Inoculation with Plant Growth-Promoting Bacteria and Nitrogen Doses Improves Wheat Productivity and Nitrogen Use Efficiency
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Area and Location
2.2. Soil Analysis
2.3. Experimental Design and Treatments
2.4. Crop Management
2.5. Field Data Collection and Sample Processing
2.6. Nitrogen Use Efficiency
2.7. Statistical Analysis
3. Results
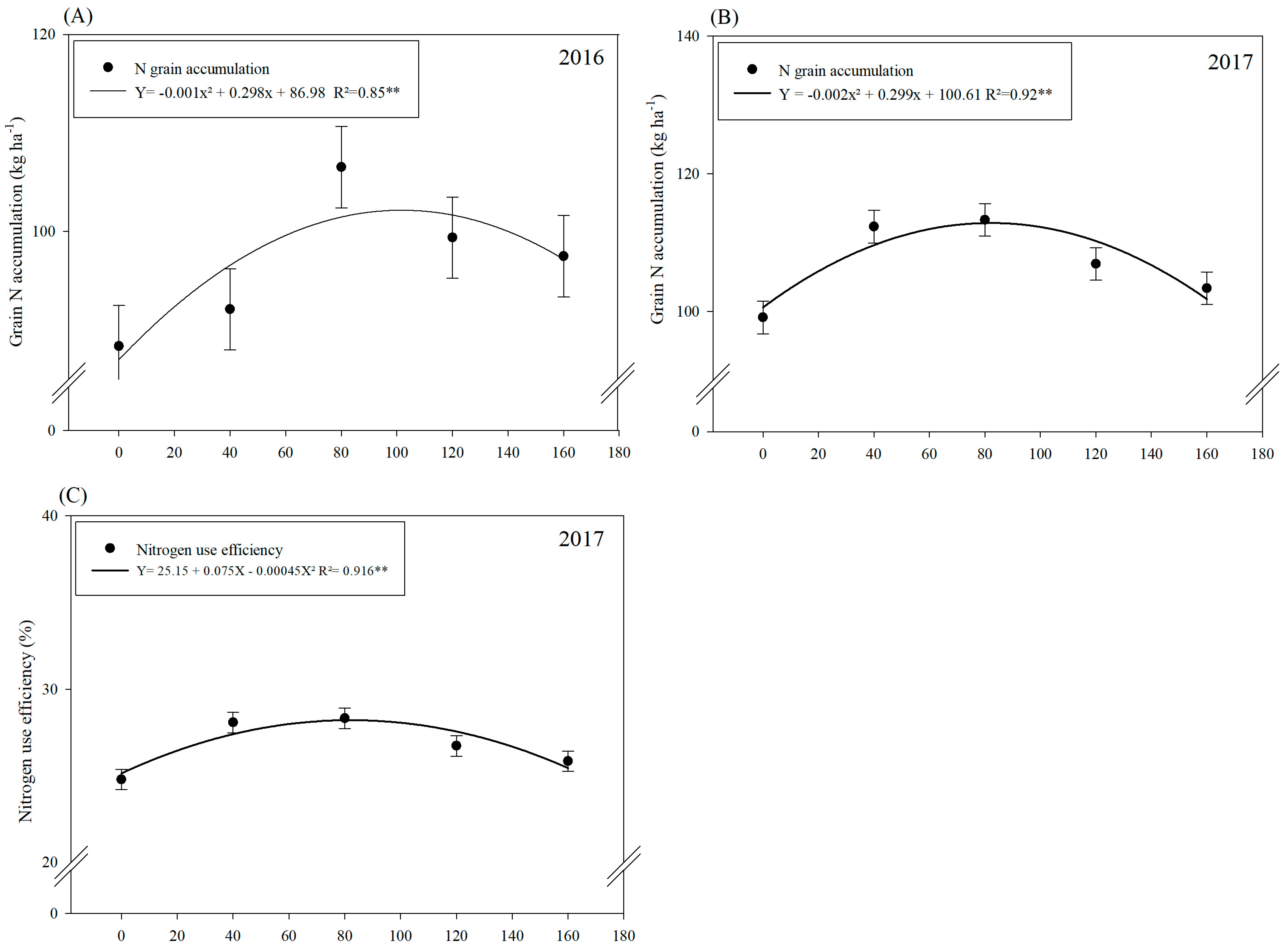
3.1. Nitrogen (N) Accumulations and Efficiencies
3.2. Leaf Chlorophyll Index (LCI), Plant Height and Productive Tillers m−1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations (FAO). Prospects of Farming and Alimentary Situation. World Quarterly Report. 2022. Available online: https://www.fao.org/worldfoodsituation/csdb/en/ (accessed on 7 March 2022).
- Galindo, F.S.; Pagliari, P.H.; Fernandes, G.C.; Rodrigues, W.L.; Boleta, E.H.M.; Jalal, A.; Céu, E.G.O.; de Lima, B.H.; Lavres, J.; Teixeira Filho, M.C.M. Improving sustainable field-grown wheat production with Azospirillum brasilense under tropical conditions: A potential tool for improving nitrogen management. Front. Environ. Sci. 2022, 10, 821628. [Google Scholar] [CrossRef]
- Marks, B.B.; Megías, M.; Nogueira, M.A.; Hungria, M. Biotechnological potential of rhizobial metabolites to enhance the performance of Bradyrhizobium spp. and Azospirillum brasilense inoculants with soybean and maize. AMB Express 2013, 3, 21. [Google Scholar] [CrossRef][Green Version]
- Galindo, F.S.; Teixeira Filho, M.C.M.; Buzetti, S.; Santini, J.M.K.; Alves, C.J.; Ludkiewicz, M.G.Z. Wheat yield in the Cerrado as affected by nitrogen fertilization and inoculation with Azospirillum brasilense. Pesqui. Agropecu. Bras. 2017, 52, 794–805. [Google Scholar] [CrossRef][Green Version]
- Galindo, F.S.; da Silva, E.C.; Pagliari, P.H.; Fernandes, G.C.; Rodrigues, W.L.; Biagini, A.L.C.; Baratella, E.B.; Silva Junior, C.A.; Moretti Neto, M.J.; Muraoka, T.; et al. Nitrogen use efficiency and recovery in a wheat-corn rotation under tropical savannah conditions. Nutr. Cycl. Agroecosyst. 2021, 119, 291–305. [Google Scholar] [CrossRef]
- Shah, S.; Hussain, M.; Jalal, A.; Khan, M.S.; Shah, T.; Ilyas, M.; Uzair, M. Nitrogen and sulfur rates and timing effects on phenology, biomass yield and economics of wheat. Sarhad J. Agric. 2018, 34, 671–679. [Google Scholar] [CrossRef]
- Tabak, M.; Lepiarczyk, A.; Filipek-Mazur, B.; Lisowska, A. Efficiency of nitrogen fertilization of winter wheat depending on sulfur fertilization. Agronomy 2020, 10, 1304. [Google Scholar] [CrossRef]
- Galindo, F.S.; Buzetti, S.; Rodrigues, W.L.; Boleta, E.H.M.; Silva, V.M.; Tavanti, R.F.R.; Fernandes, G.C.; Biagini, A.L.C.; Rosa, P.A.L.; Teixeira Filho, M.C.M. Inoculation of Azospirillum brasilense associated with silicon as a liming source to improve nitrogen fertilization in wheat crops. Sci. Rep. 2020, 10, 6160. [Google Scholar] [CrossRef][Green Version]
- Jalal, A.; Azeem, K.; Teixeira Filho, M.C.M.; Khan, A. Enhancing Soil Properties and Maize Yield through Organic and Inorganic Nitrogen and Diazotrophic Bacteria. In Sustainable Crop Production; Hasanuzzaman, M., Teixeira Filho, M.C.M., Fujita, M., Nogueira, T.A.S., Eds.; IntechOpen: London, UK, 2020; pp. 165–178. [Google Scholar] [CrossRef]
- Galindo, F.S.; Pagliari, P.H.; da Silva, E.C.; Silva, V.M.; Fernandes, G.C.; Rodrigues, W.L.; Céu, E.G.O.; de Lima, B.H.; Jalal, A.; Muraoka, T.; et al. Co-Inoculation with Azospirillum brasilense and Bradyrhizobium sp. enhances nitrogen uptake and yield in field-grown cowpea and did not change n-fertilizer recovery. Plants 2022, 11, 1847. [Google Scholar] [CrossRef]
- Cassán, F.; Coniglio, A.; López, G.; Molina, R.; Nievas, S.; Carlan, C.L.N.; Mora, V. Everything you must know about Azospirillum and its impact on agriculture and beyond. Biol. Fertil. Soils 2020, 56, 461–479. [Google Scholar] [CrossRef]
- Oliveira, C.E.D.S.; Jalal, A.; Oliveira, J.R.; Tamburi, K.V.; Teixeira Filho, M.C.M. Leaf inoculation of Azospirillum brasilense and Trichoderma harzianum in hydroponic arugula improve productive components and plant nutrition and reduce leaf nitrate. Pesqui. Agropecu. Trop. 2022, 52. [Google Scholar] [CrossRef]
- Piccinin, G.G.; Braccini, A.L.; Dan, L.G.; Scapim, C.A.; Ricci, T.T.; Bazo, G.L. Efficiency of seed inoculation with Azospirillum brasilense on agronomic characteristics and yield of wheat. Ind. Crop. Prod. 2013, 1, 393–397. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmad, I.; Hilger, T.H.; Nadeem, S.M.; Akhtar, M.F.; Jamil, M.; Hussain, A.; Zahir, Z.A. Preliminary study on phosphate solubilizing Bacillus subtilis strain Q3 and Paenibacillus sp. strain Q6 for improving cotton growth under alkaline conditions. PeerJ 2018, 6, e5122. [Google Scholar] [CrossRef][Green Version]
- Rosa, P.A.L.; Galindo, F.S.; Oliveira, C.E.D.S.; Jalal, A.; Mortinho, E.S.; Fernandes, G.C.; Marega, E.M.R.; Buzetti, S.; Teixeira Filho, M.C.M. Inoculation with plant growth-promoting bacteria to reduce phosphate fertilization requirement and enhance technological quality and yield of sugarcane. Microorganisms 2022, 10, 192. [Google Scholar] [CrossRef]
- Hussain, A.; Ahmad, M.; Nafees, M.; Iqbal, Z.; Luqman, M.; Jamil, M.; Maqsood, A.; Mora-Poblete, F.; Ahmar, S.; Chen, J.T.; et al. Plant-growth-promoting Bacillus and Paenibacillus species improve the nutritional status of Triticum aestivum L. PLoS ONE 2020, 15, e0241130. [Google Scholar] [CrossRef]
- Jalal, A.; Oliveira, C.E.dS.; Bastos, A.d.C.; Fernandes, G.C.; de Lima, B.H.; Furlani Junior, E.; de Carvalho, P.H.G.; Galindo, F.S.; Gato, I.M.B.; Teixeira Filho, M.C.M. Nanozinc and plant growth-promoting bacteria improve biochemical and metabolic attributes of maize in tropical Cerrado. Front. Plant Sci. 2023, 13, 1046642. [Google Scholar] [CrossRef]
- Sun, B.; Bai, Z.; Bao, L.; Xue, L.; Zhang, S.; Wei, Y.; Zhang, Z.; Zhuang, G.; Zhuang, X. Bacillus subtilis biofertilizer mitigating agricultural ammonia emission and shifting soil nitrogen cycling microbiomes. Environ. Int. 2020, 144, 105989. [Google Scholar] [CrossRef]
- Ribeiro, V.P.; Gomes, E.A.; de Sousa, S.M.; Lana, U.G.D.P.; Coelho, A.M.; Marriel, I.E.; de Oliveira-Paiva, C.A. Co-inoculation with tropical strains of Azospirillum and Bacillus is more efficient than single inoculation for improving plant growth and nutrient uptake in maize. Arch. Microbiol. 2022, 204, 143. [Google Scholar] [CrossRef]
- Jalal, A.; Oliveira, C.E.S.; Freitas, L.A.; Galindo, F.S.; Lima, B.H.; Boleta, E.H.M.; Silva, E.C.; Nascimento, V.; Nogueira, T.A.R.; Buzetti, S.; et al. Agronomic biofortification and productivity of wheat with soil zinc and diazotrophic bacteria in tropical savannah. Crop. Pasture Sci. 2022, 73, 817–830. [Google Scholar] [CrossRef]
- Jalal, A.; Oliveira, C.E.D.S.; Fernandes, H.B.; Galindo, F.S.; Silva, E.C.D.; Fernandes, G.C.; Nogueira, T.A.R.; De Carvalho, P.H.G.; Balbino, V.R.; de Lima, B.H.; et al. Diazotrophic bacteria is an alternative strategy for increasing grain biofortification, yield and zinc use efficiency of maize. Plants 2022, 11, 1125. [Google Scholar] [CrossRef]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef][Green Version]
- Joshi, B.; Chaudhary, A.; Singh, H.; Kumar, P.A. Prospective evaluation of individual and consortia plant growth promoting rhizobacteria for drought stress amelioration in rice (Oryza sativa L.). Plant Soil 2020, 457, 225–240. [Google Scholar] [CrossRef]
- Moretti, L.G.; Crusciol, C.A.; Kuramae, E.E.; Bossolani, J.W.; Moreira, A.; Costa, N.R.; Alves, C.J.; Pascoaloto, I.M.; Rondina, A.B.; Hungria, M. Effects of growth-promoting bacteria on soybean root activity, plant development, and yield. Agron. J. 2020, 112, 418–428. [Google Scholar] [CrossRef]
- Jalal, A.; Oliveira, C.E.S.; Galindo, F.S.; Rosa, P.A.L.; Gato, I.M.B.; de Lima, B.H.; Teixeira Filho, M.C.M. Regulatory mechanisms of plant growth-promoting rhizobacteria and plant nutrition against abiotic stresses in Brassicaceae family. Life 2023, 13, 211. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.D.M.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef][Green Version]
- Santos, H.G.; Jacomina, P.K.T.; Anjos, L.H.C.; Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; Almeida, J.A.; Araujo Filho, J.C.; Oliveira, J.B.; Cunha, T.J.F. Brazilian Soil Classification System, 5th ed.; Embrapa: Brasília, Brazil, 2018; Available online: http://www.infoteca.cnptia.embrapa.br/infoteca/handle/doc/1094001 (accessed on 5 January 2022).
- Raij, B.; van Andrade, J.C.; Cantarella, H.; Quaggio, J.A. Chemical Analysis for Fertility Evaluation of Tropical Soils; IAC: Campinas, Brazil, 2001; p. 285. (In Portuguese) [Google Scholar]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A Decimal Code for the Growth Stages of Cereals. Weed Res. 1974, 14, 415–421. Available online: https://www.cabdirect.org/cabdirect/abstract/19750730172 (accessed on 8 January 2022). [CrossRef]
- Viecelli, M.; Pagnoncelli, F.; Trezzi, M.M.; Cavalheiro, B.M.; Gobetti, R.C.R. Response of wheat plants to combinations of herbicides with insecticides and fungicides. Planta Daninha 2019, 37. [Google Scholar] [CrossRef][Green Version]
- Malavolta, E.; Vitti, G.C.; Oliveira, S.A. Evaluation of the Nutritional Status of Plants: Principles and Applications, 2nd ed.; Potafos: Piracicaba, Brazil, 1997; p. 319. (In Portuguese) [Google Scholar]
- Cowden, R.J.; Shah, A.N.; Lehmann, L.M.; Kiær, L.P.; Henriksen, C.B.; Ghaley, B.B. Nitrogen fertilizer effects on pea–barley intercrop productivity compared to sole crops in Denmark. Sustainability 2022, 12, 9335. [Google Scholar] [CrossRef]
- Ferreira, D.F. Sisvar: A computer analysis system to fixed effects split plot type designs. Rev. Bras. Biom. 2019, 37, 529–535. [Google Scholar] [CrossRef][Green Version]
- Hawkesford, M.J.; Griffiths, S. Exploiting genetic variation in nitrogen use efficiency for cereal crop improvement. Curr. Opin. Plant Biol. 2019, 49, 35–42. [Google Scholar] [CrossRef]
- Chien, S.H.; Prochnow, L.I.; Cantarella, H. Recent developments of fertilizer production and use to improve nutrient efficiency and minimize environmental impacts. Adv. Agron. 2009, 102, 267–322. [Google Scholar] [CrossRef]
- Dobermann, A. Nitrogen Use Efficiency—State of the Art. In Proceedings of the International Workshop on Enhanced-Efficiency Fertilizers, Frankfurt, Germany, 28–30 June 2005; International Fertilizer Industry Association: Paris, France, 2005. [Google Scholar]
- Fukami, J.; Cerezini, P.; Hungria, M. Azospirillum: Benefits that Go Far beyond Biological Nitrogen Fixation. AMB Express 2018, 8, 73. [Google Scholar] [CrossRef][Green Version]
- Zeffa, D.M.; Perini, L.J.; Silva, M.B.; de Sousa, N.V.; Scapim, C.A.; de Oliveira, A.L.M.; Júnior, A.T.D.A.; Gonçalves, L.S.A. Azospirillum brasilense promotes increases in growth and nitrogen use efficiency of maize genotypes. PLoS ONE 2019, 14, e0215332. [Google Scholar] [CrossRef][Green Version]
- Eckshtain-Levi, N.; Harris, S.L.; Roscios, R.Q.; Shank, E.A. Bacterial community members increase Bacillus subtilis maintenance on the roots of Arabidopsis thaliana. Phytobiomes J. 2020, 4, 303–313. [Google Scholar] [CrossRef]
- Li, X.; Zeng, R.; Liao, H. Improving crop nutrient efficiency through root architecture modifications. J. Integr. Plant Biol. 2016, 58, 193–202. [Google Scholar] [CrossRef][Green Version]
- Pii, Y.; Aldrighetti, A.; Valentinuzzi, F.; Mimmo, T.; Cesco, S. Azospirillum Brasilense inoculation Counteracts the Induction of Nitrate Uptake in maize Plants. J. Exp. Bot. 2019, 70, 1313–1324. [Google Scholar] [CrossRef]
- Singh, R.K.; Singh, P.; Li, H.B.; Song, Q.Q.; Guo, D.J.; Solanki, M.K.; Verma, K.K.; Malviya, M.K.; Song, X.P.; Lakshmanan, P.; et al. Diversity of nitrogen-fixing rhizobacteria associated with sugarcane, a comprehensive study of plant-microbe interactions for growth enhancement in Saccharum spp. BMC Plant Biol. 2020, 20, 220. [Google Scholar] [CrossRef]
- Poveda, J.; González-Andrés, F. Bacillus as a source of phytohormones for use in agriculture. Appl. Microbiol. Biotechnol. 2021, 105, 8629–8645. [Google Scholar] [CrossRef]
- Boleta, E.H.M.; Galindo, S.F.; Jalal, A.; Santini, J.M.K.; Rodrigues, W.L.; de Lima, B.H.; Arf, O.; Silva, M.R.D.; Buzetti, S.; Teixeira Filho, M.C.M. Inoculation with growth-promoting bacteria Azospirillum brasilense and its effects on productivity and nutritional accumulation of wheat cultivars. Front. Sustain. Food Syst. 2020, 4, 607262. [Google Scholar] [CrossRef]
- Lastochkina, O.; Garshina, D.; Allagulova, C.; Fedorova, K.; Koryakov, I.; Vladimirova, A. Application of Endophytic Bacillus subtilis and salicylic acid to improve wheat growth and tolerance under combined drought and Fusarium root rot stresses. Agronomy 2020, 10, 1343. [Google Scholar] [CrossRef]
- Kasim, W.A.; Osman, M.E.H.; Omar, M.N.; Salama, S. Enhancement of drought tolerance in Triticum aestivum L. seedlings using Azospirillum brasilense NO40 and Stenotrophomonas maltophilia B11. Bull. Natl. Res. Cent. 2021, 45, 95. [Google Scholar] [CrossRef]
- Silva, A.A.V.; Silva, I.A.F.; Teixeira Filho, M.C.M.; Buzetti, S.; Teixeira, M.C.M. Estimate of wheat grain yield as function of nitrogen fertilization using neuro fuzzy modeling. Rev. Bras. Eng. Agric. Ambient. 2014, 18, 80–187. [Google Scholar] [CrossRef][Green Version]
- Teixeira Filho, M.C.M.; Buzetti, S.; Andreotti, M.; Benett, C.G.S.; Arf, O.; De Sá, M.E. Wheat nitrogen fertilization under no till on the low altitude Brazilian Cerrado. J. Plant Nutr. 2014, 37, 1732–1748. [Google Scholar] [CrossRef]
- Teixeira Filho, M.C.M.; Buzetti, S.; Andreotti, M.; Arf, O.; De SÁ, M.E. Application times, sources and doses of nitrogen on wheat cultivars under no till in the Cerrado region. Ciência Rural 2011, 41, 1375–1382. [Google Scholar] [CrossRef]
- Luo, C.; Guo, Z.; Xiao, J.; Dong, J.; Dong, Y. Effects of applied ratio of nitrogen on the light environment in the canopy and growth, development and yield of wheat when intercropped. Front. Plant Sci. 2021, 12, 719850. [Google Scholar] [CrossRef]



| Layer | P resin | S-SO4 | OM | pH | K | Ca | Mg | H + Al |
|---|---|---|---|---|---|---|---|---|
| (m) | -----mg dm−3----- | g dm−3 | CaCl2 | ----------------------mmolc dm−3--------------------- | ||||
| 0.0–0.2 | 20.0 | 3.0 | 24.0 | 5.3 | 5.3 | 33.0 | 20.0 | 28.0 |
| Layer | B b | Cu c | Fe c | Mn c | Zn c | CEC | ||
| (m) | --------------------------mg dm−3-------------------------- | mmolc dm−3 | ||||||
| 0.0–0.2 | 0.19 | 3.9 | 21.0 | 63.5 | 1.6 | 86.3 | ||
| Variables | Grain N Accumulation (kg ha−1) | NUE (%) | RAN (%) | |||
|---|---|---|---|---|---|---|
| 2016 | 2017 | 2016 | 2017 | 2016 | 2017 | |
| Inoculations | ||||||
| Control | 85.13 ± 14.50 b | 97.83 ± 7.47 c | 21.28 ± 3.46 b | 24.46 ± 2.47 c | 36.12 ± 6.37 c | 40.98 ± 5.86 c |
| A. brasilense | 92.37 ± 18.92 b | 104.76 ± 10.43 b | 23.09 ± 5.81 b | 26.19 ± 2.61 bc | 42.27 ± 9.06 b | 46.27 ± 4.57 ab |
| B. subtilis | 97.75 ± 25.41 ab | 112.25 ± 11.88 a | 24.44 ± 5.58 ab | 28.32 ± 3.30 a | 38.46 ± 9.41 ab | 41.98 ± 8.31 bc |
| A. brasilense + B. subtilis | 111.86 13.32 a | 113.30 ± 9.87 a | 27.96 ± 4.17 a | 28.06 ± 2.72 ab | 49.97 ± 16.31 a | 47.67 ± 6.89 a |
| LSD | 15.46 | 6.71 | 3.86 | 1.98 | 5.11 | 4.81 |
| N Doses (kg ha−1) | ||||||
| 0 | 88.37 | 99.18 | 22.90 | 24.79 | 34.09 | 38.94 |
| 40 | 92.11 | 112.35 | 23.03 | 28.08 | 41.11 | 45.33 |
| 80 | 106.54 | 113.32 | 26.64 | 28.33 | 41.65 | 47.24 |
| 120 | 99.38 | 106.94 | 24.85 | 26.73 | 44.32 | 44.92 |
| 160 | 97.49 | 103.39 | 24.37 | 25.84 | 47.35 | 44.69 |
| F-values | ||||||
| Inoculations (I) | 7.49 ** | 11.56 ** | 7.49 ** | 11.56 ** | 19.77 ** | 6.38 ** |
| N doses (N) | 3.49 * | 6.33 ** | 2.29 ns | 6.33 ** | 10.43 ** | 4.72 ** |
| I × N | 1.29 ns | 1.85 ns | 1.28 ns | 1.86 ns | 2.31 * | 1.96 ns |
| CV(%) | 19.08 | 8.87 | 19.08 | 8.87 | 14.63 | 12.99 |
| Variables | LCI | Plant Height (cm) | Number of Tillers m−1 | |||
|---|---|---|---|---|---|---|
| 2016 | 2017 | 2016 | 2017 | 2016 | 2017 | |
| Inoculations | ||||||
| Control | 54.18 ± 5.95 b | 49.77 ± 4.03 a | 91.40 ± 3.16 bc | 97.29 ± 5.90 a | 111.80 ± 10.16 c | 151.47 ± 29.39 b |
| A. brasilense | 60.52 ± 5.44 a | 49.54 ± 19.98 a | 89.69 ± 2.81 c | 96.27 ± 2.35 a | 129.20 ± 21.07 b | 161.07 ± 14.91 ab |
| B. subtilis | 58.79 ± 5.42 ab | 49.91 ± 3.65 a | 96.11 ± 3.42 a | 97.82 ± 2.76 a | 139.55 ± 22.18 ab | 170.80 ± 25.64 ab |
| A. brasilense + B. subtilis | 57.63 ± 3.37 ab | 51.26 ± 21.22 a | 93.31 ± 3.59 b | 96.40 ± 5.05 a | 149.80 ± 14.24 a | 179.87 ± 24.31 a |
| LSD | 4.71 | 3.15 | 2.76 | 3.92 | 14.85 | 25.18 |
| N Doses (kg ha−1) | ||||||
| 0 | 54.74 | 50.05 | 92.01 | 98.14 | 131.69 | 161.60 |
| 40 | 58.36 | 50.64 | 92.88 | 97.17 | 130.75 | 171.33 |
| 80 | 56.83 | 50.67 | 93.22 | 95.89 | 133.44 | 171.33 |
| 120 | 60.71 | 49.48 | 92.35 | 96.56 | 138.50 | 159.67 |
| 160 | 58.26 | 49.77 | 92.67 | 96.97 | 128.56 | 165.17 |
| F-values | ||||||
| Inoculations (I) | 7.28 ** | 1.24 ns | 11.38 ** | 2.27 ns | 6.42 ** | 15.08 ** |
| N doses (N) | 0.58 ns | 2.01 ns | 1.47 ns | 1.92 ns | 0.98 ns | 1.18 ns |
| I x N | 1.08 ns | 0.78 ns | 0.75 ns | 1.15 ns | 1.29 ns | 0.88 ns |
| CV(%) | 8.30 | 7.50 | 3.55 | 4.12 | 13.38 | 15.48 |
| Variables | Number of Grains Spike−1 | 100-Grain Weight (g) | Grain Yield (kg ha−1) | |||
|---|---|---|---|---|---|---|
| 2016 | 2017 | 2016 | 2017 | 2016 | 2017 | |
| Inoculations | ||||||
| Control | 36.20 ± 3.48 b | 32.53 ± 1.33 a | 3.54 ± 0.28 a | 3.30 ± 0.22 a | 3502 ± 446 c | 4066 ± 250 b |
| A. brasilense | 36.90 ± 2.14 ab | 33.53 ± 1.37 a | 3.58 ± 0.22 a | 3.44 ± 0.20 a | 4037 ± 858 b | 4556 ± 245 ab |
| B. subtilis | 37.64 ± 2.01 ab | 33.80 ± 1.86 a | 3.49 ± 0.34 a | 3.42 ± 0.20 a | 4397 ± 978 ab | 4982 ± 262 a |
| A. brasilense + B. subtilis | 38.57 ± 3.39 a | 34.33 ± 2.57 a | 3.56 ± 0.22 a | 3.43 ± 0.21 a | 4947 ± 638 a | 5152 ± 358 a |
| LSD | 2.33 | 1.92 | 0.21 | 0.17 | 656.8 | 660.3 |
| N Doses (kg ha−1) | ||||||
| 0 | 36.39 | 33.42 | 3.60 | 3.37 | 4162 | 4555 |
| 40 | 37.19 | 34.33 | 3.58 | 3.41 | 4086 | 4917 |
| 80 | 38.99 | 33.17 | 3.56 | 3.45 | 4475 | 4904 |
| 120 | 37.48 | 33.42 | 3.40 | 3.34 | 4262 | 4493 |
| 160 | 36.57 | 33.42 | 3.57 | 3.35 | 4122 | 4576 |
| F-values | ||||||
| Inoculations (I) | 7.28 ** | 1.24 ns | 11.38 ** | 2.27 ns | 6.42 ** | 15.08 ** |
| N doses (N) | 5.58 ** | 1.07 ns | 1.97 ns | 2.02 ns | 1.08 ns | 1.77 ns |
| I x N | 1.00 ns | 0.88 ns | 0.55 ns | 1.07 ns | 1.09 ns | 0.38 ns |
| CV(%) | 7.45 | 5.84 | 7.07 | 6.13 | 18.59 | 14.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaspareto, R.N.; Jalal, A.; Ito, W.C.N.; Oliveira, C.E.d.S.; Garcia, C.M.d.P.; Boleta, E.H.M.; Rosa, P.A.L.; Galindo, F.S.; Buzetti, S.; Ghaley, B.B.; et al. Inoculation with Plant Growth-Promoting Bacteria and Nitrogen Doses Improves Wheat Productivity and Nitrogen Use Efficiency. Microorganisms 2023, 11, 1046. https://doi.org/10.3390/microorganisms11041046
Gaspareto RN, Jalal A, Ito WCN, Oliveira CEdS, Garcia CMdP, Boleta EHM, Rosa PAL, Galindo FS, Buzetti S, Ghaley BB, et al. Inoculation with Plant Growth-Promoting Bacteria and Nitrogen Doses Improves Wheat Productivity and Nitrogen Use Efficiency. Microorganisms. 2023; 11(4):1046. https://doi.org/10.3390/microorganisms11041046
Chicago/Turabian StyleGaspareto, Rafaela Neris, Arshad Jalal, William Cesar Nishimoto Ito, Carlos Eduardo da Silva Oliveira, Cássia Maria de Paula Garcia, Eduardo Henrique Marcandalli Boleta, Poliana Aparecida Leonel Rosa, Fernando Shintate Galindo, Salatiér Buzetti, Bhim Bahadur Ghaley, and et al. 2023. "Inoculation with Plant Growth-Promoting Bacteria and Nitrogen Doses Improves Wheat Productivity and Nitrogen Use Efficiency" Microorganisms 11, no. 4: 1046. https://doi.org/10.3390/microorganisms11041046
APA StyleGaspareto, R. N., Jalal, A., Ito, W. C. N., Oliveira, C. E. d. S., Garcia, C. M. d. P., Boleta, E. H. M., Rosa, P. A. L., Galindo, F. S., Buzetti, S., Ghaley, B. B., & Filho, M. C. M. T. (2023). Inoculation with Plant Growth-Promoting Bacteria and Nitrogen Doses Improves Wheat Productivity and Nitrogen Use Efficiency. Microorganisms, 11(4), 1046. https://doi.org/10.3390/microorganisms11041046











