Effect of a Fourth Dose of mRNA Vaccine and of Immunosuppression in Preventing SARS-CoV-2 Breakthrough Infections in Heart Transplant Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. SARS-CoV-2 Antibody Testing
2.3. Study Endpoints
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. Survival Free from SARS-CoV-2 Events
3.3. Vaccine Campaign and Its Effect on Infections and Mortality
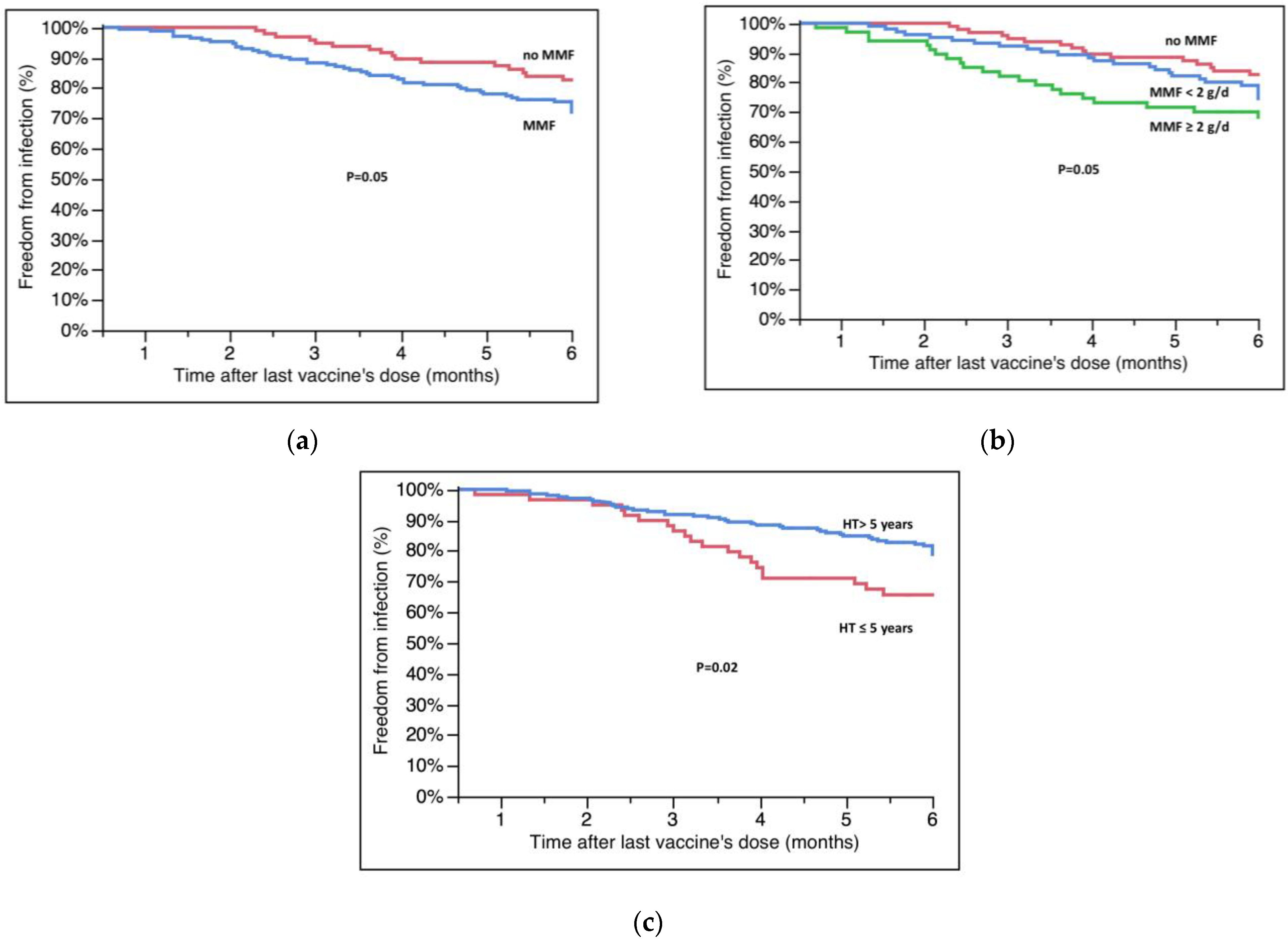
3.4. Factors Influencing Clinical Response to Vaccines
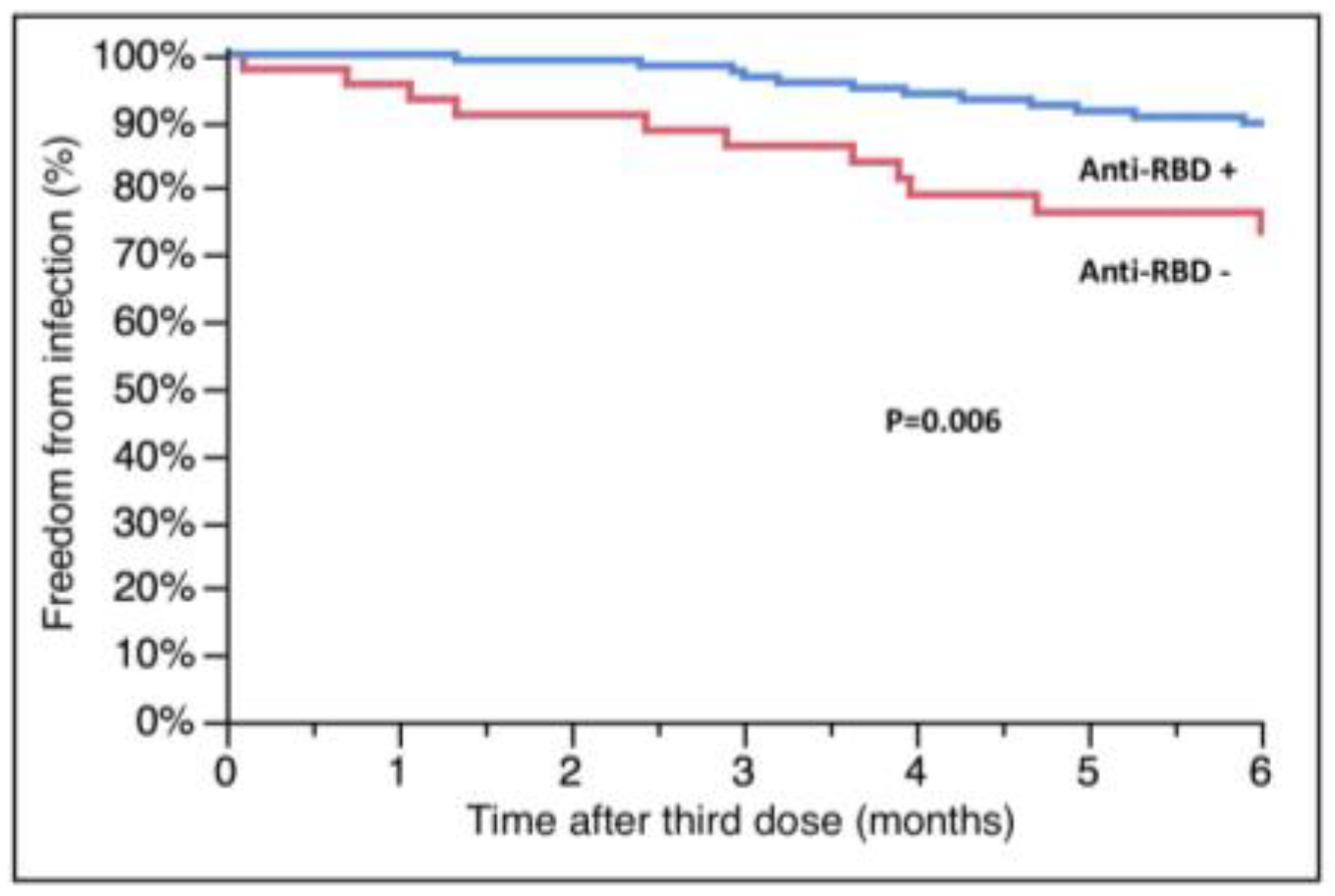
3.5. Anti-RBD Antibodies and Their Relationship with MMF and Breakthrough Infections
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ranavan, R.; Callaghan, C.J.; Mumford, L.; Ushiro-Lumb, I.; Thorburn, D.; Casey, J.; Friend, P.; Parameshwar, J.; Currie, I.; Burnapp, L.; et al. SARS-CoV-2 Infection and Early Mortality on Waitlisted and Solid organ Transplant Recipients in England: A National Cohort Study. Am. J. Transplant. 2020, 20, 3008–3018. [Google Scholar] [CrossRef]
- Aslam, S.; Adler, E.; Mekeel, K.; Little, S.J. Clinical Effectiveness of COVID-19 Vaccination in Solid Organ Transplant Recipients. Transpl. Infect. Dis. 2021, 23, e13705. [Google Scholar] [CrossRef] [PubMed]
- Embi, P.J.; Levy, M.E.; Naleway, A.L.; Patel, P.; Gaglani, M.; Natarajan, K.; Dascomb, K.; Ong, T.C.; Klein, N.P.; Liao, I.-C.; et al. Effectiveness of 2-Dose Vaccination with MRNA COVID-19 Vaccines Against COVID-19-Associated Hospitalizations Among Immunocompromised Adults—Nine States, January–September 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.X.; Moore, L.W.; Anjan, S.; Rahamimov, R.; Sifri, C.D.; Ali, N.M.; Morales, M.K.; Tsapepas, D.S.; Basic-Jukic, N.; Miller, R.A.; et al. Risk of Breakthrough SARS-CoV-2 Infections in Adult Transplant Recipients. Transplantation 2021, 105, e265–e266. [Google Scholar] [CrossRef]
- Nemet, I.; Kliker, L.; Lustig, Y.; Zuckerman, N.; Erster, O.; Cohen, C.; Kreiss, Y.; Alroy-Preis, S.; Regev-Yochay, G.; Mendelson, E.; et al. Third BNT162b2 Vaccination Neutralization of SARS-CoV-2 Omicron Infection. N. Engl. J. Med. 2022, 386, 492–494. [Google Scholar] [CrossRef] [PubMed]
- Regev-Yochay, G.; Gonen, T.; Gilboa, M.; Mandelboim, M.; Indenbaum, V.; Amit, S.; Meltzer, L.; Asraf, K.; Cohen, C.; Fluss, R.; et al. Efficacy of a Fourth Dose of COVID-19 MRNA Vaccine against Omicron. N. Engl. J. Med. 2022, 386, 1377–1380. [Google Scholar] [CrossRef]
- Peled, Y.; Ram, E.; Lavee, J.; Segev, A.; Matezki, S.; Wieder-Finesod, A.; Halperin, R.; Mandelboim, M.; Indenbaum, V.; Levy, I.; et al. Third Dose of the BNT162b2 Vaccine in Heart Transplant Recipients: Immunogenicity and Clinical Experience. J. Heart Lung Transplant. 2022, 41, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Peled, Y.; Afek, A.; Nemet, I.; Rahav, G.; Raanani, E.; Patel, J.K.; Mandelboim, M. Fourth BNT162b2 Vaccination Neutralization of Omicron Infection after Heart Transplantation. J. Heart Lung Transplant. 2022, 41, 1210–1213. [Google Scholar] [CrossRef]
- Giannella, M.; Righi, E.; Pascale, R.; Rinaldi, M.; Caroccia, N.; Gamberini, C.; Palacios-Baena, Z.R.; Caponcello, G.; Morelli, M.C.; Tamè, M.; et al. Evaluation of the Kinetics of Antibody Response to COVID-19 Vaccine in Solid Organ Transplant Recipients: The Prospective Multicenter ORCHESTRA Cohort. Microorganisms 2022, 10, 1021. [Google Scholar] [CrossRef]
- Monitoraggio COVID-19 Numero 85. Available online: https://www.salute.gov.it/portale/nuovocoronavirus/dettaglioMonitoraggioNuovoCoronavirus.jsp?lingua=italiano&menu=monitoraggi&id=94 (accessed on 15 December 2022).
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Among Hospitalized Adults Aged ≥65 Years—United States, January–March 2021. Available online: https://www.cdc.gov/mmwr/volumes/70/wr/pdfs/mm7018e1-H.pdf (accessed on 15 December 2022).
- Hall, V.; Foulkes, S.; Insalata, F.; Kirwan, P.; Saei, A.; Atti, A.; Wellington, E.; Khawam, J.; Munro, K.; Cole, M.; et al. Protection against SARS-CoV-2 after COVID-19 Vaccination and Previous Infection. N. Engl. J. Med. 2022, 386, 1207–1220. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Frenck, R.W.J.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based COVID-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef]
- Boyarsky, B.J.; Werbel, W.A.; Avery, R.K.; Tobian, A.A.R.; Massie, A.B.; Segev, D.L.; Garonzik-Wang, J.M. Antibody Response to 2-Dose SARS-CoV-2 MRNA Vaccine Series in Solid Organ Transplant Recipients. JAMA 2021, 325, 2204–2206. [Google Scholar] [CrossRef] [PubMed]
- Marinaki, S.; Adamopoulos, S.; Degiannis, D.; Roussos, S.; Pavlopoulou, I.D.; Hatzakis, A.; Boletis, I.N. Immunogenicity of SARS-CoV-2 BNT162b2 Vaccine in Solid Organ Transplant Recipients. Am. J. Transplant. 2021, 21, 2913–2915. [Google Scholar] [CrossRef]
- Hall, V.G.; Ferreira, V.H.; Ku, T.; Ierullo, M.; Majchrzak-Kita, B.; Chaparro, C.; Selzner, N.; Schiff, J.; McDonald, M.; Tomlinson, G.; et al. Randomized Trial of a Third Dose of MRNA-1273 Vaccine in Transplant Recipients. N. Engl. J. Med. 2021, 385, 1244–1246. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Abravanel, F.; Marion, O.; Couat, C.; Izopet, J.; Del Bello, A. Three Doses of an MRNA COVID-19 Vaccine in Solid-Organ Transplant Recipients. N. Engl. J. Med. 2021, 385, 661–662. [Google Scholar] [CrossRef]
- Joint Statement about COVID-19 Vaccination in Organ Transplant Candidates and Recipients. Available online: https://ishlt.org/ishlt/media/documents/ISHLT-AST-ASTS_Joint-Statement_COVID19-Vaccination_15November.pdf (accessed on 15 December 2022).
- Peled, Y.; Afek, A.; Kreiss, Y.; Rahav, G.; Nemet, I.; Kliker, L.; Indenbaum, V.; Ram, E.; Lavee, J.; Segev, A.; et al. Kinetics of Cellular and Humoral Responses to Third BNT162B2 COVID-19 Vaccine over Six Months in Heart Transplant Recipients—Implications for the Omicron Variant. J. Heart Lung Transplant. 2022, 41, 1417–1425. [Google Scholar] [CrossRef]
- Bottio, T.; Bagozzi, L.; Fiocco, A.; Nadali, M.; Caraffa, R.; Bifulco, O.; Ponzoni, M.; Lombardi, C.M.; Metra, M.; Russo, C.F.; et al. COVID-19 in Heart Transplant Recipients: A Multicenter Analysis of the Northern Italian Outbreak. JACC Heart Fail. 2021, 9, 52–61. [Google Scholar] [CrossRef]
- Mitchell, J.; Chiang, T.P.-Y.; Alejo, J.L.; Chang, A.; Abedon, A.T.; Avery, R.K.; Tobian, A.A.R.; Massie, A.B.; Levan, M.L.; Warren, D.S.; et al. Effect of Mycophenolate Mofetil Dosing on Antibody Response to SARS-CoV-2 Vaccination in Heart and Lung Transplant Recipients. Transplantation 2022, 106, e269–e270. [Google Scholar] [CrossRef]
- Itzhaki Ben Zadok, O.; Shaul, A.A.; Ben-Avraham, B.; Yaari, V.; Ben Zvi, H.; Shostak, Y.; Pertzov, B.; Eliakim-Raz, N.; Abed, G.; Abuhazira, M.; et al. Immunogenicity of the BNT162b2 MRNA Vaccine in Heart Transplant Recipients—A Prospective Cohort Study. Eur. J. Heart Fail. 2021, 23, 1555–1559. [Google Scholar] [CrossRef]
- Frölke, S.C.; Bouwmans, P.; Messchendorp, A.L.; Geerlings, S.E.; Hemmelder, M.H.; Gansevoort, R.T.; Hilbrands, L.B.; Reinders, M.E.J.; Sanders, J.-S.F.; Bemelman, F.J.; et al. Predictors of Nonseroconversion to SARS-CoV-2 Vaccination in Kidney Transplant Recipients. Transplant. Direct 2022, 8, e1397. [Google Scholar] [CrossRef] [PubMed]
- Manothummetha, K.; Chuleerarux, N.; Sanguankeo, A.; Kates, O.S.; Hirankarn, N.; Thongkam, A.; Dioverti-Prono, M.V.; Torvorapanit, P.; Langsiri, N.; Worasilchai, N.; et al. Immunogenicity and Risk Factors Associated with Poor Humoral Immune Response of SARS-CoV-2 Vaccines in Recipients of Solid Organ Transplant: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2022, 5, e226822. [Google Scholar] [CrossRef] [PubMed]
- Hallett, A.M.; Greenberg, R.S.; Boyarsky, B.J.; Shah, P.D.; Ou, M.T.; Teles, A.T.; Krach, M.R.; López, J.I.; Werbel, W.A.; Avery, R.K.; et al. SARS-CoV-2 Messenger RNA Vaccine Antibody Response and Reactogenicity in Heart and Lung Transplant Recipients. J. Heart Lung Transpl. 2021, 40, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Phillips, D.J.; White, T.; Sayal, H.; Aley, P.K.; Bibi, S.; Dold, C.; Fuskova, M.; Gilbert, S.C.; Hirsch, I.; et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 2032–2040. [Google Scholar] [CrossRef] [PubMed]
- Planas, D.; Veyer, D.; Baidaliuk, A.; Staropoli, I.; Guivel-Benhassine, F.; Rajah, M.M.; Planchais, C.; Porrot, F.; Robillard, N.; Puech, J.; et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 2021, 596, 276–280. [Google Scholar] [CrossRef]
- Lindenfeld, J.; Miller, G.G.; Shakar, S.F.; Zolty, R.; Lowes, B.D.; Wolfel, E.E.; Mestroni, L.; Page, R.L., 2nd; Kobashigawa, J. Drug Therapy in the Heart Transplant Recipient: Part I: Cardiac Rejection and Immunosuppressive Drugs. Circulation 2004, 110, 3734–3740. [Google Scholar] [CrossRef] [Green Version]
- Miele, M.; Busà, R.; Russelli, G.; Sorrentino, M.C.; Di Bella, M.; Timoneri, F.; Mularoni, A.; Panarello, G.; Vitulo, P.; Conaldi, P.G.; et al. Impaired Anti-SARS-CoV-2 Humoral and Cellular Immune Response Induced by Pfizer-BioNTech BNT162b2 MRNA Vaccine in Solid Organ Transplanted Patients. Am. J. Transplant. 2021, 21, 2919–2921. [Google Scholar] [CrossRef]
- Hall, V.G.; Ferreira, V.H.; Ierullo, M.; Ku, T.; Marinelli, T.; Majchrzak-Kita, B.; Yousuf, A.; Kulasingam, V.; Humar, A.; Kumar, D. Humoral and cellular immune response and safety of two-dose SARS-CoV-2 mRNA-1273 vaccine in solid organ transplant recipients. Am. J. Transplant. 2021, 21, 3980–3989. [Google Scholar] [CrossRef]
- Khush, K.K.; Cherikh, W.S.; Chambers, D.C.; Harhay, M.O.; Hayes, D.J.; Hsich, E.; Meiser, B.; Potena, L.; Robinson, A.; Rossano, J.W.; et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-Sixth Adult Heart Transplantation Report—2019; Focus Theme: Donor and Recipient Size Match. J. Heart Lung Transplant. 2019, 38, 1056–1066. [Google Scholar] [CrossRef]
- Sakai, A.; Morishita, T.; Suzumura, K.; Hanatate, F.; Yoshikawa, T.; Sasaki, N.; Lee, S.; Fujita, K.; Hara, T.; Araki, H.; et al. The Trajectory of the COVID-19 Vaccine Antibody Titers Over Time and the Association of Mycophenolate Mofetil in Solid Organ Transplant Recipients. Transplant. Proc. 2022, 54, 2638–2645. [Google Scholar] [CrossRef] [PubMed]
- Kahn, F.; Bonander, C.; Moghaddassi, M.; Rasmussen, M.; Malmqvist, U.; Inghammar, M.; Björk, J. Risk of severe COVID-19 from the Delta and Omicron variants in relation to vaccination status, sex, age and comorbidities—Surveillance results from southern Sweden, July 2021 to January 2022. Euro Surveill. 2022, 27, 2200121. [Google Scholar] [CrossRef] [PubMed]


| Variable 1 | (n = 268) |
|---|---|
| Demographic | |
| Age at last vaccine dose (years) | 61.4 ± 12.8 |
| Gender (males), n (%) | 198 (73.8%) |
| Distance from HT (years) | 12.3 ± 7.3 |
| Distance from HT ≤ 5 years, n (%) | 60 (22.4%) |
| Immunosuppression, n (%) | |
| Antiproliferative agents | 234 (87.3%) |
| MMF | 171 (63.8%) |
| Everolimus | 57 (21.3%) |
| Azatioprine | 6 (2.2%) |
| Calcineurin inhibitors | 267 (99.6%) |
| Cyclosporine | 174 (64.9%) |
| Tacrolimus | 93 (34.7%) |
| Steroids | 169 (63.0%) |
| Steroid dose > 5 mg/day | 39 (14.6%) |
| Comorbidities, n (%) | |
| Hypertension | 184 (69.1%) |
| Diabetes | 83 (30.9%) |
| Renal failure (GFR < 60 mL/min) | 166 (61.9%) |
| BMI > 30, n (%) | 32 (11.9%) |
| Variable | Three Doses (n = 195) | Four Doses (n = 73) | p |
|---|---|---|---|
| Demographic | |||
| Age at last vaccine dose (years) | 60.3 ± 12.9 | 64.9 ± 11.6 | 0.008 |
| Gender (males) | 142 (72.8%) | 56 (76.7%) | 0.51 |
| Distance from HT (years) | 12.3 ± 7.3 | 12.4 ± 7.1 | 0.85 |
| Distance from HT ≤ 5 years, n (%) | 43 (22.1%) | 17 (23.3%) | 0.82 |
| Immunosuppression | |||
| MMF, n (%) | 126 (64.6%) | 45 (61.6%) | 0.22 |
| MMF dose | 0.63 | ||
| No MMF | 69 (35.4%) | 28 (38.4%) | |
| MMF < 2000 mg/day | 79 (40.5%) | 25 (34.2%) | |
| MMF ≥ 2000 mg/day | 47 (24.1%) | 20 (27.4%) | |
| Everolimus, n (%) | 44 (22.8%) | 13 (17.8%) | |
| Calcineurin inhibitors, n (%) | 195 (100%) | 72 (99.6%) | 0.22 |
| Cyclosporine | 125 (64.4%) | 49 (67.1%) | |
| Tacrolimus | 70 (35.6%) | 23 (31.5%) | |
| Steroids, n (%) | 129 (66.8%) | 40 (54.8%) | 0.07 |
| Steroids dose > 5 mg/day | 31 (16.0%) | 8 (11.0%) | 0.30 |
| Comorbidities, n (%) | |||
| Hypertension | 129 (66.8%) | 55 (76.4%) | 0.12 |
| Diabetes | 61 (31.3%) | 22 (30.6%) | 0.90 |
| Renal failure (GFR < 60 mL/min) | 118 (60.5%) | 48 (65.7%) | 0.71 |
| BMI > 30 | 23 (11.8%) | 9 (12.5%) | 0.87 |
| Variable | Infected (n = 62) | Non Infected (n = 206) | p |
|---|---|---|---|
| Vaccine doses | 0.007 | ||
| Three doses, n (%) | 53 (85.5%) | 141 (68.8%) | |
| Four doses, n (%) | 9 (14.5%) | 64 (31.2%) | |
| Demographic | |||
| Age at last vaccine dose (years) | 60.5 ± 13.4 | 61.6 ± 12.7 | 0.57 |
| Gender (males) | 45 (72.6%) | 153 (74.3%) | 0.79 |
| Distance from HT (years) | 10.7 ± 7.4 | 12.7 ± 7.3 | 0.05 |
| Distance from HT ≤ 5 years | 20 (32.3%) | 40 (19.4%) | 0.03 |
| Immunosuppression | |||
| MMF, n (%) | 46 (74.2%) | 125 (60.7%) | 0.04 |
| MMF dose | 0.05 | ||
| No MMF | 16 (25.8%) | 81 (39.3%) | |
| MMF < 2000 mg/day | 25 (40.3%) | 79 (38.3%) | |
| MMF ≥ 2000 mg/day | 21 (33.8%) | 46 (22.3%) | |
| Everolimus, n (%) | 11 (17.7%) | 46 (22.3%) | 0.45 |
| Calcineurin inhibitors, n (%) | 62 (100%) | 205 (99.5%) | 0.79 |
| Cyclosporine | 42 (67.7%) | 133 (64.5%) | |
| Tacrolimus | 20 (32.3%) | 72 (34.9%) | |
| Steroids, n (%) | 37 (59.7%) | 132 (64.1%) | 0.59 |
| Steroids dose > 5 mg/day | 12 (19.4%) | 27 (13.1%) | 0.23 |
| Comorbidities, n (%) | |||
| Hypertension | 44 (71.0%) | 140 (68.3%) | 0.72 |
| Diabetes | 23 (37.1%) | 60 (29.3%) | 0.25 |
| Renal failure (GFR < 60 mL/min) | 40 (67.8%) | 128 (69.2%) | 0.71 |
| BMI > 30 | 7 (11.3%) | 25 (12.2%) | 0.85 |
| A | ||
| Variable | HR (95% CI) | p |
| Vaccine doses (four vs. three) | 0.47 (0.21–0.91) | 0.02 |
| Distance from HT ≤ 5 years | 1.97 (1.12–3.37) | 0.02 |
| Mycophenolate | 1.76 (1.02–3.20) | 0.04 |
| Age at last vaccine dose | 1.24 (0.98–1.02) | 0.99 |
| B | ||
| Variable | HR (95% CI) | p |
| Vaccine doses (four vs. three) | 0.45 (0.20–0.89) | 0.02 |
| Distance from HT ≤ 5 years | 1.93 (1.09–3.30) | 0.02 |
| Mycophenolate dose ≥ 2000 mg/day | 2.22 (1.16–4.32) | 0.02 |
| Age at last vaccine dose | 1.01 (0.98–1.02) | 0.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masetti, M.; Scuppa, M.F.; Aloisio, A.; Giovannini, L.; Borgese, L.; Manno, S.; Tazza, B.; Pascale, R.; Bonazzetti, C.; Caroccia, N.; et al. Effect of a Fourth Dose of mRNA Vaccine and of Immunosuppression in Preventing SARS-CoV-2 Breakthrough Infections in Heart Transplant Patients. Microorganisms 2023, 11, 755. https://doi.org/10.3390/microorganisms11030755
Masetti M, Scuppa MF, Aloisio A, Giovannini L, Borgese L, Manno S, Tazza B, Pascale R, Bonazzetti C, Caroccia N, et al. Effect of a Fourth Dose of mRNA Vaccine and of Immunosuppression in Preventing SARS-CoV-2 Breakthrough Infections in Heart Transplant Patients. Microorganisms. 2023; 11(3):755. https://doi.org/10.3390/microorganisms11030755
Chicago/Turabian StyleMasetti, Marco, Maria Francesca Scuppa, Alessio Aloisio, Laura Giovannini, Laura Borgese, Stefania Manno, Beatrice Tazza, Renato Pascale, Cecilia Bonazzetti, Natascia Caroccia, and et al. 2023. "Effect of a Fourth Dose of mRNA Vaccine and of Immunosuppression in Preventing SARS-CoV-2 Breakthrough Infections in Heart Transplant Patients" Microorganisms 11, no. 3: 755. https://doi.org/10.3390/microorganisms11030755
APA StyleMasetti, M., Scuppa, M. F., Aloisio, A., Giovannini, L., Borgese, L., Manno, S., Tazza, B., Pascale, R., Bonazzetti, C., Caroccia, N., Sabatino, M., Spitaleri, G., Viale, P., Giannella, M., & Potena, L. (2023). Effect of a Fourth Dose of mRNA Vaccine and of Immunosuppression in Preventing SARS-CoV-2 Breakthrough Infections in Heart Transplant Patients. Microorganisms, 11(3), 755. https://doi.org/10.3390/microorganisms11030755





