Synergistic Antibacterial Activity of Benzalkonium Bromide and Cu-Bearing Duplex Stainless Steel against Pseudomonas aeruginosa
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bacteria and Bactericide
2.3. Colony-Forming Unit (CFU) Counting
2.4. Visualization of Live/Dead Cells in Biofilms
2.5. Surface Morphology of Biofilm
2.6. Flow Cytometry (FCM)
2.7. Real-Time Cell Analyzers (RTCA)
2.8. ATP Bioluminescence Assay
2.9. Statistical Analysis
3. Results
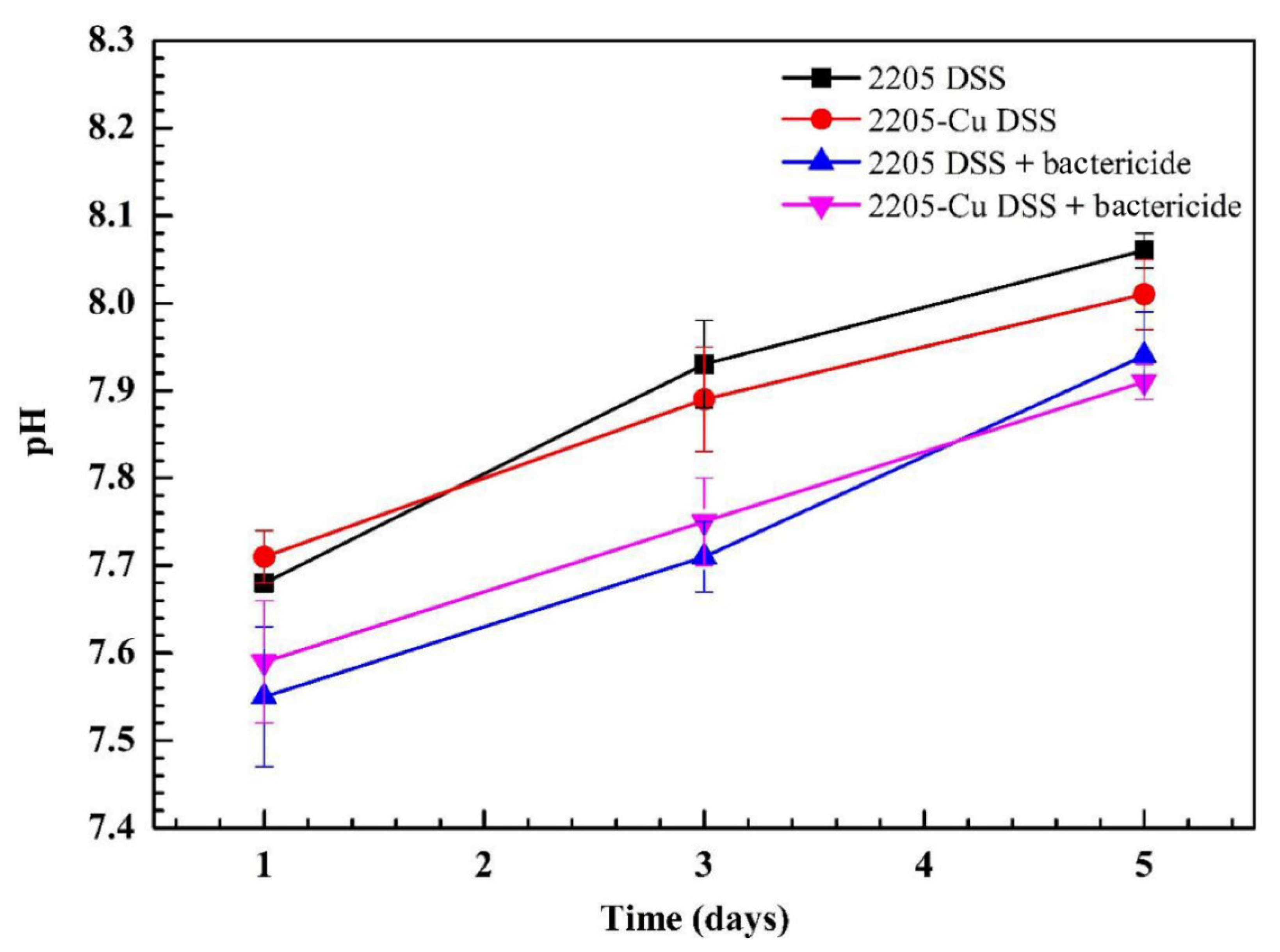
3.1. The pH Value Did Not Change Significantly in the Presence of Bactericide
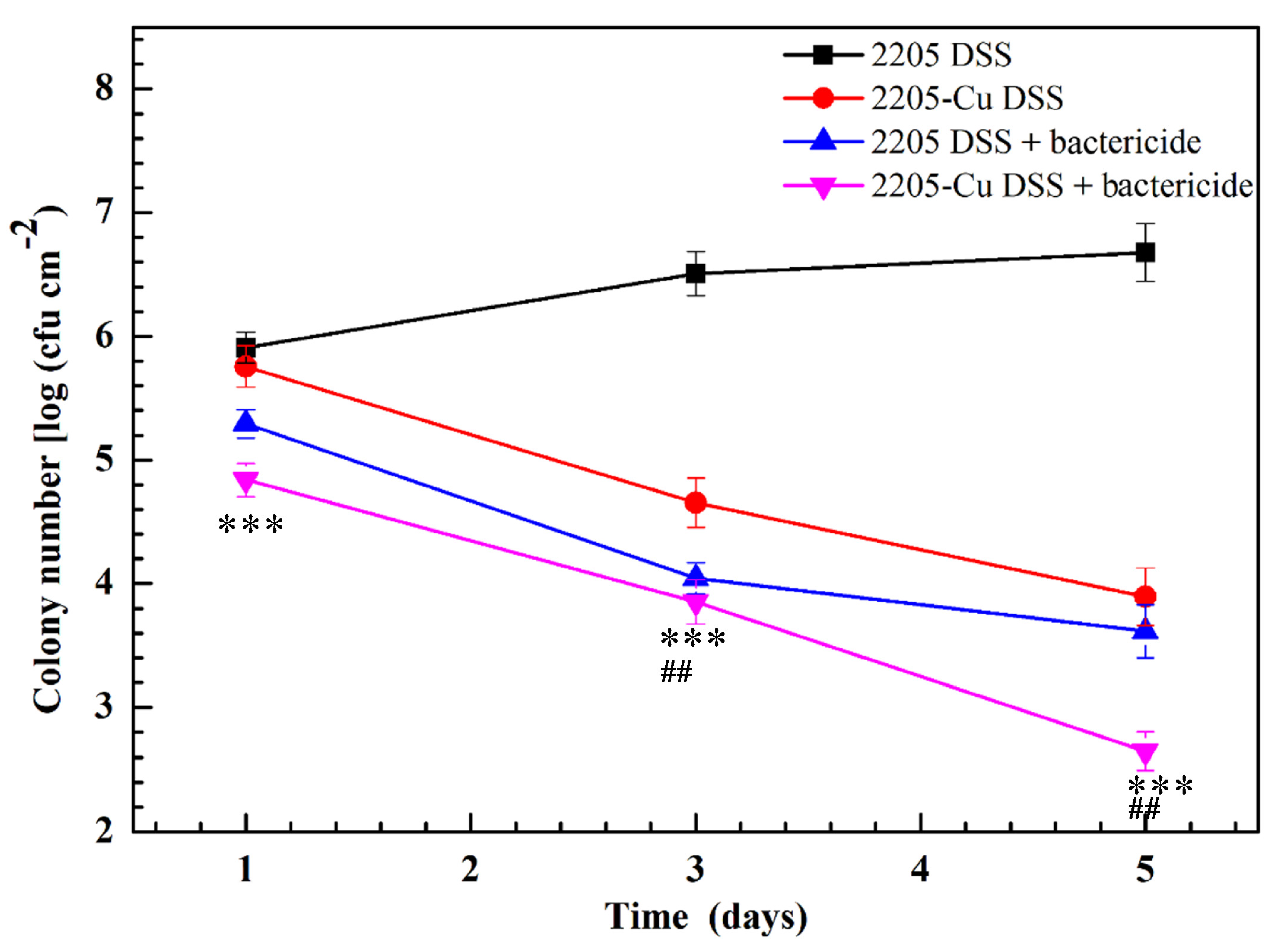
3.2. Antibacterial Effect
3.3. Morphology of Biofilm
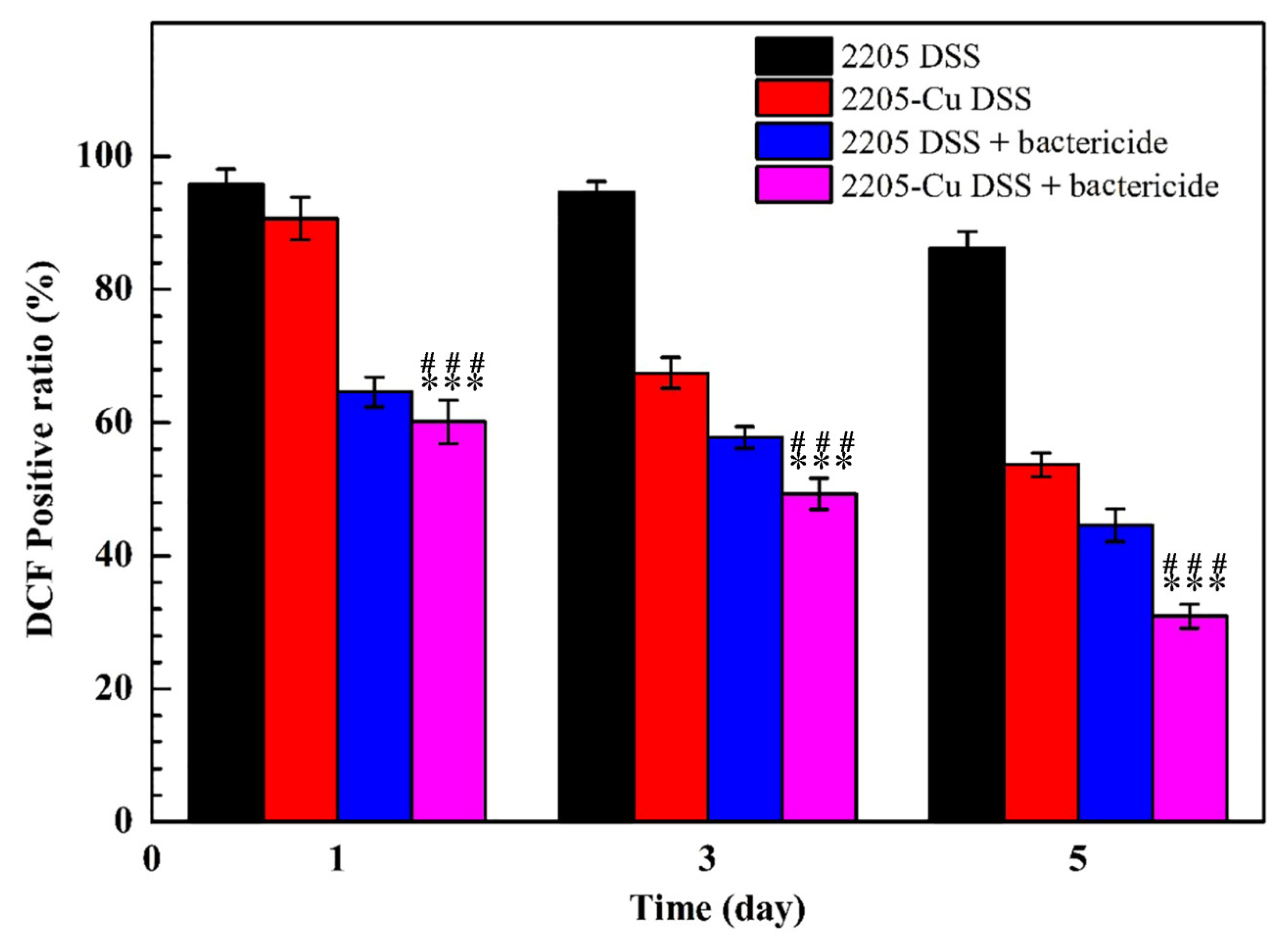
3.4. Survival Rate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Panayidou, S.; Georgiades, K.; Christofi, T.; Tamana, S.; Promponas, V.J.; Apidianakis, Y. Pseudomonas aeruginosa core metabolism exerts a widespread growth-independent control on virulence. Sci. Rep. 2020, 10, 9505. [Google Scholar] [CrossRef] [PubMed]
- Moradali, M.F.; Ghods, S.; Rehm, B.H. Pseudomonas aeruginosa Lifestyle: A Paradigm for Adaptation, Survival, and Persistence. Front. Cell. Infect. Microbiol. 2017, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Spagnolo, A.M.; Sartini, M.; Cristina, M.L. Pseudomonas aeruginosa in the healthcare facility setting. Rev. Med. Microbiol. 2021, 32, 169–175. [Google Scholar] [CrossRef]
- Sheng, X.; Ting, Y.-P.; Pehkonen, S.O. The influence of sulphate-reducing bacteria biofilm on the corrosion of stainless steel AISI 316. Corros. Sci. 2007, 49, 2159–2176. [Google Scholar] [CrossRef]
- Jeffrey, R.; Melchers, R.E. The changing topography of corroding mild steel surfaces in seawater. Corros. Sci. 2007, 49, 2270–2288. [Google Scholar] [CrossRef]
- Jia, R.; Yang, D.; Xu, D.; Gu, T. Anaerobic Corrosion of 304 Stainless Steel Caused by the Pseudomonas aeruginosa Biofilm. Front. Microbiol. 2017, 8, 2335. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Ye, X.; Hu, K.; Zhong, H.; Yuan, X.; Xiong, H.; Guo, Z. A facile one-pot method to two kinds of graphene oxide-based hydrogels with broad-spectrum antimicrobial properties. Chem. Eng. J. 2015, 260, 331–337. [Google Scholar] [CrossRef]
- Merchel Piovesan Pereira, B.; Tagkopoulos, I. Benzalkonium Chlorides: Uses, Regulatory Status, and Microbial Resistance. Appl. Environ. Microbiol. 2019, 85, e00377-19. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.; Pereira, A.M.; Pereira, M.C.; Melo, L.F.; Simoes, M. Physiological changes induced by the quaternary ammonium compound benzyldimethyldodecylammonium chloride on Pseudomonas fluorescens. J. Antimicrob. Chemother. 2011, 66, 1036–1043. [Google Scholar] [CrossRef]
- Ding, X.; Mou, S.; Zhao, S. Analysis of benzyldimethyldodecylammonium bromide in chemical disinfectants by liquid chromatography and capillary electrophoresis. J. Chromatogr. A 2004, 1039, 209–213. [Google Scholar] [CrossRef]
- Tavares, S.S.M.; Pardal, J.M.; Ponzio, E.; Loureiro, A.; de Souza, J.A. Influence of microstructure on the corrosion resistance of hyperduplex stainless steel. Mater. Corros. 2009, 61, 313–317. [Google Scholar] [CrossRef]
- Do Nascimento, A.M.; Ierardi, M.C.F.; Kina, A.Y.; Tavares, S.S.M. Pitting corrosion resistance of cast duplex stainless steels in 3.5%NaCl solution. Mater. Charact. 2008, 59, 1736–1740. [Google Scholar] [CrossRef]
- Luo, H.; Dong, C.F.; Cheng, X.Q.; Xiao, K.; Li, X.G. Electrochemical Behavior of 2205 Duplex Stainless Steel in NaCl Solution with Different Chromate Contents. J. Mater. Eng. Perform. 2011, 21, 1283–1291. [Google Scholar] [CrossRef]
- AlAbbas, F.M.; Williamson, C.; Bhola, S.M.; Spear, J.R.; Olson, D.L.; Mishra, B.; Kakpovbia, A.E. Influence of sulfate reducing bacterial biofilm on corrosion behavior of low-alloy, high-strength steel (API-5L X80). Int. Biodeterior. Biodegrad. 2013, 78, 34–42. [Google Scholar] [CrossRef]
- Heyer, A.; D’Souza, F.; Morales, C.F.L.; Ferrari, G.; Mol, J.M.C.; de Wit, J.H.W. Ship ballast tanks a review from microbial corrosion and electrochemical point of view. Ocean Eng. 2013, 70, 188–200. [Google Scholar] [CrossRef]
- Liu, J.; Jia, R.; Zhou, E.; Zhao, Y.; Dou, W.; Xu, D.; Yang, K.; Gu, T. Antimicrobial Cu-bearing 2205 duplex stainless steel against MIC by nitrate reducing Pseudomonas aeruginosa biofilm. Int. Biodeterior. Biodegrad. 2018, 132, 132–138. [Google Scholar] [CrossRef]
- Lou, Y.; Lin, L.; Xu, D.; Zhao, S.; Yang, C.; Liu, J.; Zhao, Y.; Gu, T.; Yang, K. Antibacterial ability of a novel Cu-bearing 2205 duplex stainless steel against Pseudomonas aeruginosa biofilm in artificial seawater. Int. Biodeterior. Biodegrad. 2016, 110, 199–205. [Google Scholar] [CrossRef]
- Xu, D.; Xia, J.; Zhou, E.; Zhang, D.; Li, H.; Yang, C.; Li, Q.; Lin, H.; Li, X.; Yang, K. Accelerated corrosion of 2205 duplex stainless steel caused by marine aerobic Pseudomonas aeruginosa biofilm. Bioelectrochemistry 2017, 113, 1–8. [Google Scholar] [CrossRef]
- Xia, J.; Yang, C.; Xu, D.; Sun, D.; Nan, L.; Sun, Z.; Li, Q.; Gu, T.; Yang, K. Laboratory investigation of the microbiologically influenced corrosion (MIC) resistance of a novel Cu-bearing 2205 duplex stainless steel in the presence of an aerobic marine Pseudomonas aeruginosa biofilm. Biofouling 2015, 31, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Lebourgeois, S.; Fraisse, A.; Hennechart-Collette, C.; Guillier, L.; Perelle, S.; Martin-Latil, S. Development of a Real-Time Cell Analysis (RTCA) Method as a Fast and Accurate Method for Detecting Infectious Particles of the Adapted Strain of Hepatitis A Virus. Front. Cell. Infect. Microbiol. 2018, 8, 335. [Google Scholar] [CrossRef]
- Sanna, T.; Dallolio, L.; Raggi, A.; Mazzetti, M.; Lorusso, G.; Zanni, A.; Farruggia, P.; Leoni, E. ATP bioluminescence assay for evaluating cleaning practices in operating theatres: Applicability and limitations. BMC Infect. Dis. 2018, 18, 583. [Google Scholar] [CrossRef] [PubMed]
- Abdolahi, A.; Hamzah, E.; Ibrahim, Z.; Hashim, S. Microbially influenced corrosion of steels by Pseudomonas aeruginosa. Corros. Rev. 2014, 32, 129–141. [Google Scholar] [CrossRef]
- Belsky, J.; Joshi, N.K. Effects of Fungicide and Herbicide Chemical Exposure on Apis and Non-Apis Bees in Agricultural Landscape. Front. Environ. Sci. 2020, 8, 81. [Google Scholar] [CrossRef]
- Kraemer, S.A.; Ramachandran, A.; Perron, G.G. Antibiotic Pollution in the Environment: From Microbial Ecology to Public Policy. Microorganisms 2019, 7, 180. [Google Scholar] [CrossRef]
- Ahire, J.J.; Hattingh, M.; Neveling, D.P.; Dicks, L.M. Copper-Containing Anti-Biofilm Nanofiber Scaffolds as a Wound Dressing Material. PLoS ONE 2016, 11, e0152755. [Google Scholar] [CrossRef] [PubMed]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Fazly Bazzaz, B.S. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control. 2019, 8, 118. [Google Scholar] [CrossRef]




| Component | Si | Mn | P | S | Ni | Cr | Mo | Cu | N | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| DSS (wt%) | 0.49 | 1.12 | 0.03 | <0.001 | 5.82 | 23.12 | 3.14 | - | 0.19 | Balance |
| Cu-DSS (wt%) | 0.05 | 0.02 | 0.005 | 0.0032 | 6.01 | 23.53 | 2.85 | 3.01 | 0.21 | Balance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Qiu, C.; Zhang, M.; Zhou, E.; Xu, D.; Fan, Y.; Song, Y. Synergistic Antibacterial Activity of Benzalkonium Bromide and Cu-Bearing Duplex Stainless Steel against Pseudomonas aeruginosa. Microorganisms 2023, 11, 711. https://doi.org/10.3390/microorganisms11030711
Liu X, Qiu C, Zhang M, Zhou E, Xu D, Fan Y, Song Y. Synergistic Antibacterial Activity of Benzalkonium Bromide and Cu-Bearing Duplex Stainless Steel against Pseudomonas aeruginosa. Microorganisms. 2023; 11(3):711. https://doi.org/10.3390/microorganisms11030711
Chicago/Turabian StyleLiu, Xiaomeng, Chengshuo Qiu, Mingxing Zhang, Enze Zhou, Dake Xu, Yongqiang Fan, and Yongbo Song. 2023. "Synergistic Antibacterial Activity of Benzalkonium Bromide and Cu-Bearing Duplex Stainless Steel against Pseudomonas aeruginosa" Microorganisms 11, no. 3: 711. https://doi.org/10.3390/microorganisms11030711
APA StyleLiu, X., Qiu, C., Zhang, M., Zhou, E., Xu, D., Fan, Y., & Song, Y. (2023). Synergistic Antibacterial Activity of Benzalkonium Bromide and Cu-Bearing Duplex Stainless Steel against Pseudomonas aeruginosa. Microorganisms, 11(3), 711. https://doi.org/10.3390/microorganisms11030711







