting-Lin, H.; Ming-Zheng, Z. Responses of dissolved oxygen on thermal stratification and eutrophication in lakes and reservoirs—An example in Zhoucun Reservoir in Zaozhuang City. China Environ. Sci. 2016, 36, 1547–1553. [Google Scholar]
- Yu, Z.; Yang, J.; Amalfitano, S.; Yu, X.; Liu, L. Effects of water stratification and mixing on microbial community structure in a subtropical deep reservoir. Sci. Rep. 2014, 4, srep05821. [Google Scholar] [CrossRef]
- Thompson, R.M.; Townsend, C.R. Energy availability, spatial heterogeneity and ecosystem size predict food-web structure in streams. Oikos 2005, 108, 137–148. [Google Scholar] [CrossRef]
- Wittebolle, L.; Marzorati, M.; Clement, L.; Balloi, A.; Daffonchio, D.; Heylen, K.; De Vos, P.; Verstraete, W.; Boon, N. Initial community evenness favours functionality under selective stress. Nature 2009, 458, 623–626. [Google Scholar] [CrossRef]
- Yang, X.; Huang, T.; Zhang, H. Effects of Seasonal Thermal Stratification on the Functional Diversity and Composition of the Microbial Community in a Drinking Water Reservoir. Water 2015, 7, 5525–5546. [Google Scholar] [CrossRef]
- Beisner, B.E.; Longhi, M.L. Spatial overlap in lake phytoplankton: Relations with environmental factors and consequences for diversity. Limnol. Oceanogr. 2013, 58, 1419–1430. [Google Scholar] [CrossRef]
- Walsh, E.; Kirkpatrick, J.B.; Rutherford, S.D.; Smith, D.; Sogin, M.; D’Hondt, S. Bacterial diversity and community composition from seasurface to subseafloor. ISME J. 2016, 10, 979–989. [Google Scholar] [CrossRef]
- Agogué, H.; Lamy, D.; Neal, P.R.; Sogin, M.L.; Herndl, G.J. Water mass-specificity of bacterial communities in the North Atlantic revealed by massively parallel sequencing. Mol. Ecol. 2011, 20, 258–274. [Google Scholar] [CrossRef]
- DeLong, E.F.; Preston, C.M.; Mincer, T.; Rich, V.; Hallam, S.J.; Frigaard, N.-U.; Martinez, A.; Sullivan, M.B.; Edwards, R.; Brito, B.R.; et al. Community Genomics Among Stratified Microbial Assemblages in the Ocean’s Interior. Science 2006, 311, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, J.A.; Hewson, I.; Schwalbach, M.S.; Steele, J.A.; Brown, M.V.; Naeem, S. Annually reoccurring bacterial communities are predictable from ocean conditions. Proc. Natl. Acad. Sci. USA 2006, 103, 13104–13109. [Google Scholar] [CrossRef] [PubMed]
- Zinger, L.; Amaral-Zettler, L.A.; Fuhrman, J.; Horner-Devine, M.C.; Huse, S.M.; Welch, D.B.M.; Martiny, J.; Sogin, M.; Boetius, A.; Ramette, A. Global Patterns of Bacterial Beta-Diversity in Seafloor and Seawater Ecosystems. PLoS ONE 2011, 6, e24570. [Google Scholar] [CrossRef] [PubMed]
- Longhurst, A.R. Ecological Geography of the Sea; Elsevier: Burlington, MA, USA, 2010. [Google Scholar]
- De Corte, D.; Sintes, E.; Yokokawa, T.; Reinthaler, T.; Herndl, G.J. Links between viruses and prokaryotes throughout the water column along a North Atlantic latitudinal transect. ISME J. 2012, 6, 1566–1577. [Google Scholar] [CrossRef]
- Malmstrom, R.; Coe, A.; Kettler, G.C.; Martiny, A.C.; Frias-Lopez, J.; Zinser, E.R.; Chisholm, S. Temporal dynamics of Prochlorococcus ecotypes in the Atlantic and Pacific oceans. ISME J. 2010, 4, 1252–1264. [Google Scholar] [CrossRef]
- Huisman, J.; Sharples, J.; Stroom, J.M.; Visser, P.M.; Kardinaal, W.E.A.; Verspagen, J.M.H.; Sommeijer, B. Changes in turbulent mixing shift competition for light between phytoplankton species. Ecology 2004, 85, 2960–2970. [Google Scholar] [CrossRef]
- Treusch, A.; Vergin, K.; Finlay, L.; Donatz, M.G.; Burton, R.M.; Carlson, C.; Giovannoni, S.J. Seasonality and vertical structure of microbial communities in an ocean gyre. ISME J. 2009, 3, 1148–1163. [Google Scholar] [CrossRef]
- Steiner, P.A.; Geijo, J.; Fadeev, E.; Obiol, A.; Sintes, E.; Rattei, T.; Herndl, G.J. Functional Seasonality of Free-Living and Particle-Associated Prokaryotic Communities in the Coastal Adriatic Sea. Front. Microbiol. 2020, 11, 584222. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Liu, X.; Xie, N.; Bai, M.; Liu, L.; Sen, B.; Wang, G. Subsurface Bacterioplankton Structure and Diversity in the Strongly-Stratified Water Columns within the Equatorial Eastern Indian Ocean. Microorganisms 2023, 11, 592. https://doi.org/10.3390/microorganisms11030592
Li J, Liu X, Xie N, Bai M, Liu L, Sen B, Wang G. Subsurface Bacterioplankton Structure and Diversity in the Strongly-Stratified Water Columns within the Equatorial Eastern Indian Ocean. Microorganisms. 2023; 11(3):592. https://doi.org/10.3390/microorganisms11030592
Chicago/Turabian StyleLi, Jiaqian, Xiuping Liu, Ningdong Xie, Mohan Bai, Lu Liu, Biswarup Sen, and Guangyi Wang. 2023. "Subsurface Bacterioplankton Structure and Diversity in the Strongly-Stratified Water Columns within the Equatorial Eastern Indian Ocean" Microorganisms 11, no. 3: 592. https://doi.org/10.3390/microorganisms11030592
APA StyleLi, J., Liu, X., Xie, N., Bai, M., Liu, L., Sen, B., & Wang, G. (2023). Subsurface Bacterioplankton Structure and Diversity in the Strongly-Stratified Water Columns within the Equatorial Eastern Indian Ocean. Microorganisms, 11(3), 592. https://doi.org/10.3390/microorganisms11030592









