1. Introduction
Francisella tularensis is a Gram-negative zoonotic bacterium which is classified as a class 3 biological agent and category A bioterrorism agent by the United States Centers for Disease Control and Prevention (CDC, Atlanta, GA, USA) and as category I by the European Medicine Agency (EMA).
The natural life cycle of
F. tularensis is concentrated on lagomorphs and rodents, whereas arthropods and mosquitos can play a role as vectors in transmission to humans [
1]. After handling infected carcasses or following an infected arthropod bite, ulceroglandular tularemia may arise. Consumption of contaminated water or food may result in oropharyngeal tularemia. Inhaling contaminated aerosols can result in pneumonia. The most severe form of illness is tularemia sepsis. Due to the low inoculation dose and the ability to penetrate an intact skin,
F. tularensis is a serious threat for laboratory-acquired infections [
2].
F. tularensis can be divided into four subspecies, namely,
F. tularensis subspecies (ssp.)
tularensis,
F. tularensis ssp.
holarctica,
F. tularensis ssp.
mediasiatica [
3], and
F. tularensis ssp.
novicida [
4].
F. tularensis ssp.
tularensis, which is also known as type A, is the most pathogenic subspecies and should be handled under biosafety level 3 (BSL-3) conditions. This subspecies is found in North America [
5].
F. tularensis ssp.
holarctica, which is also known as type B, displays lower virulence and can be handled under BSL-2 conditions. This subspecies is present in the Northern Hemisphere [
5]. Both
F. tularensis ssp.
mediasiatica, found in Central Asia [
3], and
F. tularensis ssp.
novicida, found in in the Northern Hemisphere and Australia, are less virulent [
6]. No human cases have been described for
F. tularensis ssp.
mediasiatica, whereas infection with
F. tularensis ssp.
novicida is rare and mainly restricted to immunocompromised patients [
6,
7].
F. tularensis ssp.
novicida is an outlier among the other
F. tularensis subspecies [
8], because it is transmitted from salt or brackish water and not found in animals [
6]. Additionally, it differs genetically from the other
F. tularensis subspecies [
6,
9,
10,
11].
Due to this wide range of clinical symptoms and virulence and the possibility of intentional dissemination, clinical laboratories need a fast and accurate method to identify
F. tularensis and distinguish its subspecies. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) could have this potential. The Bruker Biotyper MALDI-TOF MS system is increasingly used for the identification of bacteria in clinical laboratories. The Vitek MS (Biomerieux) is also available for this purpose. In the standard library of both the Vitek MS [
12] and the Bruker Biotyper system (MALDI Biotyper library),
F. tularensis is not included. The MALDI Biotyper library, however, can be supplemented with the MALDI Biotyper Security library (SR) for potential bioterrorism agents including
F. tularensis but without
F. tularensis subspecies differentiation. To overcome this, an in-house library was created to distinguish
F. tularensis subspecies [
13].
Francisella-specific biomarkers were studied for
F. tularensis, with and without subspecies differentiation, and for
F. philomiragia,
F. halioticida and
F.salimarina [
13,
14,
15,
16,
17,
18,
19]. An in-house database was also described limited to
F. tularensis ssp.
holarctica and
novicida and
F. philomiragia [
18] and one limited to different MLVA end PFGE-types of
F. tularensis ssp.
holarctica from Spain and the Czech Republic [
16].
In this study, MALDI-TOF MS was validated for fast and accurate detection of the complete range of clinically relevant Francisella species including F. tularensis and differentiation to subspecies level within a clinical setting by building an in-house Francisella library and determining specific biomarkers.
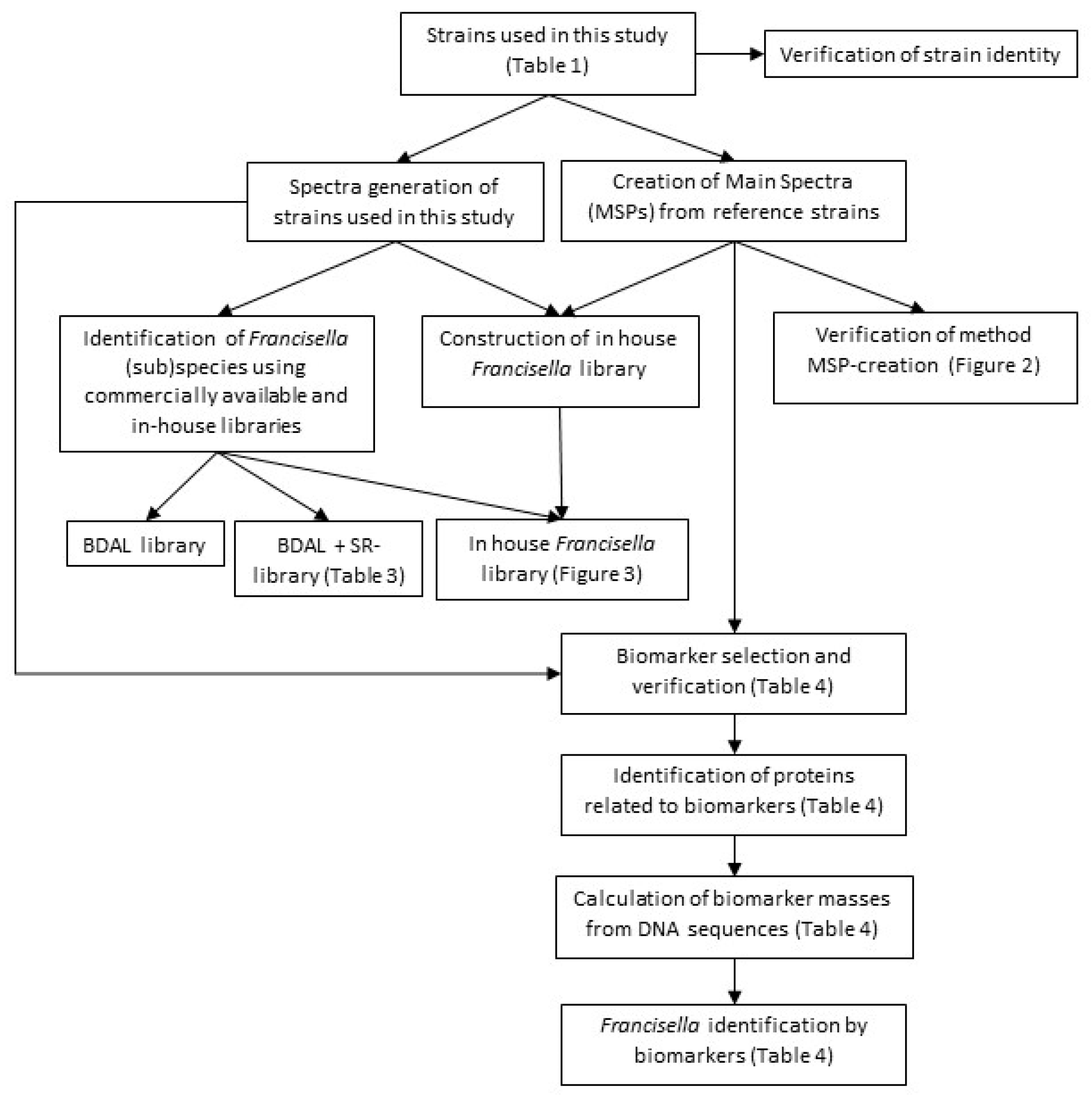
2. Materials and Methods
Both main spectra (MSPs) and regular spectra were made of the clinically relevant
Francisellaceae, including
F. tularensis with the four different subspecies that can be encountered in routine clinical practice and of the closely related
Legionella pneumophila. The in-house
Francisella-specific database was built using the MSPs and was verified, together with the BDAL and SR database, using the regular spectra. The MSPs were also used to determine differentiating biomarkers (
Figure 1).
2.1. Strains Used in This Study
All
F. tularensis strains, except for the wild-type strains, were kindly provided by the repository of the European Union project for the “Establishment of Quality Assurances for Detection of Highly Pathogenic Bacteria of Potential Bioterrorism Risk” (EQADeBa; Grant number 2007204). Strains genetically or phenotypically related to
F. tularensis were selected as negative control strains and were obtained from “Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH” (DSMZ, Braunschweig, Germany), “Culture Collection, University of Göteborg” (CCUG, Göteborg, Sweden), and “American Type Culture Collection” (ATCC, Manassas, VA, USA) (
Table 1).
F. halioticida and
F.[
Wolbachia]
persica were excluded because special culture media are required for growth [
20] and therefore they are not likely to be encountered in a routine clinical laboratory. Not validly published species were also excluded.
2.2. Verification of Strain Identity
The identity of all strains was verified by 16S rDNA Sanger sequencing and real-time PCR targeting the outer membrane gene
tul4 [
21] and the helicase gene (
FT-heli) [
22]. In addition, wild-type strains were analysed using conventional biochemical techniques and fatty acid analysis (Sherlock Microbial Identification System (MIDI Labs, Inc., Newark, NJ, USA) using the CLIN 6.1 and BTR 3.1 library) according to the polyphasic taxonomy [
23].
A loop full of bacterial culture was suspended in 100 µL of sterile water and heat inactivated at 100 °C for 30 min under BSL-3 conditions. All further molecular techniques were applied at BSL-2 conditions. PCR amplification of the DNA consisted of 10 min pre-denaturation at 95 °C, 35 cycles of denaturation at 95 °C for 30 s, annealing at 54 °C for 30 s, and elongation at 72 °C for 80 s using the primers described in
Table 2 [
24]. Partial (nt8-575 accession J01695) 16S rDNA Sanger sequence analysis was performed on the amplified DNA with a 3130XL Genetic Analyzer (Applied Biosystems, Nieuwerkerk aan de IJssel, the Netherlands). The 16S rDNA sequences were analysed using Clone Manager 9.3 (Sci-Ed Software, Cary, NC, USA). The sequence of the 16S rRNA gene of every strain was compared to the sequence of their type strain. The 16S rDNA sequence of the type strains as determined in our laboratory is compared to available 16S rDNA sequences of those strains in GenBank (National Center for Biotechnology Information (NCBI), Bethesda, USA). The in-house sequence of the type strains was compared to GenBank acessionnr. AY968223 (
F. tularensis ssp.
tularensis), AY968231 (
F. tularensis ssp.
holarctica), AY968237 (
F. tularensis ssp.
novicida), AJ698863 (
F. tularensis ssp.
mediasiatica), FN252413 (
F. hispaniensis), DQ295795 (
F. noatunensis ssp.
noatunensis), EU683030 (
F. orientalis), AY968239 (
F. philomiragia), FJ591095 (
A. guangzhouensis), M59157 (
L. pneumophila). The 16S rDNA sequences of all
F. tularensis ssp.
tularensis were compared to
F. tularensis ssp.
tularensis strain FSC 053 (AY968223) because only short stretches of the type strain sequence are deposited in GenBank [
25]. The type strain and the 16S rDNA sequence of
F. tularensis ssp.
holarctica are not available in any known culture collection [
26] or database, respectively. Strain
F. tularensis ssp.
holarctica FSC257 (AY968231) was selected for comparison of the 16S rDNA sequence.
The
F. tularensis gene
tul4 [
21] was targeted using a fluorescence resonance energy transfer (FRET)-based real-time PCR.
FT-heli [
22] was chosen to differentiate
F. tularensis ssp.
holarctica from the other subspecies. A PCR product of each target was cloned into the CR2.1-TOPO plasmid (TOPO TA Cloning Kit, Invitrogen, Breda, Netherlands) to serve as internal control. The quality of the cloned fragments was confirmed by sequence analysis. The LightCycler FastStart DNA Master
plus HybProbe kit (Roche Diagnostics GmbH, Berlin, Germany) was used with a reaction mixture containing 10 pmoles of both forward and reverse primer and 4 pmoles of the fluorescein-labelled probe and the CAL Fluor Red 635-labelled probe each (
Table 2). Four µL DNA of the described strains (
Table 1), plasmid solution, or PCR-grade water (in case of negative controls) was added to a final volume of 20 μL. All samples were run with and without plasmid control (10 fg per reaction) to check for inhibition.
The PCR was performed on the LightCycler 480 system (Roche Diagnostics GmbH, Berlin, Germany) and consists of 10 min pre-denaturation at 95 °C; 45 cycles of denaturation at 95 °C for 10 s, annealing at 59 °C for 20 s, and elongation at 72 °C for 15 s. PCR products were visualised on the QIAxcel System (QIAGEN GmbH, Hilden, Germany) using the QIAxcel DNA High Resolution Kit (QIAGEN GmbH, Hilden, Germany) with a QX Alignment Marker 15 bp/3 kb (QIAGEN GmbH, Hilden, Germany), the QX DNA Size Marker 100 bp–2.5 kb (QIAGEN GmbH, Hilden, Germany) and OM500 settings. The
tul4 fragments were sequenced using the forward and reverse primer on the 3130XL Genetic Analyzer using the real-time PCR products as template (
Table 2).
2.3. Spectra Generation of Strains Used in this Study
For each strain (
Table 1), spectra were generated using the direct transfer and the formic acid extraction method using HCCA (α-cyano-4-hydroxycinnamic acid in trifluoracetic acid) matrix (Bruker GMBH, Bremen, Germany) as described by the manufacturer. FlexControl version 3.3 (Bruker Daltonics GmbH, Bremen, Germany) was used to create spectra of the selected strains and perform baseline subtraction and smoothening of the spectra.
All F. tularensis strains were handled in a BSL-3 facility. Unviability of the organisms smeared on the MALDI target was confirmed by swapping two spots of each strain and leaving two spots for MALDI-TOF MS. The swabs were checked for growth on chocolate agar with vitox (PO5090A, Oxoid Deutschland GmbH, Wesel, Germany) aerobically (7% CO2) at 37 °C for 48 h before the MALDI target left the BSL-3 facility. Unviability of the organisms using the extraction method was confirmed by plating 25 µL of the milliQ/ethanol mixture on chocolate agar with vitox as described for the swabs.
2.4. Creation of Main Spectra (MSPs) from Reference Strains and Construction of In-House Francisella Library
To construct MSPs, the type strain of each
Francisella (sub)species was used as a reference strain. However, for the avirulent
F. tularensis ssp.
tularensis type strain [
3] and the not publicly available type strain of
F. tularensis ssp.
holarctica [
26],
F. tularensis ssp.
tularensis Schu4 and
F. tularensis ssp.
holarctica LVS were used as reference strains, respectively (
Table 1). A protein extract of each strain was applied to the target plate eightfold and analysed in triplicate, resulting in 24 spectra. MALDI Biotyper version 3.0 (Bruker Daltonics GmbH, Bremen, Germany) was applied to assemble the 24 spectra to an MSP and create an in-house
Francisella library.
2.5. Verification of the Method of MSP Creation
To verify the method of MSP creation, the MSPs of F. philomiragia strains F184, F185, F93, and F51 from the MALDI Biotyper library version 3.1.2–version 11 (BDAL; Bruker Daltonics GmbH, Bremen, Germany) and the MSPs of F. tularensis ssp. holarctica strain 10,857 (=NCTC 10857) and F. tularensis ssp. tularensis strain CAPM 5600 (=Schu4) of the MALDI Biotyper Security library version 1.0.0.0 (SR; Bruker Daltonics GmbH, Bremen, Germany) were compared to in-house MSPs of the same strains by creating a dendrogram with an arbitrary distance normalised to a maximum value of 1000 (Bruker MALDI Biotyper Compass Explorer 4.1).
2.6. Identification of Francisella (sub)Species Using Commercially Available and In-House Libraries
All spectra were verified against the BDAL library version 3.1.2 and to the BDAL+SR library (SR library version 1.0.0.0). Additionally, the in-house
Francisella library was verified using those strains. Verification was performed both with the direct transfer method and the formic acid extraction method on a Microflex LT mass spectrometer (Bruker Daltonics GmbH, Bremen, Germany) in linear positive mode measuring in a range of 2000–20,000 kDa [
27]. The SR library does not discriminate between the subspecies; however, this database includes strain (CAPM) 5600, which is
F. tularensis ssp.
tularensis, and strains (CAPM) 5151, 5536, 5537, 5540 and (NCTC) 10,857 that are
F. tularensis ssp.
holarctica.
According to the manufacturer, scores between 2.300 and 3.000 are highly probable species identifications, scores between 2.000 and 2.299 are secure genus identifications and probable species identifications, scores between 1.700 and 1.999 are probable genus identifications, and scores below 1.700 are considered “no reliable identifications”. Because the subspecies identification of F. tularensis has a clinical impact, not only the differences in score between the first and second species (not to be confused with the score of the second hit), but also the differences in scores between subspecies will be determined. Differences in scores between duplicates of the same strain higher than 0.300 were rejected.
2.7. Biomarker Selection and Verification
For the selection of biomarkers suitable for discrimination of
Francisellaceae, the MSPs of the reference strain of all species and subspecies of
Francisella (
Table 1) were imported as a text file from flexAnalysis into BioNumerics 7.1 (Applied Maths, Gent, Belgium) using the “preprocessing (strict)” method. Biomarkers were identified with the “peak class matching” method according to the standard settings except for “constant tolerance = 2” and “linear tolerance = 500”.
The selected biomarkers were validated using the spectra of all strains (
Table 1) after both the direct transfer method and the formic acid extraction method. Peaks were considered to be (sub)species-specific biomarkers when they were present in all spectra of all strains of the specific species or subspecies and absent in all spectra of most other strains. Biomarkers were compared with the MSP profiles and should be present in all individual spectra of an MSP. Extra biomarkers related in mass or DNA sequence were added.
m/
z values of the selected biomarkers were also compared in FlexAnalysis with the mass values of the identification runs, i.e., the unexpected absence or presence of masses were examined manually on peak level because biomarkers can be missed, because routine analyses are based on less spectra than MSPs.
2.8. Identification of Proteins Related to Biomarkers
To identify potential proteins, the
m/
z value of the biomarkers were compared to the Tagident and to the UniprotKB database [
28]. This was performed with 1+, 2+, and 3+ charges, as multiple charges have been observed using the HCCA matrix [
29] and for the common post-translational maturations, methylation (+15, 30 and 45 Da), acetylation (+42 Da), and methionine loss at the N-terminus of the protein (−131 Da). Incidentally, proteins start with leucine, a functional start amino acid (AA) [
30], and not the assigned methionine. Therefore, the molecular weight of the biomarkers was determined from the DNA sequence of the genes that was translated into amino acid by CloneManager. Because N-formylmethionyl-tRNA deformylase was present in the genomes of the reference strains (except CP010427), no correction was applied for the formyl mass.
2.9. Calculation of Biomarker Masses from DNA Sequences
Knowing the identity of the proteins, and as such their nucleotide sequence, made it possible to determine their presence in the relevant (sub)species and absence in the other (sub)species using the BLASTn algorithm in the NCBI Nuleotide Collection (nr/nt) database April–May 2022 against “
Francisellaceae”. The results were downloaded as a seqdump.txt file and aligned and translated to an AA sequence with CloneManager for comparison with the predicted AA sequence of the reference strains. The mass of the AA sequence was calculated with CloneManager (designated theoretical
m/
z value throughout this manuscript). For the 50S ribosomal protein L34, a BLASTp was performed to check its genus specificity. Manual adjustments were made when necessary.
F. salinarina [
19],
F. oppertunistica [
31],
A. frigiaqua,
A. inopinata [
32], and
Pseudofrancisella aestuarii [
33] are genetically related to
F. tularensis. Except for
F. oppertunistica, the species were found in cold (sea)water without any relation to human pathogenicity. However, to avoid misidentification, the theoretical presence of the biomarkers was also determined for these species.
3. Results
3.1. Verification of the Strain Identity
The identity of all strains included in this study was confirmed using 16S rDNA Sanger sequencing, detection of the
tul4 and
FT-heli genes (
Table 1), and biochemical methods. One strain of
F. tularensis ssp.
novicida was negative in the real-time PCR but demonstrated a positive
tul4 band on agarose gel. Sequencing of this product revealed a discrepancy of two bases compared to the Fl-probe sequence. Of the genetically and phenotypically related species,
tul4 was only detected in
F. hispaniensis.
The FT-heli target was positive in all
F. tularensis strains and the size of the target differed between
F. tularensis ssp.
holarctica (153 bp) and the other
F. tularensis subspecies (183 bp) as described [
22]. However, this last target was not specific for
F. tularensis because FT-heli was also detected in
F. hispaniensis and
F. philomiragia and both displayed a 183 bp PCR product.
3.2. Spectra Generation of Strains Used in this Study
The application of standard methods for direct transfer and formic acid extraction left no viable F. tularensis cells on the MALDI target plate, confirming that transport outside of the BSL3-facility is safe.
3.3. Verification of the Method of MSP Creation
The MSPs of both the Bruker databases and the in-house database formed separated clusters per species (
Figure 2), indicating differences on species level that are possibly suitable for biomarker-based identification. The SR MSPs of strains 5600 (Schu) and 10,857 did not cluster directly with the in-house MSP of the respective strains of
F. tularensis ssp.
tularensis BD13-00126 and
F. tularensis ssp.
holarctica BD13-00127. The four corresponding
F. philomiragia strains F51, F93, F184, and F185 formed database-related clusters, indicating differences in preparing MSPs.
3.4. Identification of Francisella (sub)Species Using Commercially Available and In-House Libraries
Using only the BDAL library, all Francisella strains demonstrated “no reliable identification”. This is a correct score as none of these Francisella species are present in this database. F. hispaniensis was incorrectly identified as Lactobacillus diolivorans with a borderline log score of 1.760, indicating “probable genus identification” when using the formic acid extraction method. F. philomiragia and Legionella pneumophila were correctly identified.
Using the BDAL+SR library, all
F. tularensis subspecies were correctly identified as
F. tularensis (
Supplementary Table S1), but no subspecies differentiation is possible with these libraries. The subspecies
tularensis and
holarctica could however be differentiated by their strain number (
Table 3) with both direct transfer and formic acid extraction method. The
F. tularensis ssp.
tularensis strains all scored “
F. tularensis 5600” above 2.000 and the difference with the second species was at least 0.221.
F. tularensis ssp.
holarctica and
F. philomiragia strains were correctly identified with scores higher than 1.800, and without second species scores. The strains of the other two
F. tularensis subspecies and
F. hispaniensis scored
F. tularensis below 2.000 without discriminating between subspecies
tularensis and
holarctica.
The in-house
Francisella library can detect
F. tularensis down to a subspecies level (
Figure 3) with log scores higher than 2.000 and sufficient differences with the second species. However,
F. tularensis ssp.
mediasiatica and
F. tularensis ssp.
novicida incidentally scored lower than 2.000 using the direct transfer method (>1.900, see
Supplementary Table S2). In addition, the other
Francisella species were identified correctly at species level with log scores higher than 2.500 when using the extraction method.
L. pneumophila was correctly identified as “no reliable ID” with the in-house
Francisella library, because this species was not included.
3.5. Biomarker Selection and Verification
Biomarker identification by BioNumerics from the MSPs of the reference strain of all
Francisella species and subspecies resulted in the selection of 17 biomarker masses that were potentially suitable for biomarker-based identification (
Supplementary Table S3). Verification of the selected biomarkers using the spectra of all strains included in the study (
Table 1) warranted the additional inclusion of masses 2195 and 5182–5185, resulting in a total of 19 selected biomarkers (
Table 4). The 2195 Da marker was not present in the corresponding MSP of
F. tularensis ssp.
mediasiatica, probably because it was missing in one of the twenty-four spectra used for building the MSP. The 5182–5185 mass was excluded by BioNumerics as not being (sub)species specific. It is however included as a
Francisella genus-specific marker because it was identified as such earlier [
13,
14,
15,
16,
17,
18].
Biomarker selection was further investigated by a manual check of their presence in the MSPs, their presence in the peak lists of all strains, and the theoretical
m/
z value of the matching protein calculated from the DNA sequence of the reference strain (
Table 4). This resulted in the addition of six
m/
z values to the selected biomarkers. The
m/
z value 3130–3132 of the hypothetical protein was detected in the MSP of the
F. philomiragia. A peak with
m/
z value 3119 was observed in de spectra of the
A. guangzhouensis but was not observed in the MSP of the
A. guangzhouensis.
m/
z values 5113, 5187, 9360, and 9420–9423 were also added because of close relatedness in either mass or DNA sequence.
3.6. Identification of Proteins Related to Biomarkers
The validated biomarkers were matched with possible proteins in the genome sequence of the respective reference strains (
Supplementary Table S3). The selected biomarkers could all be linked to a single protein with the exception of the
m/
z values 3119 and 4040 that could not be assigned to a gene. The selected biomarker 3679 Da (
Supplementary Tables S3 and S4) was specific for
F. orientalis was assigned to several related cold shock proteins, but BLASTn analyses indicated that this mass is less suitable as a biomarker, because similar proteins with comparable weight are found in the spectra of four (sub)species and on the genomes of
A. frigidaqua and
P. aetuarii (
Supplementary Table S4). Therefore, this biomarker was excluded from the selection of suitable biomarkers (
Table 4). Verification of the selected biomarker with
m/
z value 4387 (
Supplementary Tables S3 and S4) demonstrated that a peak was also present in the spectra of
F. tularensis ssp.
novicida. This peak could not be assigned to BolA. However, a
Bol A gene coding for a protein with similar molecular weight was present in
A. innopinata and
P. aetuarii. Therefore, this biomarker was also excluded from
Table 4 as a suitable biomarker.
3.7. Francisella Identification by Biomarker
The scheme provided in
Table 4 can be used for the identification of
Francisellaceae. According to our data and the literature [
13,
14,
15,
16,
17,
18,
19], the 5182–5185 mass of the 50S ribosomal protein L34 was specific for the
Francisella genus. Within the
Francisella genus, the species
F. tularensis could be differentiated from the other
Francisella species by the histone-like protein form Beta (Hu-beta) mass (selected biomarker 9449). The 9444 Da variant of Hu-beta was observed in the genome of subspecies
novicida. Although the 9449 Da mass was found in the MSPs of the other subspecies, a corresponding
Hu-beta gene was not present in any of investigated genomes of the subspecies
tularensis,
holarctica, or
mediasia-tica. Instead, a
Hu-beta variant with a theoretical mass of 9474 Da, caused by a single point mutation, was observed in their genome. This variant was found in only one
F. tularensis ssp.
novicida strain, namely, TCH2015. The theoretical
m/
z value of 9444 is also present in
F. opportunistica genome (
Table 4) and in that of
F. persica (
Supplementary Table S4), although sufficient expression of the protein to determine a peak was not confirmed.
The subspecies differentiation for F. tularensis was possible with the 50S ribosomal protein L36 (F. tularensis ssp. mediasiatica; 2195 Da), the unknown mass of 4040 Da (F. tularensis ssp. novicida) and the 50S ribosomal protein L27 (F. tularensis ssp. holarctica; 4455 Da). Additionally, selected biomarker 8079 was observed in the MSP of F. tularensis ssp. novicida corresponding to a DNA-directed RNA polymerase Ω subunit (theoretical m/z value 8082). Even though this last biomarker was not observed in the MSPs and spectra of the other F. tularensis subspecies, the gene was present in their genome and also in that of F. hispaniensis.
To differentiate the Francisella species other than F. tularensis, both the previously mentioned Hu-beta protein and the 50S ribosomal proteins L27 and L29 can be used. The Hu-beta protein displayed different masses for F. noatunensis subspecies noatunensis (selected biomarker 9380), F. hispaniensis and F. orientalis (m/z value 9420–9423) and F. philomiragia (m/z value 9394–9396). The HU beta protein in the F. salimarina-genome had a theoretical mass of 9392 Da. Selected biomarker 3128 was also unique for F. noatunensis ssp. noatunensis and corresponded to a hypothetical protein with a theoretical mass of 3129 Da.
The 50S ribosomal protein L27 was observed as a peak of 4477 in the MSPs of
F. orientalis and
L. pneumophila. However, in the
F. philomiragia spectrum a peak of 8923–8925 is observed, caused by the +2 charge of the same protein (
Supplementary Table S3). This difference is based on a single point mutation and was also observed in the genome sequence of
F. salimarina. Additionally, the 50S ribosomal protein L29 can be used to differentiate
F. philomiragia from the other
Francisella species. This protein is observed as 3880–3881 peak (+1 charge) and as 7759–7761 (+2 charge). The protein was also found on the genome of
F. oppertunistica and could in theory be detected with both charges as well. The 50S ribosomal protein L33 is specific for
F. orientalis (selected biomarker 3101) and a hypothetical protein (selected biomarker 3130–3132 Da) is only present in
F. philomiragia.
The F. noatunensis ssp. noatunensis biomarker of 10,215 Da matched with two different genes: the Chaporin protein GroES and the Parvulin-like peptidyprolyl isomerase. This Parvulin-like peptidyprolyl isomerase gene was also present in the genomes of F. oppertunistica and F. tularensis ssp. novicida, although a corresponding peak was not observed in the MSP or in the spectra of the latter subspecies.
F. orientalis could be identified using selected biomarkers 3101 and 4477. Although the latter was also observed in L. pneumophila, m/z 3101 is absent in this species.
The Allofrancisella species could be identified using selected biomarkers 5113 and 9360, which corresponded with the 50S ribosomal protein L34 and the Hu-beta protein, respectively. Both corresponding genes were also found in A. frigidaquae and A. innopinata (with theoretical masses of 5110 and 9357–9359, respectively), making it a suitable biomarker for the Allofrancisellaea.
An unknown protein of 3119 Da and one of 5187 Da were found in the MSPs of A. guangzhouensis and might be species specific. However, their usefulness in differentiation from the other Allofrancisella species should still be confirmed in vitro.
From the genome of Pseudofrancisella aetuarii a unique mass of 5260 Da could be identified for the 50S ribosomal protein L34, although in vitro confirmation is needed. Additionally, a specific Hu-beta gene with a calculated molecular weight of 9673 Da was found in the P. aetuarii genome.
4. Discussion
MALDI-TOF MS is increasingly used in clinical laboratories for the identification of bacteria. In this study, the application of the Bruker Biotyper system was assessed as a fast and specific method for the detection and identification of F. tularensis to the subspecies level in a clinical laboratory setting. Adding subspecies differentiation directly to this method will improve speed and certainty under which safety conditions to work.
Because MSPs were both used as basis for the in-house
Francisella library and the selection of biomarkers, the method of MSP creation was verified as shown in the dendrogram of the MSPs. This demonstrated sufficient differences between species, but the spectra as provided by Brucker did not cluster with in-house generated spectra of the same strains as already shown by Seibold et al. [
13]. The
F. tularensis ssp.
tularensis strain 5600 clusters with
F. tularensis ssp.
holarctica strains, whereas our in-house generated spectrum of this same strain (BD13-00126) clustered with
F. tularensis ssp.
mediasiatica. There was no indication for differences in the quality of the spectra. Because the same extraction procedure and matrix were used in the Bruker databases and the in-house database, growth conditions are the most probable cause for discrepancies. Particularly,
F. philomiragia was cultured on chocolate agar with vitox in this study, whereas HCA agar was used for developing the Bruker databases. The effect of the lack of standardization of culture media was recently reviewed [
34] and indicated that identification should be performed under the exact same (growth) conditions as those under which the database was built as was performed with the in-house database and the strains tested.
F. tularensis is absent in the standard libraries of the Bruker Biotyper System and Vitek MS.
F. tularensis is included in the MALDI Biotyper Security library for highly pathogenic (BSL-3) bacteria, however without the possibility of
F. tularensis subspecies differentiation. In this study, we demonstrated that
F. tularensis ssp.
tularensis and
holarctica can be identified with the SR library using both the direct transfer method and the formic acid extraction method. Care should be taken with scores below 2.300, because
F. hispaniensis and the
F. tularensis subspecies
novicida and
mediasiatica scored
F. tularensis ssp.
tularensis or
holarctica in the range 1.760–2.200. Even though the human pathogen
F. hispaniensis causes severe illness [
4] and is genetically more closely related to
F. tularensis than to other
Francisella species,
F. hispaniensis misidentification as
F. tularensis may cause unnecessary alarm. Unlike
F. tularensis,
F. hispaniensis is not associated with intentional release. Identifying
F. tularensis with the MALDI Biotyper Security library should be considered as a warning to directly verify the subspecies level using the biomarkers and when necessary, proceed handling of the bacterium under BSL-3 conditions.
Not all laboratories using the Bruker Biotyper system have obtained the SR library because of the extra costs and tedious administration. Analysis of a
Francisella tularensis isolate using only the MALDI Biotyper library will not give false positive results with other, less virulent, organisms. However, using only this library will not give a warning for the presence of
F. tularensis either. With the Vitek MS,
F. tularensis cannot be detected because the library does not contain any
Francisellaceae [
12]. To overcome the above problems, an in-house
Francisella library is an excellent alternative. The spectra are sufficiently distinctive to distinguish to
Francisellaceae at (sub)species level as previously determined for
F. tularensis ssp.
tularensis and
F. tularensis ssp.
holarctica [
15]. Spectra generated as part of the in house developed library can be exchanged with other laboratories.
The use of biomarkers excludes the need for acquiring an external database or implementing an in-house Francisella library for which highly pathogenic strains must be obtained and cultivated. Moreover, the Bruker MALDI-TOF MS has the option to create libraries; however, other MALDI-TOF MS systems such as the Vitek MS lack this possibility. The described biomarkers could potentially be useful in Vitek-MS analyses as well; however this requires thorough validation.
In this study, biomarkers were identified for all
F. tularensis subspecies and the other
Francisella species irrespective of the extraction method used. The fact that most of the selected biomarkers are highly expressed conserved proteins makes them suitable for identification of strains grown under different conditions [
33]. However, various genes coding for proteins related to specific biomarkers were present in several species but were not expressed or insufficiently expressed to reach the peak threshold under the conditions used in this setup. This indicates that under different growth conditions those peaks may be found, and specificity cannot be based on those peaks only. Additionally, masses translated from DNA do not always lead to the observation of a shifted peak in the MALDI-TOF spectrum. Therefore, a combination of multiple biomarkers is necessary for an accurate identification. Of the peaks found in this study, the Hu-beta peak was described previously [
13,
14]. Other peaks described previously were not found either because they were not differentiating within the study [
13] or due to the limited number of (sub)species tested [
14]. However, in our study we focused on smaller peaks to find peaks that were not previously included as potential biomarkers.
Combining biomarker selection with identification of the differentiating peaks makes this technique suitable to use on whole genome sequences of clinical strains to both improve the MALDI-TOF MS identification and the identification of whole genome sequences. In samples where culturing proves difficult, this could be an additional method for identification.