Pediatric Malaria with Respiratory Distress: Prognostic Significance of Point-of-Care Lactate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Inclusion Criteria
2.2. Setting
2.3. Sample Size
2.4. Study Procedures
2.5. Clinical Definitions
2.6. Statistical Analysis
2.7. Ethical Approval and Informed Consent
3. Results
3.1. Handheld Blood Lactate Predicts Subsequent Mortality in Children with Malaria and RD
3.2. Lactate Clearance as a Predictor of Mortality
3.3. Signs of Increased Work of Breathing Are Associated with Hyperlactatemia and Mortality
3.4. Elevated Lactate Is Associated with Impaired Tissue Perfusion
3.5. Elevated Lactate Is Not Associated with Hypoxemia
3.6. Elevated Lactate Is Associated with Anemia and Low Global Oxygen Delivery
3.7. Elevated Lactate Is Associated with Higher Parasite Density
3.8. Association with Acute Kidney Injury
4. Discussion
4.1. Clinical Significance of Hyperlactatemia in Malaria with RD
4.2. Mechanisms of Hyperlactatemia in Malaria with RD
4.3. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Malaria Report 2022; World Health Organization: Geneva, Switzerland, 2022.
- Hviid, L.; Jensen, A.T. PfEMP1—A Parasite Protein Family of Key Importance in Plasmodium falciparum Malaria Immunity and Pathogenesis. Adv. Parasitol. 2015, 88, 51–84. [Google Scholar]
- Miller, L.H.; Baruch, D.I.; Marsh, K.; Doumbo, O.K. The pathogenic basis of malaria. Nature 2002, 415, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Marsh, K.; Kinyanjui, S. Immune effector mechanisms in malaria. Parasite Immunol. 2006, 28, 51–60. [Google Scholar] [CrossRef]
- Marsh, K.; Forster, D.; Waruiru, C.; Mwangi, I.; Winstanley, M.; Marsh, V.; Newton, C.; Winstanley, P.; Warn, P.; Peshu, N.; et al. Indicators of life-threatening malaria in African children. N. Engl. J. Med. 1995, 332, 1399–1404. [Google Scholar] [CrossRef]
- Krishna, S.; Waller, D.W.; ter Kuile, F.; Kwiatkowski, D.; Crawley, J.; Craddock, C.F.; Nosten, F.; Chapman, D.; Brewster, D.; Holloway, P.A.; et al. Lactic acidosis and hypoglycaemia in children with severe malaria: Pathophysiological and prognostic significance. Trans. R. Soc. Trop. Med. Hyg. 1994, 88, 67–73. [Google Scholar] [CrossRef]
- Patel, H.; Dunican, C.; Cunnington, A.J. Predictors of outcome in childhood Plasmodium falciparum malaria. Virulence 2020, 11, 199–221. [Google Scholar] [CrossRef] [Green Version]
- Taylor, W.R.J.; Hanson, J.; Turner, G.D.H.; White, N.J.; Dondorp, A.M. Respiratory manifestations of malaria. Chest 2012, 142, 492–505. [Google Scholar] [CrossRef] [PubMed]
- English, M.; Waruiru, C.; Amukoye, E.; Murphy, S.; Crawley, J.; Mwangi, I.; Peshu, N.; Marsh, K. Deep breathing in children with severe malaria: Indicator of metabolic acidosis and poor outcome. Am. J. Trop. Med. Hyg. 1996, 55, 521–524. [Google Scholar] [CrossRef] [Green Version]
- Sasi, P.; Burns, S.P.; Waruiru, C.; English, M.; Hobson, C.L.; King, C.G.; Mosobo, M.; Beech, J.S.; Iles, R.A.; Boucher, B.J.; et al. Metabolic acidosis and other determinants of hemoglobin-oxygen dissociation in severe childhood Plasmodium falciparum malaria. Am. J. Trop. Med. Hyg. 2007, 77, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Herdman, M.T.; Sriboonvorakul, N.; Leopold, S.J.; Douthwaite, S.; Mohanty, S.; Hassan, M.M.; Maude, R.J.; Kingston, H.W.; Plewes, K.; Charunwatthana, P.; et al. The role of previously unmeasured organic acids in the pathogenesis of severe malaria. Crit. Care 2015, 19, 317. [Google Scholar] [CrossRef] [Green Version]
- Day, N.P.; Phu, N.H.; Mai, N.T.; Chau, T.T.; Loc, P.P.; Chuong, L.V.; Sinh, D.X.; Holloway, P.; Hien, T.T.; White, N.J. The pathophysiologic and prognostic significance of acidosis in severe adult malaria. Crit. Care Med. 2000, 28, 1833–1840. [Google Scholar] [CrossRef]
- Warrell, D.A.; White, N.J.; Veall, N.; Looareesuwan, S.; Chanthavanich, P.; Phillips, R.E.; Karbwang, J.; Pongpaew, P.; Krishna, S. Cerebral anaerobic glycolysis and reduced cerebral oxygen transport in human cerebral malaria. Lancet 1988, 2, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Newton, C.R.; Valim, C.; Krishna, S.; Wypij, D.; Olola, C.; Agbenyega, T.; Taylor, T.E.; Severe Malaria in African Children Network. The prognostic value of measures of acid/base balance in pediatric falciparum malaria, compared with other clinical and laboratory parameters. Clin. Infect. Dis. 2005, 41, 948–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Planche, T.; Agbenyega, T.; Bedu-Addo, G.; Ansong, D.; Owusu-Ofori, A.; Micah, F.; Anakwa, C.; Asafo-Agyei, E.; Hutson, A.; Stacpoole, P.W.; et al. A prospective comparison of malaria with other severe diseases in African children: Prognosis and optimization of management. Clin. Infect. Dis. 2003, 37, 890–897. [Google Scholar] [CrossRef]
- Aramburo, A.; Todd, J.; George, E.C.; Kiguli, S.; Olupot-Olupot, P.; Opoka, R.O.; Engoru, C.; Akech, S.O.; Nyeko, R.; Mtove, G.; et al. Lactate clearance as a prognostic marker of mortality in severely ill febrile children in East Africa. BMC Med. 2018, 16, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, T.E.; Borgstein, A.; Molyneux, M.E. Acid-base status in paediatric Plasmodium falciparum malaria. Q. J. Med. 1993, 86, 99–109. [Google Scholar] [PubMed]
- World Health Organization. Management of Severe Malaria: A Practical Handbook, 3rd ed.; WHO: Geneva, Switzerland, 2012. [Google Scholar]
- English, M.; Murphy, S.; Mwangi, I.; Crawley, J.; Peshu, N.; Marsh, K. Interobserver variation in respiratory signs of severe malaria. Arch. Dis. Child. 1995, 72, 334–336. [Google Scholar] [CrossRef] [Green Version]
- Jallow, M.; Casals-Pascual, C.; Ackerman, H.; Walther, B.; Walther, M.; Pinder, M.; Sisay-Joof, F.; Usen, S.; Jallow, M.; Abubakar, I.; et al. Clinical features of severe malaria associated with death: A 13-year observational study in the Gambia. PLoS ONE 2012, 7, e45645. [Google Scholar] [CrossRef] [Green Version]
- Olupot-Olupot, P.; Engoru, C.; Nteziyaremye, J.; Chebet, M.; Ssenyondo, T.; Muhindo, R.; Nyutu, G.; Macharia, A.W.; Uyoga, S.; Ndila, C.M.; et al. The clinical spectrum of severe childhood malaria in Eastern Uganda. Malar. J. 2020, 19, 322. [Google Scholar] [CrossRef]
- Ferguson, B.S.; Rogatzki, M.J.; Goodwin, M.L.; Kane, D.A.; Rightmire, Z.; Gladden, L.B. Lactate metabolism: Historical context, prior misinterpretations, and current understanding. Eur. J. Appl. Physiol. 2018, 118, 691–728. [Google Scholar] [CrossRef]
- Brand, N.R.; Opoka, R.O.; Hamre, K.E.; John, C.C. Differing Causes of Lactic Acidosis and Deep Breathing in Cerebral Malaria and Severe Malarial Anemia May Explain Differences in Acidosis-Related Mortality. PLoS ONE 2016, 11, e0163728. [Google Scholar] [CrossRef] [Green Version]
- Dondorp, A.M.; Ince, C.; Charunwatthana, P.; Hanson, J.; van Kuijen, A.; Faiz, M.A.; Rahman, M.R.; Hasan, M.; Bin Yunus, E.; Ghose, A.; et al. Direct in vivo assessment of microcirculatory dysfunction in severe falciparum malaria. J. Infect. Dis. 2008, 197, 79–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beare, N.A.; Harding, S.P.; Taylor, T.E.; Lewallen, S.; Molyneux, M.E. Perfusion abnormalities in children with cerebral malaria and malarial retinopathy. J. Infect. Dis. 2009, 199, 263–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegenthaler, N.; Giraud, R.; Bendjelid, K. Erythrocytapheresis and sublingual micro-vascular flow in severe malaria. Clin. Hemorheol. Microcirc. 2010, 46, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Vander Jagt, D.L.; Hunsaker, L.A.; Campos, N.M.; Baack, B.R. D-lactate production in erythrocytes infected with Plasmodium falciparum. Mol. Biochem. Parasitol. 1990, 42, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Possemiers, H.; Vandermosten, L.; Van den Steen, P.E. Etiology of lactic acidosis in malaria. PLoS Pathog. 2021, 17, e1009122. [Google Scholar] [CrossRef]
- Conroy, A.L.; Hawkes, M.; Elphinstone, R.E.; Morgan, C.; Hermann, L.; Barker, K.R.; Namasopo, S.; Opoka, R.O.; John, C.C.; Liles, W.C.; et al. Acute Kidney Injury Is Common in Pediatric Severe Malaria and Is Associated With Increased Mortality. In Open Forum Infectious Diseases; Oxford University Press: Oxford, UK, 2016; Volume 3, p. ofw046. [Google Scholar]
- Ouma, B.J.; Ssenkusu, J.M.; Shabani, E.; Datta, D.; Opoka, R.O.; Idro, R.; Bangirana, P.; Park, G.; Joloba, M.L.; Kain, K.C.; et al. Endothelial Activation, Acute Kidney Injury, and Cognitive Impairment in Pediatric Severe Malaria. Crit. Care Med. 2020, 48, e734–e743. [Google Scholar] [CrossRef] [PubMed]
- Batte, A.; Berrens, Z.; Murphy, K.; Mufumba, I.; Sarangam, M.L.; Hawkes, M.T.; Conroy, A.L. Malaria-Associated Acute Kidney Injury in African Children: Prevalence, Pathophysiology, Impact, and Management Challenges. Int. J. Nephrol. Renovasc. Dis. 2021, 14, 235–253. [Google Scholar] [CrossRef]
- Hawkes, M.T.; Leligdowicz, A.; Batte, A.; Situma, G.; Zhong, K.; Namasopo, S.; Opoka, R.O.; Kain, K.C.; Conroy, A.L. Pathophysiology of Acute Kidney Injury in Malaria and Non-Malarial Febrile Illness: A Prospective Cohort Study. Pathogens 2022, 11, 436. [Google Scholar] [CrossRef]
- Kamel, K.S.; Oh, M.S.; Halperin, M.L. L-lactic acidosis: Pathophysiology, classification, and causes; emphasis on biochemical and metabolic basis. Kidney Int. 2020, 97, 75–88. [Google Scholar] [CrossRef]
- Miller, B.F.; Fattor, J.A.; Jacobs, K.A.; Horning, M.A.; Navazio, F.; Lindinger, M.I.; Brooks, G.A. Lactate and glucose interactions during rest and exercise in men: Effect of exogenous lactate infusion. J. Physiol. 2002, 544, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, M.T.; Conroy, A.L.; Opoka, R.O.; Hermann, L.; Thorpe, K.E.; McDonald, C.; Kim, H.; Higgins, S.; Namasopo, S.; John, C.; et al. Inhaled nitric oxide as adjunctive therapy for severe malaria: A randomized controlled trial. Malar. J. 2015, 14, 421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leligdowicz, A.; Conroy, A.L.; Hawkes, M.; Richard-Greenblatt, M.; Zhong, K.; Opoka, R.O.; Namasopo, S.; Bell, D.; Liles, W.C.; da Costa, B.R.; et al. Risk-stratification of febrile African children at risk of sepsis using sTREM-1 as basis for a rapid triage test. Nat. Commun. 2021, 12, 6832. [Google Scholar] [CrossRef] [PubMed]
- Conradi, N.; Mian, Q.; Namasopo, S.; Conroy, A.L.; Hermann, L.L.; Olaro, C.; Amone, J.; Opoka, R.O.; Hawkes, M.T. Solar-powered oxygen delivery for the treatment of children with hypoxemia: Protocol for a cluster-randomized stepped-wedge controlled trial in Uganda. Trials 2019, 20, 679. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. World Malaria Report; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Kigozi, S.P.; Kigozi, R.N.; Sebuguzi, C.M.; Cano, J.; Rutazaana, D.; Opigo, J.; Bousema, T.; Yeka, A.; Gasasira, A.; Sartorius, B.; et al. Spatial-temporal patterns of malaria incidence in Uganda using HMIS data from 2015 to 2019. BMC Public Health 2020, 20, 1913. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K. gsDesign: Group Sequential Design. R Package Version 3.2.0. 2021. Available online: https://cran.r-project.org/web/packages/gsDesign/gsDesign.pdf (accessed on 19 November 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Ma, C.; Gunaratnam, L.C.; Ericson, A.; Conroy, A.L.; Namasopo, S.; Opoka, R.O.; Hawkes, M.T. Handheld Point-of-Care Lactate Measurement at Admission Predicts Mortality in Ugandan Children Hospitalized with Pneumonia: A Prospective Cohort Study. Am. J. Trop. Med. Hyg. 2019, 100, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Kosack, C.S.; Naing, W.T.; Piriou, E.; Shanks, L. Routine parallel diagnosis of malaria using microscopy and the malaria rapid diagnostic test SD 05FK60: The experience of Medecins Sans Frontieres in Myanmar. Malar. J. 2013, 12, 167. [Google Scholar] [CrossRef] [Green Version]
- Bonaventura, J.M.; Sharpe, K.; Knight, E.; Fuller, K.L.; Tanner, R.K.; Gore, C.J. Reliability and accuracy of six hand-held blood lactate analysers. J. Sports Sci. Med. 2015, 14, 203–214. [Google Scholar]
- American Heart Association. Pediatric Advanced Life Support Provider Manual; American Heart Association: Dallas, TX, USA, 2020. [Google Scholar]
- Leach, R.M.; Treacher, D.F. The pulmonary physician in critical care * 2: Oxygen delivery and consumption in the critically ill. Thorax 2002, 57, 170–177. [Google Scholar] [CrossRef] [Green Version]
- Nguah, S.B.; Feldt, T.; Hoffmann, S.; Pelletier, D.; Ansong, D.; Sylverken, J.; Mehrfar, P.; Herr, J.; Thiel, C.; Ehrhardt, S.; et al. Cardiac function in Ghanaian children with severe malaria. Intensive Care Med. 2012, 38, 2032–2041. [Google Scholar] [CrossRef]
- Mosteller, R.D. Simplified calculation of body-surface area. N. Engl. J. Med. 1987, 317, 1098. [Google Scholar] [PubMed]
- Ellis, R.K. Determination of PO2 from saturation. J. Appl. Physiol. 1989, 67, 902. [Google Scholar] [CrossRef] [PubMed]
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron. Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef]
- Pottel, H.; Hoste, L.; Martens, F. A simple height-independent equation for estimating glomerular filtration rate in children. Pediatr. Nephrol. 2012, 27, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Batte, A.; Starr, M.C.; Schwaderer, A.L.; Opoka, R.O.; Namazzi, R.; Phelps Nishiguchi, E.S.; Ssenkusu, J.M.; John, C.C.; Conroy, A.L. Methods to estimate baseline creatinine and define acute kidney injury in lean Ugandan children with severe malaria: A prospective cohort study. BMC Nephrol. 2020, 21, 417. [Google Scholar] [CrossRef]
- Helbok, R.; Kendjo, E.; Issifou, S.; Lackner, P.; Newton, C.R.; Kombila, M.; Agbenyega, T.; Bojang, K.; Dietz, K.; Schmutzhard, E.; et al. The Lambarene Organ Dysfunction Score (LODS) is a simple clinical predictor of fatal malaria in African children. J. Infect. Dis. 2009, 200, 1834–1841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Therneau, T.M. Coxme: Mixed Effects Cox Models. R Package Version 2.2-16. 2020. Available online: https://CRAN.R-project.org/package=coxme (accessed on 29 July 2022).
- Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). The WHO Child Growth Standards; World Health Organization: Geneva, Switzerland, 2006; Available online: http://www.who.int/childgrowth/standards/en/ (accessed on 12 February 2023).
- Fleming, S.; Thompson, M.; Stevens, R.; Heneghan, C.; Pluddemann, A.; Maconochie, I.; Tarassenko, L.; Mant, D. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: A systematic review of observational studies. Lancet 2011, 377, 1011–1018. [Google Scholar] [CrossRef] [Green Version]
- Mukanga, D.; Babirye, R.; Peterson, S.; Pariyo, G.W.; Ojiambo, G.; Tibenderana, J.K.; Nsubuga, P.; Kallander, K. Can lay community health workers be trained to use diagnostics to distinguish and treat malaria and pneumonia in children? Lessons from rural Uganda. Trop. Med. Int. Health 2011, 16, 1234–1242. [Google Scholar] [CrossRef] [Green Version]
- George, E.C.; Walker, A.S.; Kiguli, S.; Olupot-Olupot, P.; Opoka, R.O.; Engoru, C.; Akech, S.O.; Nyeko, R.; Mtove, G.; Reyburn, H.; et al. Predicting mortality in sick African children: The FEAST Paediatric Emergency Triage (PET) Score. BMC Med. 2015, 13, 174. [Google Scholar] [CrossRef] [Green Version]
- Kalkman, L.C.; Hanscheid, T.; Krishna, S.; Grobusch, M.P. Fluid therapy for severe malaria. Lancet Infect. Dis. 2022, 22, e160–e170. [Google Scholar] [CrossRef]
- Maitland, K.; Kiguli, S.; Opoka, R.O.; Engoru, C.; Olupot-Olupot, P.; Akech, S.O.; Nyeko, R.; Mtove, G.; Reyburn, H.; Lang, T.; et al. Mortality after fluid bolus in African children with severe infection. N. Engl. J. Med. 2011, 364, 2483–2495. [Google Scholar] [CrossRef] [Green Version]
- Mtove, G.; Nadjm, B.; Hendriksen, I.C.; Amos, B.; Muro, F.; Todd, J.; Reyburn, H. Point-of-care measurement of blood lactate in children admitted with febrile illness to an African District Hospital. Clin. Infect. Dis. 2011, 53, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Dienel, G.A. Brain lactate metabolism: The discoveries and the controversies. J. Cereb. Blood Flow Metab. 2012, 32, 1107–1138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gravenor, M.B.; Lloyd, A.L.; Kremsner, P.G.; Missinou, M.A.; English, M.; Marsh, K.; Kwiatkowski, D. A model for estimating total parasite load in falciparum malaria patients. J. Theor. Biol. 2002, 217, 137–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendriksen, I.C.; Mwanga-Amumpaire, J.; von Seidlein, L.; Mtove, G.; White, L.J.; Olaosebikan, R.; Lee, S.J.; Tshefu, A.K.; Woodrow, C.; Amos, B.; et al. Diagnosing severe falciparum malaria in parasitaemic African children: A prospective evaluation of plasma PfHRP2 measurement. PLoS Med. 2012, 9, e1001297. [Google Scholar] [CrossRef] [Green Version]
- Katsoulis, O.; Georgiadou, A.; Cunnington, A.J. Immunopathology of Acute Kidney Injury in Severe Malaria. Front. Immunol. 2021, 12, 651739. [Google Scholar] [CrossRef]
- Sarangam, M.L.; Namazzi, R.; Datta, D.; Bond, C.; Vanderpool, C.P.B.; Opoka, R.O.; John, C.C.; Conroy, A.L. Intestinal Injury Biomarkers Predict Mortality in Pediatric Severe Malaria. mBio 2022, 13, e0132522. [Google Scholar] [CrossRef]
- Ngai, M.; Hawkes, M.T.; Erice, C.; Weckman, A.M.; Wright, J.; Stefanova, V.; Opoka, R.O.; Namasopo, S.; Conroy, A.L.; Kain, K.C. Intestinal Injury in Ugandan Children Hospitalized With Malaria. J. Infect. Dis. 2022, 226, 2010–2020. [Google Scholar] [CrossRef]
- Leopold, S.J.; Ghose, A.; Allman, E.L.; Kingston, H.W.F.; Hossain, A.; Dutta, A.K.; Plewes, K.; Chotivanich, K.; Day, N.P.J.; Tarning, J.; et al. Identifying the Components of Acidosis in Patients With Severe Plasmodium falciparum Malaria Using Metabolomics. J. Infect. Dis. 2019, 219, 1766–1776. [Google Scholar] [CrossRef] [Green Version]
- Hawkes, M.; Conroy, A.L.; Opoka, R.O.; Namasopo, S.; Liles, W.C.; John, C.C.; Kain, K.C. Performance of point-of-care diagnostics for glucose, lactate, and hemoglobin in the management of severe malaria in a resource-constrained hospital in Uganda. Am. J. Trop. Med. Hyg. 2014, 90, 605–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agbenyega, T.; Angus, B.J.; Bedu-Addo, G.; Baffoe-Bonnie, B.; Guyton, T.; Stacpoole, P.W.; Krishna, S. Glucose and lactate kinetics in children with severe malaria. J. Clin. Endocrinol. Metab. 2000, 85, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Linderkamp, O.; Versmold, H.T.; Riegel, K.P.; Betke, K. Estimation and prediction of blood volume in infants and children. Eur. J. Pediatr. 1977, 125, 227–234. [Google Scholar] [CrossRef] [PubMed]
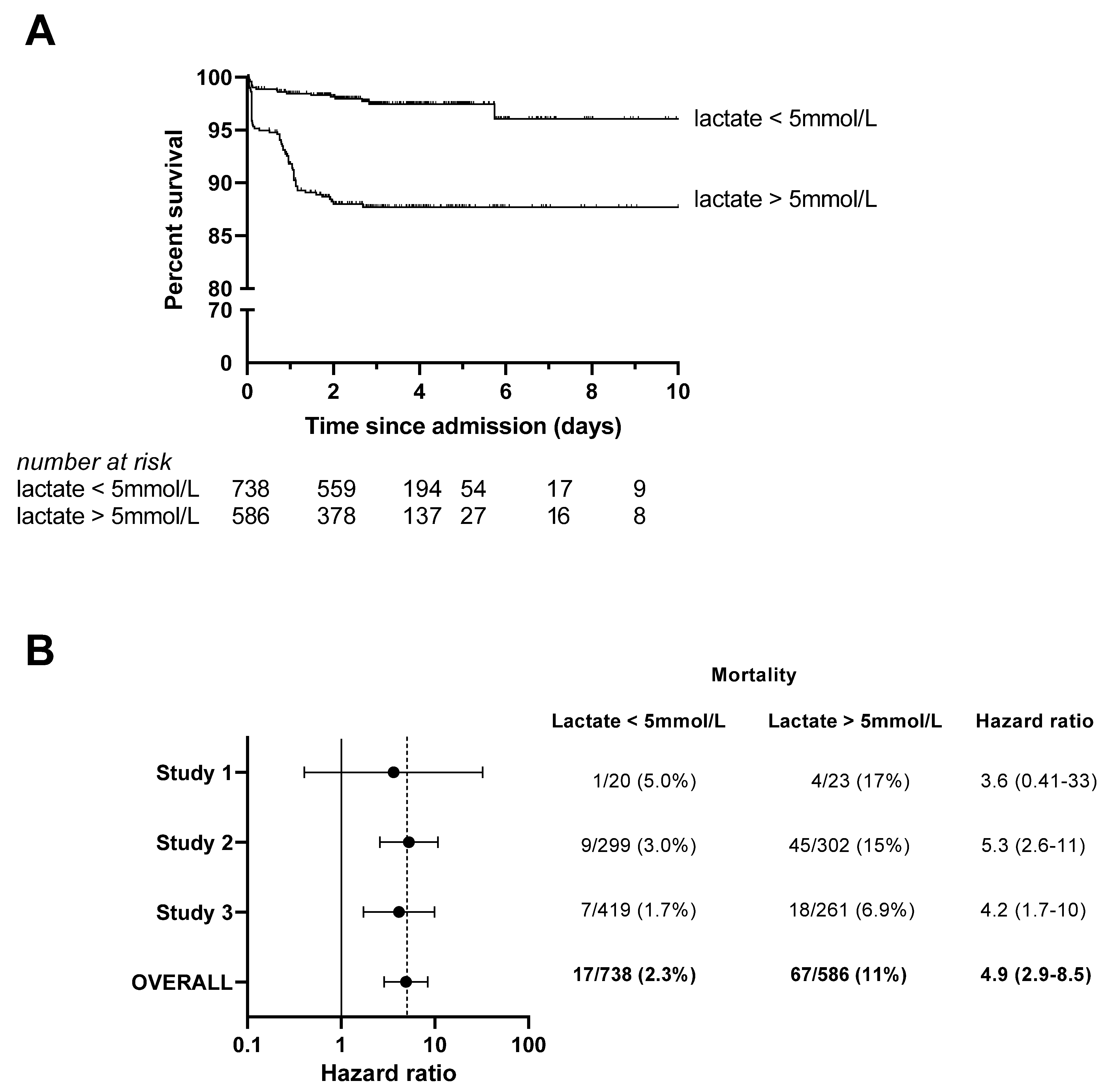
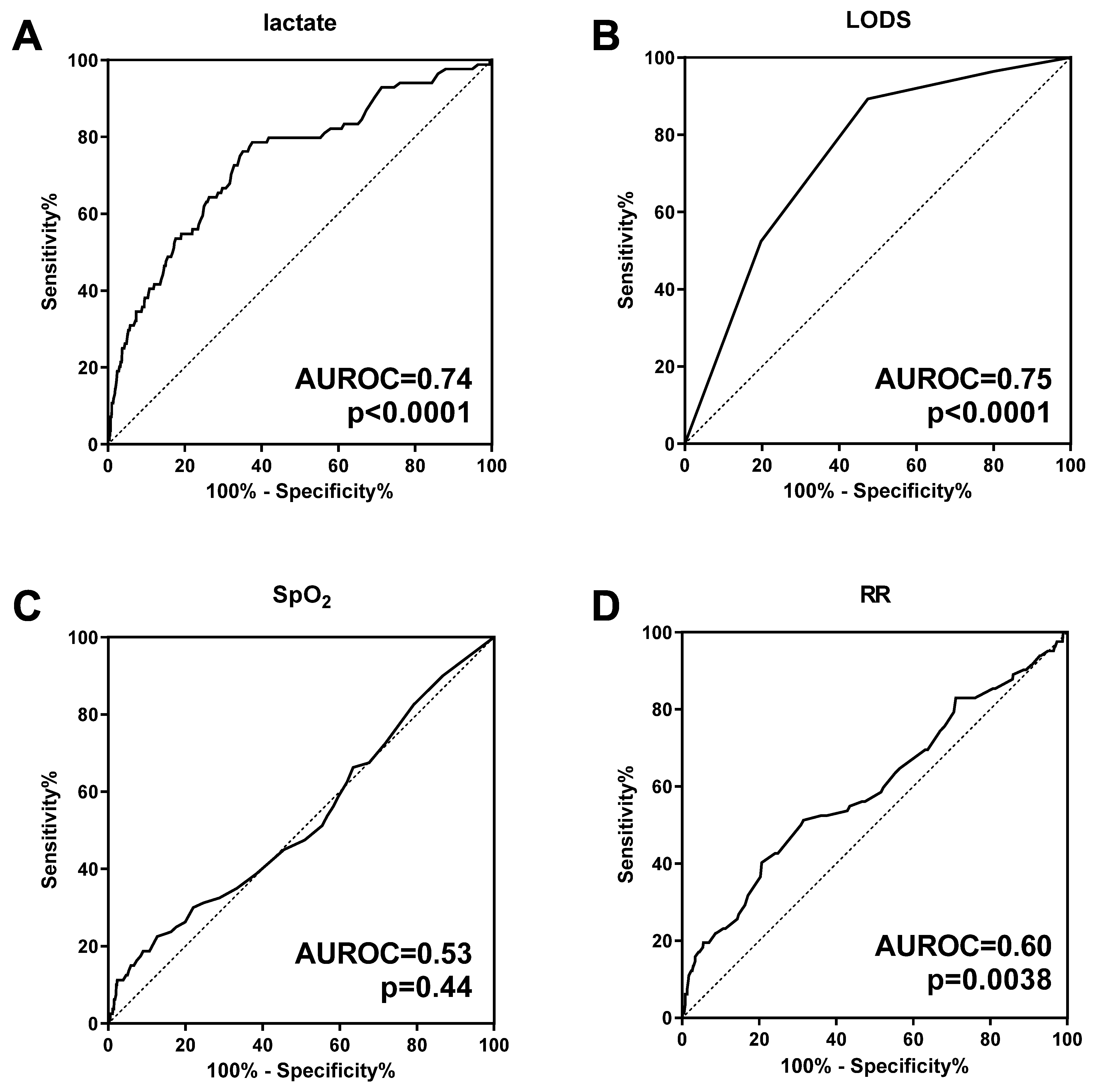

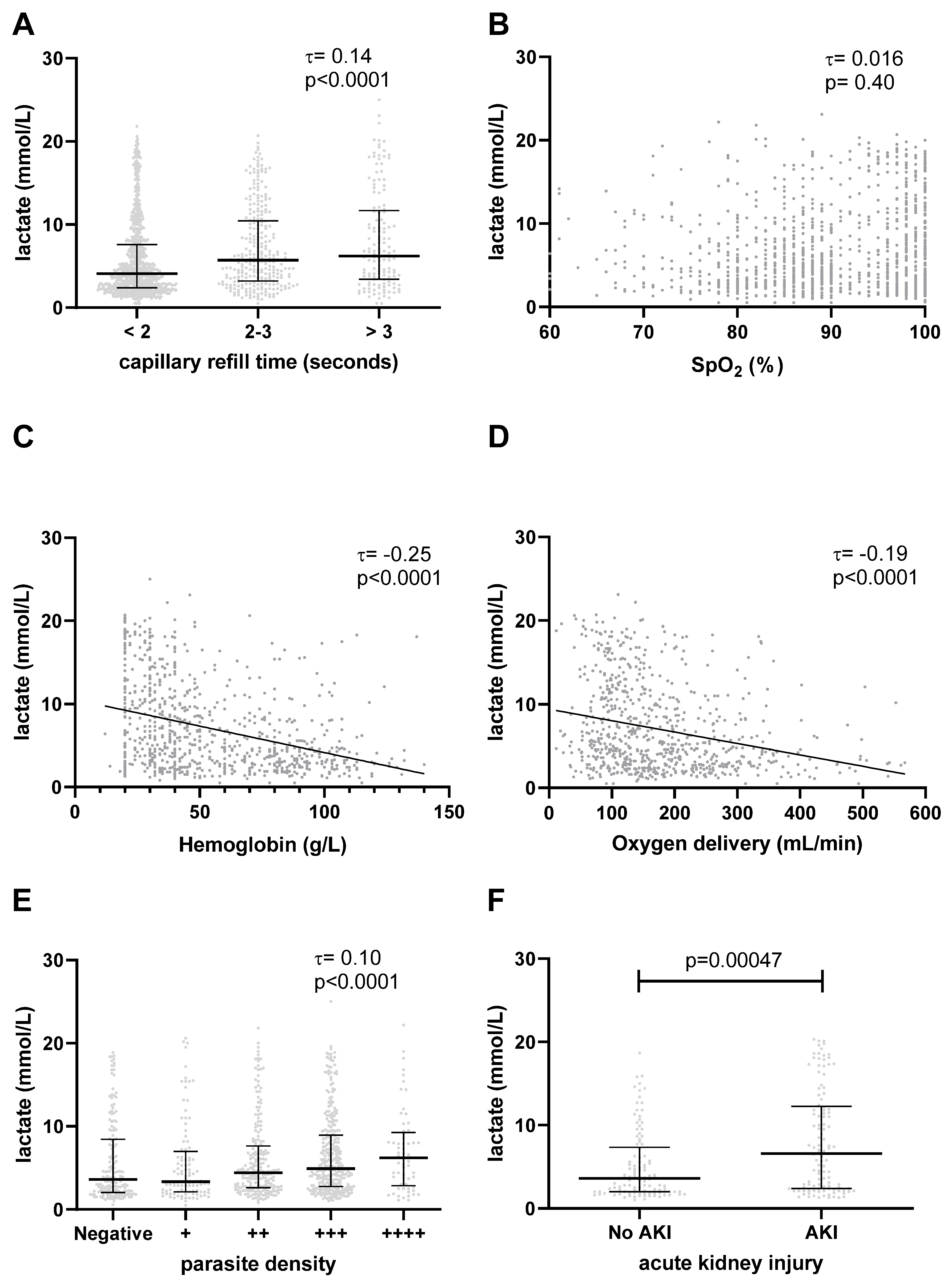
| Entire Cohort (n = 1324) | Lactate ≤5 mmol/L (n = 738) | Lactate >5 mmol/L (n = 586) | p-Value | |
|---|---|---|---|---|
| Demographics | ||||
| Female sex | 611 (46) | 354 (48) | 257 (44) | 0.14 |
| Age [years], median (IQR) | 1.4 (0.75–2.4) | 1.2 (0.75–2.3) | 1.4 (0.75–2.5) | 0.43 |
| History | ||||
| Cough | 1147 (87) | 649 (88) | 498 (85) | 0.14 |
| Difficulty breathing | 975 (74) | 537 (73) | 438 (75) | 0.48 |
| Lethargy | 438 (33) | 195 (26) | 243 (42) | <0.0001 |
| Convulsions | 329 (25) | 180 (24) | 149 (25) | 0.71 |
| Unable to feed/drink | 687 (52) | 342 (46) | 345 (59) | <0.0001 |
| Vomiting | 524 (40) | 257 (35) | 267 (46) | <0.0001 |
| Diarrhea | 352 (27) | 188 (26) | 164 (28) | 0.35 |
| Physical examination findings | ||||
| Weight [kg], median (IQR) | 9 (7.7–12) | 9 (7.5–12) | 9.5 (8–12) | 0.38 |
| Wasting 1 | 202 (16) | 114 (16) | 88 (16) | >0.99 |
| Length/height [cm], median (IQR) | 72 (65–83) | 72 (65–83) | 72 (65–84) | 0.87 |
| Stunting 2 | 651 (51) | 358 (50) | 293 (53) | 0.37 |
| Heart Rate (bpm), median (IQR) | 160 (140–170) | 150 (130–170) | 160 (140–180) | 0.00036 |
| Tachycardia 2 | 767 (58) | 390 (53) | 377 (65) | <0.0001 |
| Respiratory rate (bpm), median (IQR) | 52 (42–62) | 50 (40–60) | 54 (44–62) | 0.0015 |
| Tachypnea 3 | 834 (64) | 444 (61) | 390 (68) | 0.013 |
| Oxygen saturation (%), median (IQR) | 89 (84–98) | 89 (84–98) | 90 (85–98) | 0.098 |
| Hypoxemia (SaO2 < 90%) | 667 (51) | 392 (53) | 275 (47) | 0.033 |
| Temperature [°C], median (IQR) | 38 (37–38) | 38 (37–39) | 38 (37–38) | <0.0001 |
| Fever 4 | 798 (61) | 470 (64) | 328 (56) | 0.0045 |
| Altered level of consciousness 5 | 458 (35) | 197 (27) | 261 (45) | <0.0001 |
| Capillary refill time [seconds] | <0.0001 | |||
| <2 | 888 (68) | 545 (75) | 343 (59) | |
| 2–3 | 286 (22) | 128 (18) | 158 (27) | |
| >3 | 141 (11) | 58 (7.9) | 83 (14) | |
| Chest indrawing | 1086 (82) | 615 (83) | 471 (80) | 0.19 |
| Wheeze | 368 (54) | 247 (59) | 121 (46) | 0.0018 |
| Stridor | 206 (30) | 122 (29) | 84 (32) | 0.45 |
| Danger signs 6 | 1070 (81) | 567 (77) | 503 (86) | <0.0001 |
| Treatment | ||||
| Artesunate | 771 (58) | 440 (60) | 331 (56) | 0.27 |
| Quinine | 434 (33) | 226 (31) | 208 (35) | 0.069 |
| Artemether injection | 41 (3.1) | 14 (1.9) | 27 (4.6) | 0.0076 |
| Artemether-lumefantrine (oral) | 143 (11) | 88 (12) | 55 (9.4) | 0.16 |
| Supplemental oxygen | 681 (53) | 399 (56) | 282 (50) | 0.058 |
| Outcome | <0.0001 | |||
| Discharged | 1043 (79) | 627 (86) | 416 (71) | |
| Death | 84 (6.4) | 17 (2.3) | 67 (11) | |
| Transferred to another facility | 101 (7.7) | 46 (6.3) | 55 (9.4) | |
| Absconded | 89 (6.8) | 43 (5.9) | 46 (7.9) |
| Univariable | Multivariable * | |||
|---|---|---|---|---|
| HR | p-Value | aHR | p-Value | |
| Fixed effects | ||||
| Female sex | 0.68 (0.43–1.1) | 0.093 | 0.77 (0.48–1.2) | 0.25 |
| Age | 0.99 (0.82–1.2) | 0.38 | 1.0 (0.85–1.3) | 0.73 |
| LODS | <0.0001 | <0.0001 | ||
| 0 | 1.0 (reference) | 1.0 (reference) | ||
| 1 | 1.2 (0.29–4.8) | 1.6 (0.36–6.7) | ||
| 2 | 7.0 (2.1–23) | 9.0 (2.5–33) | ||
| 3 | 14 (4.5–47) | 18 (5.0–65) | ||
| Lactate > 5 mmol/L | 5.3 (3.1–9.0) | <0.0001 | 3.0 (1.8–5.3) | <0.0001 |
| Random effect | ||||
| Site | Variance 1.2 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitran, C.; Opoka, R.O.; Conroy, A.L.; Namasopo, S.; Kain, K.C.; Hawkes, M.T. Pediatric Malaria with Respiratory Distress: Prognostic Significance of Point-of-Care Lactate. Microorganisms 2023, 11, 923. https://doi.org/10.3390/microorganisms11040923
Mitran C, Opoka RO, Conroy AL, Namasopo S, Kain KC, Hawkes MT. Pediatric Malaria with Respiratory Distress: Prognostic Significance of Point-of-Care Lactate. Microorganisms. 2023; 11(4):923. https://doi.org/10.3390/microorganisms11040923
Chicago/Turabian StyleMitran, Catherine, Robert O. Opoka, Andrea L. Conroy, Sophie Namasopo, Kevin C. Kain, and Michael T. Hawkes. 2023. "Pediatric Malaria with Respiratory Distress: Prognostic Significance of Point-of-Care Lactate" Microorganisms 11, no. 4: 923. https://doi.org/10.3390/microorganisms11040923
APA StyleMitran, C., Opoka, R. O., Conroy, A. L., Namasopo, S., Kain, K. C., & Hawkes, M. T. (2023). Pediatric Malaria with Respiratory Distress: Prognostic Significance of Point-of-Care Lactate. Microorganisms, 11(4), 923. https://doi.org/10.3390/microorganisms11040923






