Climate Warming Does Not Override Eutrophication, but Facilitates Nutrient Release from Sediment and Motivates Eutrophic Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mesocosm Establishment
2.2. Sampling and Measurements
2.3. DNA Extraction, Sequencing, and Bioinformatics
2.4. Statistics and Data Analysis
3. Results
3.1. Experiment Water Temperature
3.2. Overall Analysis
3.3. Chl a-W and chl a-S Changes
3.4. EC, pH and DO Changes
3.5. Main Characteristics of the Sediment
3.6. Variation of Benthic Fluxes
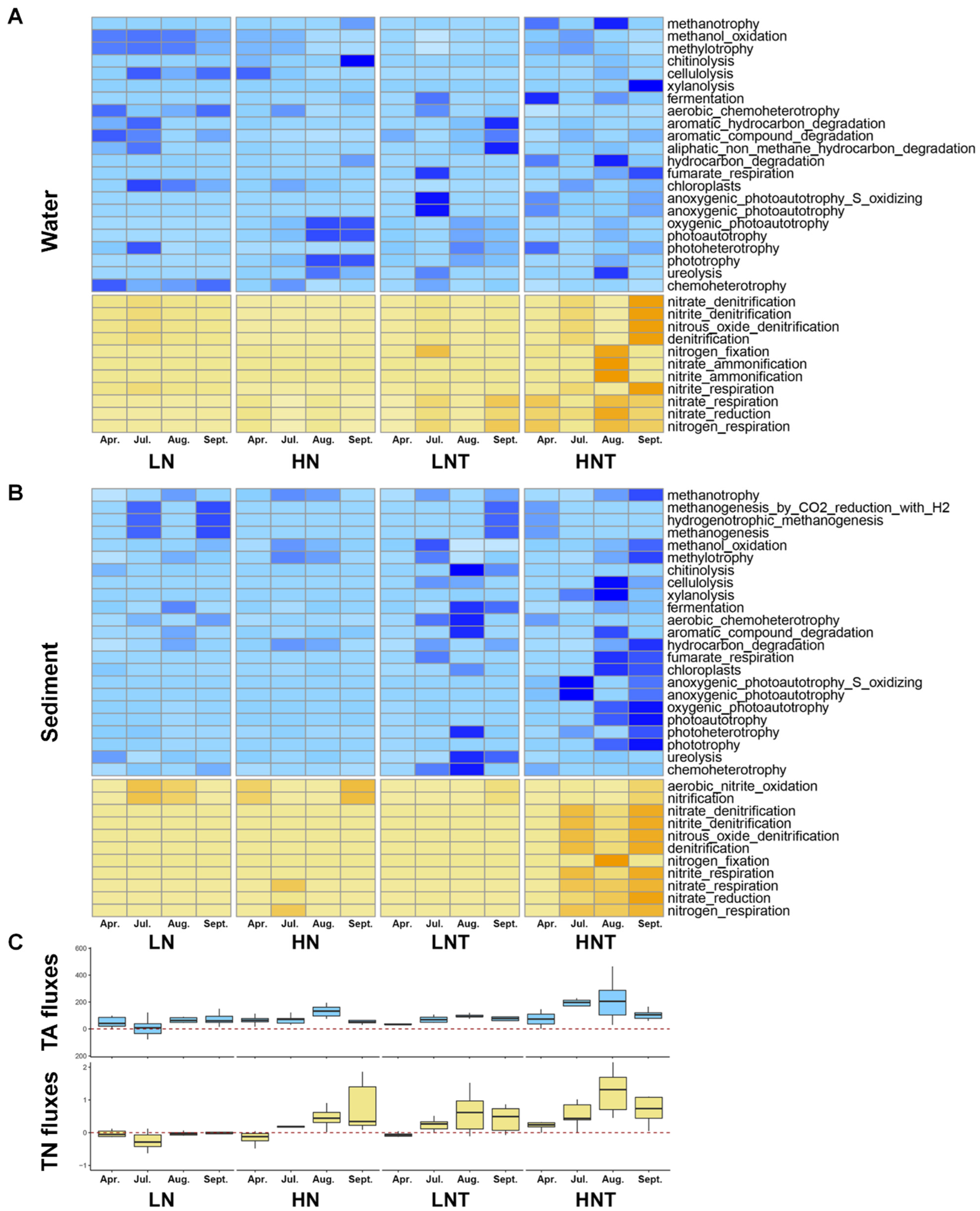
3.7. Microbial Taxonomic Composition
3.8. Metabolism Potential of Microorganisms
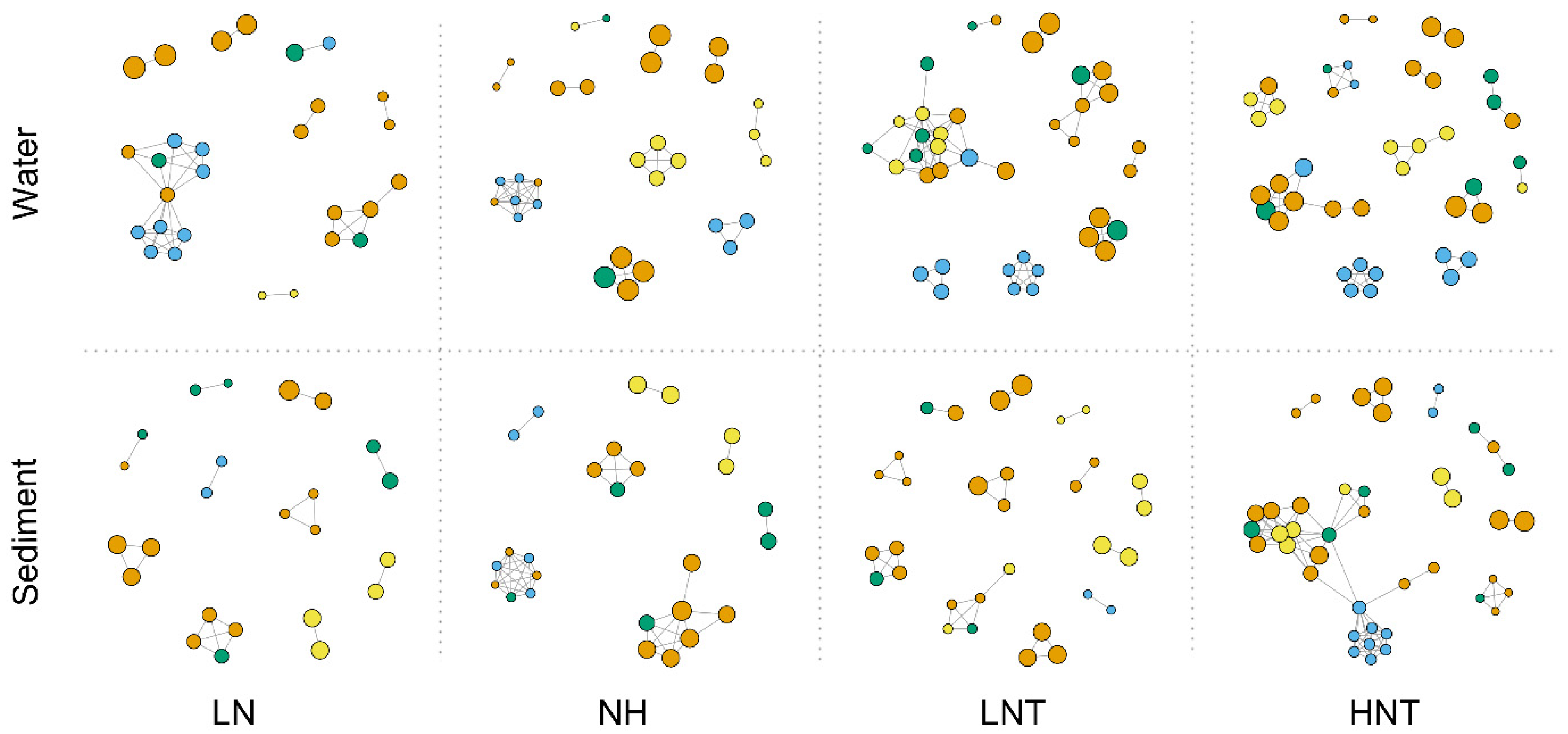
3.9. Network Analysis
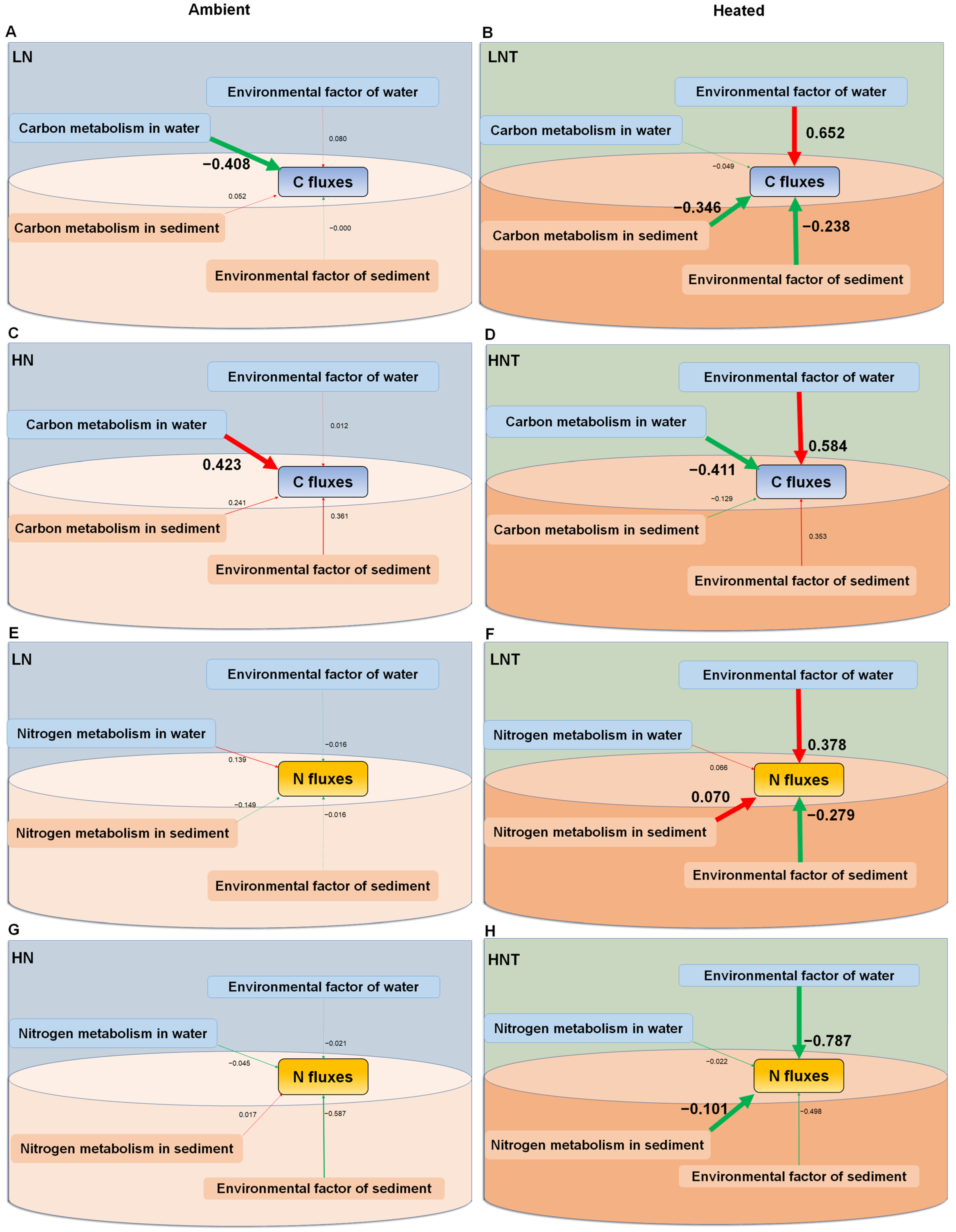
3.10. Relationship between Carbon and Nitrogen Fluxes and Functional Genes of Microbial Carbon and Nitrogen Cycling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dudgeon, D. Multiple threats imperil freshwater biodiversity in the Anthropocene. Curr. Biol. 2019, 29, R960–R967. [Google Scholar] [CrossRef]
- Richardson, J.; Feuchtmayr, H.; Miller, C.; Hunter, P.D.; Maberly, S.C.; Carvalho, L. Response of cyanobacteria and phytoplankton abundance to warming, extreme rainfall events and nutrient enrichment. Glob. Chang. Biol. 2019, 25, 3365–3380. [Google Scholar] [CrossRef]
- Feuchtmayr, H.; Moran, R.; Hatton, K.; Connor, L.; Heyes, T.; Moss, B.; Harvey, I.; Atkinson, D. Global warming and eutrophication: Effects on water chemistry and autotrophic communities in experimental hypertrophic shallow lake mesocosms. J. Appl. Ecol. 2009, 46, 713–723. [Google Scholar] [CrossRef]
- Scheffer, M.; Hosper, S.H.; Meijer, M.L.; Moss, B.; Jeppesen, E. Alternative equilibria in shallow lakes. Trends Ecol. Evol. 1993, 8, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Davidson, T.A.; Audet, J.; Jeppesen, E.; Landkildehus, F.; Lauridsen, T.L.; Søndergaard, M.; Syväranta, J. Synergy between nutrients and warming enhances methane ebullition from experimental lakes. Nat. Clim. Chang. 2018, 8, 156–160. [Google Scholar] [CrossRef]
- Greaver, T.; Clark, C.; Compton, J.; Vallano, D.; Talhelm, A.; Weaver, C.; Band, L.; Baron, J.; Davidson, E.; Tague, C. Key ecological responses to nitrogen are altered by climate change. Nat. Clim. Chang. 2016, 6, 836–843. [Google Scholar] [CrossRef]
- Tranvik, L.J.; Downing, J.A.; Cotner, J.B.; Loiselle, S.A.; Striegl, R.G.; Ballatore, T.J.; Dillon, P.; Finlay, K.; Fortino, K.; Knoll, L.B. Lakes and reservoirs as regulators of carbon cycling and climate. Limnol. Oceanogr. 2009, 54, 2298–2314. [Google Scholar] [CrossRef]
- Karlsson, J.; Serikova, S.; Vorobyev, S.N.; Rocher-Ros, G.; Denfeld, B.; Pokrovsky, O.S. Carbon emission from Western Siberian inland waters. Nat. Commun. 2021, 12, 825. [Google Scholar] [CrossRef]
- Schindler, D.W. Eutrophication and recovery in experimental lakes: Implications for lake management. Science 1974, 184, 897–899. [Google Scholar] [CrossRef]
- Schindler, D.W.; Hecky, R.E.; Findlay, D.L.; Stainton, M.P.; Parker, B.R.; Paterson, M.J.; Beaty, K.G.; Lyng, M.; Kasian, S.E.M. Eutrophication of lakes cannot be controlled by reducing nitrogen input: Results of a 37-year whole-ecosystem experiment. Proc. Natl. Acad. Sci. USA 2008, 105, 11254–11258. [Google Scholar] [CrossRef]
- Paul, S.; Küsel, K.; Alewell, C. Reduction processes in forest wetlands: Tracking down heterogeneity of source/sink functions with a combination of methods. Soil Biol. Biochem. 2006, 38, 1028–1039. [Google Scholar] [CrossRef]
- Davidson, T.A.; Audet, J.; Svenning, J.-C.; Lauridsen, T.L.; Søndergaard, M.; Landkildehus, F.; Larsen, S.E.; Jeppesen, E. Eutrophication effects on greenhouse gas fluxes from shallow-lake mesocosms override those of climate warming. Glob. Chang. Biol. 2015, 21, 4449–4463. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.J.; DelSontro, T.; Downing, J.A. Eutrophication will increase methane emissions from lakes and impoundments during the 21st century. Nat. Commun. 2019, 10, 1375. [Google Scholar] [CrossRef] [PubMed]
- Soong, J.L.; Fuchslueger, L.; Marañon-Jimenez, S.; Torn, M.S.; Janssens, I.A.; Penuelas, J.; Richter, A. Microbial carbon limitation: The need for integrating microorganisms into our understanding of ecosystem carbon cycling. Glob. Chang. Biol. 2020, 26, 1953–1961. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Z.; Chen, J.; Castellano, M.J.; Ye, C.; Zhang, N.; Miao, Y.; Zheng, H.; Li, J.; Ding, W. Oxygen availability regulates the quality of soil dissolved organic matter by mediating microbial metabolism and iron oxidation. Glob. Chang. Biol. 2022, 28, 7410–7427. [Google Scholar] [CrossRef] [PubMed]
- Curtinrich, H.J.; Sebestyen, S.D.; Griffiths, N.A.; Hall, S.J. Warming stimulates iron-mediated carbon and nutrient cycling in mineral-poor peatlands. Ecosystems 2022, 25, 44–60. [Google Scholar] [CrossRef]
- Hicks, N.; Liu, X.; Gregory, R.; Kenny, J.; Lucaci, A.; Lenzi, L.; Paterson, D.M.; Duncan, K.R. Temperature driven changes in benthic bacterial diversity influences biogeochemical cycling in coastal sediments. Front. Microbiol. 2018, 9, 1730. [Google Scholar] [CrossRef] [PubMed]
- Woodward, G.; Perkins, D.M.; Brown, L.E. Climate change and freshwater ecosystems: Impacts across multiple levels of organization. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2093–2106. [Google Scholar] [CrossRef]
- Jensen, H.S.; Andersen, F.O. Importance of temperature, nitrate, and pH for phosphate release from aerobic sediments of four shallow, eutrophic lakes. Limnol. Oceanogr. 1992, 37, 577–589. [Google Scholar] [CrossRef]
- Farrell, K.J.; Ward, N.K.; Krinos, A.I.; Hanson, P.C.; Daneshmand, V.; Figueiredo, R.J.; Carey, C.C. Ecosystem-scale nutrient cycling responses to increasing air temperatures vary with lake trophic state. Ecol. Modell. 2020, 430, 109134. [Google Scholar] [CrossRef]
- Gudasz, C.; Bastviken, D.; Steger, K.; Premke, K.; Sobek, S.; Tranvik, L.J. Temperature-controlled organic carbon mineralization in lake sediments. Nature 2010, 466, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Liikanen, A.; Murtoniemi, T.; Tanskanen, H.; Vaisanen, T.; Martikainen, P.J. Effects of temperature and oxygen availability on greenhouse gas and nutrient dynamics in sediment of a eutrophic mid-boreal lake. Biogeochemistry 2002, 59, 269–286. [Google Scholar] [CrossRef]
- Li, M.; Fang, A.; Yu, X.; Zhang, K.; He, Z.; Wang, C.; Peng, Y.; Xiao, F.; Yang, T.; Zhang, W. Microbially-driven sulfur cycling microbial communities in different mangrove sediments. Chemosphere 2021, 273, 128597. [Google Scholar] [CrossRef]
- Benbow, M.E.; Barton, P.S.; Ulyshen, M.D.; Beasley, J.C.; DeVault, T.L.; Strickland, M.S.; Tomberlin, J.K.; Jordan, H.R.; Pechal, J.L. Necrobiome framework for bridging decomposition ecology of autotrophically and heterotrophically derived organic matter. Ecol. Monogr. 2019, 89, e01331. [Google Scholar] [CrossRef]
- Zoppini, A.; Ademollo, N.; Patrolecco, L.; Langone, L.; Lungarini, S.; Dellisanti, W.; Amalfitano, S. Distribution patterns of organic pollutants and microbial processes in marine sediments across a gradient of anthropogenic impact. Environ. Pollut. 2018, 242, 1860–1870. [Google Scholar] [CrossRef] [PubMed]
- Owens, P.N. Soil erosion and sediment dynamics in the Anthropocene: A review of human impacts during a period of rapid global environmental change. J. Soils Sediments 2020, 20, 4115–4143. [Google Scholar] [CrossRef]
- Simpson, Z.; McDowell, R.; Condron, L. The biotic contribution to the benthic stream sediment phosphorus buffer. Biogeochemistry 2020, 151, 63–79. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, B.; Guan, C.-Y.; Jiang, X.; Tan, J.; Li, X. Identifying biotic and abiotic processes of reversing biochar-induced soil phosphorus leaching through biochar modification with MgAl layered (hydr) oxides. Sci. Total Environ. 2022, 843, 157037. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Y.; Sun, R.; Hu, S.; Qiao, Z.; Wang, S.; Mi, X. NH4+-N/NO3−-N ratio controlling nitrogen transformation accompanied with NO2−-N accumulation in the oxic-anoxic transition zone. Environ. Res. 2020, 189, 109962. [Google Scholar] [CrossRef]
- Elser, J.J.; Bracken, M.E.S.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef]
- Pörtner, H.-O.; Roberts, D.C.; Adams, H.; Adler, C.; Aldunce, P.; Ali, E.; Begum, R.A.; Betts, R.; Kerr, R.B.; Biesbroek, R. Climate Change 2022: Impacts, Adaptation and Vulnerability; IPCC Sixth Assessment Report; IPCC: Geneva, Switzerland, 2022. [Google Scholar]
- Woolway, R.I.; Kraemer, B.M.; Lenters, J.D.; Merchant, C.J.; O’Reilly, C.M.; Sharma, S. Global lake responses to climate change. Nat. Rev. Earth Environ. 2020, 1, 388–403. [Google Scholar] [CrossRef]
- Creed, I.F.; Bergström, A.-K.; Trick, C.G.; Grimm, N.B.; Hessen, D.O.; Karlsson, J.; Kidd, K.A.; Kritzberg, E.; McKnight, D.M.; Freeman, E.C.; et al. Global change-driven effects on dissolved organic matter composition: Implications for food webs of northern lakes. Glob. Chang. Biol. 2018, 24, 3692–3714. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Zhang, T.; Luo, Y.; Liu, J.; Lu, X.; Ye, Q.; Wang, S.; Huang, J.; Mao, Q.; Mo, J.; et al. Temporal patterns of soil carbon emission in tropical forests under long-term nitrogen deposition. Nat. Geosci. 2022, 15, 1002–1010. [Google Scholar] [CrossRef]
- Wilhelm, S.W.; Carberry, M.J.; Eldridge, M.L.; Poorvin, L.; Saxton, M.A.; Doblin, M.A. Marine and freshwater cyanophages in a Laurentian Great Lake: Evidence from infectivity assays and molecular analyses of g20 genes. Appl. Environ. Microbiol. 2006, 72, 4957–4963. [Google Scholar] [CrossRef]
- Jin, X.; Tu, Q. Investigation Specificaion on Lake Eutrophication; China Environmental Science Press: Beijing, China, 1990. [Google Scholar]
- Jespersen, A.-M.; Christoffersen, K. Measurements of chlorophyll-a from phytoplankton using ethanol as extraction solvent. Arch. Für Hydrobiol. 1987, 109, 445–454. [Google Scholar] [CrossRef]
- Haraldsson, C.; Anderson, L.G.; Hassellöv, M.; Hulth, S.; Olsson, K. Rapid, high-precision potentiometric titration of alkalinity in ocean and sediment pore waters. Deep Sea Res. Part I 1997, 44, 2031–2044. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.J.N. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P.J.N. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Caporaso, J.G.J.M. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2′s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Beule, L.; Karlovsky, P.J.P. Improved normalization of species count data in ecology by scaling with ranked subsampling (SRS): Application to microbial communities. PeerJ 2020, 8, e9593. [Google Scholar] [CrossRef]
- Louca, S.; Parfrey, L.W.; Doebeli, M.J.S. Decoupling function and taxonomy in the global ocean microbiome. Science 2016, 353, 1272–1277. [Google Scholar] [CrossRef]
- Ekvall, M.K.; Hansson, L.A. Differences in Recruitment and Life-History Strategy Alter Zooplankton Spring Dynamics Under Climate Change Conditions. PLoS ONE 2012, 7, 1–8. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing. 2013. Available online: https://cran.microsoft.com/snapshot/2014-09-08/web/packages/dplR/vignettes/xdate-dplR.pdf (accessed on 8 February 2023).
- Kolde, R. Package ‘pheatmap’. 2015. Available online: https://cran.r-project.org/web/packages/pheatmap/index.html (accessed on 8 February 2023).
- Rosseel, Y.J. Lavaan: An R package for structural equation modeling and more. J. Stat. Softw. 2012, 48, 1–36. [Google Scholar] [CrossRef]
- Epskamp, S.; Stuber, S.; Nak, J.; Veenman, M.; Jorgensen, T.D. Package ‘semPlot’. Available online: https://cran.r-project.org/web/packages/semPlot/semPlot.pdf (accessed on 8 February 2023).
- Rhoades, C.C.; Entwistle, D.; Butler, D. The influence of wildfire extent and severity on streamwater chemistry, sediment and temperature following the Hayman Fire, Colorado. Int. J. Wildland Fire 2011, 20, 430–442. [Google Scholar] [CrossRef]
- Darling, E.S.; Cote, I.M. Quantifying the evidence for ecological synergies. Ecol. Lett. 2008, 11, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Mooij, W.M.; Hülsmann, S.; Domis, L.N.D.S.; Nolet, B.A.; Bodelier, P.L.E.; Boers, P.C.M.; Pires, L.M.D.; Gons, H.J.; Ibelings, B.W.; Noordhuis, R. The impact of climate change on lakes in the Netherlands: A review. Aquat. Ecol. 2005, 39, 381–400. [Google Scholar] [CrossRef]
- Fan, Y.-Y.; Li, B.-B.; Yang, Z.-C.; Cheng, Y.-Y.; Liu, D.-F.; Yu, H.-Q. Mediation of functional gene and bacterial community profiles in the sediments of eutrophic Chaohu Lake by total nitrogen and season. Environ. Pollut. 2019, 250, 233–240. [Google Scholar] [CrossRef]
- Jiang, X.; Jin, X.; Yao, Y.; Li, L.; Wu, F. Effects of biological activity, light, temperature and oxygen on phosphorus release processes at the sediment and water interface of Taihu Lake, China. Water Res. 2008, 42, 2251–2259. [Google Scholar] [CrossRef]
- Wang, H.; Holden, J.; Spera, K.; Xu, X.; Wang, Z.; Luan, J.; Xu, X.; Zhang, Z. Phosphorus fluxes at the sediment–water interface in subtropical wetlands subjected to experimental warming: A microcosm study. Chemosphere 2013, 90, 1794–1804. [Google Scholar] [CrossRef]
- Renaud, P.E.; Morata, N.; Carroll, M.L.; Denisenko, S.G.; Reigstad, M. Pelagic–benthic coupling in the western Barents Sea: Processes and time scales. Deep Sea Res. Part II 2008, 55, 2372–2380. [Google Scholar] [CrossRef]
- Bartosiewicz, M.; Maranger, R.; Przytulska, A.; Laurion, I. Effects of phytoplankton blooms on fluxes and emissions of greenhouse gases in a eutrophic lake. Water Res. 2021, 196, 116985. [Google Scholar] [CrossRef] [PubMed]
- McGlathery, K.J.; Sundbäck, K.; Anderson, I.C. Eutrophication in shallow coastal bays and lagoons: The role of plants in the coastal filter. Mar. Ecol. Prog. Ser. 2007, 348, 1–18. [Google Scholar] [CrossRef]
- Rao, K.; Zhang, X.; Yi, X.-J.; Li, Z.-S.; Wang, P.; Huang, G.-W.; Guo, X.-X. Interactive effects of environmental factors on phytoplankton communities and benthic nutrient interactions in a shallow lake and adjoining rivers in China. Sci. Total Environ. 2018, 619, 1661–1672. [Google Scholar] [CrossRef]
- Shi, P.; Wang, H.; Feng, M.; Cheng, H.; Yang, Q.; Yan, Y.; Xu, J.; Zhang, M. The Coupling Response between Different Bacterial Metabolic Functions in Water and Sediment Improve the Ability to Mitigate Climate Change. Water 2022, 14, 1203. [Google Scholar] [CrossRef]
- Zhou, Y.; Song, K.; Han, R.; Riya, S.; Xu, X.; Yeerken, S.; Geng, S.; Ma, Y.; Terada, A. Nonlinear response of methane release to increased trophic state levels coupled with microbial processes in shallow lakes. Environ. Pollut. 2020, 265, 114919. [Google Scholar] [CrossRef]
- Alsterberg, C.; Hulth, S.; Sundbäck, K. Response of a shallow-water sediment system to warming. Limnol. Oceanogr. 2011, 56, 2147–2160. [Google Scholar] [CrossRef]
- Freitas, F.; Arndt, S.; Hendry, K.; Faust, J.; Tessin, A.; März, C. Benthic organic matter transformation drives pH and carbonate chemistry in Arctic marine sediments. Glob. Biogeochem. Cycles 2022, 36, e2021GB007187. [Google Scholar] [CrossRef]
- McLaughlin, J.W.; Webster, K.L. Alkalinity and acidity cycling and fluxes in an intermediate fen peatland in northern Ontario. Biogeochemistry 2010, 99, 143–155. [Google Scholar] [CrossRef]
- Kirk, J.T. Light and Photosynthesis in Aquatic Ecosystems; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Cantonati, M.; Lowe, R.L. Lake benthic algae: Toward an understanding of their ecology. Freshw. Sci. 2014, 33, 475–486. [Google Scholar] [CrossRef]
- Bartoli, M.; Nizzoli, D.; Zilius, M.; Bresciani, M.; Pusceddu, A.; Bianchelli, S.; Sundbäck, K.; Razinkovas-Baziukas, A.; Viaroli, P. Denitrification, nitrogen uptake, and organic matter quality undergo different seasonality in sandy and muddy sediments of a turbid estuary. Front. Microbiol. 2021, 11, 612700. [Google Scholar] [CrossRef]
- Cook, P.L.; Revill, A.T.; Butler, E.C.; Eyre, B.D. Carbon and nitrogen cycling on intertidal mudflats of a temperate Australian estuary. II. Nitrogen cycling. Mar. Ecol. Prog. Ser. 2004, 280, 39–54. [Google Scholar] [CrossRef]
- Engelsen, A.; Hulth, S.; Pihl, L.; Sundbäck, K. Benthic trophic status and nutrient fluxes in shallow-water sediments. Estuar. Coast. Shelf Sci. 2008, 78, 783–795. [Google Scholar] [CrossRef]
- Yang, C.; Yang, P.; Geng, J.; Yin, H.; Chen, K. Sediment internal nutrient loading in the most polluted area of a shallow eutrophic lake (Lake Chaohu, China) and its contribution to lake eutrophication. Environ. Pollut. 2020, 262, 114292. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Gao, S.; Zhang, Y.; Qin, B.; Xu, H.; Ding, S. Stimulation of high-concentration dissolved nitrogen and reactive phosphorus in Lake Taihu sediments on the initiation and maintenance of cyanobacterial blooms. Sci. Total Environ. 2022, 851, 158088. [Google Scholar] [CrossRef] [PubMed]
- Rabalais, N.N. Nitrogen in aquatic ecosystems. AMBIO 2002, 31, 102–112. [Google Scholar] [CrossRef]
- Han, H.; Li, Z. Effects of macrophyte-associated nitrogen cycling bacteria on ANAMMOX and denitrification in river sediments in the Taihu Lake region of China. Ecol. Eng. 2016, 93, 82–90. [Google Scholar] [CrossRef]
- Kemp, W.; Sampou, P.; Caffrey, J.; Mayer, M.; Henriksen, K.; Boynton, W.R. Ammonium recycling versus denitrification in Chesapeake Bay sediments. Limnol. Oceanogr. 1990, 35, 1545–1563. [Google Scholar] [CrossRef]
- Vadeboncoeur, Y.; Lodge, D.M.; Carpenter, S.R. Whole-lake fertilization effects on distribution of primary production between benthic and pelagic habitats. Ecology 2001, 82, 1065–1077. [Google Scholar] [CrossRef]
- Olson, C.R.; Solomon, C.T.; Jones, S.E. Shifting limitation of primary production: Experimental support for a new model in lake ecosystems. Ecol. Lett. 2020, 23, 1800–1808. [Google Scholar] [CrossRef]
- Yamamichi, M.; Kazama, T.; Tokita, K.; Katano, I.; Doi, H.; Yoshida, T.; Hairston Jr, N.G.; Urabe, J. A shady phytoplankton paradox: When phytoplankton increases under low light. Proc. R. Soc. B Biol. Sci. 2018, 285, 20181067. [Google Scholar] [CrossRef]
- Elliott, J.; Jones, I.; Thackeray, S. Testing the sensitivity of phytoplankton communities to changes in water temperature and nutrient load, in a temperate lake. Hydrobiologia 2006, 559, 401–411. [Google Scholar] [CrossRef]
| Mean Value | Probability | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | HN | HNT | LN | LNT | HN:HNT | LN:LNT | HN:LN | HNT:LNT | |
| Water column | pH | 7.93 ± 0.46 | 7.73 ± 0.27 | 7.94 ± 0.37 | 7.84 ± 0.29 | * | NS | NS | NS |
| DO (mg L−1) | 3.56 ± 0.86 | 3.00 ± 0.57 | 3.99 ± 0.99 | 3.41 ± 0.71 | NS | * | *** | ** | |
| Cond. (µs cm−1) | 413.8 ± 76.4 | 470.5 ± 60.8 | 253.7 ± 80.7 | 320.0 ± 56.6 | *** | *** | *** | *** | |
| TN (mg L−1) | 0.86 ± 0.42 | 1.15 ± 0.52 | 0.08 ± 0.08 | 0.17 ± 0.17 | * | ** | *** | *** | |
| NH4+-N (mg L−1) | 0.49 ± 0.41 | 0.53 ± 0.36 | 0.45 ± 0.33 | 0.53 ± 0.33 | NS | ** | *** | ** | |
| NO3−-N (mg L−1) | 0.34 ± 0.37 | 0.38 ± 0.31 | 0.19 ± 0.25 | 0.21 ± 0.20 | NS | NS | *** | *** | |
| TP (mg L−1) | 1.32 ± 0.50 | 1.35 ± 0.41 | 0.13 ± 0.09 | 0.23 ± 0.16 | NS | NS | *** | *** | |
| o-P (mg L−1) | 0.86 ± 0.42 | 1.15 ± 0.52 | 0.08 ± 0.08 | 0.17 ± 0.17 | *** | NS | *** | *** | |
| TA (mg L−1) | 84.2 ± 5.9 | 88.7 ± 9.2 | 44.4 ± 16.7 | 63.7 ± 9.56 | NS | *** | *** | *** | |
| SiO32− (mg L−1) | 14.2 ± 7.12 | 17.8 ± 9.9 | 14.1 ± 9.5 | 19.1 ± 9.6 | *** | ** | NS | NS | |
| Chl.a (mg L−1) | 65.0 ± 59.7 | 27.1 ± 19.3 | 2.5 ± 1.1 | 10.5 ± 10.4 | *** | *** | *** | *** | |
| Sediment | LOI (%) | 7.63 ± 0.81 | 7.59 ± 0.81 | 14.07 ± 0.64 | 13.43 ± 0.63 | NS | NS | *** | *** |
| TON (mg g−1) | 2.79 ± 0.27 | 2.58 ± 0.33 | 4.98 ± 0.49 | 4.80 ± 0.32 | ** | NS | *** | *** | |
| TN (mg g−1) | 2.90 ± 0.34 | 2.89 ± 0.34 | 5.16 ± 0.31 | 4.88 ± 0.19 | NS | NS | *** | *** | |
| TOC (mg g−1) | 27.17 ± 5.66 | 25.74 ± 5.15 | 49.58 ± 10.17 | 46.58 ± 11.42 | NS | NS | *** | *** | |
| TC (mg g−1) | 30.42 ± 3.92 | 30.89 ± 4.09 | 50.58 ± 3.72 | 47.40 ± 2.44 | NS | NS | *** | *** | |
| TP (mg g−1) | 1.55 ± 0.53 | 1.47 ± 0.56 | 0.17 ± 0.14 | 0.18 ± 0.32 | NS | NS | *** | *** | |
| APA (mg g−1 h−1) | 2.67 ± 0.34 | 2.64 ± 0.31 | 3.13 ± 0.36 | 2.98 ± 0.61 | NS | NS | *** | *** | |
| Chl.a (mg g−1) | 44.24 ± 7.68 | 42.58 ± 10.05 | 51.91 ± 15.72 | 47.45 ± 9.85 | NS | NS | ** | 0.277 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Li, Q.; Xu, J. Climate Warming Does Not Override Eutrophication, but Facilitates Nutrient Release from Sediment and Motivates Eutrophic Process. Microorganisms 2023, 11, 910. https://doi.org/10.3390/microorganisms11040910
Wang H, Li Q, Xu J. Climate Warming Does Not Override Eutrophication, but Facilitates Nutrient Release from Sediment and Motivates Eutrophic Process. Microorganisms. 2023; 11(4):910. https://doi.org/10.3390/microorganisms11040910
Chicago/Turabian StyleWang, Huan, Qi Li, and Jun Xu. 2023. "Climate Warming Does Not Override Eutrophication, but Facilitates Nutrient Release from Sediment and Motivates Eutrophic Process" Microorganisms 11, no. 4: 910. https://doi.org/10.3390/microorganisms11040910




