First Epidemiological Survey on the Prevalence and Subtypes Distribution of the Enteric Parasite Blastocystis sp. in Vietnam
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval and Questionnaire
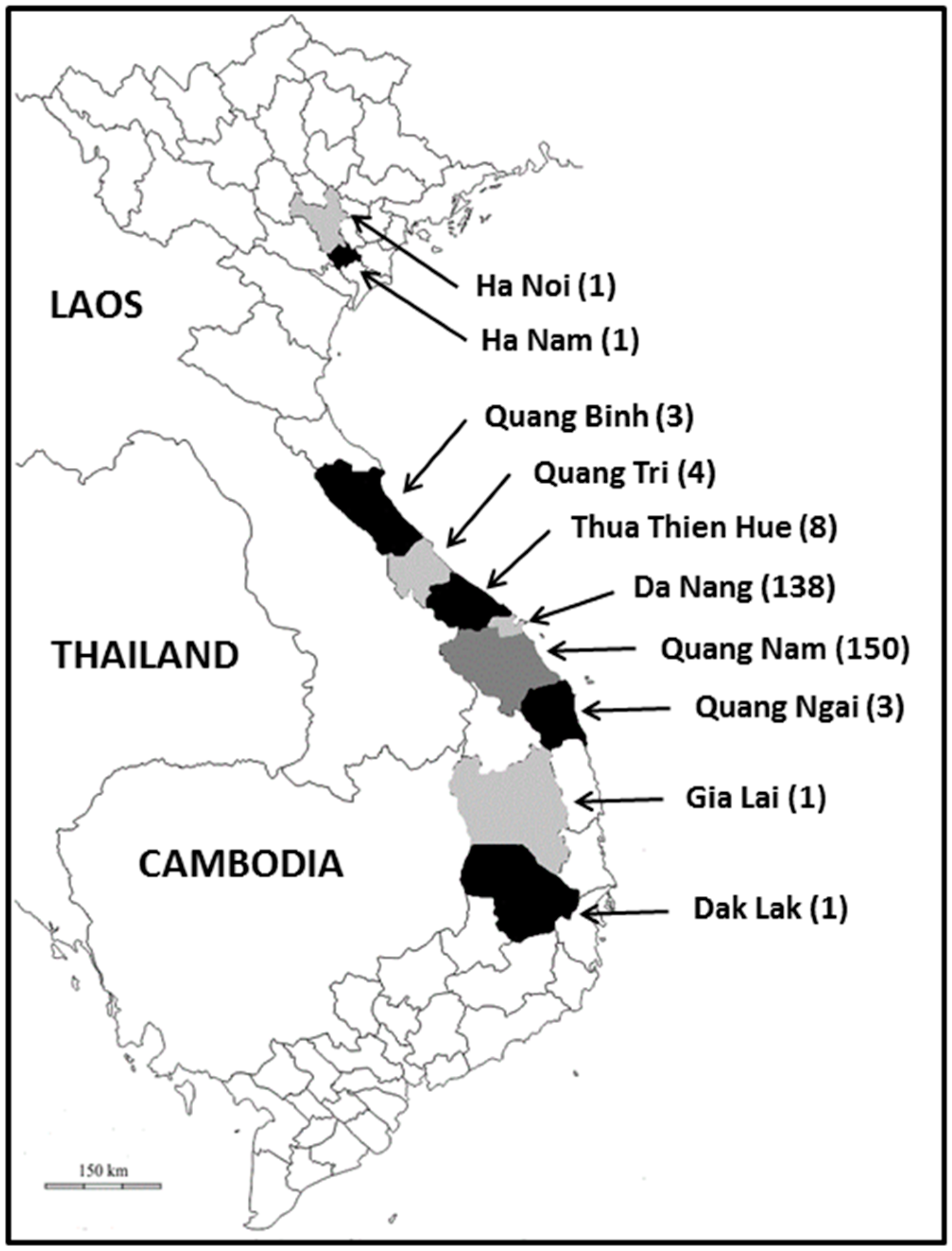
2.2. Sampling Site and Collection of Samples
2.3. DNA Extraction, PCR Assays and Molecular Subtyping/Genotyping of Blastocystis sp. Isolates
2.4. Statistical Analysis
3. Results and Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nemati, S.; Reza Zali, M.; Johnson, P.; Mirjalali, H.; Karanis, P. Molecular prevalence and subtype distribution of Blastocystis sp. in Asia and Australia. J. Water Health 2021, 19, 687. [Google Scholar] [CrossRef]
- Rauff-Adetotun, A.A.; Meor Termizi, F.H.; Shaari, N.; Lee, I.L. The coexistence of Blastocystis spp. in humans, animals and environmental sources from 2010–2021 in Asia. Biology 2021, 10, 990. [Google Scholar] [CrossRef]
- Jimenez, P.; Munoz, M.; Ramirez, J.D. An update on the distribution of Blastocystis subtypes in the Americas. Helyon 2023, 8, e12592. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.-Q.; Hu, Z.-H.; Chen, J.-H.; Tian, L.-G. Epidemiology of Blastocystis infection from 1990 to 2019 in China. Infect. Dis. Poverty 2020, 9, 168. [Google Scholar] [CrossRef] [PubMed]
- Lokmer, A.; Cian, A.; Froment, A.; Gantois, N.; Viscogliosi, E.; Chabé, M.; Ségurel, L. Use of shotgun metagenomics for the identification of protozoa in the gut microbiota of healthy individuals from worldwide populations with various industrialization levels. PLoS ONE 2019, 14, e0211139. [Google Scholar] [CrossRef]
- Khaled, S.; Gantois, N.; Tidjani Ly, A.; Senghor, S.; Even, G.; Dautel, E.; Dejager, R.; Sawant, M.; Baydoun, M.; Benamrouz-Vanneste, S.; et al. Prevalence and subtype distribution of Blastocystis sp. in Senegalese school children. Microorganisms 2020, 8, 1408. [Google Scholar] [CrossRef] [PubMed]
- Guilavogui, T.; Gantois, N.; Even, G.; Desramaut, J.; Dautel, E.; Denoyelle, C.; Cissé, F.I.; Touré, S.C.; Kourouma, B.L.; Sawant, M.; et al. Detection, Molecular identification and transmission of the intestinal protozoa Blastocystis sp. in Guinea from a large-scale epidemiological study conducted in the Conakry area. Microorganisms 2022, 10, 446. [Google Scholar] [CrossRef]
- Hublin, J.S.Y.; Maloney, J.G.; Santin, M. Blastocystis in domesticated and wild mammals and birds. Res. Vet. Sci. 2021, 135, 260–282. [Google Scholar] [CrossRef] [PubMed]
- Rauff-Adedotun, A.A.; Mohd Zain, S.N.; Farah Haziqah, M.T. Current status of Blastocystis sp. in animals from Southeast Asia: A review. Parasitol. Res. 2020, 119, 3559–3570. [Google Scholar] [CrossRef]
- Sanggari, A.; Komala, T.; Rauff-Adetotun, A.A.; Awosolu, O.B.; Attah, O.A.; Farah Haziqah, M.T. Blastocystis in captivated and free-ranging wild animals worldwide: A review. Trop. Biomed. 2022, 39, 338–372. [Google Scholar]
- Gantois, N.; Lamot, A.; Seesao, Y.; Creusy, C.; Li, L.L.; Monchy, S.; Benamrouz-Vanneste, S.; Karpouzopoulos, J.; Bourgain, J.L.; Rault, C.; et al. First report on the prevalence and subtype distribution of Blastocystis sp. in edible marine fish and marine mammals: A large scale-study conducted in Atlantic Northeast and on the coasts of Northern France. Microorganisms 2020, 8, 460. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.S.W. New insights on classification, identification, and clinical relevance of Blastocystis spp. Clin. Microbiol. Rev. 2008, 21, 639–665. [Google Scholar] [CrossRef]
- Attah, A.O.; Sanggari, A.; Li, L.I.; Nik Him, N.A.I.I.; Ismail, A.H.; Meor Termizi, F.H. Blastocystis occurrence in water sources worldwide from 2005 to 2022: A review. Parasitol. Res. 2023, 122, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Z.; Karim, M.R.; Zhang, L. Detection of human intestinal protozoan parasites in vegetables and fruits: A review. Parasit. Vectors 2020, 13, 380. [Google Scholar] [CrossRef]
- Stensvold, C.R.; Alfellani, M.A.; Norskov-Lauritsen, S.; Prip, K.; Victory, E.L.; Maddox, C.; Nielsen, H.V.; Clark, C.G. Subtype distribution of Blastocystis isolates from synanthropic and zoo animals and identification of a new subtype. Int. J. Parasitol. 2009, 39, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.O.; Stensvold, C.R. Blastocystis in health and disease: Are we moving from a clinical to a public health perspective? J. Clin. Microbiol. 2016, 54, 524–528. [Google Scholar] [CrossRef]
- Audebert, C.; Even, G.; Cian, A.; Blastocystis Investigation Group; Loywick, A.; Merlin, S.; Viscogliosi, E.; Chabé, M. Colonization with the enteric protozoa Blastocystis is associated with increased diversity of human gut bacterial microbiota. Sci. Rep. 2016, 6, 25255. [Google Scholar] [CrossRef]
- Tito, R.Y.; Chaffron, S.; Caenepeel, C.; Lima-Mendez, G.; Wang, J.; Vieira-Silva, S.; Falony, G.; Hildebrand, F.; Darzi, Y.; Rymenans, L.; et al. Population-level analysis of Blastocystis subtype prevalence and variation in the human gut microbiota. Gut 2019, 68, 1180–1189. [Google Scholar] [CrossRef]
- Even, G.; Lokmer, A.; Rodrigues, J.; Audebert, C.; Viscogliosi, E.; Ségurel, L.; Chabé, M. Changes in the human gut microbiota associated with colonization by Blastocystis sp. and Entamoeba spp. in non-industrialized populations. Front. Cell. Infect. Microbiol. 2021, 11, 533528. [Google Scholar] [CrossRef]
- Yason, J.A.; Liang, Y.R.; Png, C.W.; Zhang, Y.; Tan, K.S.W. Interactions between a pathogenic Blastocystis subtype and gut microbiota: In vitro and in vivo studies. Microbiome 2019, 7, 30. [Google Scholar] [CrossRef]
- Fréalle, E.; El Safadi, D.; Cian, A.; Aubry, E.; Certad, G.; Osman, M.; Wacrenier, A.; Dutoit, E.; Creusy, C.; Dubos, F.; et al. Acute Blastocystis-associated appendicular peritonitis in a child, Casablanca, Morocco. Emerg. Infect. Dis. 2015, 21, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Aykur, M.; Camyar, A.; Türk, B.G.; Sin, A.Z.; Dagci, H. Evaluation of association with subtypes and alleles of Blastocystis with chronic spontaneous urticarial. Acta Trop. 2022, 231, 106455. [Google Scholar] [CrossRef]
- Taghipour, A.; Rayatdoost, E.; Bairami, A.; Bahadory, S.; Abdoli, A. Are Blastocystis hominis and Cryptosporidium spp. playing a positive role in colorectal cancer risk? A systematic review and meta-analysis. Infect. J. Agents Cancer 2022, 17, 32. [Google Scholar] [CrossRef] [PubMed]
- Ajjampur, S.S.; Tan, K.S.W. Pathogenic mechanisms in Blastocystis spp.—Interpreting results from in vitro and in vivo studies. Parasitol. Int. 2016, 65, 772–779. [Google Scholar] [CrossRef]
- Deng, L.; Wojciech, L.; Gascoigne, N.R.J.; Peng, G.; Tan, K.S.W. New insights into the interactions between Blastocystis, the gut microbiota, and host immunity. PLoS Pathog. 2021, 17, e1009253. [Google Scholar] [CrossRef]
- Baek, S.; Maloney, J.G.; Molokin, A.; George, N.S.; Vecino, J.A.C.; Santin, M. Diversity of Blastocystis subtypes in horses in Colombia and identification of two new subtypes. Microorganisms 2022, 10, 1693. [Google Scholar] [CrossRef]
- Maloney, J.G.; Molokin, A.; Segui, R.; Maravilla, P.; Martinez-Hernandez, F.; Villalobos, G.; Tsaousis, A.D.; Gentekaki, E.; Munoz-Antoli, C.; Klisiowicz, D.R.; et al. Identification and molecular characterization of four new Blastocystis subtypes designated ST35-ST38. Microorganisms 2023, 11, 46. [Google Scholar] [CrossRef]
- Stensvold, C.R.; Clark, C.G. Pre-empting Pandora’s box: Blastocystis subtypes revisited. Trends Parasitol. 2020, 36, 229–232. [Google Scholar] [CrossRef]
- Alfellani, M.A.; Stensvold, C.R.; Vidal-Lapiedra, A.; Onuoha, E.S.; Fagbenro-Beyioku, A.F.; Clark, C.G. Variable geographic distribution of Blastocystis subtypes and its potential implications. Acta Trop. 2013, 126, 11–18. [Google Scholar] [CrossRef]
- Ramírez, J.D.; Sánchez, A.; Hernández, C.; Florez, C.; Bernal, M.C.; Giraldo, J.C.; Reyes, P.; Lopez, M.C.; Garcia, L.; Cooper, P.J.; et al. Geographic distribution of human Blastocystis subtypes in South America. Infect. Genet. Evol. 2016, 41, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.; Gantois, N.; Ayoubi, A.; Even, G.; Sawant, M.; El Houmayraa, J.; Nabot, M.; Benamrouz-Vanneste, S.; Chabé, M.; Certad, G.; et al. Blastocystis sp. prevalence and subtypes distribution amongst Syrian refugee communities living in North Lebanon. Microorganisms 2021, 9, 184. [Google Scholar] [CrossRef]
- Osario-Pulgarin, M.I.; Higuera, A.; Beltran-Alzate, J.C.; Sanchez-Jimenez, M.; Ramirez, J.D. Epidemiological and molecular characterization of Blastocystis infection in children attending daycare centers in Medellin, Colombia. Biology 2021, 10, 669. [Google Scholar] [CrossRef] [PubMed]
- Jinatham, V.; Maxamhud, S.; Popluechai, S.; Tsaousis, A.D.; Gentekaki, E. Blastocystis One Health approach in a rural community of Northern Thailand: Prevalence, subtypes and novel transmission routes. Front. Microbiol. 2021, 12, 746340. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Owen, H.; Traub, R.J.; Cuttell, L.; Inpankaew, T.; Bielefeldt-Ohmann, H. Molecular epidemiology of Blastocystis in pigs and their in-contact humans in Southeast Queensland, Australia, and Cambodia. Vet. Parasitol. 2014, 203, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Greige, S.; El Safadi, D.; Bécu, N.; Gantois, N.; Pereira, B.; Chabé, M.; Benamrouz-Vanneste, S.; Certad, G.; El Hage, R.; Chemaly, M.; et al. Prevalence and subtype distribution of Blastocystis sp. isolates from poultry in Lebanon and evidence of zoonotic potential. Parasit. Vectors 2018, 11, 389. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.-S.; Haung, Z.-F.; Lan, W.-H.; Kuo, T.-C.; Shin, J.-W. Epidemiology of Blastocystis hominis and other intestinal parasites in a Vietnamese female immigrant population in southern Taiwan. Kaohsiung J. Med. Sci. 2006, 22, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.T.; Sung, Y.J. Epidemiology of Blastocystis hominis and other intestinal parasites among the immigrant population in northeastern Taiwan by routine physical examination for residence approval. J. Microbiol. Immunol. Infect. 2009, 42, 505–509. [Google Scholar]
- Stensvold, C.R.; Arendrup, M.C.; Jespersgaard, C.; Molbak, K.; Nielsen, H.V. Detecting Blastocystis using parasitologic and DNA-based methods: A comparative study. Diagn. Microbiol. Infect. Dis. 2007, 59, 303–307. [Google Scholar] [CrossRef]
- Poirier, P.; Wawrzyniak, I.; Albert, A.; El Alaoui, H.; Delbac, F.; Livrelli, V. Development and evaluation of a real-time PCR assay for detection and quantification of Blastocystis parasites in human stool samples: Prospective study of patients with hematological malignancies. J. Clin. Microbiol. 2011, 49, 975–983. [Google Scholar] [CrossRef]
- Naguib, D.; Gantois, N.; Desramaut, J.; Arafat, N.; Even, G.; Certad, G.; Chabé, M.; Viscogliosi, E. Prevalence, subtype distribution and zoonotic significance of Blastocystis sp. isolates from poultry, cattle and pets in Northern Egypt. Microorganisms 2022, 10, 2259. [Google Scholar] [CrossRef]
- Noradilah, S.A.; Lee, I.L.; Anuar, T.S.; Salleh, F.M.; Manap, S.N.A.A.; Mohtar, N.S.H.M.; Azrul, S.M.; Abdullah, W.O.; Moktar, N. Occurrence of Blastocystis sp. in water catchments at Malay villages and aboriginal settlement during wet and dry seasons in Peninsular Malaysia. Peer J. 2016, 4, e2541. [Google Scholar] [CrossRef] [PubMed]

| Blastocystis sp. STs | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Provinces/ Municipalities | Samples (n) | Positive Samples (n) | Prevalence (%) | ST1 | ST3 | ST4 | ST6 | ST7 | ST8 | ST10 | ST14 | MI a |
| Da Nang | 138 | 49 | 35.5 | 5 | 14 | 0 | 1 | 2 | 1 | 1 | 1 | 24 |
| Quang Nam | 150 | 52 | 34.7 | 1 | 9 | 2 | 1 | 5 | 0 | 6 | 5 | 23 |
| Thua Thien Hué | 8 | 2 | 25.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Quang Tri | 4 | 0 | 0 | - | - | - | - | - | - | - | - | - |
| Quang Ngai | 3 | 1 | 33.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Quang Binh | 3 | 2 | 66.7 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Dak Lak | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - |
| Gia Lai | 1 | 1 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Ha Nam | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - |
| Ha Noi | 1 | 0 | 0 | - | - | - | - | - | - | - | - | - |
| Total | 310 | 107 | 34.5 | 6 | 23 | 2 | 2 | 7 | 1 | 8 | 6 | 52 |
| Blastocystis sp. STs | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Provinces/ Municipalities | ST1 | ST3 | ST4 | ST6 | ST7 | ST8 | ST10 | ST14 | MI a |
| Da Nang | 6 | 18 | 0 | 1 | 3 | 1 | 4 | 4 | 19 |
| Quang Nam | 1 | 14 | 2 | 1 | 7 | 0 | 12 | 11 | 16 |
| Thua Thien Hué | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Quang Tri | - | - | - | - | - | - | - | - | - |
| Quang Ngai | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| Quang Binh | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Dak Lak | - | - | - | - | - | - | - | - | - |
| Gia Lai | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Ha Nam | - | - | - | - | - | - | - | - | - |
| Ha Noi | - | - | - | - | - | - | - | - | - |
| Total | 7 | 32 | 2 | 2 | 11 | 1 | 17 | 16 | 39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, L.D.N.; Gantois, N.; Hoang, T.T.; Do, B.T.; Desramaut, J.; Naguib, D.; Tran, T.N.; Truong, A.D.; Even, G.; Certad, G.; et al. First Epidemiological Survey on the Prevalence and Subtypes Distribution of the Enteric Parasite Blastocystis sp. in Vietnam. Microorganisms 2023, 11, 731. https://doi.org/10.3390/microorganisms11030731
Nguyen LDN, Gantois N, Hoang TT, Do BT, Desramaut J, Naguib D, Tran TN, Truong AD, Even G, Certad G, et al. First Epidemiological Survey on the Prevalence and Subtypes Distribution of the Enteric Parasite Blastocystis sp. in Vietnam. Microorganisms. 2023; 11(3):731. https://doi.org/10.3390/microorganisms11030731
Chicago/Turabian StyleNguyen, Linh Do Ngoc, Nausicaa Gantois, Trung Thanh Hoang, Bong Thi Do, Jeremy Desramaut, Doaa Naguib, Tuan Ngoc Tran, Anh Duc Truong, Gaël Even, Gabriela Certad, and et al. 2023. "First Epidemiological Survey on the Prevalence and Subtypes Distribution of the Enteric Parasite Blastocystis sp. in Vietnam" Microorganisms 11, no. 3: 731. https://doi.org/10.3390/microorganisms11030731
APA StyleNguyen, L. D. N., Gantois, N., Hoang, T. T., Do, B. T., Desramaut, J., Naguib, D., Tran, T. N., Truong, A. D., Even, G., Certad, G., Chabé, M., & Viscogliosi, E. (2023). First Epidemiological Survey on the Prevalence and Subtypes Distribution of the Enteric Parasite Blastocystis sp. in Vietnam. Microorganisms, 11(3), 731. https://doi.org/10.3390/microorganisms11030731








