Ways of Long-Term Survival of Hydrocarbon-Oxidizing Bacteria in a New Biocomposite Material—Silanol-Humate Gel
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Physiological Characteristics of SHG-Immobilized Cells
3.1.1. Preservation of Cell Viability
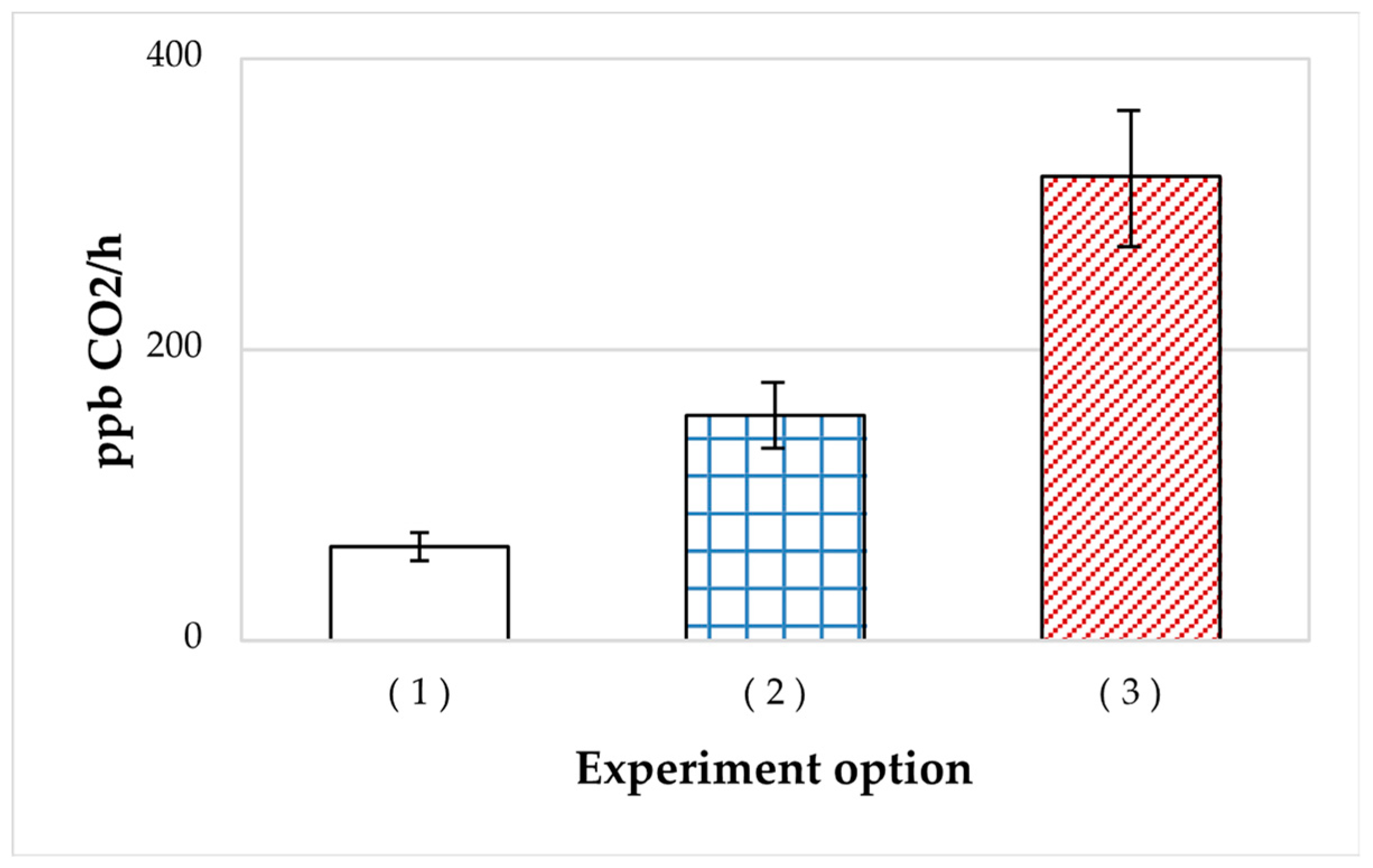
3.1.2. Respiration of the HOB Cultures on Oil Paraffins
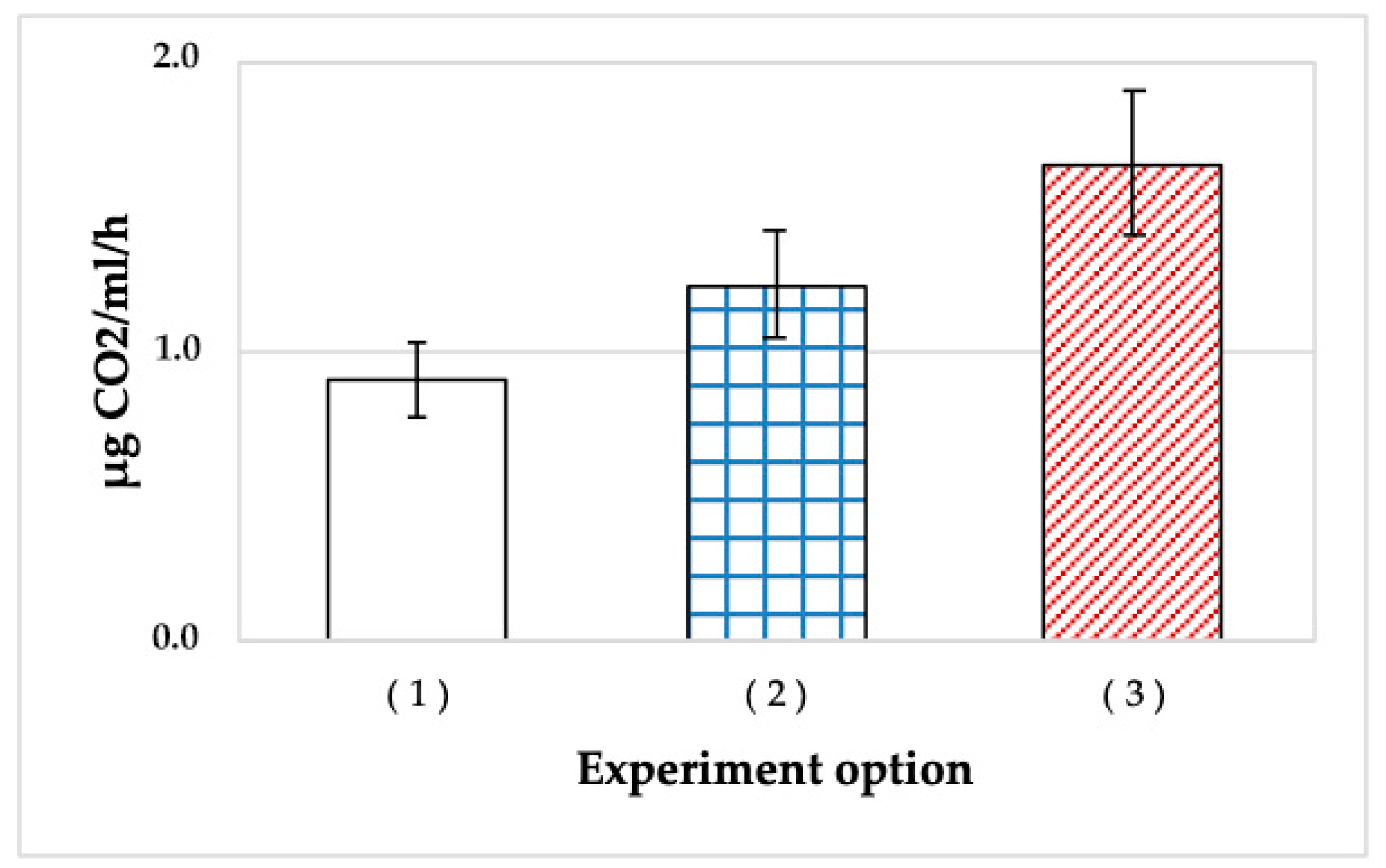
3.1.3. Endogenous Respiration of HOB Cells
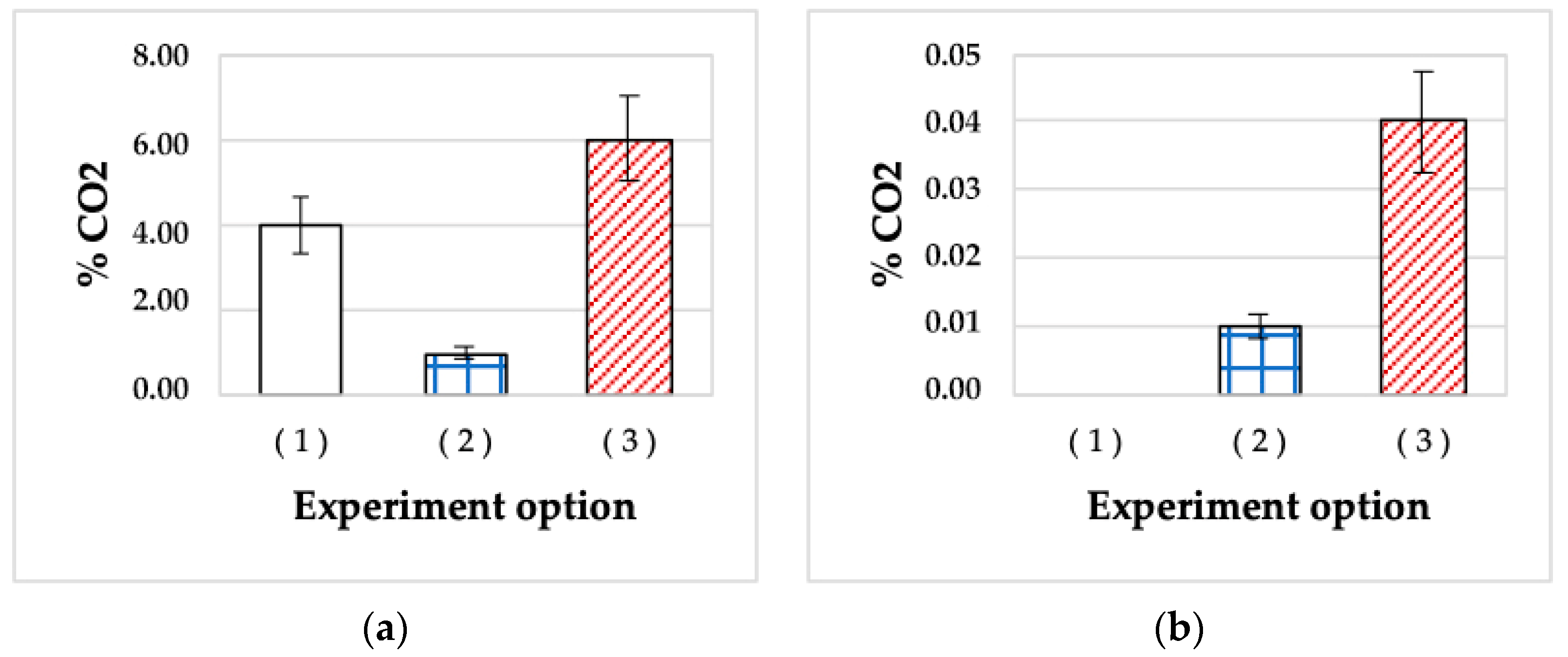
3.1.4. Detergent Formation
3.1.5. Dynamics of Acetate and Ethanol Content in SHG Samples with HOB during Long-Term Storage
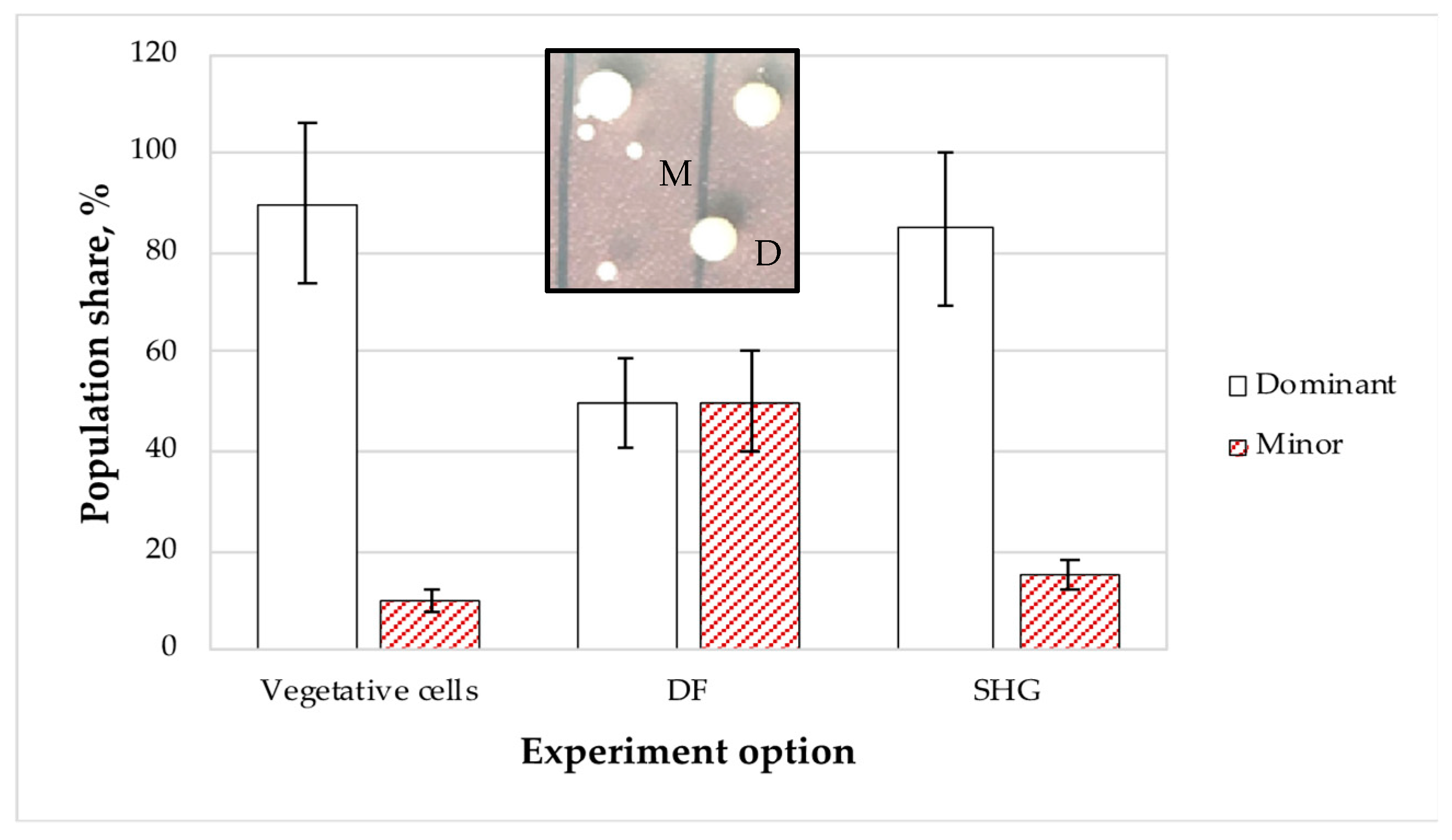
3.1.6. Phase Variations of the P. extremaustralis Populations
3.1.7. Ability of SHG-Stored HOB Cells to Oxidize Oil in Liquid Media under Stress Conditions
3.1.8. Stress Resistance of HOB Phase Variants Grown on Solid Media
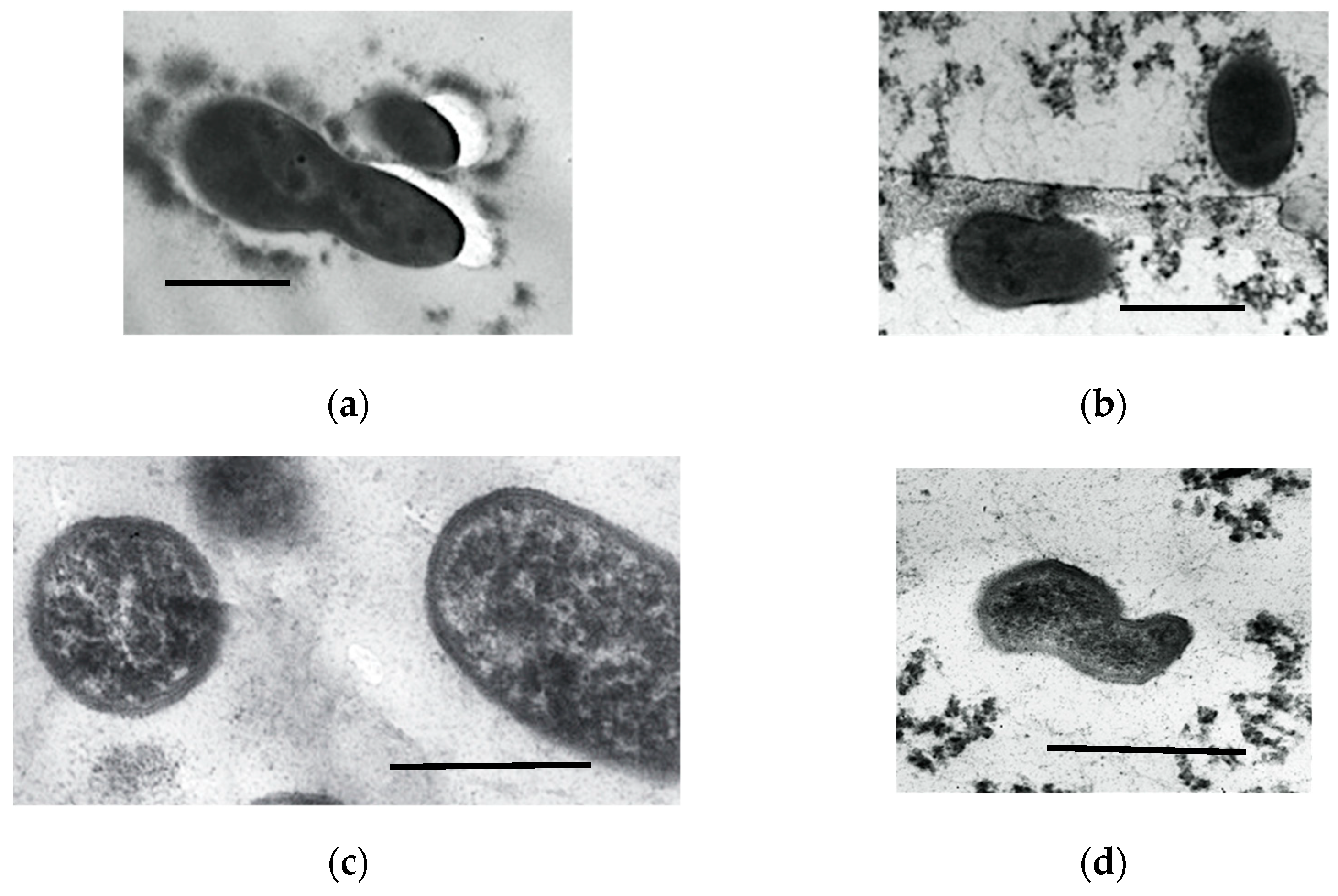
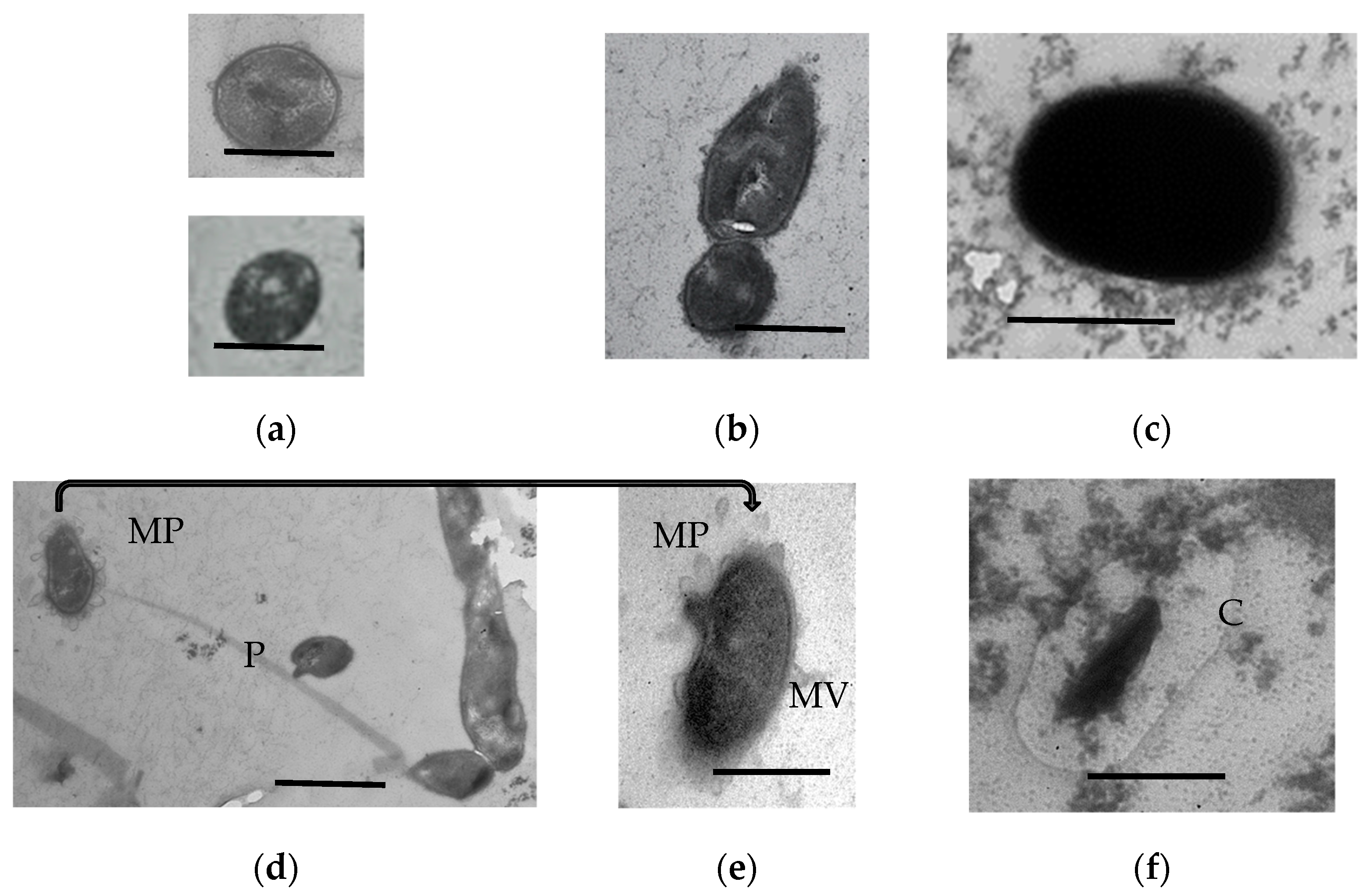
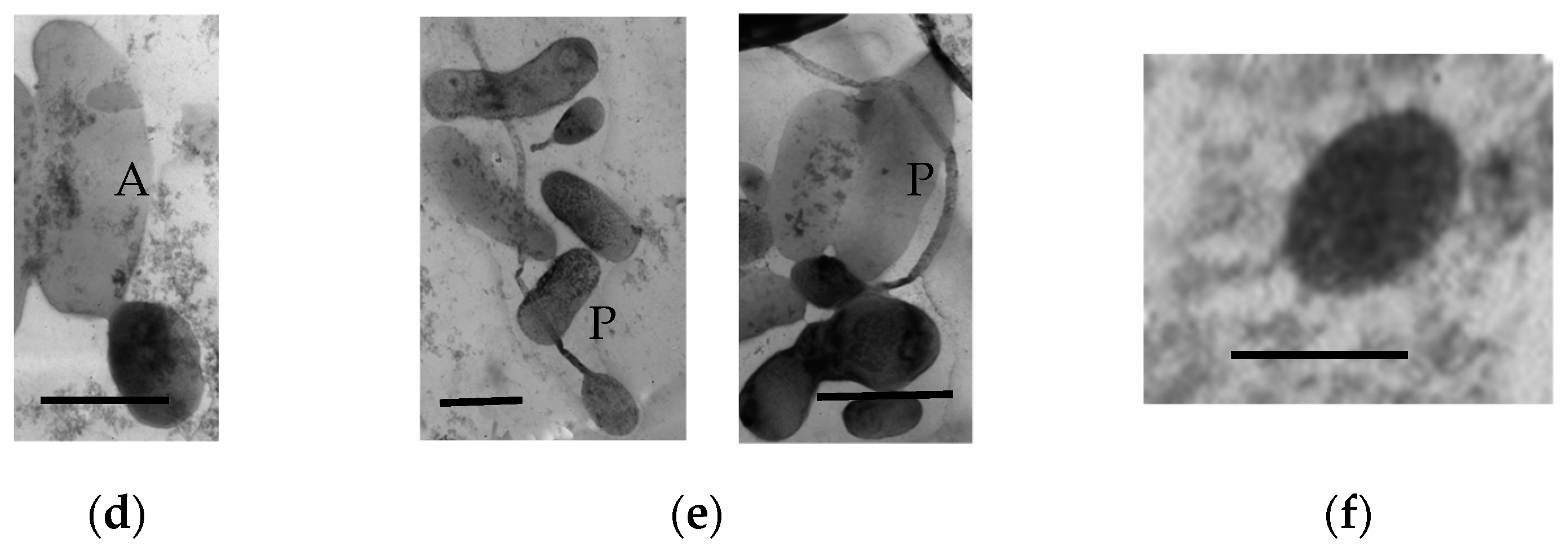
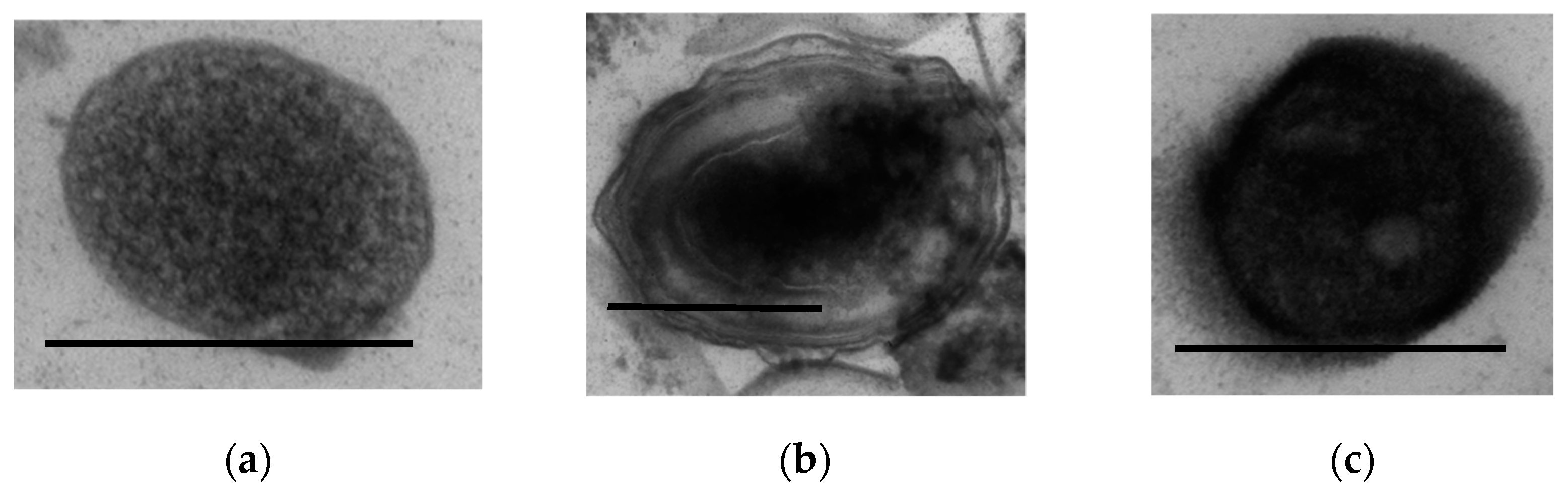
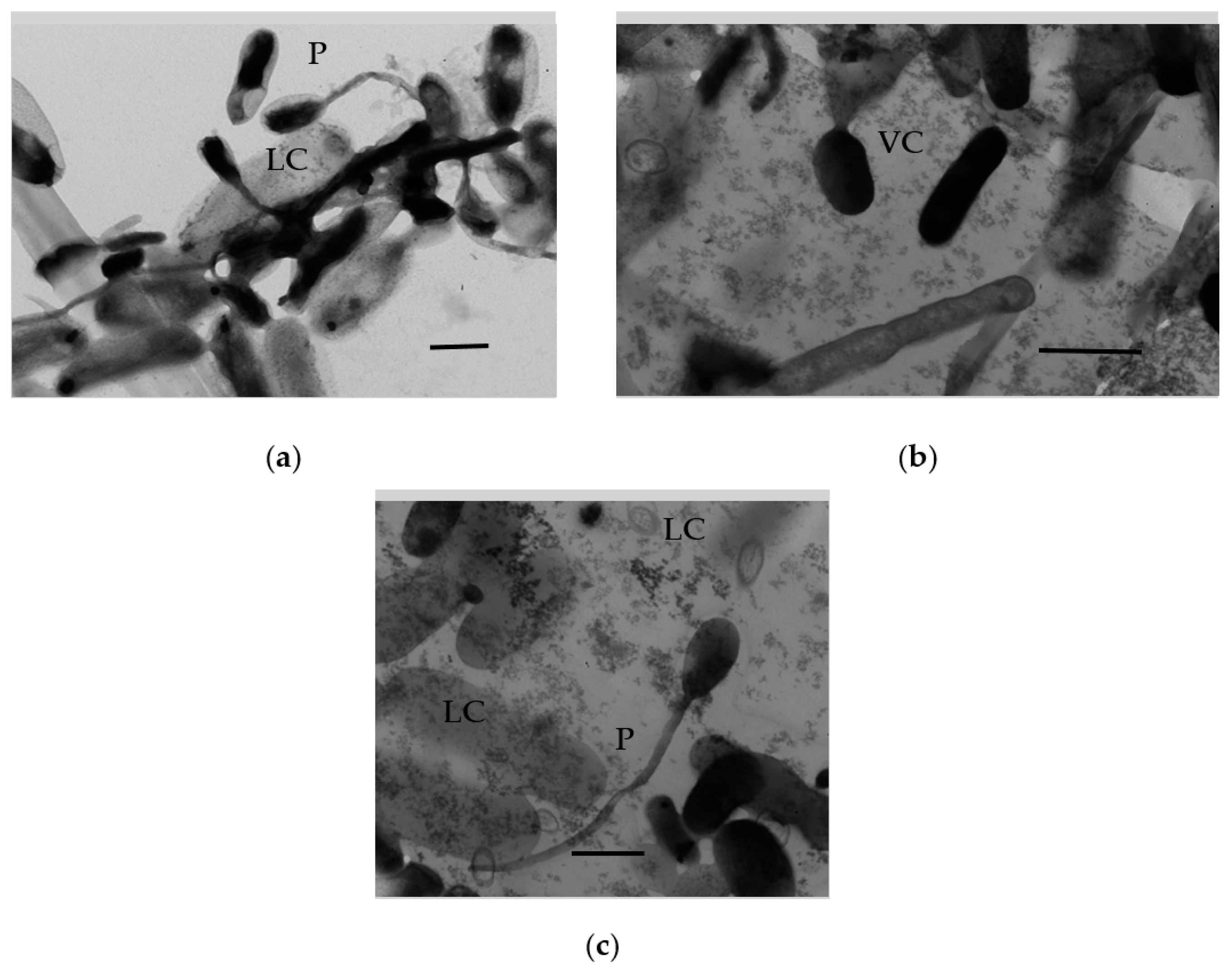
3.1.9. Ultrastructural Characteristics of the HOB in Long-Stored SHG Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teal, J.M.; Howarth, R.W. Oil spill studies: A review of ecological effects. Environ. Manag. 1984, 8, 27–43. [Google Scholar] [CrossRef]
- Sihag, S.; Pathak, H.; Jaroli, D. Factors affecting the rate of biodegradation of polyaromatic hydrocarbons. Int. J. Pure Appl. Biosci. 2014, 2, 185–202. [Google Scholar]
- Das, N.; Chandran, P. Microbial degradation of petroleum hydrocarbon contaminants: An overview. Biotechnol. Res. Int. 2011, 2011, 941810. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, W.; Tian, S.; Wang, W.; Qi, Q.; Jiang, P.; Gao, X.; Li, F.; Li, H.; Yu, H. Petroleum Hydrocarbon-Degrading Bacteria for the Remediation of Oil Pollution Under Aerobic Conditions: A Perspective Analysis. Front. Microbiol. 2018, 9, 2885. [Google Scholar] [CrossRef]
- Krivoruchko, A.; Kuyukina, M.; Ivshina, I. Advanced Rhodococcus Biocatalysts for Environmental Biotechnologies. Catalysts 2019, 9, 236. [Google Scholar] [CrossRef]
- Nikolaev, Y.A.; Borzenkov, I.A.; Demkina, E.V.; Loiko, N.G.; Kanapatsky, T.A.; Perminova, I.V.; Khreptugova, A.N.; Grigoryeva, N.V.; Bliznets, I.V.; Manucharova, N.A.; et al. New biocomposite materials including hydrocarbon-oxidizing microorganisms and their potential for degradation of petroleum products. Microbiology 2021, 90, 692–705. [Google Scholar] [CrossRef]
- Naloka, K.; Jaroonrunganan, J.; Woratecha, N.; Khondee, N.; Nojiri, H.; Pinyakong, O. Physiological changes in Rhodococcus ruber S103 immobilized on biobooms using low-cost media enhance stress tolerance and crude oil-degrading activity. Sci. Rep. 2022, 12, 10474. [Google Scholar] [CrossRef]
- Fu, X.; Zhang, Q.; Gao, Y.; Wu, Y.; Xiao, X.; Li, L.; Xue, J.; Liu, B. Degradation potential of petroleum hydrocarbon-degrading bacteria immobilized on different carriers in marine environment. Pet. Sci. Technol. 2019, 37, 1417–1424. [Google Scholar] [CrossRef]
- Nedovic, V.; Willaert, R. Fundamentals of Cell Immobilization Biotechnology; Springer Science & Business Media: Berlin, Germany, 2013; Volume 8A, p. 550. [Google Scholar]
- Ge, X.; Yang, L.; Xu, J. Cell Immobilization: Fundamentals, Technologies, and Applications. Ind. Biotechnol. 2016, 7, 205–235. [Google Scholar] [CrossRef]
- Su, Q.; Yu, J.; Fang, K.; Dong, P.; Li, Z.; Zhang, W.; Liu, M.; Xiang, L.; Cai, J. Microbial Removal of Petroleum Hydrocarbons from Contaminated Soil under Arsenic Stress. Toxics 2023, 11, 143. [Google Scholar] [CrossRef]
- Akbari, A.; David, C.; Abdul Rahim, A.; Ghoshal, S. Salt selected for hydrocarbon-degrading bacteria and enhanced hydrocarbon biodegradation in slurry bioreactors. Water Res. 2021, 202, 117424. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Song, X.; Ding, D.; Wang, Q.; Zhang, Z.; Tang, Z. Bioremediation of PAHs and heavy metals co-contaminated soils: Challenges and enhancement strategies. Environ. Pollut. 2022, 295, 118686. [Google Scholar] [CrossRef]
- Nikolaev, Y.A.; Demkina, E.V.; Borzenkov, I.A.; Ivanova, A.E.; Kanapatsky, T.A.; Konstantinov, A.I.; Volikov, A.B.; Perminova, I.V.; El-Registan, G.I. Role of the Structure of Humic Substances in Increasing Bacterial Survival. Open. Access J. Microbiol. Biotechnol. 2020, 5, 4. [Google Scholar] [CrossRef]
- Perminova, I.V.; Ponomarenko, S.A.; Volikov, A.B. Humic Silanol Derivatives: Method of Production and Method of Application. RU Patent 2,530,024, 10 October 2014. [Google Scholar]
- Volikov, A.B.; Ponomarenko, S.A.; Gutsche, A.; Nirschl, H.; Hatfield, K.; Perminova, I.V. Targeted design of water-based humic substances-silsesquioxane soft materials for nature-inspired remedial. RSC Adv. 2016, 6, 48222–48230. [Google Scholar] [CrossRef]
- Zajic, J.E.; Guignard, H.; Gerson, D.F. Emulsifying and surface active agents from Corynebacterium hydrocarboclastus. Biotech. Bioeng. 1977, 19, 1285–1301. [Google Scholar] [CrossRef] [PubMed]
- Mulyukin, A.L.; Suzina, N.E.; Melnikov, V.G.; Galchenko, V.F.; El-Registan, G.I. Dormant state and phenotypic variability of Staphylococcus aureus and Corynebacterium pseudodiphtheriticum. Microbiology 2014, 83, 149–159. [Google Scholar] [CrossRef]
- Solyanikova, I.P.; Suzina, N.E.; Egozarjan, N.S.; Polivtseva, V.N.; Mulyukin, A.L.; Egorova, D.O.; El-Registan, G.I.; Golovleva, L.A. Structural and functional rearrangements in the cells of actinobacteria Microbacterium foliorum BN52 during transition from vegetative growth to a dormant state and during germination of dormant forms. Microbiology 2017, 86, 476–486. [Google Scholar] [CrossRef]
- Solyanikova, I.P.; Suzina, N.E.; Egozarjan, N.S.; Polivtseva, V.N.; Prisyazhnaya, N.V.; Golovleva, L.A.; El-Registan, G.I.; Mulyukin, A.L. The response of soil Arthrobacter agilis lush13 to changing conditions: Transition between vegetative and dormant state. J. Environ. Sci. Health. Part B Pestic. Food Contam. Agric. Wastes 2017, 52, 745–751. [Google Scholar] [CrossRef]
- Schooling, S.R.; Beveridge, T.J. Membrane vesicles: An overlooked component of the matrices of biofilms. J. Bacteriol. 2006, 88, 5945–5957. [Google Scholar] [CrossRef]
- Smirnova, T.A.; Didenko, L.V.; Tiganova, I.G.; Andreevskaya, S.G.; Alekseeva, N.V.; Stepanova, T.V.; Romanova, Y.M. Study of the Structures of Biofilms Formed by Salmonella typhimurium Bacteria on Abiotic Surfaces by the Methods of Light and Transmission Electron Microscopy. Appl. Biochem. Microbiol. 2010, 46, 706–711. [Google Scholar] [CrossRef]
- Zhurina, M.V.; Kostrikina, N.A.; Parshina, E.Y.; Strelkova, E.A.; Yusipovich, A.I.; Maksimov, G.V.; Plakunov, V.K. Visualization of the extracellular polymeric matrix of Chromobacteriumviolaceum biofilms by microscopic methods. Microbiology 2013, 82, 517–524. [Google Scholar] [CrossRef]
- Vitse, J.; Devreese, B. The Contribution of Membrane Vesicles to Bacterial Pathogenicity in Cystic Fibrosis Infections and Healthcare Associated Pneumonia. Front. Microbiol. 2020, 11, 630. [Google Scholar] [CrossRef] [PubMed]
- Nikolaev, Y.; Yushina, Y.; Mardanov, A.; Gruzdev, E.; Tikhonova, E.; El-Registan, G.; Beletskiy, A.; Semenova, A.; Zaiko, E.; Bataeva, D.; et al. Microbial Biofilms at Meat-Processing Plant as Possible Places of Bacteria Survival. Microorganisms 2022, 10, 1583. [Google Scholar] [CrossRef] [PubMed]
- Pajic-Lijakovic, I.; Milivojevic, M.; Levicb, S.; Trifkovic, K.; Stevanovic-Dajic, Z.; Radosevic, R.; Nedovicb, V.; Bugarski, B. Matrix resistance stress: A key parameter for immobilized cell growth regulation. Process Biochem. 2017, 52, 30–43. [Google Scholar] [CrossRef]
- Lamboley, L.; St-Gelais, D.; Champagne, C.P.; Lamoureux, M. Growth and morphology of thermophilic dairy starters in alginate beads. J. Gen. Appl. Microbiol. 2003, 49, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Mater, D.D.G.; Craynest, M.; Barbotin, J.-N.; Truffaut, N.; Thomas, D. Bacterial conjugation within k-carraginan gel beads: Biotic and abiotic factors affecting plasmid transfer. Immobil. Cells Basics Appl. 1996, 11, 444–451. [Google Scholar] [CrossRef]
- Barbotin, J.N. Plasmid Stability in Immobilized Cells. In Immobilized Cells; Wijffels, R.H., Ed.; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar] [CrossRef]
- Buitelaar, R.M.; Bucke, C.; Tramper, J.; Wijffels, R.H. Immobilized Cells: Basics and Applications; Elsevier: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Park, S.; Kim, K.S.; Kim, J.-T.; Kang, D.; Sung, K. Effects of humic acid on phytodegradation of petroleum hydrocarbons in soil simultaneously contaminated with heavy metals. J. Environ. Sci. 2011, 23, 2034–2041. [Google Scholar] [CrossRef]
- Kuráň, P.; Trögl, J.; Nováková, J.; Pilařová, V.; Dáňová, P.; Pavlorková, J.; Kozler, J.; Novák, F.; Popelka, J. Biodegradation of spilled diesel fuel in agricultural soil: Effect of humates, zeolite, and bioaugmentation. Sci. World J. 2014, 2014, 642427. [Google Scholar] [CrossRef]
- Almiron, M.; Link, A.J.; Furlong, D.; Kolter, R. A novel DNA-binding protein with regulatory and protective roles in starved Escherichia coli. Genes. Dev. 1992, 6, 2646–2654. [Google Scholar] [CrossRef]
- Calhoun, L.N.; Kwon, Y.M. Structure, function and regulation of the DNA-binding protein Dps and its role in acid and oxidative stress resistance in Escherichia coli: A review. J. Appl. Microbiol. 2011, 110, 375–386. [Google Scholar] [CrossRef]
- Janissen, R.; Arens, M.M.A.; Vtyurina, N.N.; Rivai, Z.; Sunday, N.D.; Eslami-Mossallam, B.; Gritsenko, A.A.; Laan, L.; de Ridder, D.; Artsimovitch, I.; et al. Global DNA Compaction in Stationary-Phase Bacteria Does Not Affect Transcription. Cell 2018, 174, 1188–1199. [Google Scholar] [CrossRef] [PubMed]
- Frenkiel-Krispin, D.; Ben-Avraham, I.; Englander, J.; Shimoni, E.; Wolf, S.G.; Minsky, A. Nucleoid restructuring in stationary-state bacteria. Mol. Microbiol. 2004, 51, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Balaban, N.Q.; Merrin, J.; Chait, R.; Kowalik, L.; Leibler, S. Bacterial persistence as a phenotypic switch. Science 2004, 305, 1622–1625. [Google Scholar] [CrossRef]
- Balaban, N.Q.; Helaine, S.; Lewis, K.; Ackermann, M.; Aldridge, B.; Andersson, D.I.; Brynildsen, M.P.; Bumann, D.; Camilli, A.; Collins, J.J.; et al. Definitions and guidelines for research on antibiotic persistence. Nat. Rev. Microbiol. 2019, 17, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Mulyukin, A.L.; Kozlova, A.N.; Sorokin, V.V.; El-Registan, G.I.; Suzina, N.E.; Cherdyntseva, T.A.; Kotova, I.B.; Gaponov, A.M.; Tutelyan, A.V. Surviving forms in antibiotic-treated Pseudomonas aeruginosa. Microbiology 2015, 84, 751–763. [Google Scholar] [CrossRef]
- Loiko, N.G.; Kozlova, A.N.; Nikolaev, Y.A.; Gaponov, A.M.; Tuteljan, A.V.; El-Registan, G.I. Effect of stress on antibiotic tolerant cell formation in Escherichia coli. Microbiology 2015, 84, 595–609. [Google Scholar] [CrossRef]
- Fuqua, W.C.; Winans, S.C.; Greenberg, E.P. Quorum sensing in bacteria: The LuxR-LuxI family of cell density-responsive transcriptional regulators. J. Bacteriol. 1994, 176, 269–275. [Google Scholar] [CrossRef]
- El-Registan, G.I.; Mulyukin, A.L.; Nikolaev, Y.A.; Suzina, N.E.; Galchenko, V.F.; Duda, V.I. Adaptogenic functions of extracellular autoregulators of microorganisms. Microbiology 2006, 75, 446–456. [Google Scholar] [CrossRef]
- Tuomanen, E.; Tomasz, A. Induction of autolysis in nongrowing Escherichia coli. J. Bacteriol. 1986, 167, 1077–1080. [Google Scholar] [CrossRef]
- Podlesek, Z.; Žgur Bertok, D. The DNA Damage Inducible SOS Response Is a Key Player in the Generation of Bacterial Persister Cells and Population Wide Tolerance. Front. Microbiol. 2020, 4, 1785. [Google Scholar] [CrossRef]
- Ksiazek, K. Let’s stop overlooking bacterial aging. Biogerontology 2010, 11, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Zur, J.; Wojcieszynska, D.; Guzik, U. Metabolic Responses of Bacterial Cells to Immobilization. Molecules 2016, 21, 958. [Google Scholar] [CrossRef] [PubMed]
- Mulyukin, A.L.; Pogorelova, A.Y.; El-Registan, G.I.; Suzina, N.E.; Duda, V.I.; Antonyuk, L.P. Diverse morphological types of dormant cells and conditions for their formation in Azospirillum brasilense. Microbiology 2009, 78, 33–41. [Google Scholar] [CrossRef]
- van der Woude, M.W.; Baumler, A.J. Phase and Antigenic Variation in Bacteria. Clin. Microbiol. Rev. 2004, 17, 581–611. [Google Scholar] [CrossRef] [PubMed]
- Magdanova, L.A.; Golyasnaya, N.V. Heterogeneity as an adaptive property of a bacterial population. Microbiology 2013, 82, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Phillips, Z.N.; Tram, G.; Seib, K.L.; Atack, J.M. Phase-variable bacterial loci: How bacteria gamble to maximise fitness in changing environments. Biochem. Soc. Trans. 2019, 47, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Loiko, N.G.; Kryazhevskikh, N.A.; Demkina, E.V.; Kozlova, A.N.; Galchenko, V.F.; El-Registan, G.I.; Suzina, N.E.; Muratova, A.Y.; Turkovskaya, O.V. Resting Forms of Sinorhizobium meliloti. Microbiology 2011, 80, 472–482. [Google Scholar] [CrossRef]
- MacArthur, R.H.; Wilson, E.O. The Theory of Island Biogeography; Princeton University Press: Princeton, NJ, USA, 1967; ISBN 978-0691088365. [Google Scholar]
- Pianka, E.R. On r- and K-Selection. Am. Naturalist. 1970, 104, 592–597. [Google Scholar] [CrossRef]
- Andrews, J.H.; Harris, R.F. R-and K-Selection and Microbial Ecology. Adv. Microb. Ecol. 1986, 9, 99–147. [Google Scholar] [CrossRef]
- Lempp, M.; Lubrano, P.; Bange, G.; Link, H. Metabolism of non-growing bacteria. Biol. Chem. 2020, 401, 1479–1485. [Google Scholar] [CrossRef]
- Jaishankar, J.; Srivastava, P. Molecular Basis of Stationary Phase Survival and Applications. Front. Microbiol. 2017, 8, 2000. [Google Scholar] [CrossRef] [PubMed]
- Rittershaus, E.S.C.; Baek, S.-H.; Krieger, I.V.; Nelson, S.J.; Cheng, Y.-S.; Nambi, S.; Baker, R.E.; Leszyk, J.D.; Shaffer, S.A.; Sacchettini, J.C.; et al. A Lysine Acetyltransferase Contributes to the Metabolic Adaptation to Hypoxia in Mycobacterium tuberculosis. Cell Chem. Biol. 2018, 25, 1495–1505. [Google Scholar] [CrossRef] [PubMed]
- Pletnev, P.; Osterman, I.; Sergiev, P.; Bogdanov, A.; Survival, O. Guide: Escherichia coli in the Stationary Phase. Acta Nat. 2015, 7, 22–33. [Google Scholar] [CrossRef]
- Mailloux, R.J.; Lemire, J.; Appanna, V.D. Metabolic networks to combat oxidative stress in Pseudomonas fluorescens. Antonie Leeuwenhoek 2011, 99, 433–442. [Google Scholar] [CrossRef] [PubMed]

| ST, mN/m | IT, mN/m | E24, % | |
|---|---|---|---|
| Control (SHG without cells) | 58.0 | 28.9 | 0 |
| A. seifertii, 1:20 | 55.4 | 32.4 | 0 |
| A. seifertii, undiluted | 50.3 | 18.2 | 25 |
| H2O | 59.2 | 29.2 | 0 |
| Shg-Immobilized Culture | Storage in SHG, Months | Acetate, mM | Ethanol, mM | CO2, % |
|---|---|---|---|---|
| Control (SHG without cells) | 0 and 18 | 225 | 213 | 0 |
| R. qingshengii | 6 | 91 | 184 | 63 |
| 9 | 2 | 4 | 58 | |
| A. seifertii | 9 | 32 | 153 | 67 |
| 18 | 7 | 155 | 100 | |
| P. extremoaustralis | 18 | 5 | 2 | 71 |
| Cell Type | NaCl 3% | NaCl 6% | NaCl 9% |
|---|---|---|---|
| A. seifertii | |||
| Stationary | 0.1% | 0.0% | 0.0% |
| DF | 1.5% | 0.0% | 0.0% |
| R. qingshengii | |||
| Stationary | 100% | 100% Growth on day 21 | 0.1% |
| DF | 100% | 100% Growth on day 21 | 10.0% |
| Cell Type | 10−4 M CuSO4∙5H2O | 10−3 M CuSO4∙5H2O | 2 × 10−3 M CuSO4∙5H2O |
|---|---|---|---|
| A. seifertii | |||
| Stationary | 100% | 4.9% | 0.03% |
| DF | 100% | 17.5% | 0.70% |
| R. qingshengii | |||
| Stationary | 100% | 10% | 0.00% |
| DF | 100% | 100% | 1.00% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolaev, Y.A.; Demkina, E.V.; Ilicheva, E.A.; Kanapatskiy, T.A.; Borzenkov, I.A.; Ivanova, A.E.; Tikhonova, E.N.; Sokolova, D.S.; Ruzhitsky, A.O.; El-Registan, G.I. Ways of Long-Term Survival of Hydrocarbon-Oxidizing Bacteria in a New Biocomposite Material—Silanol-Humate Gel. Microorganisms 2023, 11, 1133. https://doi.org/10.3390/microorganisms11051133
Nikolaev YA, Demkina EV, Ilicheva EA, Kanapatskiy TA, Borzenkov IA, Ivanova AE, Tikhonova EN, Sokolova DS, Ruzhitsky AO, El-Registan GI. Ways of Long-Term Survival of Hydrocarbon-Oxidizing Bacteria in a New Biocomposite Material—Silanol-Humate Gel. Microorganisms. 2023; 11(5):1133. https://doi.org/10.3390/microorganisms11051133
Chicago/Turabian StyleNikolaev, Yury A., Elena V. Demkina, Ekaterina A. Ilicheva, Timur A. Kanapatskiy, Igor A. Borzenkov, Anna E. Ivanova, Ekaterina N. Tikhonova, Diyana S. Sokolova, Alexander O. Ruzhitsky, and Galina I. El-Registan. 2023. "Ways of Long-Term Survival of Hydrocarbon-Oxidizing Bacteria in a New Biocomposite Material—Silanol-Humate Gel" Microorganisms 11, no. 5: 1133. https://doi.org/10.3390/microorganisms11051133
APA StyleNikolaev, Y. A., Demkina, E. V., Ilicheva, E. A., Kanapatskiy, T. A., Borzenkov, I. A., Ivanova, A. E., Tikhonova, E. N., Sokolova, D. S., Ruzhitsky, A. O., & El-Registan, G. I. (2023). Ways of Long-Term Survival of Hydrocarbon-Oxidizing Bacteria in a New Biocomposite Material—Silanol-Humate Gel. Microorganisms, 11(5), 1133. https://doi.org/10.3390/microorganisms11051133






