Oral Microbiota in Children and Adolescents with Type 1 Diabetes Mellitus: Novel Insights into the Pathogenesis of Dental and Periodontal Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical Data Collection
2.3. Glucometabolic Parameters
2.4. Microbiological Analysis
2.5. Statistical Methods
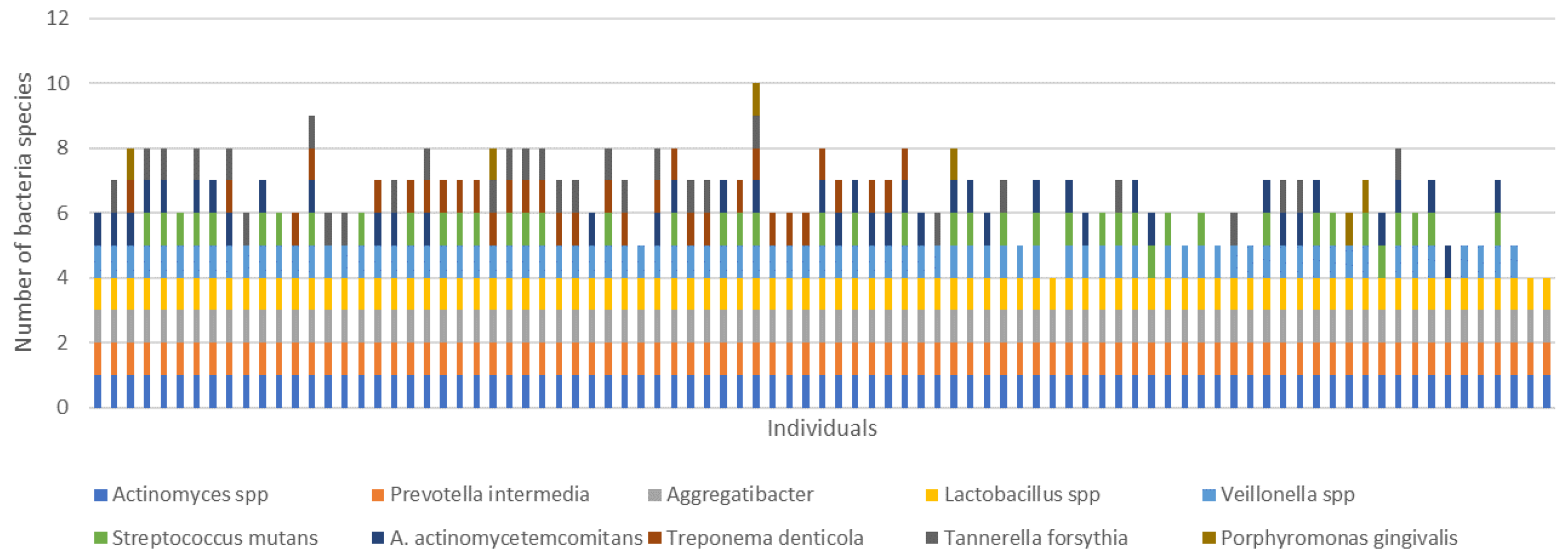
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atkinson, M.A.; Eisenbarth, G.S.; Michels, A.W. Type 1 diabetes. Lancet 2014, 383, 69–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulton, A.J.; Vileikyte, L.; Ragnarson-Tennvall, G.; Apelqvist, J. The global burden of diabetic foot disease. Lancet 2005, 366, 1719–1724. [Google Scholar] [CrossRef] [PubMed]
- Coresh, J.; Astor, B.C.; Greene, T.; Eknoyan, G.; Levey, A.S. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third national health and nutrition examination survey. Am. J. Kidney Dis. 2003, 41, 1–12. [Google Scholar] [CrossRef]
- Yau, J.W.Y.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.-J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global Prevalence and Major Risk Factors of Diabetic Retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 2018, 17, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Löe, H. Periodontal Disease: The sixth complication of diabetes mellitus. Diabetes Care 1993, 16, 329–334. [Google Scholar] [CrossRef]
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef] [Green Version]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89, S159–S172. [Google Scholar] [CrossRef] [Green Version]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S.; et al. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Periodontol. 2018, 89 (Suppl. S1), S1–S8. [Google Scholar] [CrossRef] [Green Version]
- Al-Khabbaz, A.K.; Al-Shammari, K.F.; Hasan, A.; Abdul-Rasoul, M. Periodontal Health of Children with Type 1 Diabetes Mellitus in Kuwait: A Case-Control Study. Med. Princ. Pract. 2012, 22, 144–149. [Google Scholar] [CrossRef]
- Carneiro, V.L.; Fraiz, F.C.; Ferreira, F.D.M.; Pintarelli, T.P.; Oliveira, A.C.B.; Boguszewski, M.C.D.S. The influence of glycemic control on the oral health of children and adolescents with diabetes mellitus type 1. Arq. Bras. Endocrinol. Metabol. 2015, 59, 535–540. [Google Scholar] [CrossRef] [Green Version]
- Salvi, G.E.; Carollo-Bittel, B.; Lang, N.P. Effects of diabetes mellitus on periodontal and peri-implant conditions: Update on associations and risks. J. Clin. Periodontol. 2008, 35, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Stöhr, J.; Barbaresko, J.; Neuenschwander, M.; Schlesinger, S. Bidirectional association between periodontal disease and diabetes mellitus: A systematic review and meta-analysis of cohort studies. Sci. Rep. 2021, 11, 13686. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, G.G.; Leite, F.R.M.; Vestergaard, P.; Scheutz, F.; López, R. Does diabetes increase the risk of periodontitis? A systematic review and meta-regression analysis of longitudinal prospective studies. Acta Diabetol. 2018, 55, 653–667. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.J.; Preshaw, P.M.; Lalla, E. A review of the evidence for pathogenic mechanisms that may link periodontitis and diabetes. J. Periodontol. 2013, 84 (Suppl. S4), S113–S134. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.; Goel, K. Interrelationship between diabetes and periodontitis: A review. J. Nepal Med. Assoc. 2011, 51, 144–153. [Google Scholar] [CrossRef]
- Tetè, G.; D’orto, B.; Ferrante, L.; Polizzi, E.; Cattoni, F. Role of mast cells in oral inflammation. J. Biol. Regul. Homeost Agents. 2021, 35 (Suppl. S1), 65–70. [Google Scholar] [CrossRef]
- Paju, S.; Pussinen, P.; Suominen-Taipale, L.; Hyvönen, M.; Knuuttila, M.; Könönen, E. Detection of Multiple Pathogenic Species in Saliva Is Associated with Periodontal Infection in Adults. J. Clin. Microbiol. 2009, 47, 235–238. [Google Scholar] [CrossRef] [Green Version]
- Mineoka, T.; Awano, S.; Rikimaru, T.; Kurata, H.; Yoshida, A.; Ansai, T.; Takehara, T. Site-Specific Development of Periodontal Disease Is Associated with Increased Levels of Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia in Subgingival Plaque. J. Periodontol. 2008, 79, 670–676. [Google Scholar] [CrossRef]
- Deng, Z.-L.; Szafrański, S.P.; Jarek, M.; Bhuju, S.; Wagner-Döbler, I. Dysbiosis in chronic periodontitis: Key microbial players and interactions with the human host. Sci. Rep. 2017, 7, 3703. [Google Scholar] [CrossRef] [Green Version]
- Siudikiene, J.; Machiulskiene, V.; Nyvad, B.; Tenovuo, J.; Nedzelskiene, I. Dental Caries Increments and Related Factors in Children with Type 1 Diabetes Mellitus. Caries Res. 2008, 42, 354–362. [Google Scholar] [CrossRef] [PubMed]
- El-Tekeya, M.; El Tantawi, M.; Fetouh, H.; Mowafy, E.; Khedr, N.A. Caries risk indicators in children with type 1 diabetes mellitus in relation to metabolic control. Dent. Traumatol. 2012, 34, 510–516. [Google Scholar]
- Ferizi, L.; Dragidella, F.; Spahiu, L.; Begzati, A.; Kotori, V. The Influence of Type 1 Diabetes Mellitus on Dental Caries and Salivary Composition. Int. J. Dent. 2018, 2018, 5780916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, M.R.; Paster, B.J.; Leys, E.J.; Moeschberger, M.L.; Kenyon, S.G.; Galvin, J.L.; Boches, S.K.; Dewhirst, F.E.; Griffen, A.L. Molecular Analysis of Bacterial Species Associated with Childhood Caries. J. Clin. Microbiol. 2002, 40, 1001–1009. [Google Scholar] [CrossRef] [Green Version]
- Mashima, I.; Nakazawa, F. Interaction between Streptococcus spp. and Veillonella tobetsuensis in the Early Stages of Oral Biofilm Formation. J. Bacteriol. 2015, 197, 2104–2111. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Wang, Y.; Zhao, L.; Sun, X.; Feng, Q. Microbiome succession with increasing age in three oral sites. Aging 2020, 12, 7874–7907. [Google Scholar] [CrossRef]
- Crielaard, W.; Zaura, E.; Schuller, A.A.; Huse, S.M.; Montijn, R.C.; Keijser, B.J.F. Exploring the oral microbiota of children at various developmental stages of their dentition in the relation to their oral health. BMC Med. Genom. 2011, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Burcham, Z.M.; Garneau, N.L.; Comstock, S.S.; Tucker, R.M.; Knight, R.; Metcalf, J.L.; Miranda, A.; Reinhart, B.; Meyers, D.; Woltkamp, D.; et al. Patterns of Oral Microbiota Diversity in Adults and Children: A Crowdsourced Population Study. Sci. Rep. 2020, 10, 2133. [Google Scholar] [CrossRef] [Green Version]
- Rodenburg, J.P.; Winkelhoff, A.J.; Winkel, E.G.; Goene, R.J.; Abbas, F.; Graaff, J. Occurrence of Bacteroides gingivalis, Bacteroides intermedius and Actinobacillus actinomycetemcomitans in severe periodontitis in relation to age and treatment history. J. Clin. Periodontol. 1990, 17, 392–399. [Google Scholar] [CrossRef]
- Cattoni, F.; Tetè, G.; D’orto, B.; Bergamaschi, A.; Polizzi, E.; Gastaldi, G. Comparison of hygiene levels in metal-ceramic and stratified zirconia in prosthetic rehabilitation on teeth and implants: A retrospective clinical study of a three-year follow-up. J. Biol. Regul. Homeost. Agents 2021, 35, 41–49. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, S.; Wang, Y.; Wang, Z.; Ding, W.; Sun, X.; He, K.; Feng, Q.; Zhang, X. Changes of saliva microbiota in the onset and after the treatment of diabetes in patients with periodontitis. Aging 2020, 12, 13090–13114. [Google Scholar] [CrossRef]
- de Groot, P.F.; Belzer, C.; Aydin, Ö.; Levin, E.; Levels, J.H.; Aalvink, S.; Boot, F.; Holleman, F.; van Raalte, D.H.; Scheithauer, T.P.; et al. Distinct fecal and oral microbiota composition in human type 1 diabetes, an observational study. PLoS ONE 2017, 12, e0188475. [Google Scholar] [CrossRef] [Green Version]
- Jensen, E.D.; Selway, C.A.; Allen, G.; Bednarz, J.; Weyrich, L.S.; Gue, S.; Peña, A.S.; Couper, J. Early markers of periodontal disease and altered oral microbiota are associated with glycemic control in children with type 1 diabetes. Pediatr. Diabetes 2020, 22, 474–481. [Google Scholar] [CrossRef]
- Garn, S.M. Growth at adolescence. By J. M. Tanner. Pp. vii + 212. Blackwell Scientific Publications, Oxford. Publisher simultaneously by Charles C Thomas and the Ryerson Press. 1955. Am. J. Phys. Anthropol. 1956, 14, 120–122. [Google Scholar] [CrossRef]
- Maguolo, A.; Rioda, M.; Zusi, C.; Emiliani, F.; Olivieri, F.; Piona, C.; Marigliano, M.; Orsi, S.; Morandi, A.; Maffeis, C. Cardiovascular risk factors in children and adolescents with type 1 diabetes mellitus: The role of insulin resistance and associated genetic variants. Horm. Res. Paediatr. 2022. [Google Scholar] [CrossRef]
- De Onis, M.; Onyango, A.W.; Borghi, E.; Siyam, A.; Nishida, C.; Siekmann, J. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.T.; Kaelber, D.C.; Baker-Smith, C.M.; Blowey, D.; Carroll, A.E.; Daniels, S.R.; De Ferranti, S.D.; Dionne, J.M.; Falkner, B.; Flinn, S.K.; et al. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics 2017, 140, e20171904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danne, T.; Nimri, R.; Battelino, T.; Bergenstal, R.M.; Close, K.L.; DeVries, J.H.; Garg, S.; Heinemann, L.; Hirsch, I.; Amiel, S.A.; et al. International Consensus on Use of Continuous Glucose Monitoring. Diabetes Care 2017, 40, 1631–1640. [Google Scholar] [CrossRef] [Green Version]
- Garcia, L.; Tercero, J.C.; Legido, B.; Ramos, J.A.; Alemany, J.; Sanz, M. Rapid detection of Actinobacillus actinomycetemcomitans, Prevotella intermedia and Porphyromona gingivalis by multiplex PCR. J. Periodontal Res. 1998, 33, 59–64. [Google Scholar] [CrossRef]
- Pardo, A.; Signoriello, A.; Signoretto, C.; Messina, E.; Carelli, M.; Tessari, M.; De Manna, N.D.; Rossetti, C.; Albanese, M.; Lombardo, G.; et al. Detection of Periodontal Pathogens in Oral Samples and Cardiac Specimens in Patients Undergoing Aortic Valve Replacement: A Pilot Study. J. Clin. Med. 2021, 10, 3874. [Google Scholar] [CrossRef]
- Sedghi, L.M.; Bacino, M.; Kapila, Y.L. Periodontal Disease: The Good, The Bad, and The Unknown. Front. Cell. Infect. Microbiol. 2021, 11, 1210. [Google Scholar] [CrossRef]
- Longo, P.L.; Dabdoub, S.; Kumar, P.; Artese, H.P.C.; Dib, S.A.; Romito, G.A.; Mayer, M.P.A. Glycaemic status affects the subgingival microbiome of diabetic patients. J. Clin. Periodontol. 2018, 45, 932–940. [Google Scholar] [CrossRef]
- Schara, R.; Skaleric, E.; Seme, K.; Skaleric, U. Prevalence of periodontal pathogens and metabolic control of type 1 diabetes patients. J. Int. Acad. Periodontol. 2013, 15, 29–34. [Google Scholar]
- Genco, R.J.; Borgnakke, W.S. Risk factors for periodontal disease. Periodontology 2000 2013, 62, 59–94. [Google Scholar] [CrossRef] [PubMed]
- Akherati, M.; Shafaei, E.; Salehiniya, H.; Abbaszadeh, H. Comparison of the frequency of periodontal pathogenic species of diabetics and non-diabetics and its relation to periodontitis severity, glycemic control and body mass index. Clin. Exp. Dent. Res. 2021, 7, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Chowdhury, R.; Bhakta, A.; Mukhopahyay, P.; Ghosh, S. Microbiology of periodontal disease in adolescents with Type 1 diabetes. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 102333. [Google Scholar] [CrossRef] [PubMed]
- Castrillon, C.A.; Hincapie, J.P.; Yepes, F.L.; Roldan, N.; Moreno, S.M.; Contreras, A.; Botero, J.E. Occurrence of red complex microorganisms and Aggregatibacter actinomycetemcomitans in patients with diabetes. J. Investig. Clin. Dent. 2013, 6, 25–31. [Google Scholar] [CrossRef]
- Raja, M. Aggregatibacter Actinomycetemcomitans—A Tooth Killer? J. Clin. Diagn. Res. 2014, 8, ZE13–ZE16. [Google Scholar] [CrossRef]
- Mitrakul, K.; Asvanund, Y.; Vongsavan, K. Prevalence of Five Biofilm-Related Oral Streptococci Species from Plaque. J. Clin. Pediatr. Dent. 2011, 36, 161–166. [Google Scholar] [CrossRef]
- Lemos, J.A.; Palmer, S.R.; Zeng, L.; Wen, Z.T.; Kajfasz, J.K.; Freires, I.A.; Abranches, J.; Brady, L.J. The Biology of Streptococcus mutans. Microbiol. Spectr. 2019, 7, 7. [Google Scholar] [CrossRef]
- Gross, E.L.; Beall, C.; Kutsch, S.R.; Firestone, N.D.; Leys, E.J.; Griffen, A.L. Beyond Streptococcus mutans: Dental Caries Onset Linked to Multiple Species by 16S rRNA Community Analysis. PLoS ONE 2012, 7, e47722. [Google Scholar] [CrossRef] [PubMed]
- Thorstensson, H. Periodontal disease in adult insulin-dependent diabetics. Swed. Dent. J. Suppl. 1995, 107, 1–68. [Google Scholar]
- Dusková, J.; Broukal, Z. Compensation criteria of basal disease in the prevention and treatment of periodontal disease in patients with diabetes mellitus. Prakt. Zubn. Lek. 1991, 39, 51–54. [Google Scholar]
- Lai, S.; Cagetti, M.G.; Cocco, F.; Cossellu, D.; Meloni, G.; Campus, G.; Lingström, P. Evaluation of the difference in caries experience in diabetic and non-diabetic children—A case control study. PLoS ONE 2017, 12, e0188451. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wu, J.; Chen, R.; Chen, Z.; Su, Z.; Ni, J.; Zhang, M.; Sun, C.; Zhang, F.; Liu, Y.; et al. Characterization of the oral microbiome of children with type 1 diabetes in the acute and chronic phases. J. Oral Microbiol. 2022, 14, 2094048. [Google Scholar] [CrossRef] [PubMed]
- Nansel, T.R.; Haynie, D.; Lipsky, L.; Laffel, L.M.; Mehta, S.N. Multiple Indicators of Poor Diet Quality in Children and Adolescents with Type 1 Diabetes Are Associated with Higher Body Mass Index Percentile but not Glycemic Control. J. Acad. Nutr. Diet. 2012, 112, 1728–1735. [Google Scholar] [CrossRef] [Green Version]
- Zalewska, A.; Knaś, M.; Kuźmiuk, A.; Waszkiewicz, N.; Niczyporuk, M.; Waszkiel, D.; Zwierz, K. Salivary innate defense system in type 1 diabetes mellitus in children with mixed and permanent dentition. Acta Odontol. Scand. 2013, 71, 1493–1500. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.; Passos, I.; Sampaio, F.; Soares, M.; Oliveira, R. Flow rate, pH and calcium concentration of saliva of children and adolescents with type 1 diabetes mellitus. Braz. J. Med. Biol. Res. 2009, 42, 707–711. [Google Scholar] [CrossRef] [Green Version]
- Giuca, M.R.; Pasini, M.; Giuca, G.; Caruso, S.; Necozione, S.; Gatto, R. Investigation of periodontal status in type 1 diabetic adolescents. Eur. J. Paediatr. Dent. 2015, 16, 319. [Google Scholar]
- Sas, B. Anti-discoloration system: A new chlorhexidine mouthwash. J. Biol. Regul. Homeost. Agents 2021, 35, 113–118. [Google Scholar] [CrossRef]
- Chugh, P.; Dutt, R.; Sharma, A.; Bhagat, N.; Dhar, M.S. A critical appraisal of the effects of probiotics on oral health. J. Funct. Foods 2020, 70, 103985. [Google Scholar] [CrossRef]
- Butera, A.; Maiorani, C.; Gallo, S.; Pascadopoli, M.; Venugopal, A.; Marya, A.; Scribante, A. Evaluation of Adjuvant Systems in Non-Surgical Peri-Implant Treatment: A Literature Review. Healthcare 2022, 10, 886. [Google Scholar] [CrossRef]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Maiorani, C.; Milone, A.; Alovisi, M.; Scribante, A. Paraprobiotics in Non-Surgical Periodontal Therapy: Clinical and Microbiological Aspects in a 6-Month Follow-Up Domiciliary Protocol for Oral Hygiene. Microorganisms 2022, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Baumgartner, J.C. Occurrence of Actinomyces in Infections of Endodontic Origin. J. Endod. 2003, 29, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Mashima, I.; Theodorea, C.F.; Thaweboon, B.; Thaweboon, S.; Nakazawa, F. Identification of Veillonella species in the tongue biofilm by using a novel one-step polymerase chain reaction method. PLoS ONE 2016, 11, e0157516. [Google Scholar] [CrossRef] [Green Version]
- Nakano, K.; Inaba, H.; Nomura, R.; Nemoto, H.; Takeda, M.; Yoshioka, H.; Matsue, H.; Takahashi, T.; Taniguchi, K.; Amano, A.; et al. Detection of cariogenic Streptococcus mutans in extirpated heart valve and atheromatous plaque specimens. J. Clin. Microbiol. 2006, 44, 3313–3317. [Google Scholar] [CrossRef] [Green Version]
- Byun, R.; Nadkarni, M.A.; Chhour, K.L.; Martin, F.E.; Jacques, N.A.; Hunter, N. Quantitative analysis of diverse Lactobacillus species present in advanced dental caries. J. Clin. Microbiol. 2004, 42, 3128–3136. [Google Scholar] [CrossRef] [Green Version]
| All (n = 89) | Males (n = 55) | Females (n = 34) | p-Value | HbA1c ≤ 7.5% (n = 47) | HbA1c > 7.5% (n = 42) | p-Value | |
|---|---|---|---|---|---|---|---|
| Female (%) | 34 (38.2) | 19 (40.4) | 15 (35.7) | 0.781 | |||
| Male (%) | 55 (61.8) | 28 (59.6) | 27 (64.3) | ||||
| Age (years) | 12.56 ± 2.17 | 12.60 ± 2.18 | 12.50 ± 2.17 | 0.824 | 12.55 ± 2.11 | 12.58 ± 2.24 | 0.986 |
| Diabetes duration (years) | 6.22 ± 2.97 | 6.08 ± 2.82 | 6.43 ± 3.23 | 0.596 | 6.26 ± 3.00 | 6.17 ± 2.97 | 0.736 |
| BMI (kg/m2) | 20.12 ± 3.00 | 19.68 ± 3.03 | 20.82 ± 2.83 | 0.080 | 19.85 ± 3.04 | 20.41 ± 2.94 | 0.342 |
| HbA1c (%) | 7.54 ± 0.84 | 7.52 ± 0.87 | 7.57 ± 0.81 | 0.813 | |||
| GMI (%) | 7.42 ± 0.71 | 7.42 ± 0.73 | 7.41 ± 0.68 | 0.911 | 7.04 ± 0.46 | 7.87 ± 0.69 | <0.001 |
| Pubertal status, n (%) | 0.003 | 0.697 | |||||
| Prepubertal | 21 (23.6) | 15 (27.3) | 4 (11.8) | 10 (21.3) | 9 (21.4) | ||
| Pubertal | 44 (49.4) | 32 (58.2) | 14 (41.2) | 26 (55.3) | 20 (47.6) | ||
| Post-pubertal | 24 (27.0) | 8 (14.5) | 16 (47.1) | 11 (23.4) | 13 (31.0) | ||
| Total Insulin (U/kg/die) | 0.88 ± 0.25 | 0.84 ± 0.23 | 0.94 ± 0.27 | 0.064 | 0.88 ± 0.22 | 0.88 ± 0.27 | 0.818 |
| Basal Insulin (U/kg/die) | 0.43 ± 0.15 | 0.41 ± 0.15 | 0.47 ± 0.14 | 0.064 | 0.44 ± 0.15 | 0.42 ± 0.14 | 0.696 |
| Prandial Insulin (U/kg/die) | 0.40 [0.29–0.55] | 0.38 [0.28–0.54] | 0.43 [0.33–0.58] | 0.293 | 0.38 [0.28–0.54] | 0.42 [0.33–0.56] | 0.479 |
| Time below range (%) | 3.90 ± 3.61 | 4.28 ± 3.75 | 3.27 ± 3.31 | 0.177 | 4.04 ± 3.69 | 3.73 ± 3.55 | 0.690 |
| Time in range (%) | 56.64 ± 15.15 | 55.96 ± 14.87 | 57.76 ± 15.76 | 0.595 | 65.83 ± 11.03 | 46.34 ± 12.28 | <0.001 |
| Time above range (%) | 39.40 ± 16.22 | 39.48 ± 16.04 | 39.27 ± 16.75 | 0.954 | 29.91 ± 11.96 | 50.05 ± 13.60 | <0.001 |
| Mean glycemia (sensor) | 172.1 ± 30.0 | 171.9 ± 30.5 | 172.5 ± 29.5 | 0.922 | 156.1 ± 19.1 | 191.5 ± 29.4 | <0.001 |
| CV (%) | 37.89 ± 5.49 | 38.64 ± 5.69 | 36.59 ± 4.95 | 0.104 | 36.65 ± 4.72 | 39.48 ± 6.04 | 0.020 |
| Blood agar, n = 82 (CFU × 108/mL) | 10.4 ± 43.8 | 4.47 ± 4.17 | 19.7 ± 69.6 | 0.005 | 6.57 ± 5.42 | 14.9 ± 64.3 | 0.161 |
| Sabouraud agar, n = 81 (CFU × 104/mL) | 0.35 ± 1.59 | 0.35 ± 1.49 | 0.36 ± 3.62 | 0.347 | 0.56 ± 2.13 | 0.13 ± 0.45 | 0.890 |
| Mannitol Salt agar, n = 82 (CFU × 108/mL) | 0.10 ±0.89 | 1.84 ± 1.15 | 0.02 ± 0.035 | 0.133 | 0.19 ± 0.12 | 0.026 ± 0.16 | 0.860 |
| Mitis Salivarius Agar, n = 82 (CFU × 107/mL) | 10.2 ± 21.5 | 7.59 ± 14.2 | 14.3 ± 29.1 | 0.034 | 11.9 ± 25.6 | 8.28 ± 1.55 | 0.262 |
| MSB, N = 82 (CFU × 107/mL) | 1.36 ± 3.24 | 0.78 ± 1.58 | 2.22 ± 4.65 | 0.092 | 1.87 ± 4.16 | 0.82 ± 1.75 | 0.450 |
| Veillonella spp., n (%) | 83 (93.3) | 51 (92.7) | 32 (94.1) | 0.583 | 42 (89.4) | 41 (97.6) | 0.129 |
| Actinomyces spp., n (%) | 89 (100) | 55 (100) | 34 (100) | 1 | 47 (100) | 42 (100) | 1 |
| Actinomyces naeslundii, n (%) | 42 (47.2) | 29 (52.7) | 13 (38.2) | 0.133 | 22 (46.8) | 20 (47.6) | 0.554 |
| Treponema denticola, n (%) | 32 (36.0) | 17 (30.9) | 15 (44.1) | 0.151 | 19 (40.4) | 13 (31.0) | 0.240 |
| A. actinomycetemcomitans, n (%) | 89 (100) | 55 (100) | 34 (100) | 1 | 47 (100) | 42 (100) | 1 |
| Prevotella intermedia, n (%) | 89 (100) | 55 (100) | 34 (100) | 1 | 47 (100) | 42 (100) | 1 |
| Porphyromonas gingivalis, n (%) | 6 (6.7) | 2 (3.6) | 4 (11.8) | 0.147 | 3 (6.4) | 3 (7.1) | 0.606 |
| Tannerella forsythia, n (%) | 30 (33.7) | 19 (34.5) | 11 (32.4) | 0.510 | 14 (29.8) | 13 (38.1) | 0.273 |
| Streptococcus mutans, n (%) | 44 (49.4) | 31 (56.4) | 13 (38.2) | 0.074 | 19 (40.4) | 25 (59.5) | 0.031 |
| Lactobacillus spp., n (%) | 89 (100) | 55 (100) | 34 (100) | 1 | 47 (100) | 42 (100) | 1 |
| Veillonella spp+ Streptococcus mutans., n (%) | 42 (47.2) | 29 (52.7) | 13 (38.2) | 0.133 | 17 (36.2) | 25 (59.5) | 0.028 |
| T. forsythia + T. denticola + P. gingivalis (at least 2 of them), n(%) | 16 (17.9) | 10 (18.1) | 6 (17.6) | 0.592 | 8 (17.0) | 8 (19.1) | 0.510 |
| Questions | Frequency | Percent | |
|---|---|---|---|
| 1. Dentistry frequency | Never | 1/74 | 1.35 |
| Only if necessary | 29/74 | 39.19 | |
| Once a year | 16/74 | 21.62 | |
| Twice a year | 20/74 | 27.03 | |
| More frequently | 8/74 | 10.81 | |
| 2. Professional hygiene frequency | Never | 12/74 | 16.22 |
| Only if necessary | 24/74 | 32.43 | |
| Once a year | 22/74 | 29.73 | |
| Twice a year | 16/74 | 21.62 | |
| 3. Brushing frequency | Once a day | 14/75 | 18.67 |
| Twice a day | 46/75 | 61.33 | |
| More often | 15/75 | 20.00 | |
| 4. Brushing time | Less than 1 min | 15/73 | 20.55 |
| From 1 to 2 min | 38/73 | 52.05 | |
| More than 2 min | 20/73 | 27.40 | |
| 5. If I see blood when I brush my teeth | Normal | 4/75 | 5.33 |
| It rarely happens | 25/75 | 33.33 | |
| It never happens | 46/75 | 61.33 | |
| 6. Brushing technique | I don’t know | 16/69 | 23.19 |
| Horizontal movements | 16/69 | 18.84 | |
| Horizontal and vertical movements | 97/69 | 53.62 | |
| Others | 3/69 | 4.35 | |
| 7. Types of toothbrushes | Manual | 37/75 | 49.33 |
| Electric | 38/75 | 50.67 | |
| 8. Frequency of toothbrush/toothbrush head replace | 2–3 months | 47/72 | 65.28 |
| 6 months | 7/72 | 9.72 | |
| 1 year | 2/72 | 2.78 | |
| Until is not working | 16/72 | 22.22 | |
| 9. Dental floss use | No | 61/74 | 82.43 |
| Yes | 13/74 | 17.57 | |
| 10. Dental brush use | No | 70/74 | 94.54 |
| Yes | 4/74 | 5.41 | |
| 11. Mouthwash use | No | 43/74 | 58.11 |
| Yes | 31/74 | 41.89 | |
| 12. Do you brush your teeth when correcting hypoglycemia | Never | 57/73 | 78.08 |
| Sometimes | 15/73 | 20.55 | |
| Always during the day | 1/73 | 1.37 | |
| Variable | OR | p-Value | [95% CI] | |
|---|---|---|---|---|
| Dependent | Independent | |||
| Streptococcus mutans and Veionella spp. | HbA1c | 3.83 | 0.018 | 1.26;11.65 |
| Gender | 0.40 | 0.080 | 0.14;1.12 | |
| Age | 0.97 | 0.952 | 0.35;2.69 | |
| Professional hygiene frequency | 1.10 | 0.865 | 0.35;3.48 | |
| Bleeding during brushing | 1.56 | 0.407 | 0.55;4.45 | |
| Pseudo R2 = 0.0969 | ||||
| GMI | 4.35 | 0.021 | 1.25;15.15 | |
| Gender | 0.42 | 0.101 | 0.15;1.19 | |
| Age | 1.29 | 0.631 | 0.46;3.63 | |
| Professional hygiene frequency | 0.89 | 0.835 | 0.31;2.58 | |
| Bleeding during brushing | 1.86 | 0.254 | 0.64;5.40 | |
| Pseudo R2 = 0.0883 | ||||
| TAR | 6.48 | 0.009 | 1.60;26.20 | |
| Gender | 0.32 | 0.040 | 0.11;0.95 | |
| Age | 1.55 | 0.416 | 0.54;4.44 | |
| Professional hygiene frequency | 0.84 | 0.750 | 0.29;2.44 | |
| Bleeding during brushing | 1.79 | 0.291 | 0.61;5.24 | |
| Pseudo R2 = 0.1153 | ||||
| TIR | 0.15 | 0.007 | 0.04;0.60 | |
| Gender | 0.33 | 0.048 | 0.11;0.99 | |
| Age | 1.50 | 0.454 | 0.52;4.30 | |
| Professional hygiene frequency | 0.87 | 0.798 | 0.30;2.53 | |
| Bleeding during brushing | 1.75 | 0.309 | 0.60;5.12 | |
| Pseudo R2 = 0.1212 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carelli, M.; Maguolo, A.; Zusi, C.; Olivieri, F.; Emiliani, F.; De Grandi, G.; Unali, I.; Zerman, N.; Signoretto, C.; Maffeis, C. Oral Microbiota in Children and Adolescents with Type 1 Diabetes Mellitus: Novel Insights into the Pathogenesis of Dental and Periodontal Disease. Microorganisms 2023, 11, 668. https://doi.org/10.3390/microorganisms11030668
Carelli M, Maguolo A, Zusi C, Olivieri F, Emiliani F, De Grandi G, Unali I, Zerman N, Signoretto C, Maffeis C. Oral Microbiota in Children and Adolescents with Type 1 Diabetes Mellitus: Novel Insights into the Pathogenesis of Dental and Periodontal Disease. Microorganisms. 2023; 11(3):668. https://doi.org/10.3390/microorganisms11030668
Chicago/Turabian StyleCarelli, Maria, Alice Maguolo, Chiara Zusi, Francesca Olivieri, Federica Emiliani, Gelinda De Grandi, Ilaria Unali, Nicoletta Zerman, Caterina Signoretto, and Claudio Maffeis. 2023. "Oral Microbiota in Children and Adolescents with Type 1 Diabetes Mellitus: Novel Insights into the Pathogenesis of Dental and Periodontal Disease" Microorganisms 11, no. 3: 668. https://doi.org/10.3390/microorganisms11030668







