Responses of Nitrous Oxide Emissions and Bacterial Communities to Experimental Freeze–Thaw Cycles in Contrasting Soil Types
Abstract
1. Introduction
2. Materials and Methods
2.1. Soils
2.2. Incubation Experiment and Gas Measurements
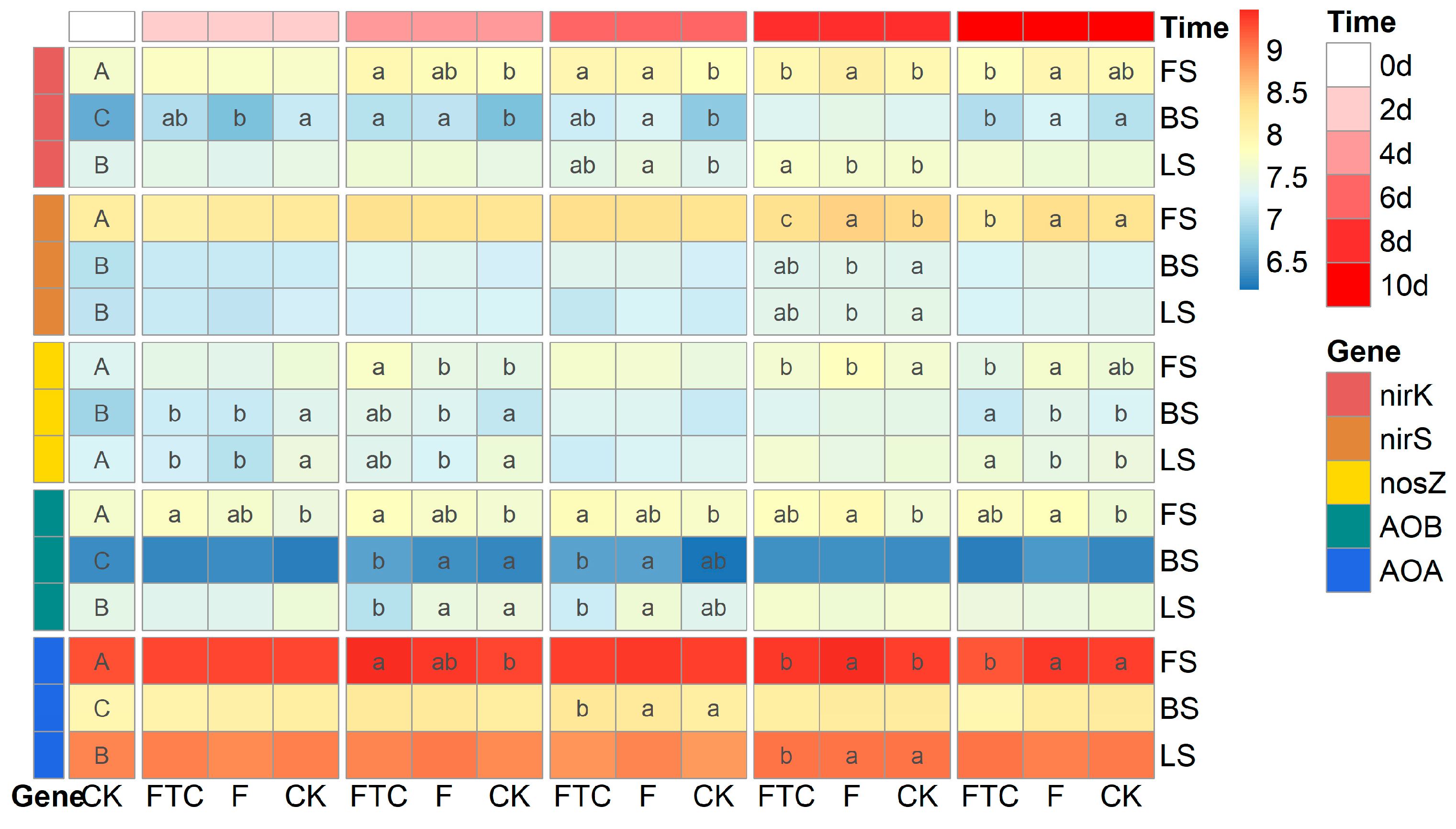
2.3. Quantification of N-Cycling Genes
2.4. Amplicon Sequencing and Bioinformatics Analysis
2.5. Statistical Analysis
3. Results
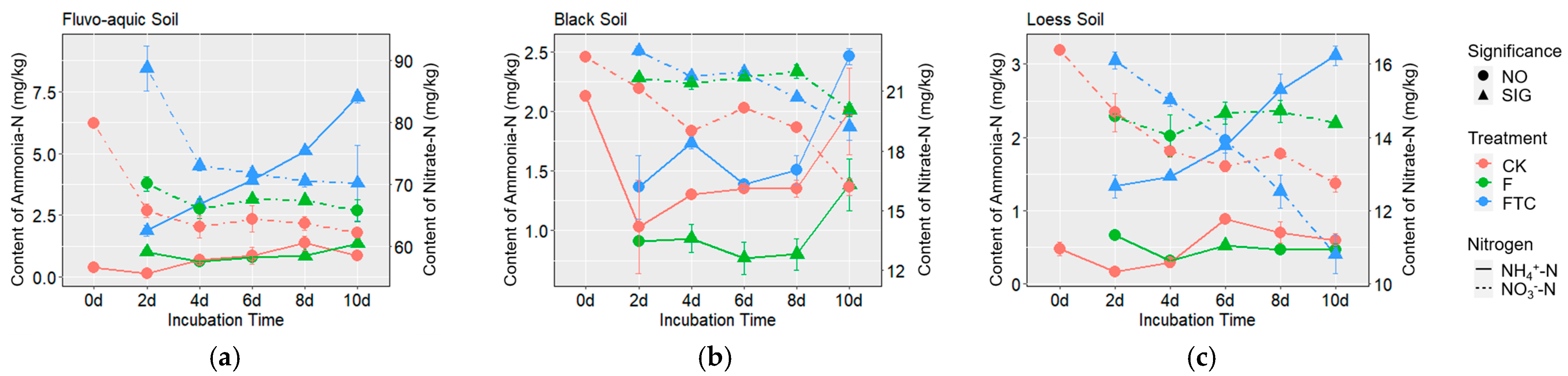
3.1. Soil Properties
3.2. Effects of Freeze–Thaw Cycles on Soil Denitrification
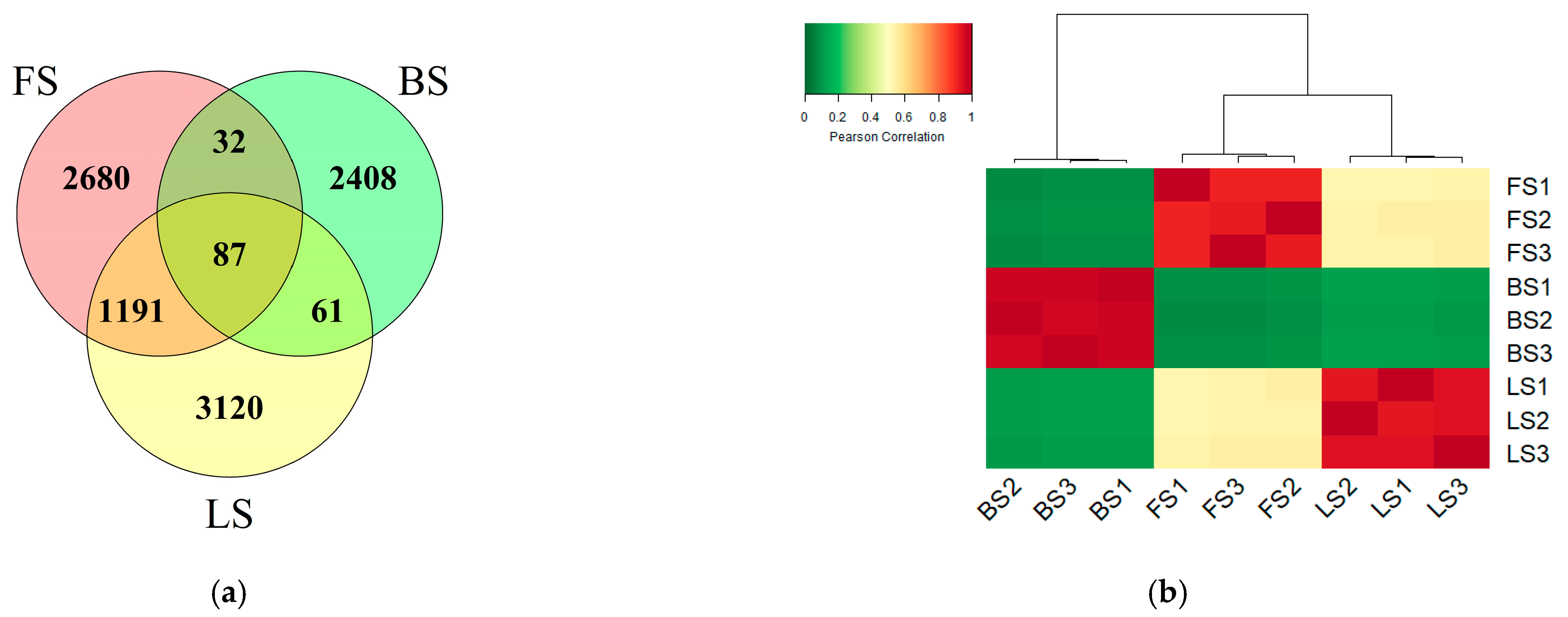
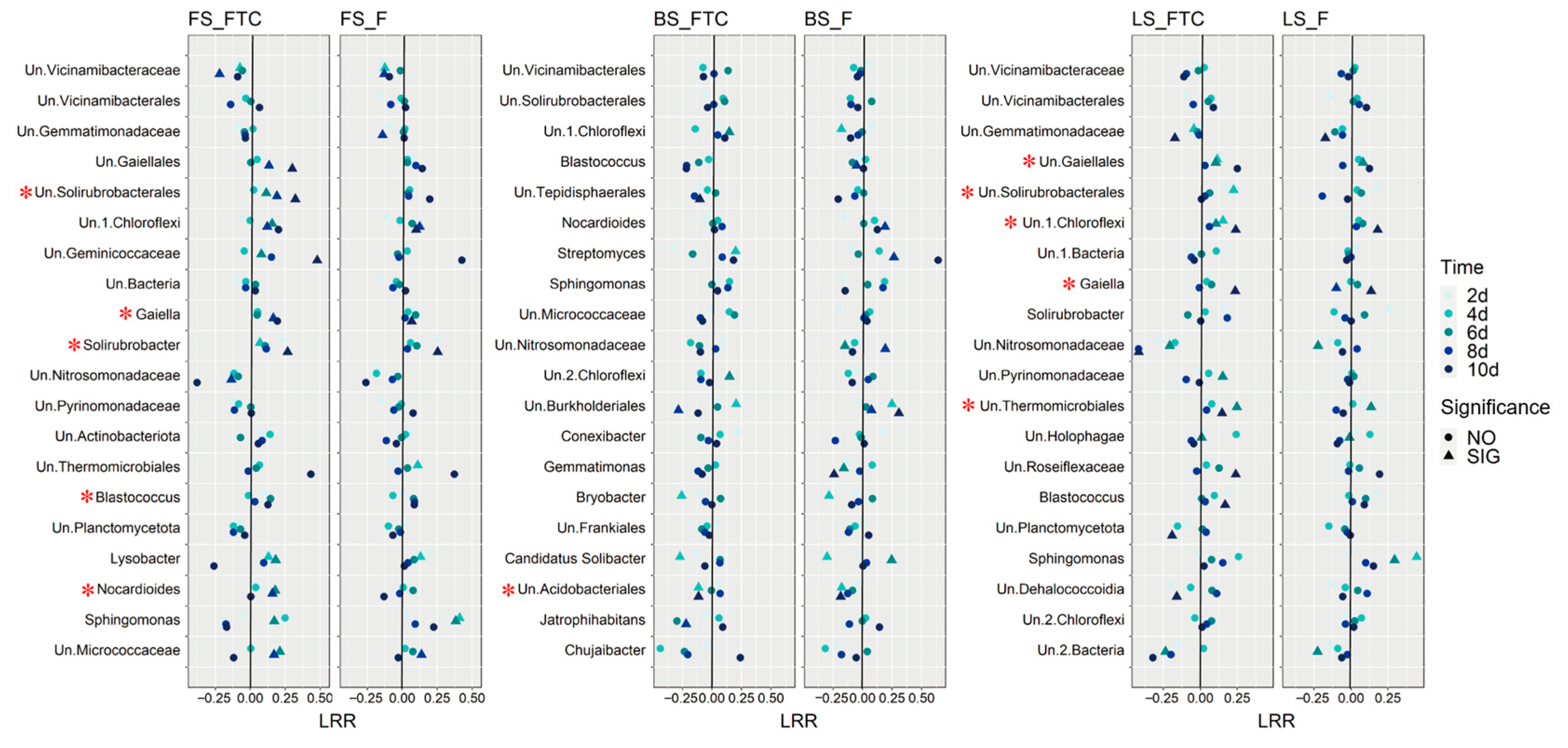
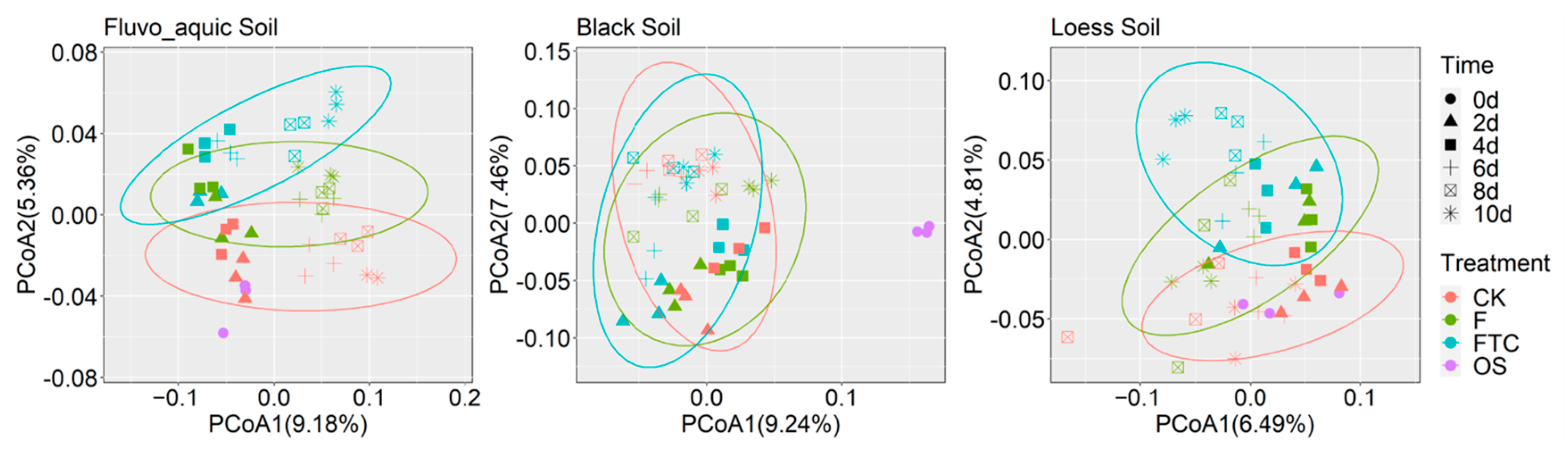
3.3. Effects of Freeze–Thaw Cycles on the Bacterial Community Structure and Diversity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matzner, E.; Borken, W. Do freeze-thaw events enhance C and N losses from soils of different ecosystems? A review. Eur. J. Soil Sci. 2008, 59, 274–284. [Google Scholar] [CrossRef]
- Kreyling, J.; Beierkuhnlein, C.; Pritsch, K.; Schloter, M.; Jentsch, A. Recurrent soil freeze-thaw cycles enhance grassland productivity. New Phytol. 2008, 177, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Grogan, P.; Michelsen, A.; Ambus, P.; Jonasson, S. Freeze-thaw regime effects on carbon and nitrogen dynamics in sub-arctic heath tundra mesocosms. Soil Biol. Biochem. 2004, 36, 641–654. [Google Scholar] [CrossRef]
- Henry, H.A.L. Soil freeze–thaw cycle experiments: Trends, methodological weaknesses and suggested improvements. Soil Biol. Biochem. 2007, 39, 977–986. [Google Scholar] [CrossRef]
- Zhang, S.; Qu, F.; Wang, X.; Xiao, Z.; Hao, X.; Wang, L. Freeze-thaw cycles changes soil nitrogen in a Mollisol sloping field in Northeast China. Nutr. Cycl. Agroecosyst. 2020, 116, 345–364. [Google Scholar] [CrossRef]
- Song, Y.; Zou, Y.C.; Wang, G.P.; Yu, X.F. Altered soil carbon and nitrogen cycles due to the freeze-thaw effect: A meta-analysis. Soil Biol. Biochem. 2017, 109, 35–49. [Google Scholar] [CrossRef]
- Christensen, S.; Christensen, B.T. Organic matter available for denitrification in different soil fractions: Effect of freeze/thaw cycles and straw disposal. J. Soil Sci. 1991, 42, 637–647. [Google Scholar] [CrossRef]
- Tenuta, M.; Sparling, B. A laboratory study of soil conditions affecting emissions of nitrous oxide from packed cores subjected to freezing and thawing. Can. J. Soil Sci. 2011, 91, 223–233. [Google Scholar] [CrossRef]
- van Bochove, E.; Theriault, G.; Rochette, P.; Jones, H.G.; Pomeroy, J.W. Thick ice layers in snow and frozen soil affecting gas emissions from agricultural soils during winter. J. Geophys. Res.-Atmos. 2001, 106, 23061–23071. [Google Scholar] [CrossRef]
- Risk, N.; Snider, D.; Wagner-Riddle, C. Mechanisms leading to enhanced soil nitrous oxide fluxes induced by freeze–thaw cycles. Can. J. Soil Sci. 2013, 93, 401–414. [Google Scholar] [CrossRef]
- Christensen, S.; Tiedje, J.M. Brief and vigorous N2O production by soil at spring thaw. J. Soil Sci. 1990, 41, 1–4. [Google Scholar] [CrossRef]
- Smith, J.; Wagner-Riddle, C.; Dunfield, K. Season and management related changes in the diversity of nitrifying and denitrifying bacteria over winter and spring. Appl. Soil Ecol. 2010, 44, 138–146. [Google Scholar] [CrossRef]
- King, A.E.; Rezanezhad, F.; Wagner-Riddle, C. Evidence for microbial rather than aggregate origin of substrates fueling freeze-thaw induced N2O emissions. Soil Biol. Biochem. 2021, 160, 108352. [Google Scholar] [CrossRef]
- Li, Y.; Moinet, G.Y.K.; Clough, T.J.; Whitehead, D. Organic matter contributions to nitrous oxide emissions following nitrate addition are not proportional to substrate-induced soil carbon priming. Sci. Total Environ. 2022, 851, 158274. [Google Scholar] [CrossRef] [PubMed]
- Green, D.; Rezanezhad, F.; Jordan, S.; Wagner-Riddle, C.; Henry, H.A.L.; Slowinski, S.; Van Cappellen, P. Effects of winter pulsed warming and snowmelt on soil nitrogen cycling in agricultural soils: A lysimeter study. Front. Environ. Sci. 2022, 10. [Google Scholar] [CrossRef]
- Wrage, N.; Velthof, G.L.; van Beusichem, M.L.; Oenema, O. Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol. Biochem. 2001, 33, 1723–1732. [Google Scholar] [CrossRef]
- Tian, H.; Xu, R.; Canadell, J.G.; Thompson, R.L.; Winiwarter, W.; Suntharalingam, P.; Davidson, E.A.; Ciais, P.; Jackson, R.B.; Janssens-Maenhout, G.; et al. A comprehensive quantification of global nitrous oxide sources and sinks. Nature 2020, 586, 248–256. [Google Scholar] [CrossRef]
- Randall, K.; Scarratt, M.; Levasseur, M.; Michaud, S.; Xie, H.; Gosselin, M. First measurements of nitrous oxide in Arctic sea ice. J. Geophys. Res.-Ocean. 2012, 117, 1–8. [Google Scholar] [CrossRef]
- Davidson, E.A. Fluxes of nitrous oxide and nitric oxide from terrestrial ecosystems. Am. Soc. Microbiol. 1991, 219–235. [Google Scholar]
- Vilain, G.; Garnier, J.; Decuq, C.; Lugnot, M. Nitrous oxide production from soil experiments: Denitrification prevails over nitrification. Nutr. Cycl. Agroecosyst. 2014, 98, 169–186. [Google Scholar] [CrossRef]
- Bizimana, F.; Timilsina, A.; Dong, W.; Uwamungu, J.Y.; Li, X.; Wang, Y.; Pandey, B.; Qin, S.; Hu, C. Effects of long-term nitrogen fertilization on N2O, N2 and their yield-scaled emissions in a temperate semi-arid agro-ecosystem. J. Soils Sediments 2021, 21, 1659–1671. [Google Scholar] [CrossRef]
- Muller, C.; Martin, M.; Stevens, R.J.; Laughlin, R.J.; Kammann, C.; Ottow, J.C.G.; Jager, H.J. Processes leading to N2O emissions in grassland soil during freezing and thawing. Soil Biol. Biochem. 2002, 34, 1325–1331. [Google Scholar] [CrossRef]
- Oquist, M.G.; Nilsson, M.; Sorensson, F.; Kasimir-Klemedtsson, A.; Persson, T.; Weslien, P.; Klemedtsson, L. Nitrous oxide production in a forest soil at low temperatures processes and environmental controls. FEMS Microbiol. Ecol. 2004, 49, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Rochette, P.; Angers, D.A.; Chantigny, M.H.; Bertrand, N. Nitrous oxide emissions respond differently to no-till in a loam and a heavy clay soil. Soil Sci. Soc. Am. J. 2008, 72, 1363–1369. [Google Scholar] [CrossRef]
- Pelster, D.E.; Chantigny, M.H.; Rochette, P.; Angers, D.A.; Rieux, C.; Vanasse, A. Nitrous Oxide Emissions Respond Differently to Mineral and Organic Nitrogen Sources in Contrasting Soil Types. J. Environ. Qual. 2012, 41, 427–435. [Google Scholar] [CrossRef]
- van Bruggen, A.H.C.; He, M.; Zelenev, V.V.; Semenov, V.M.; Semenov, A.M.; Semenova, E.V.; Kuznetsova, T.V.; Khodzaeva, A.K.; Kuznetsov, A.M.; Semenov, M.V. Relationships between greenhouse gas emissions and cultivable bacterial populations in conventional, organic and long-term grass plots as affected by environmental variables and disturbances. Soil Biol. Biochem. 2017, 114, 145–159. [Google Scholar] [CrossRef]
- He, M.; Ma, W.; Zelenev, V.V.; Khodzaeva, A.K.; Kuznetsov, A.M.; Semenov, A.M.; Semenov, V.M.; Blok, W.; van Bruggen, A.H.C. Short-term dynamics of greenhouse gas emissions and cultivable bacterial populations in response to induced and natural disturbances in organically and conventionally managed soils. Appl. Soil Ecol. 2017, 119, 294–306. [Google Scholar] [CrossRef]
- Koponen, H.T.; Jaakkola, T.; Keinänen-Toivola, M.M.; Kaipainen, S.; Tuomainen, J.; Servomaa, K.; Martikainen, P.J. Microbial communities, biomass, and activities in soils as affected by freeze thaw cycles. Soil Biol. Biochem. 2006, 38, 1861–1871. [Google Scholar] [CrossRef]
- Sharma, S.; Szele, Z.; Schilling, R.; Munch, J.C.; Schloter, M. Influence of freeze-thaw stress on the structure and function of microbial communities and denitrifying populations in soil. Appl. Environ. Microbiol. 2006, 72, 2148–2154. [Google Scholar] [CrossRef] [PubMed]
- Sawicka, J.E.; Robador, A.; Hubert, C.; Jorgensen, B.B.; Bruchert, V. Effects of freeze-thaw cycles on anaerobic microbial processes in an Arctic intertidal mud flat. ISME J. 2010, 4, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.M.; Deng, M.W.; Yuan, A.Q.; Wang, J.H.; Li, H.; Ma, J.C. Vertical variation of a black soil’s properties in response to freeze-thaw cycles and its links to shift of microbial community structure. Sci. Total Environ. 2018, 625, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Wieder, W.R.; Boehnert, J.; Bonan, G.B.; Langseth, M. Regridded Harmonized World Soil Database v1.2.
- Molstad, L.; Dorsch, P.; Bakken, L.R. Robotized incubation system for monitoring gases (O2, NO, N2O N2) in denitrifying cultures. J. Microbiol. Methods 2007, 71, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.M.; Wang, F.H.; Zhang, Y.M.; Qin, S.P.; Wei, S.C.; Wang, S.Q.; Hu, C.S.; Liu, B.B. Organic carbon availability limiting microbial denitrification in the deep vadose zone. Environ. Microbiol. 2018, 20, 980–992. [Google Scholar] [CrossRef] [PubMed]
- Francis, C.A.; Roberts, K.J.; Beman, J.M.; Santoro, A.E.; Oakley, B.B. Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc. Natl. Acad. Sci. USA 2005, 102, 14683–14688. [Google Scholar] [CrossRef]
- Rotthauwe, J.H.; Witzel, K.P.; Liesack, W. The ammonia monooxygenase structural gene amoA as a functional marker: Molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl. Environ. Microbiol. 1997, 63, 4704–4712. [Google Scholar] [CrossRef]
- Throback, I.N.; Enwall, K.; Jarvis, A.; Hallin, S. Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. Fems Microbiol. Ecol. 2004, 49, 401–417. [Google Scholar] [CrossRef]
- Wang, F.H.; Chen, S.M.; Wang, Y.Y.; Zhang, Y.M.; Hui, C.S.; Liu, B.B. Long-Term Nitrogen Fertilization Elevates the Activity and Abundance of Nitrifying and Denitrifying Microbial Communities in an Upland Soil: Implications for Nitrogen Loss From Intensive Agricultural Systems. Front. Microbiol. 2018, 9, 12. [Google Scholar] [CrossRef]
- Parada, A.E.; Needham, D.M.; Fuhrman, J.A. Every base matters: Assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ. Microbiol. 2016, 18, 1403–1414. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Robeson, M.S.; O’Rourke, D.R.; Kaehler, B.D.; Ziemski, M.; Dillon, M.R.; Foster, J.T.; Bokulich, N.A. RESCRIPt: Reproducible sequence taxonomy reference database management for the masses. bioRxiv 2020. [Google Scholar] [CrossRef]
- Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. R Package Version 1.3-5. 2021. Available online: https://CRAN.R-project.org/package-agricolae (accessed on 11 December 2022).
- Revelle, W. psych: Procedures for Personality and Psychological Research; Version 2.1.9; Northwestern University: Evanston, IL, USA, 2021; Available online: https://cran.r-project.org/web/packages/psych/index.html (accessed on 11 December 2022).
- Chen, H. VennDiagram: Generate High-Resolution venn and Euler Plots, R Package Version 1.7.1. 2021. Available online: https://CRAN.R-project.org/package=vennDiagram (accessed on 11 December 2022).
- Gao, D.C.; Zhang, L.; Liu, J.; Peng, B.; Fan, Z.Z.; Dai, W.W.; Jiang, P.; Bai, E. Responses of terrestrial nitrogen pools and dynamics to different patterns of freeze-thaw cycle: A meta-analysis. Glob. Change Biol. 2018, 24, 2377–2389. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.M.; Wang, J.L. Various electron donors for biological nitrate removal: A review. Sci. Total Environ. 2021, 794, 148699. [Google Scholar] [CrossRef] [PubMed]
- Swerts, M.; Merckx, R.; Vlassak, K. Influence of carbon availability on the production of NO, N2O, N2 and CO2 by soil cores during anaerobic incubation. Plant Soil 1996, 181, 145–151. [Google Scholar] [CrossRef]
- Nemeth, D.D.; Wagner-Riddle, C.; Dunfield, K.E. Abundance and gene expression in nitrifier and denitrifier communities associated with a field scale spring thaw N2O flux event. Soil Biol. Biochem. 2014, 73, 1–9. [Google Scholar] [CrossRef]
- Levy-Booth, D.J.; Prescott, C.E.; Grayston, S.J. Microbial functional genes involved in nitrogen fixation, nitrification and denitrification in forest ecosystems. Soil Biol. Biochem. 2014, 75, 11–25. [Google Scholar] [CrossRef]
- Sun, R.; Wang, F.; Hu, C.; Liu, B. Metagenomics reveals taxon-specific responses of the nitrogen-cycling microbial community to long-term nitrogen fertilization. Soil Biol. Biochem. 2021, 156, 108214. [Google Scholar] [CrossRef]
- Wu, X.; Chen, Z.; Kiese, R.; Fu, J.; Gschwendter, S.; Schloter, M.; Liu, C.; Butterbach-Bahl, K.; Wolf, B.; Dannenmann, M. Dinitrogen (N2) pulse emissions during freeze-thaw cycles from montane grassland soil. Biol. Fertil. Soils 2020, 56, 959–972. [Google Scholar] [CrossRef]
- de Bruijn, A.M.G.; Butterbach-Bahl, K.; Blagodatsky, S.; Grote, R. Model evaluation of different mechanisms driving freeze-thaw N2O emissions. Agric. Ecosyst. Environ. 2009, 133, 196–207. [Google Scholar] [CrossRef]
- Peng, B.; Sun, J.F.; Liu, J.; Dai, W.W.; Sun, L.F.; Pei, G.T.; Gao, D.C.; Wang, C.; Jiang, P.; Bai, E. N2O emission from a temperate forest soil during the freeze-thaw period: A mesocosm study. Sci. Total Environ. 2019, 648, 350–357. [Google Scholar] [CrossRef] [PubMed]

| Soil | TC mg/g | TN mg/g | C/N | DOC mg/kg | SOC mg/g | DON mg/kg | NO3−-N mg/kg | NH4+-N mg/kg | pH |
|---|---|---|---|---|---|---|---|---|---|
| FS | 19.52 a | 1.69 a | 11.54 a | 46.44 a | 11.44 a | 99.97 a | 47.84 a | 0.35 a | 7.94 a |
| BS | 11.75 b | 1.28 b | 9.20 b | 47.23 a | 8.47 b | 46.92 b | 19.56 b | 1.01 b | 5.40 b |
| LS | 17.38 c | 0.89 c | 19.32 c | 43.98 b | 5.34 c | 58.79 c | 11.40 c | 0.47 c | 8.25 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Mosongo, P.S.; Dong, W.; Timilsina, A.; Sun, R.; Wang, F.; Walkiewicz, A.; Liu, B.; Hu, C. Responses of Nitrous Oxide Emissions and Bacterial Communities to Experimental Freeze–Thaw Cycles in Contrasting Soil Types. Microorganisms 2023, 11, 593. https://doi.org/10.3390/microorganisms11030593
Li W, Mosongo PS, Dong W, Timilsina A, Sun R, Wang F, Walkiewicz A, Liu B, Hu C. Responses of Nitrous Oxide Emissions and Bacterial Communities to Experimental Freeze–Thaw Cycles in Contrasting Soil Types. Microorganisms. 2023; 11(3):593. https://doi.org/10.3390/microorganisms11030593
Chicago/Turabian StyleLi, Wenyan, Peter Semba Mosongo, Wenxu Dong, Arbindra Timilsina, Ruibo Sun, Fenghua Wang, Anna Walkiewicz, Binbin Liu, and Chunsheng Hu. 2023. "Responses of Nitrous Oxide Emissions and Bacterial Communities to Experimental Freeze–Thaw Cycles in Contrasting Soil Types" Microorganisms 11, no. 3: 593. https://doi.org/10.3390/microorganisms11030593
APA StyleLi, W., Mosongo, P. S., Dong, W., Timilsina, A., Sun, R., Wang, F., Walkiewicz, A., Liu, B., & Hu, C. (2023). Responses of Nitrous Oxide Emissions and Bacterial Communities to Experimental Freeze–Thaw Cycles in Contrasting Soil Types. Microorganisms, 11(3), 593. https://doi.org/10.3390/microorganisms11030593









