From In Vitro to In Vivo: A Rational Flowchart for the Selection and Characterization of Candidate Probiotic Strains in Intestinal Disorders
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Growth Conditions
2.2. Cell Culture Conditions
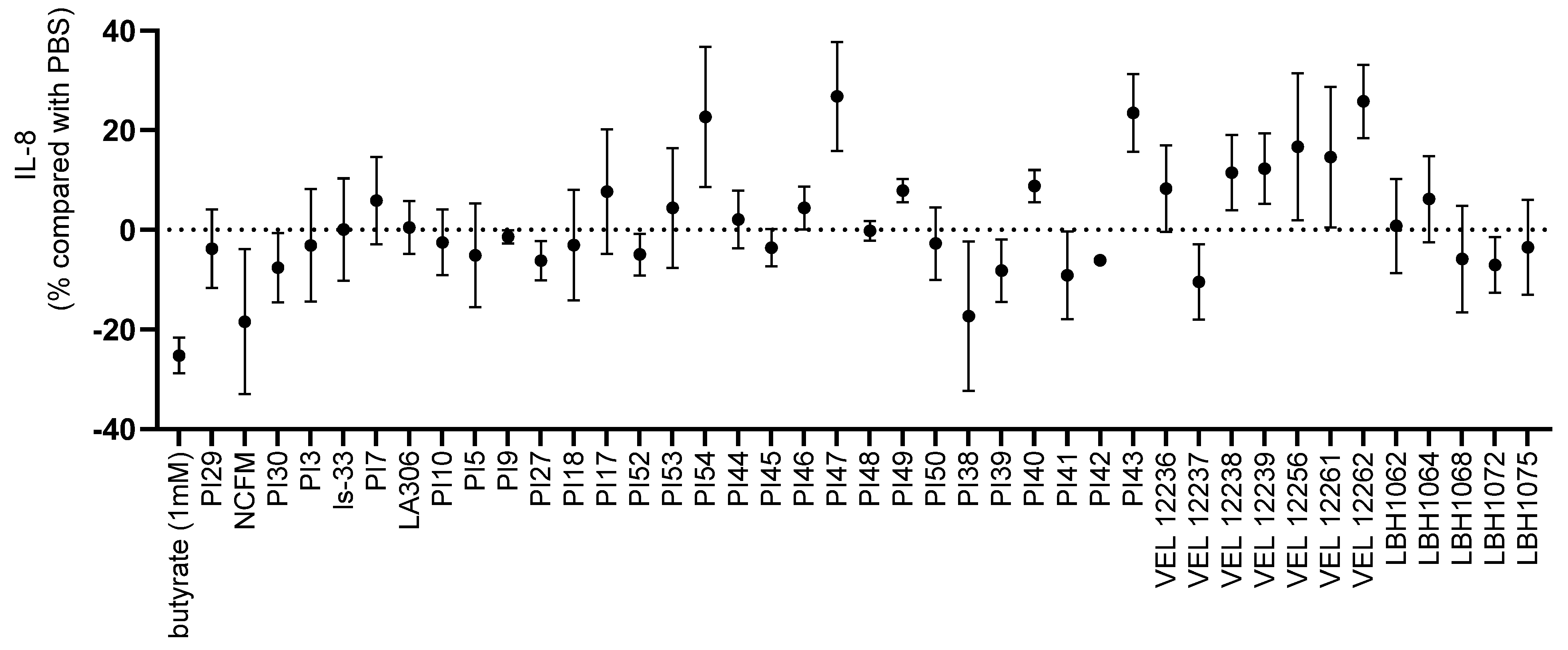
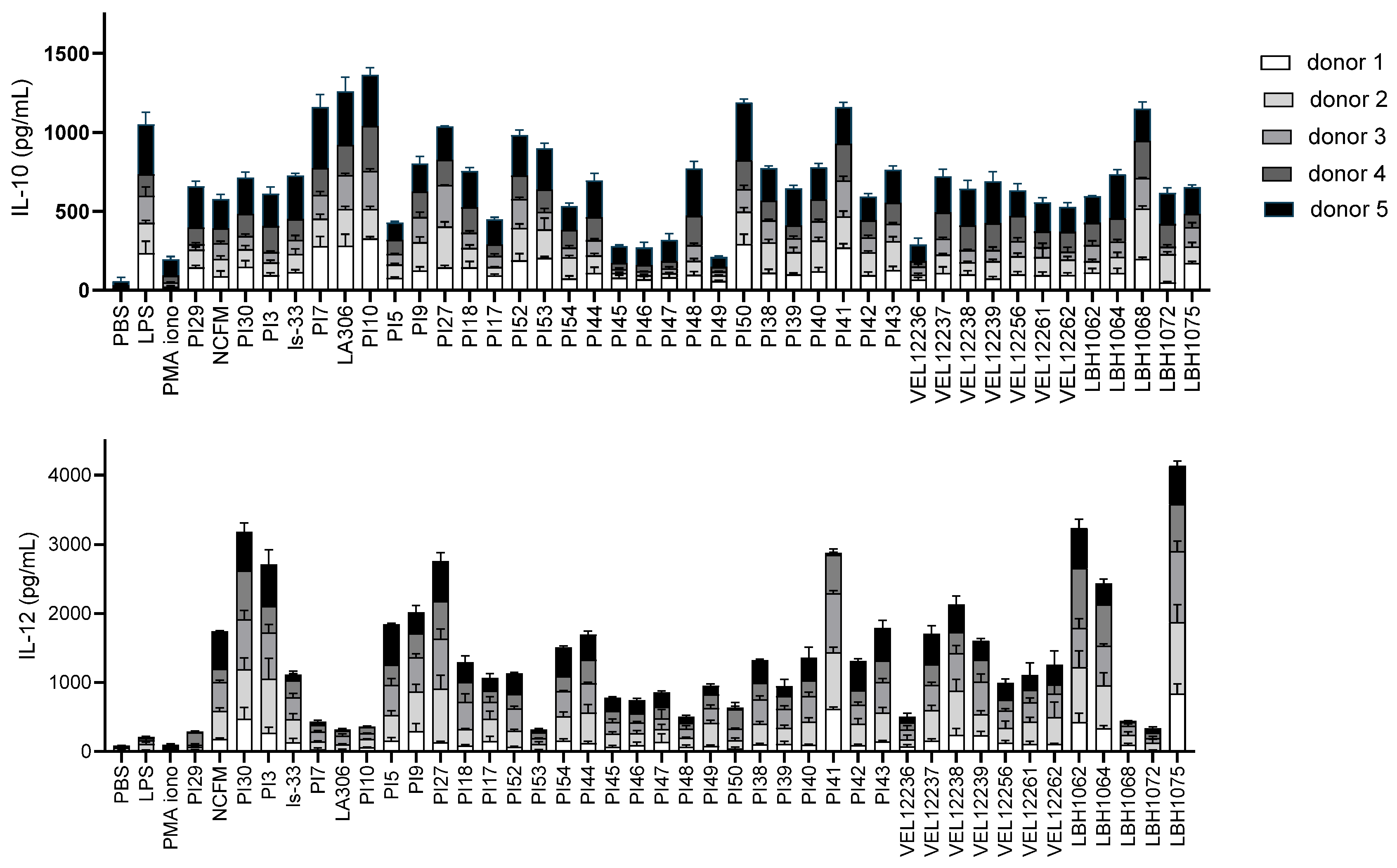
2.3. In Vitro Immunomodulation Assays
2.4. In Vitro Permeability Assay
2.5. Metabolite Quantification in Bacterial Supernatants
2.6. Animal Studies
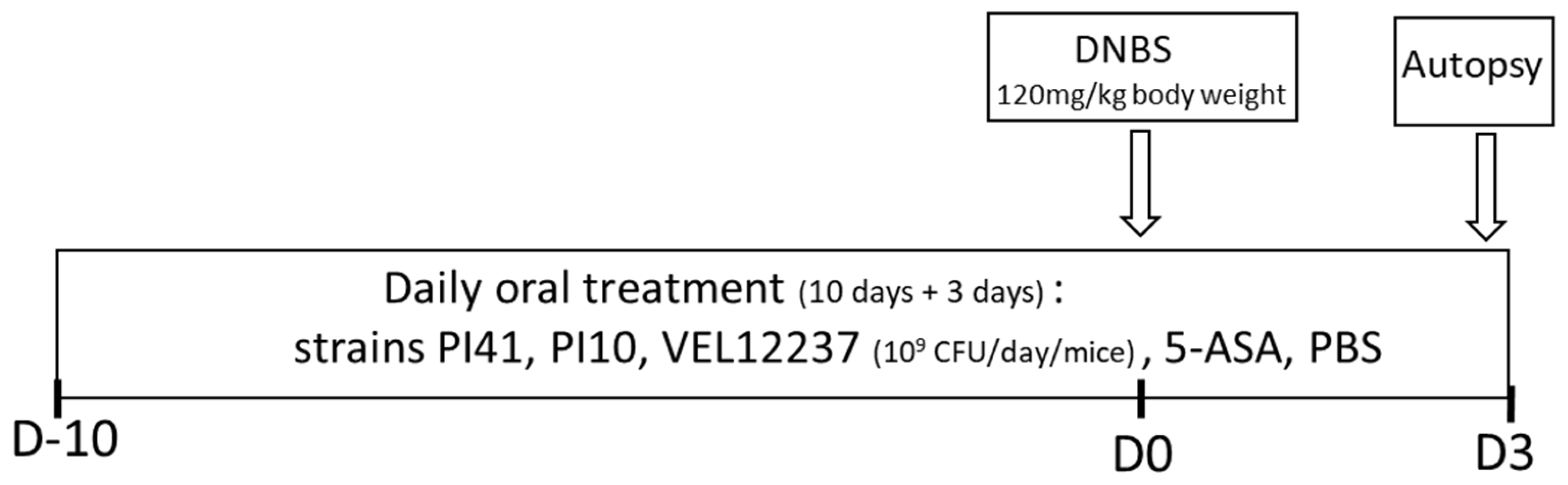
2.7. Chemically Induced Colitis Study Design
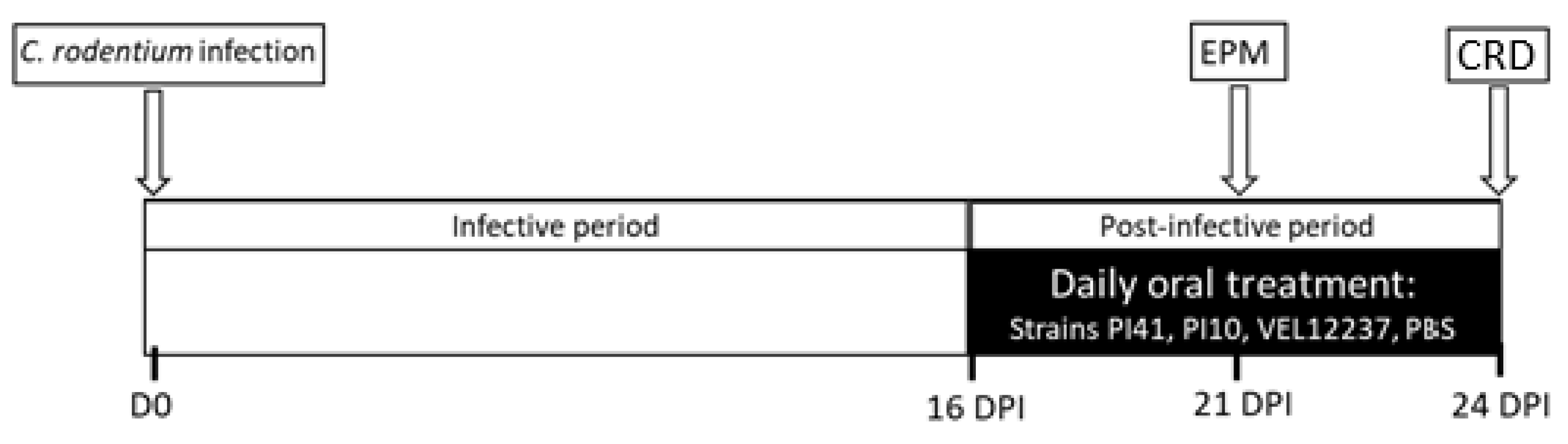
2.8. Citrobacter Rodentium-Induced IBS Study Design
2.9. Statistical Analyses
3. Results
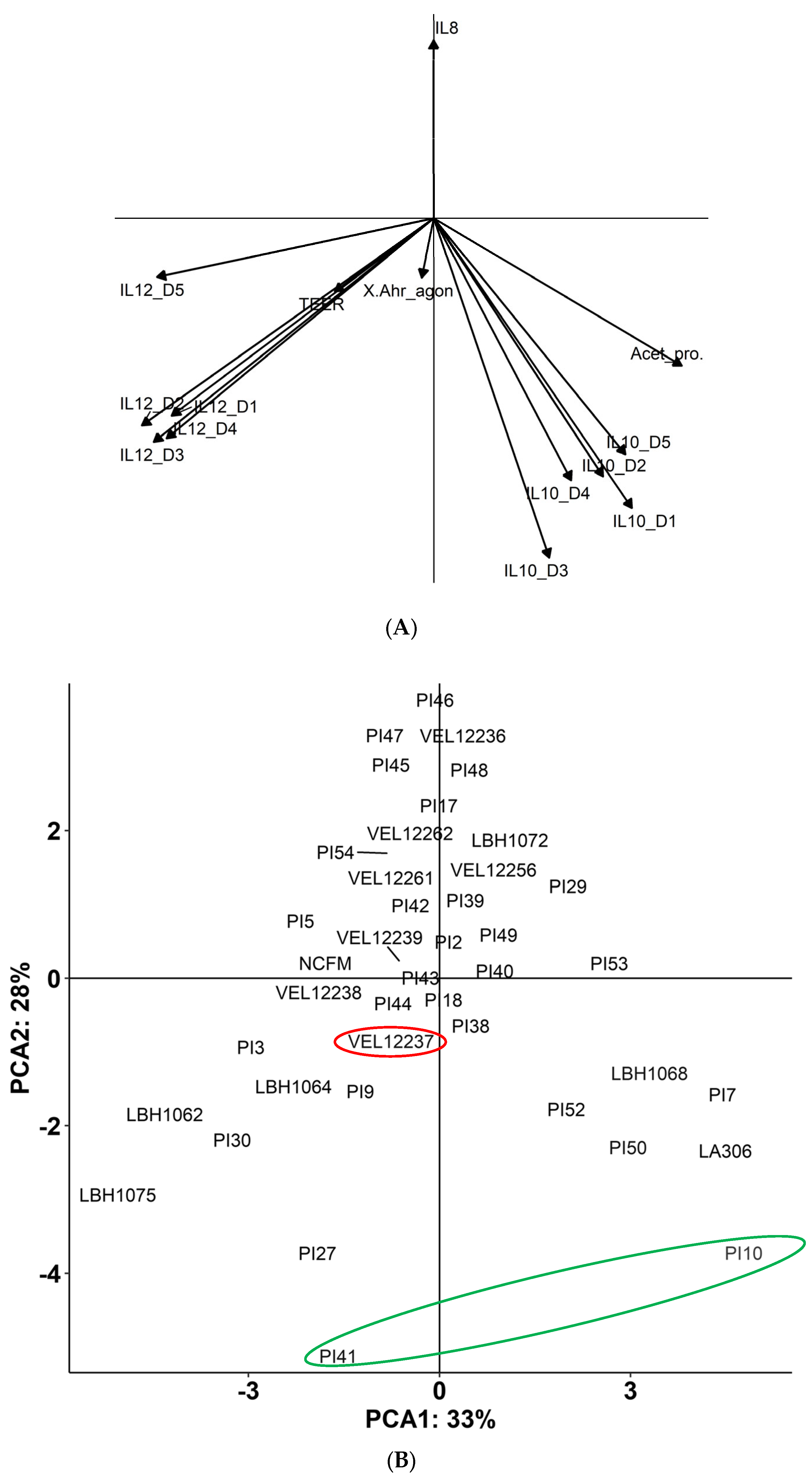
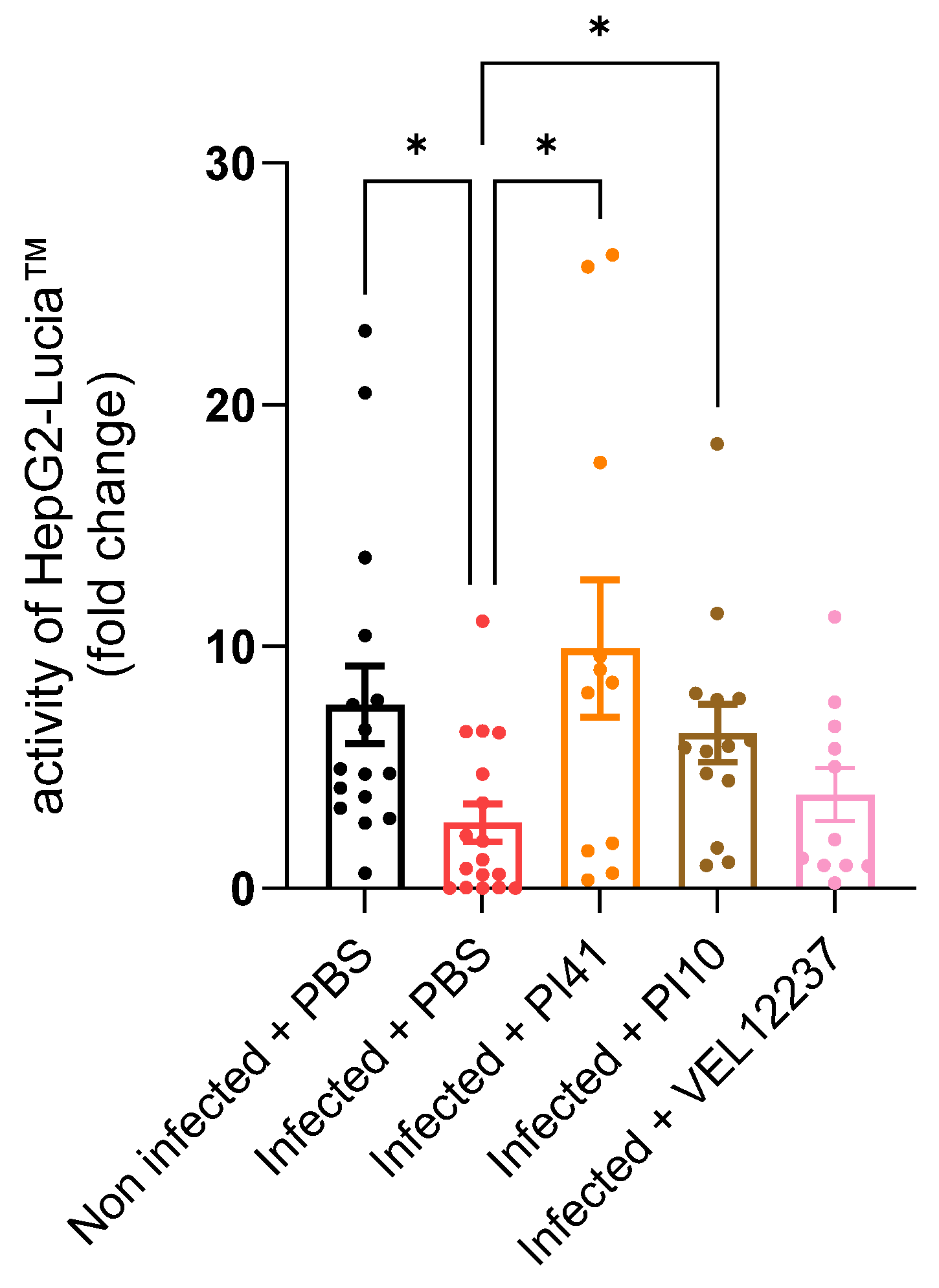
3.1. In Vitro Immunomodulation Evaluation
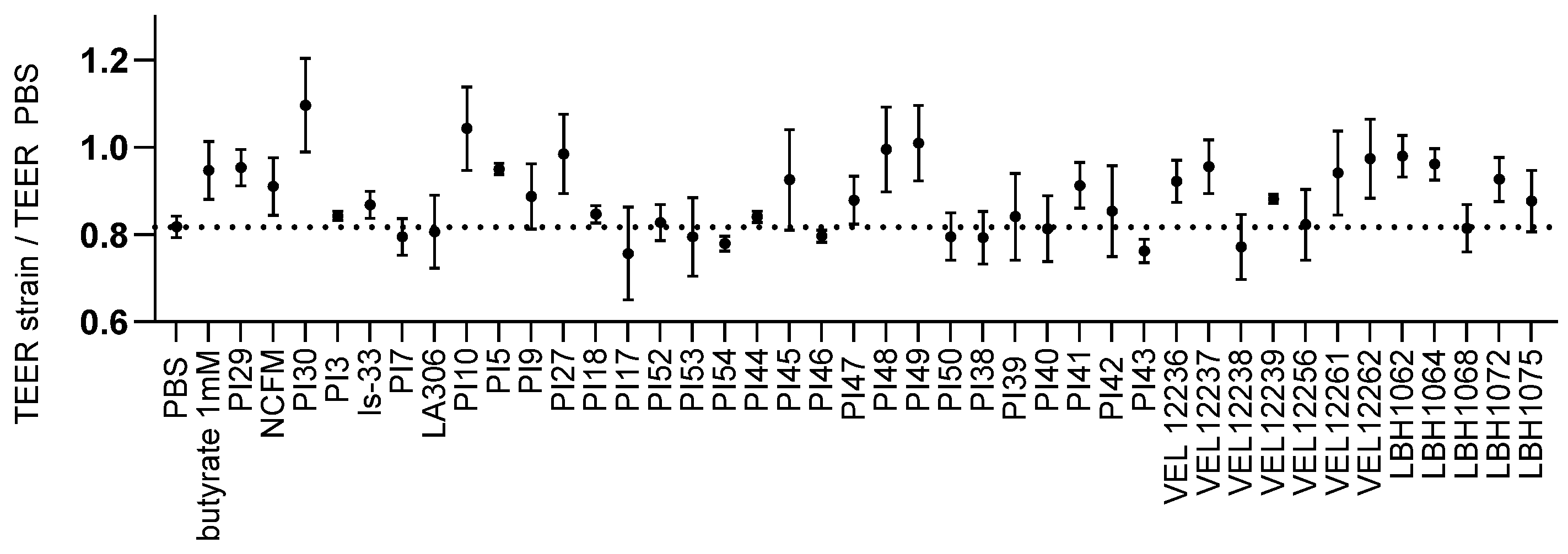
3.2. Evaluation of the Transepithelial Electric Resistance (TEER)
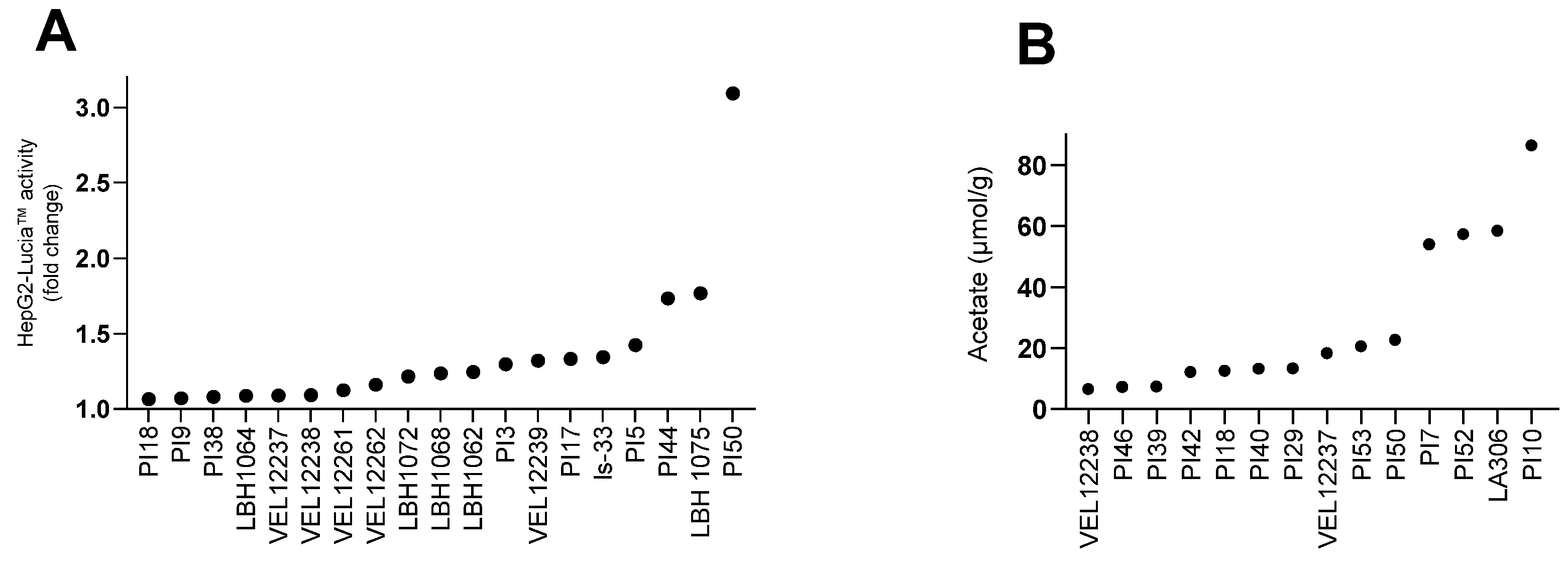
3.3. Production of Metabolites
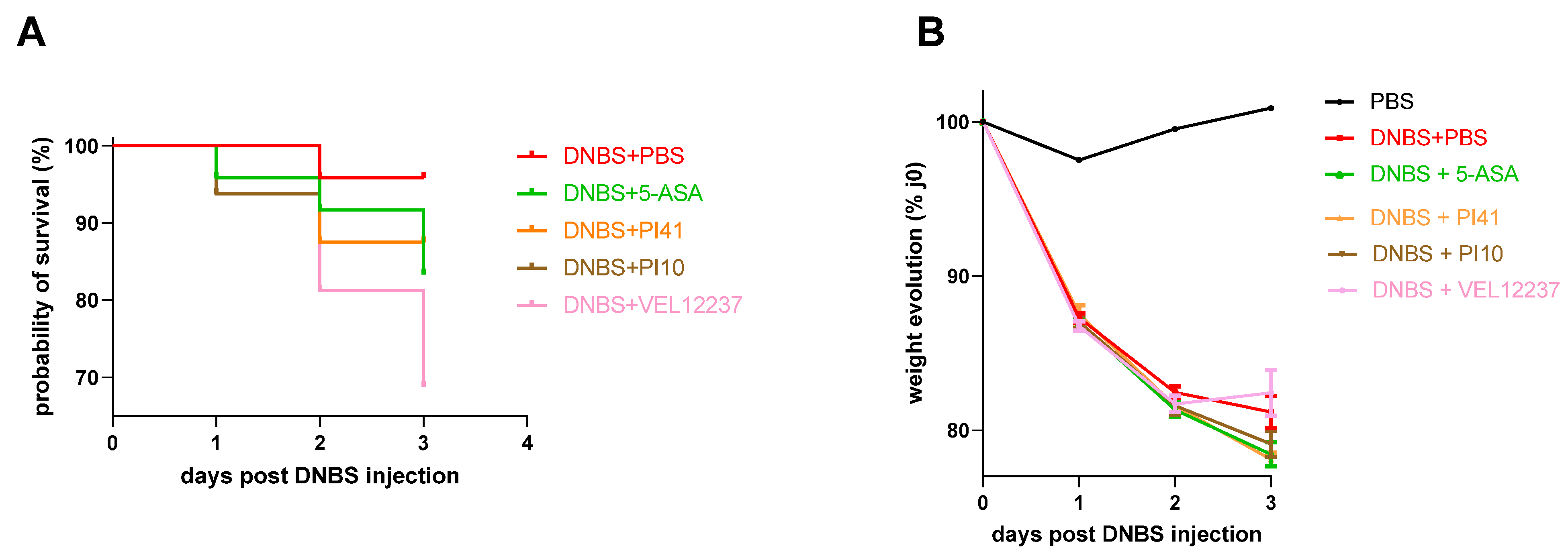
3.4. Evaluation of the Selected Strains in a Chemically Induced Colitis Model
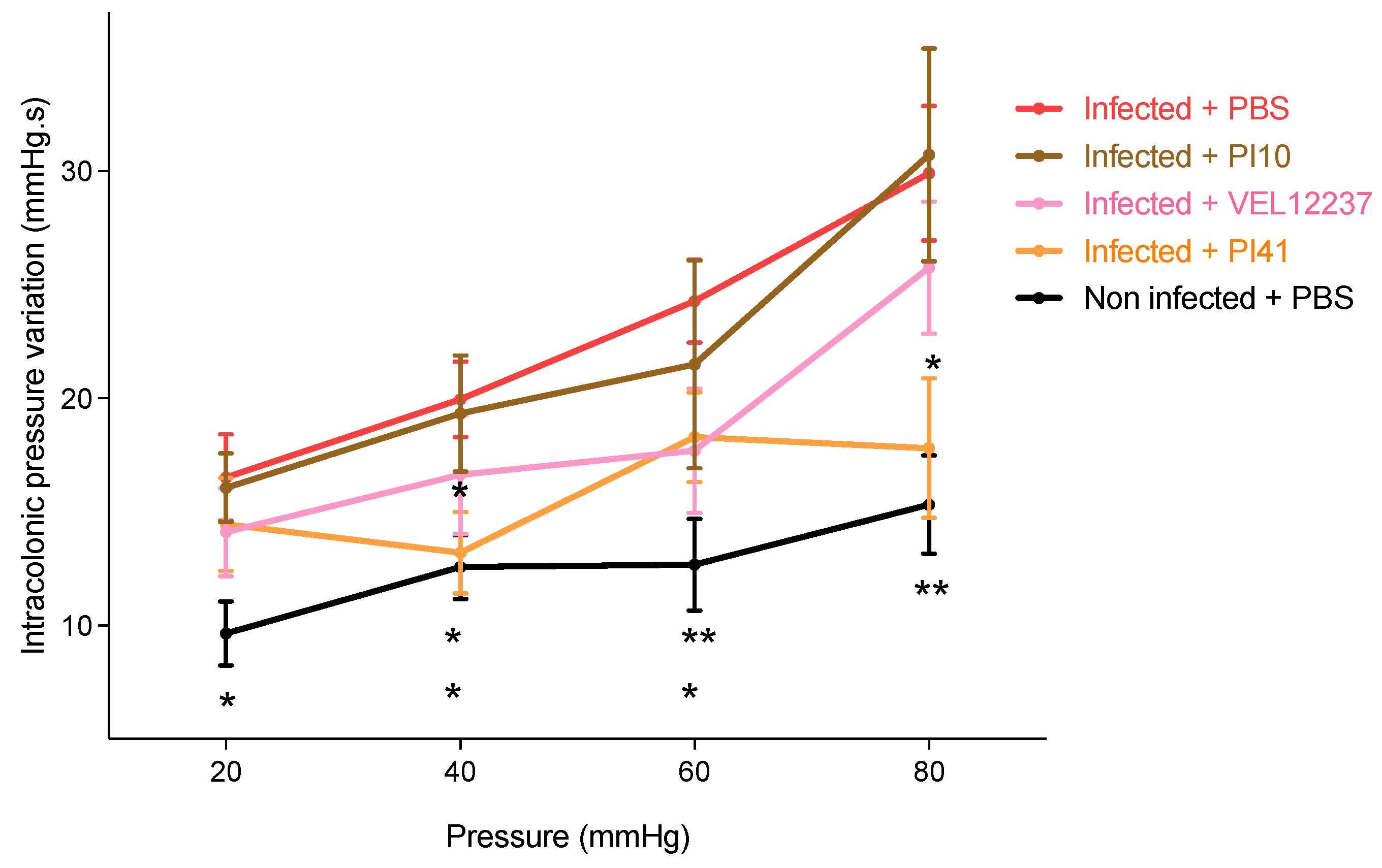
3.5. Evaluation of the Selected Strains in a Post-Infectious IBS Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jairath, V.; Feagan, B.G. Global burden of inflammatory bowel disease. Lancet Gastroenterol. Hepatol. 2019, 5, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, G.; Trapasso, F.; Parrello, T.; Biancone, L.; Stella, A.; Iuliano, R.; Luzza, F.; Fusco, A.; Pallone, F. Bioactive IL-18 Expression Is Up-Regulated in Crohn’s Disease. J. Immunol. 1999, 163, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Heller, F.; Florian, P.; Bojarski, C.; Richter, J.; Christ, M.; Hillenbrand, B.; Schulzke, J.D. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology 2005, 129, 550–564. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.C.-H.; Ng, S.C. Emerging biologics in inflammatory bowel disease. J. Gastroenterol. 2016, 52, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Dave, M.; Purohit, T.; Razonable, R.; Loftus, E.V., Jr. Opportunistic Infections Due to Inflammatory Bowel Disease Therapy. Inflamm. Bowel Dis. 2014, 20, 196–212. [Google Scholar] [CrossRef]
- Sperber, A.D.; Bangdiwala, S.I.; Drossman, D.A.; Ghoshal, U.C.; Simren, M.; Tack, J.; Whitehead, W.E.; Dumitrascu, D.L.; Fang, X.; Fukudo, S.; et al. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology 2021, 160, 99–114.e3. [Google Scholar] [CrossRef]
- Bashashati, M.; Moossavi, S.; Cremon, C.; Barbaro, M.R.; Moraveji, S.; Talmon, G.; Rezaei, N.; Hughes, P.A.; Bian, Z.X.; Choi, C.H.; et al. Colonic immune cells in irritable bowel syndrome: A systematic review and meta-analysis. Neurogastroenterol. Motil. 2017, 30, e13192. [Google Scholar] [CrossRef]
- Spiller, R.C.; Jenkins, D.; Thornley, J.P.; Hebden, J.M.; Wright, T.; Skinner, M.; Neal, K.R. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut 2000, 47, 804–811. [Google Scholar] [CrossRef]
- Card, T.; Enck, P.; Barbara, G.; Boeckxstaens, G.E.; Santos, J.; Azpiroz, F.; Mearin, F.; Aziz, Q.; Marshall, J.; Spiller, R. Post-infectious IBS: Defining its clinical features and prognosis using an internet-based survey. United Eur. Gastroenterol. J. 2018, 6, 1245–1253. [Google Scholar] [CrossRef]
- Wang, L.; Alammar, N.; Singh, R.; Nanavati, J.; Song, Y.; Chaudhary, R.; Mullin, G.E. Gut Microbial Dysbiosis in the Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis of Case-Control Studies. J. Acad. Nutr. Diet. 2019, 120, 565–586. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.-H.; Park, J.H.; Jeon, W.-M.; Han, K.-S. Butyrate modulates bacterial adherence on LS174T human colorectal cells by stimulating mucin secretion and MAPK signaling pathway. Nutr. Res. Pract. 2015, 9, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Fu, L.; Wang, J. Protocol for Fecal Microbiota Transplantation in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2018, 2018, e8941340. [Google Scholar] [CrossRef]
- Xu, D.; Chen, V.; Steiner, C.A.; Berinstein, J.; Eswaran, S.; Waljee, A.K.; Higgins, P.D.; Owyang, C. Efficacy of Fecal Microbiota Transplantation in Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2019, 114, 1043–1050. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Fernandez, E.M.; Valenti, V.; Rockel, C.; Hermann, C.; Pot, B.; Boneca, I.G.; Grangette, C. Anti-inflammatory capacity of selected lactobacilli in experimental colitis is driven by NOD2-mediated recognition of a specific peptidoglycan-derived muropeptide. Gut 2011, 60, 1050–1059. [Google Scholar] [CrossRef]
- Rousseaux, C.; Thuru, X.; Gelot, A.; Barnich, N.; Neut, C.; Dubuquoy, L.; Dubuquoy, C.; Merour, E.; Geboes, K.; Chamaillard, M.; et al. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat. Med. 2006, 13, 35–37. [Google Scholar] [CrossRef]
- Harmon, C.M.; Yongyi, F.; Mattar, A.F.; Drongowski, R.A.; Teitelbaum, D.H.; Coran, A.G. Probiotics up-regulate MUC-2 mucin gene expression in a Caco-2 cell-culture model. Pediatr. Surg. Int. 2002, 18, 586–590. [Google Scholar]
- Derwa, Y.; Gracie, D.J.; Hamlin, P.J.; Ford, A.C. Systematic review with meta-analysis: The efficacy of probiotics in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2017, 46, 389–400. [Google Scholar] [CrossRef]
- FAO; WHO. Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation; Food and Agriculture Organization of the United Nations: Rome, Italy; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Chamignon, C.; Guéneau, V.; Medina, S.; Deschamps, J.; Gil-Izquierdo, A.; Briandet, R.; Mousset, P.-Y.; Langella, P.; Lafay, S.; Bermúdez-Humarán, L.G. Evaluation of the Probiotic Properties and the Capacity to Form Biofilms of Various Lactobacillus Strains. Microorganisms 2020, 8, 1053. [Google Scholar] [CrossRef]
- Ottenstein, D.M.; Bartley, D.A. Improved gas chromatography separation of free acids C2-C5 in dilute solution. Anal. Chem. 1971, 43, 952–955. [Google Scholar] [CrossRef]
- Szylit, O.; Dabard, J.; Durand, M.; Dumay, C.; Bensaada, M.; Raibaud, P. Production of volatile fatty acids as a result of bacterial interactions in the cecum of gnotobiotic rats and chickens fed a lactose-containing diet. Reprod. Nutr. Dev. 1988, 28, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L.; MacNaughton, W.K.; Morris, G.P.; Beck, P.L. Inhibition of leukotriene synthesis markedly accelerates healing in a rat model of inflammatory bowel disease. Gastroenterology 1989, 96, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Ameho, C.K.; Adjei, A.A.; Harrison, E.K.; Takeshita, K.; Morioka, T.; Arakaki, Y.; Ito, E.; Suzuki, I.; Kulkarni, A.D.; Kawajiri, A.; et al. Prophylactic effect of dietary glutamine supplementation on interleukin 8 and tumour necrosis factor α production in trinitrobenzene sulphonic acid induced colitis. Gut 1997, 41, 487–493. [Google Scholar] [CrossRef]
- Meynier, M.; Baudu, E.; Rolhion, N.; Defaye, M.; Straube, M.; Daugey, V.; Modoux, M.; Wawrzyniak, I.; Delbac, F.; Villéger, R.; et al. AhR/IL-22 pathway as new target for the treatment of post-infectious irritable bowel syndrome symptoms. Gut Microbes 2022, 14, 2022997. [Google Scholar] [CrossRef]
- Alard, J.; Peucelle, V.; Boutillier, D.; Breton, J.; Kuylle, S.; Pot, B.; Holowacz, S.; Grangette, C. New probiotic strains for inflammatory bowel disease management identified by combining in vitro and in vivo approaches. Benef. Microbes 2018, 9, 317–331. [Google Scholar] [CrossRef]
- Zaylaa, M.; Al Kassaa, I.; Alard, J.; Peucelle, V.; Boutillier, D.; Desramaut, J.; Dabboussi, F.; Pot, B.; Grangette, C. Probiotics in IBD: Combining in vitro and in vivo models for selecting strains with both anti-inflammatory potential as well as a capacity to restore the gut epithelial barrier. J. Funct. Foods 2018, 47, 304–315. [Google Scholar] [CrossRef]
- Cuffaro, B.; Assohoun, A.; Boutillier, D.; Peucelle, V.; Desramaut, J.; Boudebbouze, S.; Croyal, M.; Waligora-Dupriet, A.-J.; Rhimi, M.; Grangette, C.; et al. Identification of New Potential Biotherapeutics from Human Gut Microbiota-Derived Bacteria. Microorganisms 2021, 9, 565. [Google Scholar] [CrossRef]
- Rogler, G.; Andus, T. Cytokines in Inflammatory Bowel Disease. World J. Surg. 1998, 22, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Martín, R.; Chamignon, C.; Mhedbi-Hajri, N.; Chain, F.; Derrien, M.; Escribano-Vázquez, U.; Garault, P.; Cotillard, A.; Pham, H.P.; Chervaux, C.; et al. The potential probiotic Lactobacillus rhamnosus CNCM I-3690 strain protects the intestinal barrier by stimulating both mucus production and cytoprotective response. Sci. Rep. 2019, 9, 5398. [Google Scholar] [CrossRef] [PubMed]
- Lammers, K.M.; Helwig, U.; Swennen, E.; Rizzello, F.; Venturi, A.; Caramelli, E.; Kamm, M.A.; Brigidi, P.; Gionchetti, P.; Campieri, M. Effect of probiotic strains on interleukin 8 production by HT29/19A cells. Am. J. Gastroenterol. 2002, 97, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-C.; Hsu, W.-F.; Chang, J.-S.; Shih, C.-K. Combination of Lactobacillus acidophilus and Bifidobacterium animalis subsp. lactis Shows a Stronger Anti-Inflammatory Effect than Individual Strains in HT-29 Cells. Nutrients 2019, 11, 969. [Google Scholar] [CrossRef] [PubMed]
- Torres-Maravilla, E.; Lenoir, M.; Mayorga-Reyes, L.; Allain, T.; Sokol, H.; Langella, P.; Sánchez-Pardo, M.E.; Bermúdez-Humarán, L.G. Identification of novel anti-inflammatory probiotic strains isolated from pulque. Appl. Microbiol. Biotechnol. 2016, 100, 385–396. [Google Scholar] [CrossRef]
- Li, M.-C.; He, S.-H. IL-10 and its related cytokines for treatment of inflammatory bowel disease. World J. Gastroenterol. 2004, 10, 620–625. [Google Scholar] [CrossRef]
- Bashashati, M.; Rezaei, N.; Shafieyoun, A.; McKernan, D.P.; Chang, L.; Öhman, L.; Quigley, E.M.; Schmulson, M.; Sharkey, K.A.; Simrén, M. Cytokine imbalance in irritable bowel syndrome: A systematic review and meta-analysis. Neurogastroenterol. Motil. 2014, 26, 1036–1048. [Google Scholar] [CrossRef]
- Panaccione, R.; Sandborn, W.J.; Gordon, G.L.; Lee, S.D.; Safdi, A.; Sedghi, S.; Feagan, B.G.; Hanauer, S.; Reinisch, W.; Valentine, J.F.; et al. Briakinumab for Treatment of Crohn’s Disease: Results of a Randomized Trial. Inflamm. Bowel Dis. 2015, 21, 1329–1340. [Google Scholar]
- Sands, B.E.; Sandborn, W.J.; Panaccione, R.; O’Brien, C.D.; Zhang, H.; Johanns, J.; Adedokun, O.J.; Li, K.; Peyrin-Biroulet, L.; Van Assche, G.; et al. Ustekinumab as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2019, 381, 1201–1214. [Google Scholar] [CrossRef]
- Foligne, B.; Nutten, S.; Grangette, C.; Dennin, V.; Goudercourt, D.; Poiret, S.; Dewulf, J.; Brassart, D.; Mercenier, A.; Pot, B. Correlation between in vitro and in vivo immunomodulatory properties of lactic acid bacteria. World J. Gastroenterol. 2007, 13, 236–243. [Google Scholar] [CrossRef]
- O’Mahony, L.; McCarthy, J.; Kelly, P.; Hurley, G.; Luo, F.; Chen, K.; O’Sullivan, G.C.; Kiely, B.; Collins, J.K.; Shanahan, F.; et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: Symptom responses and relationship to cytokine profiles. Gastroenterology 2005, 128, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Meijerink, M.; Wells, J.M.; Taverne, N.; Brouwer, M.-L.D.Z.; Hilhorst, B.; Venema, K.; van Bilsen, J. Immunomodulatory effects of potential probiotics in a mouse peanut sensitization model. FEMS Immunol. Med. Microbiol. 2012, 65, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Neau, E.; Delannoy, J.; Marion, C.; Cottart, C.-H.; Labellie, C.; Holowacz, S.; Butel, M.-J.; Kapel, N.; Waligora-Dupriet, A.-J. Three Novel Candidate Probiotic Strains with Prophylactic Properties in a Murine Model of Cow’s Milk Allergy. Appl. Environ. Microbiol. 2016, 82, 1722–1733. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermúdez-Humaran, L.G.; Gratadoux, J.-J.; Blugeon, S.; Bridonneau, C.; Furet, J.-P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef] [PubMed]
- Kechaou, N.; Chain, F.; Gratadoux, J.-J.; Blugeon, S.; Bertho, N.; Chevalier, C.; Le Goffic, R.; Courau, S.; Molimard, P.; Chatel, J.M.; et al. Identification of One Novel Candidate Probiotic Lactobacillus plantarum Strain Active against Influenza Virus Infection in Mice by a Large-Scale Screening. Appl. Environ. Microbiol. 2013, 79, 1491–1499. [Google Scholar] [CrossRef]
- Hanning, N.; Edwinson, A.L.; Ceuleers, H.; Peters, S.A.; De Man, J.G.; Hassett, L.C.; De Winter, B.Y.; Grover, M. Intestinal barrier dysfunction in irritable bowel syndrome: A systematic review. Ther. Adv. Gastroenterol. 2021, 14, 1756284821993586. [Google Scholar] [CrossRef]
- Zeissig, S.; Bürgel, N.; Günzel, D.; Richter, J.; Mankertz, J.; Wahnschaffe, U.; Kroesen, A.J.; Zeitz, M.; Fromm, M.; Schulzke, J.-D. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut 2007, 56, 61–72. [Google Scholar] [CrossRef]
- McKay, D.M.; Singh, P.K. Superantigen activation of immune cells evokes epithelial (T84) transport and barrier abnormalities via IFN-gamma and TNF alpha: Inhibition of increased permeability, but not diminished secretory responses by TGF-beta2. J. Immunol. 1997, 159, 2382–2390. [Google Scholar] [CrossRef]
- Van der Hee, B.; Wells, J.M. Microbial Regulation of Host Physiology by Short-chain Fatty Acids. Trends Microbiol. 2021, 29, 700–712. [Google Scholar] [CrossRef]
- Sun, Q.; Jia, Q.; Song, L.; Duan, L. Alterations in fecal short-chain fatty acids in patients with irritable bowel syndrome: A systematic review and meta-analysis. Medicine 2019, 98, e14513. [Google Scholar] [CrossRef]
- Zhuang, X.; Li, T.; Li, M.; Huang, S.; Qiu, Y.; Feng, R.; Zhang, S.; Chen, M.; Xiong, L.; Zeng, Z. Systematic Review and Meta-analysis: Short-Chain Fatty Acid Characterization in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, 1751–1763. [Google Scholar] [CrossRef] [PubMed]
- Eeckhaut, V.; Machiels, K.; Perrier, C.; Romero, C.; Maes, S.; Flahou, B.; Steppe, M.; Haesebrouck, F.; Sas, B.; Ducatelle, R.; et al. Butyricicoccus pullicaecorum in inflammatory bowel disease. Gut 2013, 62, 1745–1752. [Google Scholar] [CrossRef]
- Rios-Covian, D.; Gueimonde, M.; Duncan, S.H.; Flint, H.J.; de los Reyes-Gavilan, C.G. Enhanced butyrate formation by cross-feeding between Faecalibacterium prausnitzii and Bifidobacterium adolescentis. FEMS Microbiol. Lett. 2015, 362, fnv176. [Google Scholar] [CrossRef] [PubMed]
- Markowiak-Kopeć, P.; Śliżewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- Lamas, B.; Natividad, J.M.; Sokol, H. Aryl hydrocarbon receptor and intestinal immunity. Mucosal Immunol. 2018, 11, 1024–1038. [Google Scholar] [CrossRef]
- Lamas, B.; Richard, M.L.; Leducq, V.; Pham, H.-P.; Michel, M.-L.; Da Costa, G.; Bridonneau, C.; Jegou, S.; Hoffmann, T.W.; Natividad, J.M.; et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat. Med. 2016, 22, 598–605. [Google Scholar] [CrossRef]
- Monteleone, I.; Rizzo, A.; Sarra, M.; Sica, G.; Sileri, P.; Biancone, L.; Macdonald, T.T.; Pallone, F.; Monteleone, G. Aryl Hydrocarbon Receptor-Induced Signals Up-regulate IL-22 Production and Inhibit Inflammation in the Gastrointestinal Tract. Gastroenterology 2011, 141, 237–248.e1. [Google Scholar] [CrossRef]
- Noguès, E.B.; Kropp, C.; Bétemps, L.; de Sousa, C.; Chain, F.; Auger, S.; Azevedo, V.; Langella, P.; Chatel, J.-M. Lactococcus lactis engineered to deliver hCAP18 cDNA alleviates DNBS-induced colitis in C57BL/6 mice by promoting IL17A and IL10 cytokine expression. Sci. Rep. 2022, 12, 15641. [Google Scholar] [CrossRef]
- Sonu, I.; Lin, M.V.; Blonski, W.; Lichtenstein, G.R. Clinical Pharmacology of 5-ASA Compounds in Inflammatory Bowel Disease. Gastroenterol. Clin. N. Am. 2010, 39, 559–599. [Google Scholar] [CrossRef]
- The importance of no evidence. Nat. Hum. Behav. 2019, 3, 197. [CrossRef]
- Mileti, E.; Matteoli, G.; Iliev, I.D.; Rescigno, M. Comparison of the Immunomodulatory Properties of Three Probiotic Strains of Lactobacilli Using Complex Culture Systems: Prediction for In Vivo Efficacy. PLoS ONE 2009, 4, e7056. [Google Scholar] [CrossRef] [PubMed]
- Delvaux, M. Role of visceral sensitivity in the pathophysiology of irritable bowel syndrome. Gut 2002, 51, i67–i71. [Google Scholar] [CrossRef] [PubMed]
- Midenfjord, I.; Polster, A.; Sjövall, H.; Törnblom, H.; Simrén, M. Anxiety and depression in irritable bowel syndrome: Exploring the interaction with other symptoms and pathophysiology using multivariate analyses. Neurogastroenterol. Motil. 2019, 31, e13619. [Google Scholar] [CrossRef] [PubMed]
- Obata, Y.; Castaño, Á.; Boeing, S.; Bon-Frauches, A.C.; Fung, C.; Fallesen, T.; de Agüero, M.G.; Yilmaz, B.; Lopes, R.; Huseynova, A.; et al. Neuronal programming by microbiota regulates intestinal physiology. Nature 2020, 578, 284–289. [Google Scholar] [CrossRef]

| Bacterial Species | Strain Designation | Owner | |
|---|---|---|---|
| Bifidobacterium | animalis subsp. lactis | PI50 | PiLeJe |
| Lacticaseibacillus | casei | PI45 | PiLeJe |
| Lacticaseibacillus | casei | PI46 | PiLeJe |
| Lacticaseibacillus | casei | PI47 | PiLeJe |
| Lactobacillus | gasseri | PI38 | PiLeJe |
| Lactobacillus | gasseri | PI39 | PiLeJe |
| Lactobacillus | gasseri | PI40 | PiLeJe |
| Lactobacillus | gasseri | PI41 | PiLeJe |
| Lactobacillus | gasseri | PI42 | PiLeJe |
| Lactobacillus | gasseri | PI43 | PiLeJe |
| Lactiplantibacillus | plantarum | PI44 | PiLeJe |
| Limosilactobacillus | reuteri | PI49 | PiLeJe |
| Lacticaseibacillus | rhamnosus | PI48 | PiLeJe |
| Lactobacillus | delbrueckii subsp. bulgaricus | VEL12236 | INRAE |
| Lacticaseibacillus | paracasei subsp. paracasei | VEL12237 | INRAE |
| Lactiplantibacillus | plantarum | VEL12238 | INRAE |
| Lactiplantibacillus | plantarum | VEL12239 | INRAE |
| Lactococcus | lactis subsp. cremoris | VEL12256 | INRAE |
| Lactococcus | lactis subsp. cremoris | VEL12261 | INRAE |
| Lactococcus | lactis subsp. lactis | VEL12262 | INRAE |
| Lactiplantibacillus | plantarum | LBH1062 | INRAE, Escuela Nacional de Ciencias Biológicas, IPN, Departamento de Ingeniería Bioquímica, Laboratorio de Investigación en Alimentos, Distrito Federal, México |
| Lactiplantibacillus | plantarum | LBH1064 | INRAE, Escuela Nacional de Ciencias Biológicas, IPN, Departamento de Ingeniería Bioquímica, Laboratorio de Investigación en Alimentos, Distrito Federal, México |
| Fructilactobacillus | sanfranciscensis | LBH1068 | INRAE, Escuela Nacional de Ciencias Biológicas, IPN, Departamento de Ingeniería Bioquímica, Laboratorio de Investigación en Alimentos, Distrito Federal, México |
| Agrilactobacillus | composti | LBH1072 | INRAE, Escuela Nacional de Ciencias Biológicas, IPN, Departamento de Ingeniería Bioquímica, Laboratorio de Investigación en Alimentos, Distrito Federal, México |
| Lactiplantibacillus | plantarum | LBH1075 | INRAE, Escuela Nacional de Ciencias Biológicas, IPN, Departamento de Ingeniería Bioquímica, Laboratorio de Investigación en Alimentos, Distrito Federal, México |
| Bifidobacterium | bifidum | PI29 | PiLeJe |
| Lactiplantibacillus | plantarum | PI30 | PiLeJe |
| Bifidobacterium | longum | PI52 | PiLeJe |
| Bifidobacterium | bifidum | PI53 | PiLeJe |
| Lactobacillus | gasseri | PI54 | PiLeJe |
| Lactiplantibacillus | plantarum | PI3 | PiLeJe |
| Bifidobacterium | animalis subsp. lactis | PI7 | PiLeJe |
| Bifidobacterium | animalis subsp. lactis | LA306 | PiLeJe |
| Bifidobacterium | longum | PI10 | PiLeJe |
| Lactobacillus | helveticus | PI5 | PiLeJe |
| Lactococcus | lactis | PI9 | PiLeJe |
| Streptococcus | salivarius subsp. thermophilus | PI27 | PiLeJe |
| Lacticaseibacillus | paracasei | PI18 | PiLeJe |
| Lactobacillus | gasseri | PI17 | PiLeJe |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maillard, F.; Meynier, M.; Mondot, S.; Pepke, F.; Galbert, C.; Torres Maravilla, E.; Kropp, C.; Sokol, H.; Carvalho, F.A.; Jacouton, E.; et al. From In Vitro to In Vivo: A Rational Flowchart for the Selection and Characterization of Candidate Probiotic Strains in Intestinal Disorders. Microorganisms 2023, 11, 906. https://doi.org/10.3390/microorganisms11040906
Maillard F, Meynier M, Mondot S, Pepke F, Galbert C, Torres Maravilla E, Kropp C, Sokol H, Carvalho FA, Jacouton E, et al. From In Vitro to In Vivo: A Rational Flowchart for the Selection and Characterization of Candidate Probiotic Strains in Intestinal Disorders. Microorganisms. 2023; 11(4):906. https://doi.org/10.3390/microorganisms11040906
Chicago/Turabian StyleMaillard, Flore, Maëva Meynier, Stanislas Mondot, Frederic Pepke, Chloé Galbert, Edgar Torres Maravilla, Camille Kropp, Harry Sokol, Frédéric Antonio Carvalho, Elsa Jacouton, and et al. 2023. "From In Vitro to In Vivo: A Rational Flowchart for the Selection and Characterization of Candidate Probiotic Strains in Intestinal Disorders" Microorganisms 11, no. 4: 906. https://doi.org/10.3390/microorganisms11040906
APA StyleMaillard, F., Meynier, M., Mondot, S., Pepke, F., Galbert, C., Torres Maravilla, E., Kropp, C., Sokol, H., Carvalho, F. A., Jacouton, E., Holowacz, S., Langella, P., Chain, F., & Martín, R. (2023). From In Vitro to In Vivo: A Rational Flowchart for the Selection and Characterization of Candidate Probiotic Strains in Intestinal Disorders. Microorganisms, 11(4), 906. https://doi.org/10.3390/microorganisms11040906






