The Interplay between Candida albicans, Vaginal Mucosa, Host Immunity and Resident Microbiota in Health and Disease: An Overview and Future Perspectives
Abstract
1. Introduction
2. Role of Vaginal Epithelial Cells in the Pathogenesis of VVC
2.1. Vaginal Epithelial Cells: More Than a Mere Barrier
2.2. Response of VECs to C. albicans
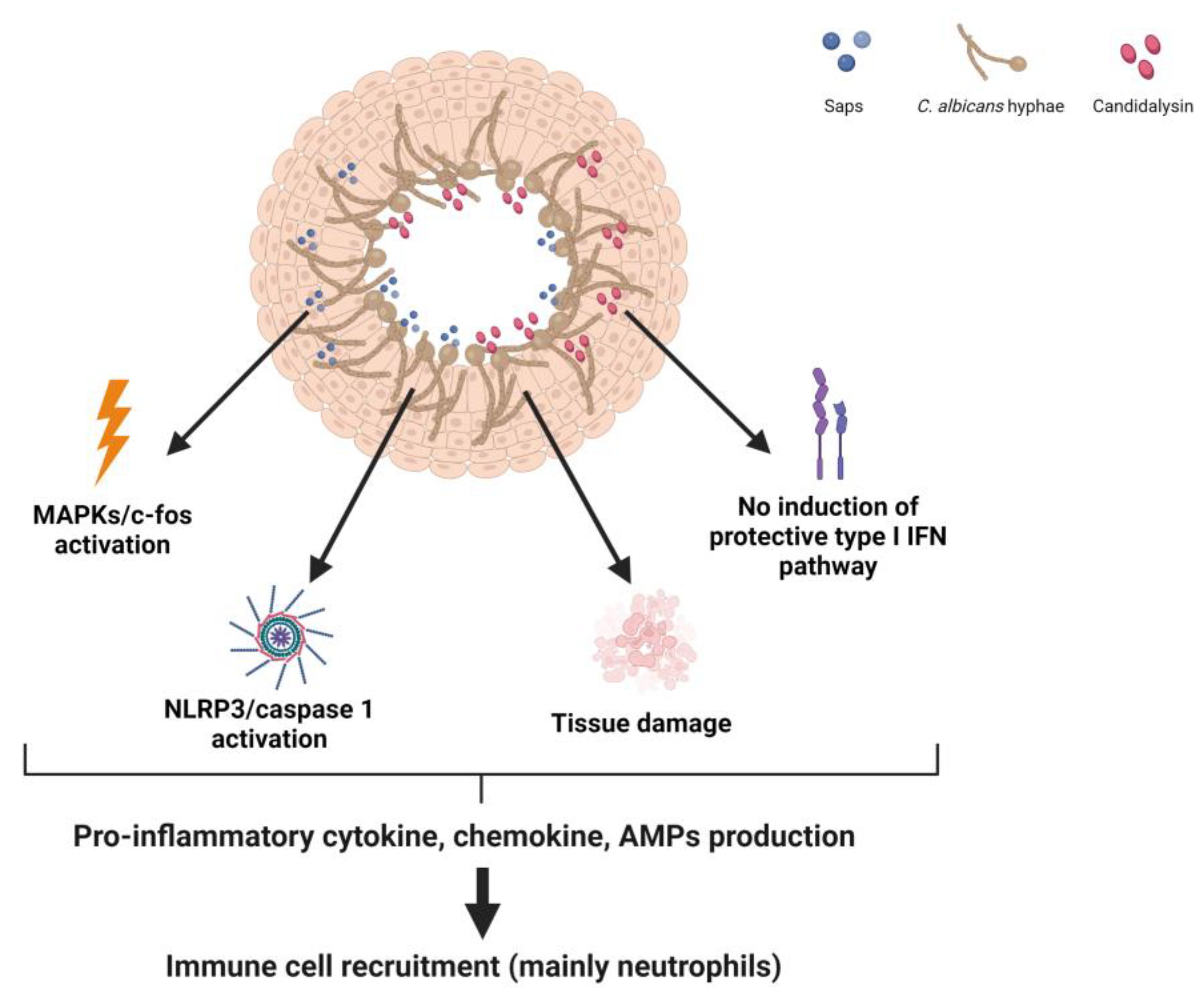
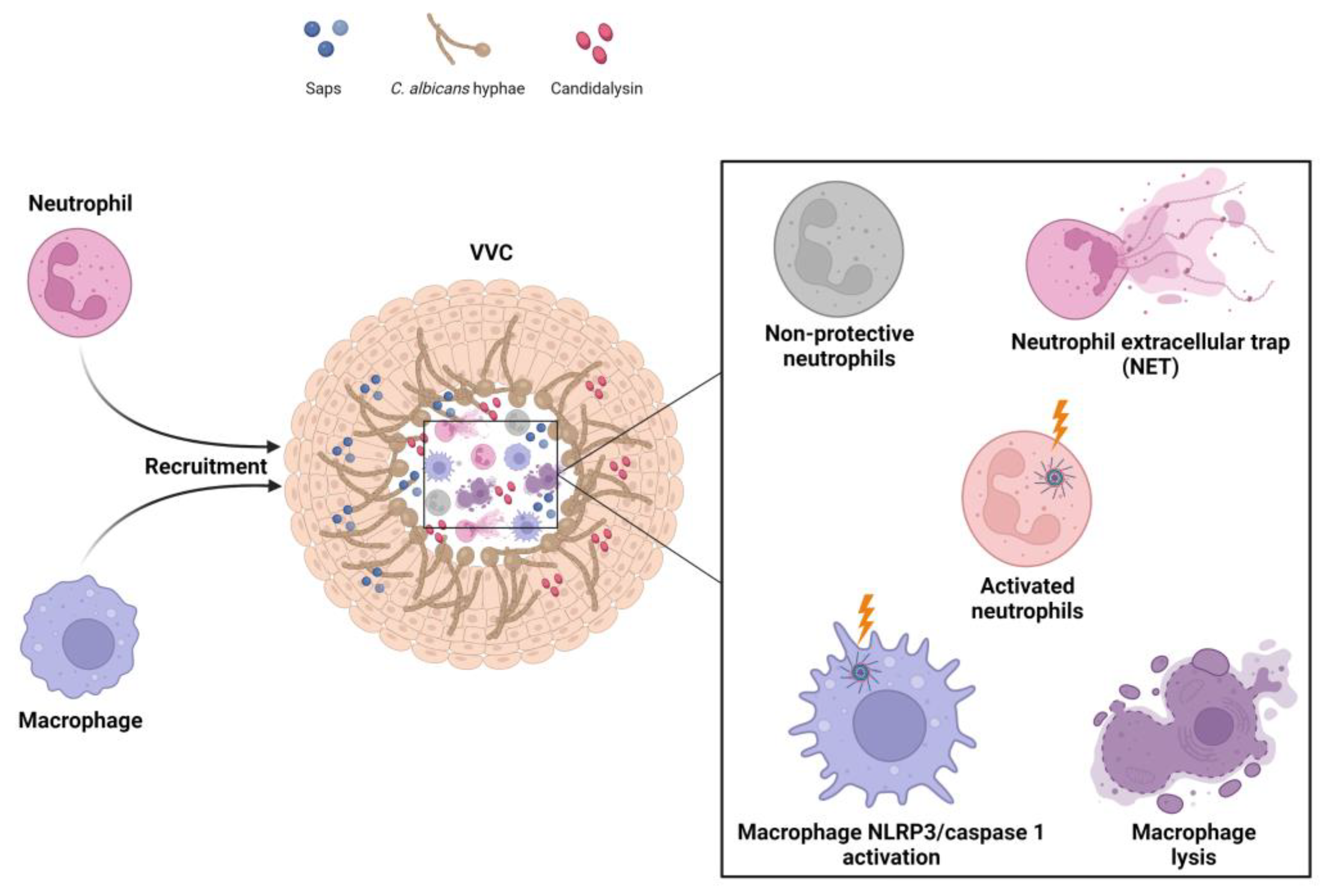
3. Host Immune Response in VVC Pathogenicity
3.1. Vaginal Host Immune Response to C. albicans
3.2. NLRP3 Inflammasome Dysregulation as a Key Factor in the Immunopathogenesis of VVC
4. Relationship between Vaginal Microbial Communities and C. albicans
4.1. Vaginal Microbial Communities in Healthy Women
4.2. Impact of Vaginal Dysbiosis on the Onset of VVC
5. C. albicans-Related Key Virulence Factors in the Pathogenesis of VVC/RVVC
5.1. Morphological Transition of Candida
5.2. Secretory Factors
5.3. Biofilm Formation
5.4. Potential Role of β-Glucan Unmasking in the Pathogenesis of VVC
6. Potential Therapeutic Strategies for the Prevention and/or Treatment of VVC
7. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Richardson, J.P. Candida albicans: A Major Fungal Pathogen of Humans. Pathogens 2022, 11, 459. [Google Scholar] [CrossRef]
- Lopes, J.P.; Lionakis, M.S. Pathogenesis and virulence of Candida albicans. Virulence 2022, 13, 89–121. [Google Scholar] [CrossRef] [PubMed]
- d’Enfert, C.; Kaune, A.K.; Alaban, L.R.; Chakraborty, S.; Cole, N.; Delavy, M.; Kosmala, D.; Marsaux, B.; Frois-Martins, R.; Morelli, M.; et al. The impact of the Fungus-Host-Microbiota interplay upon Candida albicans infections: Current knowledge and new perspectives. FEMS Microbiol. Rev. 2021, 45, fuaa060. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.D. Vaginitis. N. Engl. J. Med. 1997, 337, 1896–1903. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Kneale, M.; Sobel, J.D.; Rautemaa-Richardson, R. Global burden of recurrent vulvovaginal candidiasis: A systematic review. Lancet Infect Dis 2018, 18, e339–e347. [Google Scholar] [CrossRef]
- Rosentul, D.C.; Delsing, C.E.; Jaeger, M.; Plantinga, T.S.; Oosting, M.; Costantini, I.; Venselaar, H.; Joosten, L.A.; van der Meer, J.W.; Dupont, B.; et al. Gene polymorphisms in pattern recognition receptors and susceptibility to idiopathic recurrent vulvovaginal candidiasis. Front. Microbiol. 2014, 5, 483. [Google Scholar] [CrossRef]
- Jaeger, M.; Carvalho, A.; Cunha, C.; Plantinga, T.S.; van de Veerdonk, F.; Puccetti, M.; Galosi, C.; Joosten, L.A.; Dupont, B.; Kullberg, B.J.; et al. Association of a variable number tandem repeat in the NLRP3 gene in women with susceptibility to RVVC. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 797–801. [Google Scholar] [CrossRef]
- Camilli, G.; Griffiths, J.S.; Ho, J.; Richardson, J.P.; Naglik, J.R. Some like it hot: Candida activation of inflammasomes. PLoS Pathog. 2020, 16, e1008975. [Google Scholar] [CrossRef]
- Rodriguez-Cerdeira, C.; Martinez-Herrera, E.; Carnero-Gregorio, M.; Lopez-Barcenas, A.; Fabbrocini, G.; Fida, M.; El-Samahy, M.; Gonzalez-Cespon, J.L. Pathogenesis and Clinical Relevance of Candida Biofilms in Vulvovaginal Candidiasis. Front. Microbiol. 2020, 11, 544480. [Google Scholar] [CrossRef]
- Ardizzoni, A.; Wheeler, R.T.; Pericolini, E. It Takes Two to Tango: How a Dysregulation of the Innate Immunity, Coupled With Candida Virulence, Triggers VVC Onset. Front. Microbiol. 2021, 12, 692491. [Google Scholar] [CrossRef]
- Zheng, N.X.; Wang, Y.; Hu, D.D.; Yan, L.; Jiang, Y.Y. The role of pattern recognition receptors in the innate recognition of Candida albicans. Virulence 2015, 6, 347–361. [Google Scholar] [CrossRef]
- Fazeli, A.; Bruce, C.; Anumba, D.O. Characterization of Toll-like receptors in the female reproductive tract in humans. Hum. Reprod. 2005, 20, 1372–1378. [Google Scholar] [CrossRef]
- Linhares, I.M.; Sisti, G.; Minis, E.; de Freitas, G.B.; Moron, A.F.; Witkin, S.S. Contribution of Epithelial Cells to Defense Mechanisms in the Human Vagina. Curr. Infect. Dis. Rep. 2019, 21, 30. [Google Scholar] [CrossRef]
- Babula, O.; Lazdane, G.; Kroica, J.; Ledger, W.J.; Witkin, S.S. Relation between recurrent vulvovaginal candidiasis, vaginal concentrations of mannose-binding lectin, and a mannose-binding lectin gene polymorphism in Latvian women. Clin. Infect. Dis. 2003, 37, 733–737. [Google Scholar] [CrossRef]
- Amabebe, E.; Anumba, D.O.C. The Vaginal Microenvironment: The Physiologic Role of Lactobacilli. Front. Med. 2018, 5, 181. [Google Scholar] [CrossRef]
- Netea, M.G.; Joosten, L.A.; van der Meer, J.W.; Kullberg, B.J.; van de Veerdonk, F.L. Immune defence against Candida fungal infections. Nat. Rev. Immunol. 2015, 15, 630–642. [Google Scholar] [CrossRef]
- Moyes, D.L.; Runglall, M.; Murciano, C.; Shen, C.; Nayar, D.; Thavaraj, S.; Kohli, A.; Islam, A.; Mora-Montes, H.; Challacombe, S.J.; et al. A biphasic innate immune MAPK response discriminates between the yeast and hyphal forms of Candida albicans in epithelial cells. Cell Host Microbe 2010, 8, 225–235. [Google Scholar] [CrossRef]
- Moyes, D.L.; Murciano, C.; Runglall, M.; Islam, A.; Thavaraj, S.; Naglik, J.R. Candida albicans yeast and hyphae are discriminated by MAPK signaling in vaginal epithelial cells. PLoS ONE 2011, 6, e26580. [Google Scholar] [CrossRef]
- Moyes, D.L.; Richardson, J.P.; Naglik, J.R. Candida albicans-epithelial interactions and pathogenicity mechanisms: Scratching the surface. Virulence 2015, 6, 338–346. [Google Scholar] [CrossRef]
- Nikou, S.A.; Kichik, N.; Brown, R.; Ponde, N.O.; Ho, J.; Naglik, J.R.; Richardson, J.P. Candida albicans Interactions with Mucosal Surfaces during Health and Disease. Pathogens 2019, 8, 53. [Google Scholar] [CrossRef]
- Roselletti, E.; Perito, S.; Sabbatini, S.; Monari, C.; Vecchiarelli, A. Vaginal Epithelial Cells Discriminate Between Yeast and Hyphae of Candida albicans in Women Who Are Colonized or Have Vaginal Candidiasis. J. Infect. Dis. 2019, 220, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Peng, J.; Li, D.; Mei, H.; Yu, Y.; Li, X.; She, X.; Liu, W. Divergent EGFR/MAPK-Mediated Immune Responses to Clinical Candida Pathogens in Vulvovaginal Candidiasis. Front. Immunol. 2022, 13, 894069. [Google Scholar] [CrossRef] [PubMed]
- Green, C.B.; Cheng, G.; Chandra, J.; Mukherjee, P.; Ghannoum, M.A.; Hoyer, L.L. RT-PCR detection of Candida albicans ALS gene expression in the reconstituted human epithelium (RHE) model of oral candidiasis and in model biofilms. Microbiology 2004, 150, 267–275. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cheng, G.; Wozniak, K.; Wallig, M.A.; Fidel, P.L., Jr.; Trupin, S.R.; Hoyer, L.L. Comparison between Candida albicans agglutinin-like sequence gene expression patterns in human clinical specimens and models of vaginal candidiasis. Infect. Immun. 2005, 73, 1656–1663. [Google Scholar] [CrossRef]
- Richardson, J.P.; Ho, J.; Naglik, J.R. Candida-Epithelial Interactions. J. Fungi 2018, 4, 22. [Google Scholar] [CrossRef]
- Liu, Y.; Filler, S.G. Candida albicans Als3, a multifunctional adhesin and invasin. Eukaryot. Cell 2011, 10, 168–173. [Google Scholar] [CrossRef]
- Bu, Q.R.; Bao, M.Y.; Yang, Y.; Wang, T.M.; Wang, C.Z. Targeting Virulence Factors of Candida albicans with Natural Products. Foods 2022, 11, 2951. [Google Scholar] [CrossRef]
- Phan, Q.T.; Myers, C.L.; Fu, Y.; Sheppard, D.C.; Yeaman, M.R.; Welch, W.H.; Ibrahim, A.S.; Edwards, J.E., Jr.; Filler, S.G. Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLoS Biol. 2007, 5, e64. [Google Scholar] [CrossRef]
- Zhu, W.; Phan, Q.T.; Boontheung, P.; Solis, N.V.; Loo, J.A.; Filler, S.G. EGFR and HER2 receptor kinase signaling mediate epithelial cell invasion by Candida albicans during oropharyngeal infection. Proc. Natl. Acad. Sci. USA 2012, 109, 14194–14199. [Google Scholar] [CrossRef]
- Roselletti, E.; Monari, C.; Sabbatini, S.; Perito, S.; Vecchiarelli, A.; Sobel, J.D.; Cassone, A. A Role for Yeast/Pseudohyphal Cells of Candida albicans in the Correlated Expression of NLRP3 Inflammasome Inducers in Women With Acute Vulvovaginal Candidiasis. Front. Microbiol. 2019, 10, 2669. [Google Scholar] [CrossRef]
- Naglik, J.R.; Konig, A.; Hube, B.; Gaffen, S.L. Candida albicans-epithelial interactions and induction of mucosal innate immunity. Curr. Opin. Microbiol. 2017, 40, 104–112. [Google Scholar] [CrossRef]
- Winkle, S.M.; Throop, A.L.; Herbst-Kralovetz, M.M. IL-36gamma Augments Host Defense and Immune Responses in Human Female Reproductive Tract Epithelial Cells. Front. Microbiol. 2016, 7, 955. [Google Scholar] [CrossRef]
- Pellon, A.; Sadeghi Nasab, S.D.; Moyes, D.L. New Insights in Candida albicans Innate Immunity at the Mucosa: Toxins, Epithelium, Metabolism, and Beyond. Front. Cell. Infect. Microbiol. 2020, 10, 81. [Google Scholar] [CrossRef]
- Wang, X.; Yi, P.; Liang, Y. The Role of IL-36 in Infectious Diseases: Potential Target for COVID-19? Front. Immunol. 2021, 12, 662266. [Google Scholar] [CrossRef]
- Moyes, D.L.; Wilson, D.; Richardson, J.P.; Mogavero, S.; Tang, S.X.; Wernecke, J.; Hofs, S.; Gratacap, R.L.; Robbins, J.; Runglall, M.; et al. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature 2016, 532, 64–68. [Google Scholar] [CrossRef]
- Richardson, J.P.; Willems, H.M.E.; Moyes, D.L.; Shoaie, S.; Barker, K.S.; Tan, S.L.; Palmer, G.E.; Hube, B.; Naglik, J.R.; Peters, B.M. Candidalysin Drives Epithelial Signaling, Neutrophil Recruitment, and Immunopathology at the Vaginal Mucosa. Infect. Immun. 2018, 86, e00645-17. [Google Scholar] [CrossRef]
- Richardson, J.P.; Brown, R.; Kichik, N.; Lee, S.; Priest, E.; Mogavero, S.; Maufrais, C.; Wickramasinghe, D.N.; Tsavou, A.; Kotowicz, N.K.; et al. Candidalysins Are a New Family of Cytolytic Fungal Peptide Toxins. mBio 2022, 13, e0351021. [Google Scholar] [CrossRef]
- Bruno, V.M.; Shetty, A.C.; Yano, J.; Fidel, P.L., Jr.; Noverr, M.C.; Peters, B.M. Transcriptomic analysis of vulvovaginal candidiasis identifies a role for the NLRP3 inflammasome. mBio 2015, 6, e00182-15. [Google Scholar] [CrossRef]
- Pericolini, E.; Gabrielli, E.; Amacker, M.; Kasper, L.; Roselletti, E.; Luciano, E.; Sabbatini, S.; Kaeser, M.; Moser, C.; Hube, B.; et al. Secretory Aspartyl Proteinases Cause Vaginitis and Can Mediate Vaginitis Caused by Candida albicans in Mice. mBio 2015, 6, e00724. [Google Scholar] [CrossRef]
- Roselletti, E.; Perito, S.; Gabrielli, E.; Mencacci, A.; Pericolini, E.; Sabbatini, S.; Cassone, A.; Vecchiarelli, A. NLRP3 inflammasome is a key player in human vulvovaginal disease caused by Candida albicans. Sci. Rep. 2017, 7, 17877. [Google Scholar] [CrossRef]
- Gabrielli, E.; Sabbatini, S.; Roselletti, E.; Kasper, L.; Perito, S.; Hube, B.; Cassone, A.; Vecchiarelli, A.; Pericolini, E. In vivo induction of neutrophil chemotaxis by secretory aspartyl proteinases of Candida albicans. Virulence 2016, 7, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.; Peters, B.M.; Noverr, M.C.; Fidel, P.L., Jr. Novel Mechanism behind the Immunopathogenesis of Vulvovaginal Candidiasis: “Neutrophil Anergy”. Infect. Immun. 2018, 86, e00684-17. [Google Scholar] [CrossRef] [PubMed]
- Pekmezovic, M.; Hovhannisyan, H.; Gresnigt, M.S.; Iracane, E.; Oliveira-Pacheco, J.; Siscar-Lewin, S.; Seemann, E.; Qualmann, B.; Kalkreuter, T.; Muller, S.; et al. Candida pathogens induce protective mitochondria-associated type I interferon signalling and a damage-driven response in vaginal epithelial cells. Nat. Microbiol. 2021, 6, 643–657. [Google Scholar] [CrossRef] [PubMed]
- Sala, A.; Ardizzoni, A.; Spaggiari, L.; Vaidya, N.; van der Schaaf, J.; Rizzato, C.; Cermelli, C.; Mogavero, S.; Kruger, T.; Himmel, M.; et al. A New Phenotype in Candida-Epithelial Cell Interaction Distinguishes Colonization- versus Vulvovaginal Candidiasis-Associated Strains. mBio 2023, 14, e0010723. [Google Scholar] [CrossRef] [PubMed]
- Bojang, E.; Ghuman, H.; Kumwenda, P.; Hall, R.A. Immune Sensing of Candida albicans. J. Fungi 2021, 7, 119. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Swidergall, M. Candida albicans at Host Barrier Sites: Pattern Recognition Receptors and Beyond. Pathogens 2019, 8, 40. [Google Scholar] [CrossRef]
- van de Veerdonk, F.L.; Kullberg, B.J.; van der Meer, J.W.; Gow, N.A.; Netea, M.G. Host-microbe interactions: Innate pattern recognition of fungal pathogens. Curr. Opin. Microbiol. 2008, 11, 305–312. [Google Scholar] [CrossRef]
- Kankkunen, P.; Teirila, L.; Rintahaka, J.; Alenius, H.; Wolff, H.; Matikainen, S. (1,3)-beta-glucans activate both dectin-1 and NLRP3 inflammasome in human macrophages. J. Immunol. 2010, 184, 6335–6342. [Google Scholar] [CrossRef]
- Goodridge, H.S.; Underhill, D.M.; Touret, N. Mechanisms of Fc receptor and dectin-1 activation for phagocytosis. Traffic 2012, 13, 1062–1071. [Google Scholar] [CrossRef]
- Drummond, R.A.; Collar, A.L.; Swamydas, M.; Rodriguez, C.A.; Lim, J.K.; Mendez, L.M.; Fink, D.L.; Hsu, A.P.; Zhai, B.; Karauzum, H.; et al. CARD9-Dependent Neutrophil Recruitment Protects against Fungal Invasion of the Central Nervous System. PLoS Pathog. 2015, 11, e1005293. [Google Scholar] [CrossRef]
- Li, D.D.; Jawale, C.V.; Zhou, C.; Lin, L.; Trevejo-Nunez, G.J.; Rahman, S.A.; Mullet, S.J.; Das, J.; Wendell, S.G.; Delgoffe, G.M.; et al. Fungal sensing enhances neutrophil metabolic fitness by regulating antifungal Glut1 activity. Cell Host Microbe 2022, 30, 530–544.e6. [Google Scholar] [CrossRef]
- McGreal, E.P.; Rosas, M.; Brown, G.D.; Zamze, S.; Wong, S.Y.; Gordon, S.; Martinez-Pomares, L.; Taylor, P.R. The carbohydrate-recognition domain of Dectin-2 is a C-type lectin with specificity for high mannose. Glycobiology 2006, 16, 422–430. [Google Scholar] [CrossRef]
- Saijo, S.; Ikeda, S.; Yamabe, K.; Kakuta, S.; Ishigame, H.; Akitsu, A.; Fujikado, N.; Kusaka, T.; Kubo, S.; Chung, S.H.; et al. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity 2010, 32, 681–691. [Google Scholar] [CrossRef]
- Wu, S.Y.; Weng, C.L.; Jheng, M.J.; Kan, H.W.; Hsieh, S.T.; Liu, F.T.; Wu-Hsieh, B.A. Candida albicans triggers NADPH oxidase-independent neutrophil extracellular traps through dectin-2. PLoS Pathog. 2019, 15, e1008096. [Google Scholar] [CrossRef]
- Plantinga, T.S.; Johnson, M.D.; Scott, W.K.; Joosten, L.A.; van der Meer, J.W.; Perfect, J.R.; Kullberg, B.J.; Netea, M.G. Human genetic susceptibility to Candida infections. Med. Mycol. 2012, 50, 785–794. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, L.; Xu, Z.; Zhang, J.; Jiang, Y.Y.; Cao, Y.; Yan, T. Innate immune cell response upon Candida albicans infection. Virulence 2016, 7, 512–526. [Google Scholar] [CrossRef]
- Cheng, S.C.; Joosten, L.A.; Kullberg, B.J.; Netea, M.G. Interplay between Candida albicans and the mammalian innate host defense. Infect. Immun. 2012, 80, 1304–1313. [Google Scholar] [CrossRef]
- Urban, C.F.; Ermert, D.; Schmid, M.; Abu-Abed, U.; Goosmann, C.; Nacken, W.; Brinkmann, V.; Jungblut, P.R.; Zychlinsky, A. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009, 5, e1000639. [Google Scholar] [CrossRef]
- Urban, C.F.; Nett, J.E. Neutrophil extracellular traps in fungal infection. Semin. Cell Dev. Biol. 2019, 89, 47–57. [Google Scholar] [CrossRef]
- Rosales, C. Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types? Front. Physiol. 2018, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Selders, G.S.; Fetz, A.E.; Radic, M.Z.; Bowlin, G.L. An overview of the role of neutrophils in innate immunity, inflammation and host-biomaterial integration. Regen. Biomater. 2017, 4, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Kenno, S.; Perito, S.; Mosci, P.; Vecchiarelli, A.; Monari, C. Autophagy and Reactive Oxygen Species Are Involved in Neutrophil Extracellular Traps Release Induced by C. albicans Morphotypes. Front. Microbiol. 2016, 7, 879. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, F.; Melo, A.; Rivera-Concha, R.; Schulz, M.; Uribe, P.; Fonseca-Salamanca, F.; Ossa, X.; Taubert, A.; Hermosilla, C.; Sanchez, R. High Presence of NETotic Cells and Neutrophil Extracellular Traps in Vaginal Discharges of Women with Vaginitis: An Exploratory Study. Cells 2022, 11, 3185. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.; Palmer, G.E.; Eberle, K.E.; Peters, B.M.; Vogl, T.; McKenzie, A.N.; Fidel, P.L., Jr. Vaginal epithelial cell-derived S100 alarmins induced by Candida albicans via pattern recognition receptor interactions are sufficient but not necessary for the acute neutrophil response during experimental vaginal candidiasis. Infect. Immun. 2014, 82, 783–792. [Google Scholar] [CrossRef]
- Willems, H.M.E.; Lowes, D.J.; Barker, K.S.; Palmer, G.E.; Peters, B.M. Comparative Analysis of the Capacity of the Candida Species To Elicit Vaginal Immunopathology. Infect. Immun. 2018, 86, e00527-18. [Google Scholar] [CrossRef]
- Verma, A.; Gaffen, S.L.; Swidergall, M. Innate Immunity to Mucosal Candida Infections. J. Fungi 2017, 3, 60. [Google Scholar] [CrossRef]
- Peters, B.M.; Yano, J.; Noverr, M.C.; Fidel, P.L., Jr. Candida vaginitis: When opportunism knocks, the host responds. PLoS Pathog. 2014, 10, e1003965. [Google Scholar] [CrossRef]
- Yano, J.; Noverr, M.C.; Fidel, P.L., Jr. Vaginal Heparan Sulfate Linked to Neutrophil Dysfunction in the Acute Inflammatory Response Associated with Experimental Vulvovaginal Candidiasis. mBio 2017, 8, e00211-17. [Google Scholar] [CrossRef]
- Ardizzoni, A.; Sala, A.; Colombari, B.; Giva, L.B.; Cermelli, C.; Peppoloni, S.; Vecchiarelli, A.; Roselletti, E.; Blasi, E.; Wheeler, R.T.; et al. Perinuclear Anti-Neutrophil Cytoplasmic Antibodies (pANCA) Impair Neutrophil Candidacidal Activity and Are Increased in the Cellular Fraction of Vaginal Samples from Women with Vulvovaginal Candidiasis. J. Fungi 2020, 6, 225. [Google Scholar] [CrossRef]
- Rudkin, F.M.; Bain, J.M.; Walls, C.; Lewis, L.E.; Gow, N.A.; Erwig, L.P. Altered dynamics of Candida albicans phagocytosis by macrophages and PMNs when both phagocyte subsets are present. mBio 2013, 4, e00810-13. [Google Scholar] [CrossRef]
- Leonhardt, J.; Grosse, S.; Marx, C.; Siwczak, F.; Stengel, S.; Bruns, T.; Bauer, R.; Kiehntopf, M.; Williams, D.L.; Wang, Z.Q.; et al. Candida albicans beta-Glucan Differentiates Human Monocytes Into a Specific Subset of Macrophages. Front. Immunol. 2018, 9, 2818. [Google Scholar] [CrossRef]
- Olivier, F.A.B.; Hilsenstein, V.; Weerasinghe, H.; Weir, A.; Hughes, S.; Crawford, S.; Vince, J.E.; Hickey, M.J.; Traven, A. The escape of Candida albicans from macrophages is enabled by the fungal toxin candidalysin and two host cell death pathways. Cell Rep. 2022, 40, 111374. [Google Scholar] [CrossRef]
- Ding, X.; Kambara, H.; Guo, R.; Kanneganti, A.; Acosta-Zaldivar, M.; Li, J.; Liu, F.; Bei, T.; Qi, W.; Xie, X.; et al. Inflammasome-mediated GSDMD activation facilitates escape of Candida albicans from macrophages. Nat. Commun. 2021, 12, 6699. [Google Scholar] [CrossRef]
- Fidel, P.L., Jr.; Sobel, J.D. Immunopathogenesis of recurrent vulvovaginal candidiasis. Clin. Microbiol. Rev. 1996, 9, 335–348. [Google Scholar] [CrossRef]
- Mendling, W.; Koldovsky, U. Investigations by cell-mediated immunologic tests and therapeutic trials with thymopentin in vaginal mycoses. Infect. Dis. Obstet. Gynecol. 1996, 4, 225–231. [Google Scholar] [CrossRef]
- Fong, I.W.; McCleary, P.; Read, S. Cellular immunity of patients with recurrent or refractory vulvovaginal moniliasis. Am. J. Obstet. Gynecol. 1992, 166, 887–890. [Google Scholar] [CrossRef]
- Pietrella, D.; Rachini, A.; Pines, M.; Pandey, N.; Mosci, P.; Bistoni, F.; d’Enfert, C.; Vecchiarelli, A. Th17 cells and IL-17 in protective immunity to vaginal candidiasis. PLoS ONE 2011, 6, e22770. [Google Scholar] [CrossRef]
- Cypowyj, S.; Picard, C.; Marodi, L.; Casanova, J.L.; Puel, A. Immunity to infection in IL-17-deficient mice and humans. Eur. J. Immunol. 2012, 42, 2246–2254. [Google Scholar] [CrossRef]
- Ling, Y.; Puel, A. IL-17 and infections. Actas Dermosifiliogr. 2014, 105 (Suppl. S1), 34–40. [Google Scholar] [CrossRef]
- Campione, E.; Cosio, T.; Lanna, C.; Mazzilli, S.; Ventura, A.; Dika, E.; Gaziano, R.; Dattola, A.; Candi, E.; Bianchi, L. Predictive role of vitamin A serum concentration in psoriatic patients treated with IL-17 inhibitors to prevent skin and systemic fungal infections. J. Pharmacol. Sci. 2020, 144, 52–56. [Google Scholar] [CrossRef]
- Yano, J.; Kolls, J.K.; Happel, K.I.; Wormley, F.; Wozniak, K.L.; Fidel, P.L., Jr. The acute neutrophil response mediated by S100 alarmins during vaginal Candida infections is independent of the Th17-pathway. PLoS ONE 2012, 7, e46311. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.M.; Coleman, B.M.; Willems, H.M.E.; Barker, K.S.; Aggor, F.E.Y.; Cipolla, E.; Verma, A.H.; Bishu, S.; Huppler, A.H.; Bruno, V.M.; et al. The Interleukin (IL) 17R/IL-22R Signaling Axis Is Dispensable for Vulvovaginal Candidiasis Regardless of Estrogen Status. J. Infect. Dis. 2020, 221, 1554–1563. [Google Scholar] [CrossRef] [PubMed]
- Kummer, J.A.; Broekhuizen, R.; Everett, H.; Agostini, L.; Kuijk, L.; Martinon, F.; van Bruggen, R.; Tschopp, J. Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J. Histochem. Cytochem. 2007, 55, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, E.; Pericolini, E.; Luciano, E.; Sabbatini, S.; Roselletti, E.; Perito, S.; Kasper, L.; Hube, B.; Vecchiarelli, A. Induction of caspase-11 by aspartyl proteinases of Candida albicans and implication in promoting inflammatory response. Infect. Immun. 2015, 83, 1940–1948. [Google Scholar] [CrossRef]
- Pietrella, D.; Pandey, N.; Gabrielli, E.; Pericolini, E.; Perito, S.; Kasper, L.; Bistoni, F.; Cassone, A.; Hube, B.; Vecchiarelli, A. Secreted aspartic proteases of Candida albicans activate the NLRP3 inflammasome. Eur. J. Immunol. 2013, 43, 679–692. [Google Scholar] [CrossRef]
- Borghi, M.; De Luca, A.; Puccetti, M.; Jaeger, M.; Mencacci, A.; Oikonomou, V.; Pariano, M.; Garlanda, C.; Moretti, S.; Bartoli, A.; et al. Pathogenic NLRP3 Inflammasome Activity during Candida Infection Is Negatively Regulated by IL-22 via Activation of NLRC4 and IL-1Ra. Cell Host Microbe 2015, 18, 198–209. [Google Scholar] [CrossRef]
- Lupfer, C.; Anand, P.K. Integrating Inflammasome Signaling in Sexually Transmitted Infections. Trends Immunol. 2016, 37, 703–714. [Google Scholar] [CrossRef]
- Netea, M.G.; Quintin, J.; van der Meer, J.W. Trained immunity: A memory for innate host defense. Cell Host Microbe 2011, 9, 355–361. [Google Scholar] [CrossRef]
- Netea, M.G. Training innate immunity: The changing concept of immunological memory in innate host defence. Eur. J. Clin. Investig. 2013, 43, 881–884. [Google Scholar] [CrossRef]
- Cassone, A. The Case for an Expanded Concept of Trained Immunity. mBio 2018, 9, e00570-18. [Google Scholar] [CrossRef]
- Saeed, S.; Quintin, J.; Kerstens, H.H.; Rao, N.A.; Aghajanirefah, A.; Matarese, F.; Cheng, S.C.; Ratter, J.; Berentsen, K.; van der Ent, M.A.; et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 2014, 345, 1251086. [Google Scholar] [CrossRef]
- De Santa, F.; Totaro, M.G.; Prosperini, E.; Notarbartolo, S.; Testa, G.; Natoli, G. The histone H3 lysine-27 demethylase Jmjd3 links inflammation to inhibition of polycomb-mediated gene silencing. Cell 2007, 130, 1083–1094. [Google Scholar] [CrossRef]
- Netea, M.G.; Joosten, L.A.; Latz, E.; Mills, K.H.; Natoli, G.; Stunnenberg, H.G.; O’Neill, L.A.; Xavier, R.J. Trained immunity: A program of innate immune memory in health and disease. Science 2016, 352, aaf1098. [Google Scholar] [CrossRef]
- Netea, M.G.; Dominguez-Andres, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; van der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef]
- Wellington, M.; Koselny, K.; Sutterwala, F.S.; Krysan, D.J. Candida albicans triggers NLRP3-mediated pyroptosis in macrophages. Eukaryot. Cell 2014, 13, 329–340. [Google Scholar] [CrossRef]
- Chee, W.J.Y.; Chew, S.Y.; Than, L.T.L. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb. Cell Fact. 2020, 19, 203. [Google Scholar] [CrossRef]
- Kalia, N.; Singh, J.; Kaur, M. Microbiota in vaginal health and pathogenesis of recurrent vulvovaginal infections: A critical review. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 5. [Google Scholar] [CrossRef]
- Alonzo Martinez, M.C.; Cazorla, E.; Canovas, E.; Martinez-Blanch, J.F.; Chenoll, E.; Climent, E.; Navarro-Lopez, V. Study of the Vaginal Microbiota in Healthy Women of Reproductive Age. Microorganisms 2021, 9, 1069. [Google Scholar] [CrossRef]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4680–4687. [Google Scholar] [CrossRef]
- Gajer, P.; Brotman, R.M.; Bai, G.; Sakamoto, J.; Schutte, U.M.; Zhong, X.; Koenig, S.S.; Fu, L.; Ma, Z.S.; Zhou, X.; et al. Temporal dynamics of the human vaginal microbiota. Sci. Transl. Med. 2012, 4, 132ra152. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.X.; Li, T.; Zhang, X.; Wang, S.X.; Liu, Z.H. Lactobacillus crispatus Modulates Vaginal Epithelial Cell Innate Response to Candida albicans. Chin. Med. J. 2017, 130, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Bradford, L.L.; Ravel, J. The vaginal mycobiome: A contemporary perspective on fungi in women’s health and diseases. Virulence 2017, 8, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Ramon, A.M.; Porta, A.; Fonzi, W.A. Effect of environmental pH on morphological development of Candida albicans is mediated via the PacC-related transcription factor encoded by PRR2. J. Bacteriol. 1999, 181, 7524–7530. [Google Scholar] [CrossRef]
- Hearps, A.C.; Tyssen, D.; Srbinovski, D.; Bayigga, L.; Diaz, D.J.D.; Aldunate, M.; Cone, R.A.; Gugasyan, R.; Anderson, D.J.; Tachedjian, G. Vaginal lactic acid elicits an anti-inflammatory response from human cervicovaginal epithelial cells and inhibits production of pro-inflammatory mediators associated with HIV acquisition. Mucosal. Immunol. 2017, 10, 1480–1490. [Google Scholar] [CrossRef]
- O’Hanlon, D.E.; Moench, T.R.; Cone, R.A. Vaginal pH and microbicidal lactic acid when lactobacilli dominate the microbiota. PLoS ONE 2013, 8, e80074. [Google Scholar] [CrossRef]
- Witkin, S.S.; Mendes-Soares, H.; Linhares, I.M.; Jayaram, A.; Ledger, W.J.; Forney, L.J. Influence of vaginal bacteria and D- and L-lactic acid isomers on vaginal extracellular matrix metalloproteinase inducer: Implications for protection against upper genital tract infections. mBio 2013, 4, e00460-13. [Google Scholar] [CrossRef]
- Parolin, C.; Marangoni, A.; Laghi, L.; Foschi, C.; Nahui Palomino, R.A.; Calonghi, N.; Cevenini, R.; Vitali, B. Isolation of Vaginal Lactobacilli and Characterization of Anti-Candida Activity. PLoS ONE 2015, 10, e0131220. [Google Scholar] [CrossRef]
- Kumherova, M.; Vesela, K.; Kosova, M.; Masata, J.; Horackova, S.; Smidrkal, J. Novel Potential Probiotic Lactobacilli for Prevention and Treatment of Vulvovaginal Infections. Probiotics Antimicrob. Proteins 2021, 13, 163–172. [Google Scholar] [CrossRef]
- Boris, S.; Barbes, C. Role played by lactobacilli in controlling the population of vaginal pathogens. Microbes Infect. 2000, 2, 543–546. [Google Scholar] [CrossRef]
- Vazquez-Munoz, R.; Dongari-Bagtzoglou, A. Anticandidal Activities by Lactobacillus Species: An Update on Mechanisms of Action. Front. Oral Health 2021, 2, 689382. [Google Scholar] [CrossRef]
- Tachedjian, G.; O’Hanlon, D.E.; Ravel, J. The implausible “in vivo” role of hydrogen peroxide as an antimicrobial factor produced by vaginal microbiota. Microbiome 2018, 6, 29. [Google Scholar] [CrossRef]
- O’Hanlon, D.E.; Moench, T.R.; Cone, R.A. In vaginal fluid, bacteria associated with bacterial vaginosis can be suppressed with lactic acid but not hydrogen peroxide. BMC Infect. Dis. 2011, 11, 200. [Google Scholar] [CrossRef]
- O’Hanlon, D.E.; Lanier, B.R.; Moench, T.R.; Cone, R.A. Cervicovaginal fluid and semen block the microbicidal activity of hydrogen peroxide produced by vaginal lactobacilli. BMC Infect. Dis. 2010, 10, 120. [Google Scholar] [CrossRef]
- Bracey, D.; Holyoak, C.D.; Coote, P.J. Comparison of the inhibitory effect of sorbic acid and amphotericin B on Saccharomyces cerevisiae: Is growth inhibition dependent on reduced intracellular pH? J. Appl. Microbiol. 1998, 85, 1056–1066. [Google Scholar] [CrossRef]
- Guldfeldt, L.U.; Arneborg, N. Measurement of the effects of acetic acid and extracellular pH on intracellular pH of nonfermenting, individual Saccharomyces cerevisiae cells by fluorescence microscopy. Appl. Environ. Microbiol. 1998, 64, 530–534. [Google Scholar] [CrossRef]
- Stratford, M.; Anslow, P.A. Evidence that sorbic acid does not inhibit yeast as a classic ‘weak acid preservative’. Lett. Appl. Microbiol. 1998, 27, 203–206. [Google Scholar] [CrossRef]
- Mollapour, M.; Shepherd, A.; Piper, P.W. Novel stress responses facilitate Saccharomyces cerevisiae growth in the presence of the monocarboxylate preservatives. Yeast 2008, 25, 169–177. [Google Scholar] [CrossRef]
- Ullah, A.; Orij, R.; Brul, S.; Smits, G.J. Quantitative analysis of the modes of growth inhibition by weak organic acids in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2012, 78, 8377–8387. [Google Scholar] [CrossRef]
- Lourenco, A.; Pedro, N.A.; Salazar, S.B.; Mira, N.P. Effect of Acetic Acid and Lactic Acid at Low pH in Growth and Azole Resistance of Candida albicans and Candida glabrata. Front. Microbiol. 2018, 9, 3265. [Google Scholar] [CrossRef]
- Bergsson, G.; Arnfinnsson, J.; Steingrimsson, O.; Thormar, H. In vitro killing of Candida albicans by fatty acids and monoglycerides. Antimicrob. Agents Chemother. 2001, 45, 3209–3212. [Google Scholar] [CrossRef] [PubMed]
- Murzyn, A.; Krasowska, A.; Stefanowicz, P.; Dziadkowiec, D.; Lukaszewicz, M. Capric acid secreted by S. boulardii inhibits C. albicans filamentous growth, adhesion and biofilm formation. PLoS ONE 2010, 5, e12050. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Inoue, S.; Hayama, K.; Ninomiya, K.; Abe, S. Inhibition of Candida mycelia growth by a medium chain fatty acids, capric acid in vitro and its therapeutic efficacy in murine oral candidiasis. Med. Mycol. J. 2012, 53, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Clitherow, K.H.; Binaljadm, T.M.; Hansen, J.; Spain, S.G.; Hatton, P.V.; Murdoch, C. Medium-Chain Fatty Acids Released from Polymeric Electrospun Patches Inhibit Candida albicans Growth and Reduce the Biofilm Viability. ACS Biomater. Sci. Eng. 2020, 6, 4087–4095. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.G.; Khadke, S.K.; Lee, J. Antibiofilm and antifungal activities of medium-chain fatty acids against Candida albicans via mimicking of the quorum-sensing molecule farnesol. Microb. Biotechnol. 2021, 14, 1353–1366. [Google Scholar] [CrossRef]
- Suchodolski, J.; Derkacz, D.; Bernat, P.; Krasowska, A. Capric acid secreted by Saccharomyces boulardii influences the susceptibility of Candida albicans to fluconazole and amphotericin B. Sci. Rep. 2021, 11, 6519. [Google Scholar] [CrossRef]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef]
- Wu, H.; Xie, S.; Miao, J.; Li, Y.; Wang, Z.; Wang, M.; Yu, Q. Lactobacillus reuteri maintains intestinal epithelial regeneration and repairs damaged intestinal mucosa. Gut Microbes 2020, 11, 997–1014. [Google Scholar] [CrossRef]
- Cheng, S.C.; van de Veerdonk, F.; Smeekens, S.; Joosten, L.A.; van der Meer, J.W.; Kullberg, B.J.; Netea, M.G. Candida albicans dampens host defense by downregulating IL-17 production. J. Immunol. 2010, 185, 2450–2457. [Google Scholar] [CrossRef]
- Drell, T.; Lillsaar, T.; Tummeleht, L.; Simm, J.; Aaspollu, A.; Vain, E.; Saarma, I.; Salumets, A.; Donders, G.G.; Metsis, M. Characterization of the vaginal micro- and mycobiome in asymptomatic reproductive-age Estonian women. PLoS ONE 2013, 8, e54379. [Google Scholar] [CrossRef]
- Lehtoranta, L.; Hibberd, A.A.; Yeung, N.; Laitila, A.; Maukonen, J.; Ouwehand, A.C. Characterization of vaginal fungal communities in healthy women and women with bacterial vaginosis (BV); a pilot study. Microb. Pathog. 2021, 161, 105055. [Google Scholar] [CrossRef]
- Holland, J.; Young, M.L.; Lee, O.; Chen, S.C.A. Vulvovaginal carriage of yeasts other than Candida albicans. Sex. Transm. Infect. 2003, 79, 249–250. [Google Scholar] [CrossRef]
- France, M.; Alizadeh, M.; Brown, S.; Ma, B.; Ravel, J. Towards a deeper understanding of the vaginal microbiota. Nat. Microbiol. 2022, 7, 367–378. [Google Scholar] [CrossRef]
- Barrientos-Duran, A.; Fuentes-Lopez, A.; de Salazar, A.; Plaza-Diaz, J.; Garcia, F. Reviewing the Composition of Vaginal Microbiota: Inclusion of Nutrition and Probiotic Factors in the Maintenance of Eubiosis. Nutrients 2020, 12, 419. [Google Scholar] [CrossRef]
- Moreno, I.; Codoner, F.M.; Vilella, F.; Valbuena, D.; Martinez-Blanch, J.F.; Jimenez-Almazan, J.; Alonso, R.; Alama, P.; Remohi, J.; Pellicer, A.; et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am. J. Obstet. Gynecol. 2016, 215, 684–703. [Google Scholar] [CrossRef]
- Tanaka, S.E.; Sakuraba, Y.; Kitaya, K.; Ishikawa, T. Differential Vaginal Microbiota Profiling in Lactic-Acid-Producing Bacteria between Infertile Women with and without Chronic Endometritis. Diagnostics 2022, 12, 878. [Google Scholar] [CrossRef]
- Kitaya, K.; Nagai, Y.; Arai, W.; Sakuraba, Y.; Ishikawa, T. Characterization of Microbiota in Endometrial Fluid and Vaginal Secretions in Infertile Women with Repeated Implantation Failure. Mediators Inflamm. 2019, 2019, 4893437. [Google Scholar] [CrossRef]
- Ling, Z.; Kong, J.; Liu, F.; Zhu, H.; Chen, X.; Wang, Y.; Li, L.; Nelson, K.E.; Xia, Y.; Xiang, C. Molecular analysis of the diversity of vaginal microbiota associated with bacterial vaginosis. BMC Genom. 2010, 11, 488. [Google Scholar] [CrossRef]
- Nasioudis, D.; Linhares, I.M.; Ledger, W.J.; Witkin, S.S. Bacterial vaginosis: A critical analysis of current knowledge. BJOG 2017, 124, 61–69. [Google Scholar] [CrossRef]
- Han, Y.; Liu, Z.; Chen, T. Role of Vaginal Microbiota Dysbiosis in Gynecological Diseases and the Potential Interventions. Front. Microbiol. 2021, 12, 643422. [Google Scholar] [CrossRef]
- Schwebke, J.R. Abnormal vaginal flora as a biological risk factor for acquisition of HIV infection and sexually transmitted diseases. J. Infect. Dis. 2005, 192, 1315–1317. [Google Scholar] [CrossRef] [PubMed]
- van de Wijgert, J. The vaginal microbiome and sexually transmitted infections are interlinked: Consequences for treatment and prevention. PLoS Med. 2017, 14, e1002478. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Westman, R.; Hickey, R.; Hansmann, M.A.; Kennedy, C.; Osborn, T.W.; Forney, L.J. Vaginal microbiota of women with frequent vulvovaginal candidiasis. Infect. Immun. 2009, 77, 4130–4135. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Ge, X.; Qiu, B.; Xiang, Z.; Jiang, C.; Wu, J.; Li, Y. Vulvovaginal candidiasis and vaginal microflora interaction: Microflora changes and probiotic therapy. Front. Cell. Infect. Microbiol. 2023, 13, 1123026. [Google Scholar] [CrossRef] [PubMed]
- Petrova, M.I.; Reid, G.; Vaneechoutte, M.; Lebeer, S. Lactobacillus iners: Friend or Foe? Trends Microbiol. 2017, 25, 182–191. [Google Scholar] [CrossRef]
- Tachedjian, G.; Aldunate, M.; Bradshaw, C.S.; Cone, R.A. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res. Microbiol. 2017, 168, 782–792. [Google Scholar] [CrossRef]
- Zheng, N.; Guo, R.; Wang, J.; Zhou, W.; Ling, Z. Contribution of Lactobacillus iners to Vaginal Health and Diseases: A Systematic Review. Front. Cell. Infect. Microbiol. 2021, 11, 792787. [Google Scholar] [CrossRef]
- Rampersaud, R.; Planet, P.J.; Randis, T.M.; Kulkarni, R.; Aguilar, J.L.; Lehrer, R.I.; Ratner, A.J. Inerolysin, a cholesterol-dependent cytolysin produced by Lactobacillus iners. J. Bacteriol. 2011, 193, 1034–1041. [Google Scholar] [CrossRef]
- Macklaim, J.M.; Fernandes, A.D.; Di Bella, J.M.; Hammond, J.A.; Reid, G.; Gloor, G.B. Comparative meta-RNA-seq of the vaginal microbiota and differential expression by Lactobacillus iners in health and dysbiosis. Microbiome 2013, 1, 12. [Google Scholar] [CrossRef]
- Zhou, R.; Lu, J.; Wang, J.; Xiao, B. Vaginal Lactobacillus iners abundance is associated with outcome in antibiotic treatment of bacterial vaginosis and capable of inhibiting Gardnerella. Front. Cell. Infect. Microbiol. 2022, 12, 1033431. [Google Scholar] [CrossRef]
- Tortelli, B.A.; Lewis, W.G.; Allsworth, J.E.; Member-Meneh, N.; Foster, L.R.; Reno, H.E.; Peipert, J.F.; Fay, J.C.; Lewis, A.L. Associations between the vaginal microbiome and Candida colonization in women of reproductive age. Am. J. Obstet. Gynecol. 2020, 222, 471.e1–471.e9. [Google Scholar] [CrossRef]
- Ceccarani, C.; Foschi, C.; Parolin, C.; D’Antuono, A.; Gaspari, V.; Consolandi, C.; Laghi, L.; Camboni, T.; Vitali, B.; Severgnini, M.; et al. Diversity of vaginal microbiome and metabolome during genital infections. Sci. Rep. 2019, 9, 14095. [Google Scholar] [CrossRef]
- Novak, J.; Ravel, J.; Ma, B.; Ferreira, C.S.T.; Tristao, A.D.R.; Silva, M.G.; Marconi, C. Characteristics associated with Lactobacillus iners-dominated vaginal microbiota. Sex. Transm. Infect. 2022, 98, 353–359. [Google Scholar] [CrossRef]
- Harriott, M.M.; Lilly, E.A.; Rodriguez, T.E.; Fidel, P.L.; Noverr, M.C. Candida albicans forms biofilms on the vaginal mucosa. Microbiology 2010, 156, 3635–3644. [Google Scholar] [CrossRef]
- Gao, M.; Wang, H.; Zhu, L. Quercetin Assists Fluconazole to Inhibit Biofilm Formations of Fluconazole-Resistant Candida albicans in In Vitro and In Vivo Antifungal Managements of Vulvovaginal Candidiasis. Cell. Physiol. Biochem. 2016, 40, 727–742. [Google Scholar] [CrossRef]
- Muzny, C.A.; Schwebke, J.R. Biofilms: An Underappreciated Mechanism of Treatment Failure and Recurrence in Vaginal Infections. Clin. Infect. Dis. 2015, 61, 601–606. [Google Scholar] [CrossRef]
- Sherry, L.; Rajendran, R.; Lappin, D.F.; Borghi, E.; Perdoni, F.; Falleni, M.; Tosi, D.; Smith, K.; Williams, C.; Jones, B.; et al. Biofilms formed by Candida albicans bloodstream isolates display phenotypic and transcriptional heterogeneity that are associated with resistance and pathogenicity. BMC Microbiol. 2014, 14, 182. [Google Scholar] [CrossRef]
- McKloud, E.; Delaney, C.; Sherry, L.; Kean, R.; Williams, S.; Metcalfe, R.; Thomas, R.; Richardson, R.; Gerasimidis, K.; Nile, C.J.; et al. Recurrent Vulvovaginal Candidiasis: A Dynamic Interkingdom Biofilm Disease of Candida and Lactobacillus. mSystems 2021, 6, e0062221. [Google Scholar] [CrossRef]
- Balakrishnan, S.N.; Yamang, H.; Lorenz, M.C.; Chew, S.Y.; Than, L.T.L. Role of Vaginal Mucosa, Host Immunity and Microbiota in Vulvovaginal Candidiasis. Pathogens 2022, 11, 618. [Google Scholar] [CrossRef]
- Sabbatini, S.; Visconti, S.; Gentili, M.; Lusenti, E.; Nunzi, E.; Ronchetti, S.; Perito, S.; Gaziano, R.; Monari, C. Lactobacillus iners Cell-Free Supernatant Enhances Biofilm Formation and Hyphal/Pseudohyphal Growth by Candida albicans Vaginal Isolates. Microorganisms 2021, 9, 2577. [Google Scholar] [CrossRef]
- Mba, I.E.; Nweze, E.I. Mechanism of Candida pathogenesis: Revisiting the vital drivers. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1797–1819. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Yang, X.; Nikou, S.A.; Kichik, N.; Donkin, A.; Ponde, N.O.; Richardson, J.P.; Gratacap, R.L.; Archambault, L.S.; Zwirner, C.P.; et al. Candidalysin activates innate epithelial immune responses via epidermal growth factor receptor. Nat. Commun. 2019, 10, 2297. [Google Scholar] [CrossRef] [PubMed]
- Naglik, J.R.; Gaffen, S.L.; Hube, B. Candidalysin: Discovery and function in Candida albicans infections. Curr. Opin. Microbiol. 2019, 52, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Russell, C.M.; Schaefer, K.G.; Dixson, A.; Gray, A.L.H.; Pyron, R.J.; Alves, D.S.; Moore, N.; Conley, E.A.; Schuck, R.J.; White, T.A.; et al. The Candida albicans virulence factor candidalysin polymerizes in solution to form membrane pores and damage epithelial cells. Elife 2022, 11, e75490. [Google Scholar] [CrossRef] [PubMed]
- Russell, C.M.; Rybak, J.A.; Miao, J.; Peters, B.M.; Barrera, F.N. Candidalysin: Connecting the pore forming mechanism of this virulence factor to its immunostimulatory properties. J. Biol. Chem. 2023, 299, 102829. [Google Scholar] [CrossRef]
- Kasper, L.; Konig, A.; Koenig, P.A.; Gresnigt, M.S.; Westman, J.; Drummond, R.A.; Lionakis, M.S.; Gross, O.; Ruland, J.; Naglik, J.R.; et al. The fungal peptide toxin Candidalysin activates the NLRP3 inflammasome and causes cytolysis in mononuclear phagocytes. Nat. Commun. 2018, 9, 4260. [Google Scholar] [CrossRef]
- Rogiers, O.; Frising, U.C.; Kucharikova, S.; Jabra-Rizk, M.A.; van Loo, G.; Van Dijck, P.; Wullaert, A. Candidalysin Crucially Contributes to Nlrp3 Inflammasome Activation by Candida albicans Hyphae. mBio 2019, 10, e02221-18. [Google Scholar] [CrossRef]
- Lowes, D.J.; Hevener, K.E.; Peters, B.M. Second-Generation Antidiabetic Sulfonylureas Inhibit Candida albicans and Candidalysin-Mediated Activation of the NLRP3 Inflammasome. Antimicrob. Agents Chemother. 2020, 64, e01777-19. [Google Scholar] [CrossRef]
- Liu, J.; Willems, H.M.E.; Sansevere, E.A.; Allert, S.; Barker, K.S.; Lowes, D.J.; Dixson, A.C.; Xu, Z.; Miao, J.; DeJarnette, C.; et al. A variant ECE1 allele contributes to reduced pathogenicity of Candida albicans during vulvovaginal candidiasis. PLoS Pathog. 2021, 17, e1009884. [Google Scholar] [CrossRef]
- Mogavero, S.; Hofs, S.; Lauer, A.N.; Muller, R.; Brunke, S.; Allert, S.; Gerwien, F.; Groth, S.; Dolk, E.; Wilson, D.; et al. Candidalysin Is the Hemolytic Factor of Candida albicans. Toxins 2022, 14, 874. [Google Scholar] [CrossRef]
- Mak, P. Hemocidins in a functional and structural context of human antimicrobial peptides. Front. Biosci. 2008, 13, 6859–6871. [Google Scholar] [CrossRef]
- Mak, P.; Wojcik, K.; Wicherek, L.; Suder, P.; Dubin, A. Antibacterial hemoglobin peptides in human menstrual blood. Peptides 2004, 25, 1839–1847. [Google Scholar] [CrossRef]
- Naglik, J.R.; Challacombe, S.J.; Hube, B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol. Mol. Biol. Rev. 2003, 67, 400–428, table of contents. [Google Scholar] [CrossRef]
- Schaller, M.; Korting, H.C.; Borelli, C.; Hamm, G.; Hube, B. Candida albicans-secreted aspartic proteinases modify the epithelial cytokine response in an in vitro model of vaginal candidiasis. Infect. Immun. 2005, 73, 2758–2765. [Google Scholar] [CrossRef]
- Hube, B.; Ruchel, R.; Monod, M.; Sanglard, D.; Odds, F.C. Functional aspects of secreted Candida proteinases. Adv. Exp. Med. Biol. 1998, 436, 339–344. [Google Scholar] [CrossRef]
- Gropp, K.; Schild, L.; Schindler, S.; Hube, B.; Zipfel, P.F.; Skerka, C. The yeast Candida albicans evades human complement attack by secretion of aspartic proteases. Mol. Immunol. 2009, 47, 465–475. [Google Scholar] [CrossRef]
- Seifi, Z.; Zarei Mahmoudabadi, A.; Zarrin, M. Extracellular enzymes and susceptibility to fluconazole in Candida strains isolated from patients with vaginitis and healthy individuals. Jundishapur. J. Microbiol. 2015, 8, e20162. [Google Scholar] [CrossRef]
- De Bernardis, F.; Amacker, M.; Arancia, S.; Sandini, S.; Gremion, C.; Zurbriggen, R.; Moser, C.; Cassone, A. A virosomal vaccine against candidal vaginitis: Immunogenicity, efficacy and safety profile in animal models. Vaccine 2012, 30, 4490–4498. [Google Scholar] [CrossRef]
- Gulati, M.; Nobile, C.J. Candida albicans biofilms: Development, regulation, and molecular mechanisms. Microbes Infect. 2016, 18, 310–321. [Google Scholar] [CrossRef]
- Nobile, C.J.; Nett, J.E.; Andes, D.R.; Mitchell, A.P. Function of Candida albicans adhesin Hwp1 in biofilm formation. Eukaryot. Cell 2006, 5, 1604–1610. [Google Scholar] [CrossRef]
- Ramage, G.; VandeWalle, K.; Lopez-Ribot, J.L.; Wickes, B.L. The filamentation pathway controlled by the Efg1 regulator protein is required for normal biofilm formation and development in Candida albicans. FEMS Microbiol. Lett. 2002, 214, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, S.; Li, H.; Shen, L.; Dong, C.; Sun, Y.; Chen, H.; Xu, B.; Zhuang, W.; Deighton, M.; et al. Biofilm Formation of Candida albicans Facilitates Fungal Infiltration and Persister Cell Formation in Vaginal Candidiasis. Front. Microbiol. 2020, 11, 1117. [Google Scholar] [CrossRef] [PubMed]
- Auler, M.E.; Morreira, D.; Rodrigues, F.F.; Abr Ao, M.S.; Margarido, P.F.; Matsumoto, F.E.; Silva, E.G.; Silva, B.C.; Schneider, R.P.; Paula, C.R. Biofilm formation on intrauterine devices in patients with recurrent vulvovaginal candidiasis. Med. Mycol. 2010, 48, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Sherry, L.; Kean, R.; McKloud, E.; O’Donnell, L.E.; Metcalfe, R.; Jones, B.L.; Ramage, G. Biofilms Formed by Isolates from Recurrent Vulvovaginal Candidiasis Patients Are Heterogeneous and Insensitive to Fluconazole. Antimicrob. Agents Chemother. 2017, 61, e01065-17. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, R.; May, A.; Sherry, L.; Kean, R.; Williams, C.; Jones, B.L.; Burgess, K.V.; Heringa, J.; Abeln, S.; Brandt, B.W.; et al. Integrating Candida albicans metabolism with biofilm heterogeneity by transcriptome mapping. Sci. Rep. 2016, 6, 35436. [Google Scholar] [CrossRef]
- Kean, R.; Rajendran, R.; Haggarty, J.; Townsend, E.M.; Short, B.; Burgess, K.E.; Lang, S.; Millington, O.; Mackay, W.G.; Williams, C.; et al. Candida albicans Mycofilms Support Staphylococcus aureus Colonization and Enhances Miconazole Resistance in Dual-Species Interactions. Front. Microbiol. 2017, 8, 258. [Google Scholar] [CrossRef]
- Li, H.; Miao, M.X.; Jia, C.L.; Cao, Y.B.; Yan, T.H.; Jiang, Y.Y.; Yang, F. Interactions between Candida albicans and the resident microbiota. Front. Microbiol. 2022, 13, 930495. [Google Scholar] [CrossRef]
- Ponde, N.O.; Lortal, L.; Ramage, G.; Naglik, J.R.; Richardson, J.P. Candida albicans biofilms and polymicrobial interactions. Crit Rev. Microbiol. 2021, 47, 91–111. [Google Scholar] [CrossRef]
- Goncalves, B.; Ferreira, C.; Alves, C.T.; Henriques, M.; Azeredo, J.; Silva, S. Vulvovaginal candidiasis: Epidemiology, microbiology and risk factors. Crit. Rev. Microbiol. 2016, 42, 905–927. [Google Scholar] [CrossRef]
- Pal, Z.; Urban, E.; Dosa, E.; Pal, A.; Nagy, E. Biofilm formation on intrauterine devices in relation to duration of use. J. Med. Microbiol. 2005, 54, 1199–1203. [Google Scholar] [CrossRef]
- Anju, V.T.; Busi, S.; Imchen, M.; Kumavath, R.; Mohan, M.S.; Salim, S.A.; Subhaswaraj, P.; Dyavaiah, M. Polymicrobial Infections and Biofilms: Clinical Significance and Eradication Strategies. Antibiotics 2022, 11, 1731. [Google Scholar] [CrossRef]
- Wheeler, R.T.; Kombe, D.; Agarwala, S.D.; Fink, G.R. Dynamic, morphotype-specific Candida albicans beta-glucan exposure during infection and drug treatment. PLoS Pathog. 2008, 4, e1000227. [Google Scholar] [CrossRef]
- Galan-Diez, M.; Arana, D.M.; Serrano-Gomez, D.; Kremer, L.; Casasnovas, J.M.; Ortega, M.; Cuesta-Dominguez, A.; Corbi, A.L.; Pla, J.; Fernandez-Ruiz, E. Candida albicans beta-glucan exposure is controlled by the fungal CEK1-mediated mitogen-activated protein kinase pathway that modulates immune responses triggered through dectin-1. Infect. Immun. 2010, 78, 1426–1436. [Google Scholar] [CrossRef]
- Wagner, A.S.; Vogel, A.K.; Lumsdaine, S.W.; Phillips, E.K.; Willems, H.M.E.; Peters, B.M.; Reynolds, T.B. Mucosal Infection with Unmasked Candida albicans Cells Impacts Disease Progression in a Host Niche-Specific Manner. Infect. Immun. 2022, 90, e0034222. [Google Scholar] [CrossRef] [PubMed]
- Pericolini, E.; Perito, S.; Castagnoli, A.; Gabrielli, E.; Mencacci, A.; Blasi, E.; Vecchiarelli, A.; Wheeler, R.T. Epitope unmasking in vulvovaginal candidiasis is associated with hyphal growth and neutrophilic infiltration. PLoS ONE 2018, 13, e0201436. [Google Scholar] [CrossRef]
- Gerwien, F.; Dunker, C.; Brandt, P.; Garbe, E.; Jacobsen, I.D.; Vylkova, S. Clinical Candida albicans Vaginal Isolates and a Laboratory Strain Show Divergent Behaviors during Macrophage Interactions. mSphere 2020, 5, e00393-20. [Google Scholar] [CrossRef]
- Sobel, J.D. Recurrent vulvovaginal candidiasis. Am. J. Obstet. Gynecol. 2016, 214, 15–21. [Google Scholar] [CrossRef]
- Marchaim, D.; Lemanek, L.; Bheemreddy, S.; Kaye, K.S.; Sobel, J.D. Fluconazole-resistant Candida albicans vulvovaginitis. Obstet. Gynecol. 2012, 120, 1407–1414. [Google Scholar] [CrossRef]
- White, D.J.; Vanthuyne, A.; Wood, P.M.; Ayres, J.G. Zafirlukast for severe recurrent vulvovaginal candidiasis: An open label pilot study. Sex. Transm. Infect. 2004, 80, 219–222. [Google Scholar] [CrossRef]
- Yano, J.; White, D.J.; Sampson, A.P.; Wormley, F.L., Jr.; Fidel, P.L., Jr. Leukotrienes Are Dispensable for Vaginal Neutrophil Recruitment as Part of the Immunopathological Response During Experimental Vulvovaginal Candidiasis. Front. Microbiol. 2021, 12, 739385. [Google Scholar] [CrossRef]
- Willems, H.M.E.; Ahmed, S.S.; Liu, J.; Xu, Z.; Peters, B.M. Vulvovaginal Candidiasis: A Current Understanding and Burning Questions. J. Fungi 2020, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Borghi, M.; Pariano, M.; Solito, V.; Puccetti, M.; Bellet, M.M.; Stincardini, C.; Renga, G.; Vacca, C.; Sellitto, F.; Mosci, P.; et al. Targeting the Aryl Hydrocarbon Receptor With Indole-3-Aldehyde Protects From Vulvovaginal Candidiasis via the IL-22-IL-18 Cross-Talk. Front. Immunol. 2019, 10, 2364. [Google Scholar] [CrossRef] [PubMed]
- Cosio, T.; Gaziano, R.; Zuccari, G.; Costanza, G.; Grelli, S.; Di Francesco, P.; Bianchi, L.; Campione, E. Retinoids in Fungal Infections: From Bench to Bedside. Pharmaceuticals 2021, 14, 962. [Google Scholar] [CrossRef] [PubMed]
- Campione, E.; Gaziano, R.; Marino, D.; Orlandi, A. Fungistatic activity of all-trans retinoic acid against Aspergillus fumigatus and Candida albicans. Drug Des. Dev. Ther. 2016, 10, 1551–1555. [Google Scholar] [CrossRef] [PubMed]
- Pistoia, E.S.; Cosio, T.; Campione, E.; Pica, F.; Volpe, A.; Marino, D.; Di Francesco, P.; Monari, C.; Fontana, C.; Favaro, M.; et al. All-Trans Retinoic Acid Effect on Candida albicans Growth and Biofilm Formation. J. Fungi 2022, 8, 1049. [Google Scholar] [CrossRef]
- Vladareanu, R.; Mihu, D.; Mitran, M.; Mehedintu, C.; Boiangiu, A.; Manolache, M.; Vladareanu, S. New evidence on oral L. plantarum P17630 product in women with history of recurrent vulvovaginal candidiasis (RVVC): A randomized double-blind placebo-controlled study. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 262–267. [Google Scholar] [CrossRef]
- Li, T.; Liu, Z.; Zhang, X.; Chen, X.; Wang, S. Local Probiotic Lactobacillus crispatus and Lactobacillus delbrueckii Exhibit Strong Antifungal Effects Against Vulvovaginal Candidiasis in a Rat Model. Front. Microbiol. 2019, 10, 1033. [Google Scholar] [CrossRef]
- Falagas, M.E.; Betsi, G.I.; Athanasiou, S. Probiotics for prevention of recurrent vulvovaginal candidiasis: A review. J. Antimicrob. Chemother. 2006, 58, 266–272. [Google Scholar] [CrossRef]
- Vahedpoor, Z.; Abastabar, M.; Sehat, M.; Talebian, P.; Felfelian Fini, T.; Dastanpour, Z.; Haghani, I.; Chelongarian, R.; Nazeri, M. Vaginal and oral use of probiotics as adjunctive therapy to fluconazole in patients with vulvovaginal candidiasis: A clinical trial on Iranian women. Curr. Med. Mycol. 2021, 7, 36–43. [Google Scholar] [CrossRef]
- Pericolini, E.; Gabrielli, E.; Ballet, N.; Sabbatini, S.; Roselletti, E.; Cayzeele Decherf, A.; Pelerin, F.; Luciano, E.; Perito, S.; Justen, P.; et al. Therapeutic activity of a Saccharomyces cerevisiae-based probiotic and inactivated whole yeast on vaginal candidiasis. Virulence 2017, 8, 74–90. [Google Scholar] [CrossRef]
- Gaziano, R.; Sabbatini, S.; Roselletti, E.; Perito, S.; Monari, C. Saccharomyces cerevisiae-Based Probiotics as Novel Antimicrobial Agents to Prevent and Treat Vaginal Infections. Front. Microbiol. 2020, 11, 718. [Google Scholar] [CrossRef]
- Gabrielli, E.; Pericolini, E.; Ballet, N.; Roselletti, E.; Sabbatini, S.; Mosci, P.; Decherf, A.C.; Pelerin, F.; Perito, S.; Justen, P.; et al. Saccharomyces cerevisiae-based probiotic as novel anti-fungal and anti-inflammatory agent for therapy of vaginal candidiasis. Benef. Microbes 2018, 9, 219–230. [Google Scholar] [CrossRef]
- Decherf, A.; Dehay, E.; Boyer, M.; Clement-Ziza, M.; Rodriguez, B.; Legrain-Raspaud, S. Recovery of Saccharomyces cerevisiae CNCM I-3856 in Vaginal Samples of Healthy Women after Oral Administration. Nutrients 2020, 12, 2211. [Google Scholar] [CrossRef]
- Chen, T.; Xia, C.; Hu, H.; Wang, H.; Tan, B.; Tian, P.; Zhao, X.; Wang, L.; Han, Y.; Deng, K.Y.; et al. Dysbiosis of the rat vagina is efficiently rescued by vaginal microbiota transplantation or probiotic combination. Int. J. Antimicrob. Agents 2021, 57, 106277. [Google Scholar] [CrossRef]
- Lu, F.; Wei, J.; Zhong, Y.; Feng, Y.; Ma, B.; Xiong, Y.; Wei, K.; Tan, B.; Chen, T. Antibiotic Therapy and Vaginal Microbiota Transplantation Reduce Endometriosis Disease Progression in Female Mice via NF-kappaB Signaling Pathway. Front. Med. 2022, 9, 831115. [Google Scholar] [CrossRef]
- Lev-Sagie, A.; Goldman-Wohl, D.; Cohen, Y.; Dori-Bachash, M.; Leshem, A.; Mor, U.; Strahilevitz, J.; Moses, A.E.; Shapiro, H.; Yagel, S.; et al. Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat. Med. 2019, 25, 1500–1504. [Google Scholar] [CrossRef]


| Potential Therapeutic Strategies | Effects | References |
|---|---|---|
| Immunomodulators | ||
| Zafirlukast (LTRA) | Symptom relief in women with RVVC | [199] |
| Anakinra (IL-1Ra), Indole-3-Aldehyde (AhR agonist) | Reduction of inflammation in murine models of VVC | [87,202] |
| ATRA | Fungistatic effect against C. albicans growth and biofilm formation in in vitro model | [205] |
| Lactobacilli-based probiotics | ||
| L. plantarum P17630 | Vaginal colonization by lactobacilli and improvement of clinical signs in women with RVVC | [206] |
| L. crispatus L. delbrueckii | Antifungal efficacy in a rat model of VVC | [207] |
| L. acidophilus L. rhamnosus GR-1 L. fermentum RC-14 | Effectiveness in preventing C. albicans colonization and infection in women with RVVC | [208,209] |
| Yeast-based probiotics | ||
| S. cerevisiae (CNCM I-3856 strain) | Reduction of C. albicans load and vaginal inflammation in a murine model of VVC; | [210,211,212] |
| Colonization of human vagina after oral administration | [213] | |
| VMT | Therapeutic efficacy in rat models of vaginal dysbiosis; | [214] |
| Reduction of endometriosis disease progression in female mice; | [215] | |
| Improvement of symptoms in patients with RBV | [216] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaziano, R.; Sabbatini, S.; Monari, C. The Interplay between Candida albicans, Vaginal Mucosa, Host Immunity and Resident Microbiota in Health and Disease: An Overview and Future Perspectives. Microorganisms 2023, 11, 1211. https://doi.org/10.3390/microorganisms11051211
Gaziano R, Sabbatini S, Monari C. The Interplay between Candida albicans, Vaginal Mucosa, Host Immunity and Resident Microbiota in Health and Disease: An Overview and Future Perspectives. Microorganisms. 2023; 11(5):1211. https://doi.org/10.3390/microorganisms11051211
Chicago/Turabian StyleGaziano, Roberta, Samuele Sabbatini, and Claudia Monari. 2023. "The Interplay between Candida albicans, Vaginal Mucosa, Host Immunity and Resident Microbiota in Health and Disease: An Overview and Future Perspectives" Microorganisms 11, no. 5: 1211. https://doi.org/10.3390/microorganisms11051211
APA StyleGaziano, R., Sabbatini, S., & Monari, C. (2023). The Interplay between Candida albicans, Vaginal Mucosa, Host Immunity and Resident Microbiota in Health and Disease: An Overview and Future Perspectives. Microorganisms, 11(5), 1211. https://doi.org/10.3390/microorganisms11051211








