Development of the Neonatal Intestinal Barrier, Microbiome, and Susceptibility to NEC
Abstract
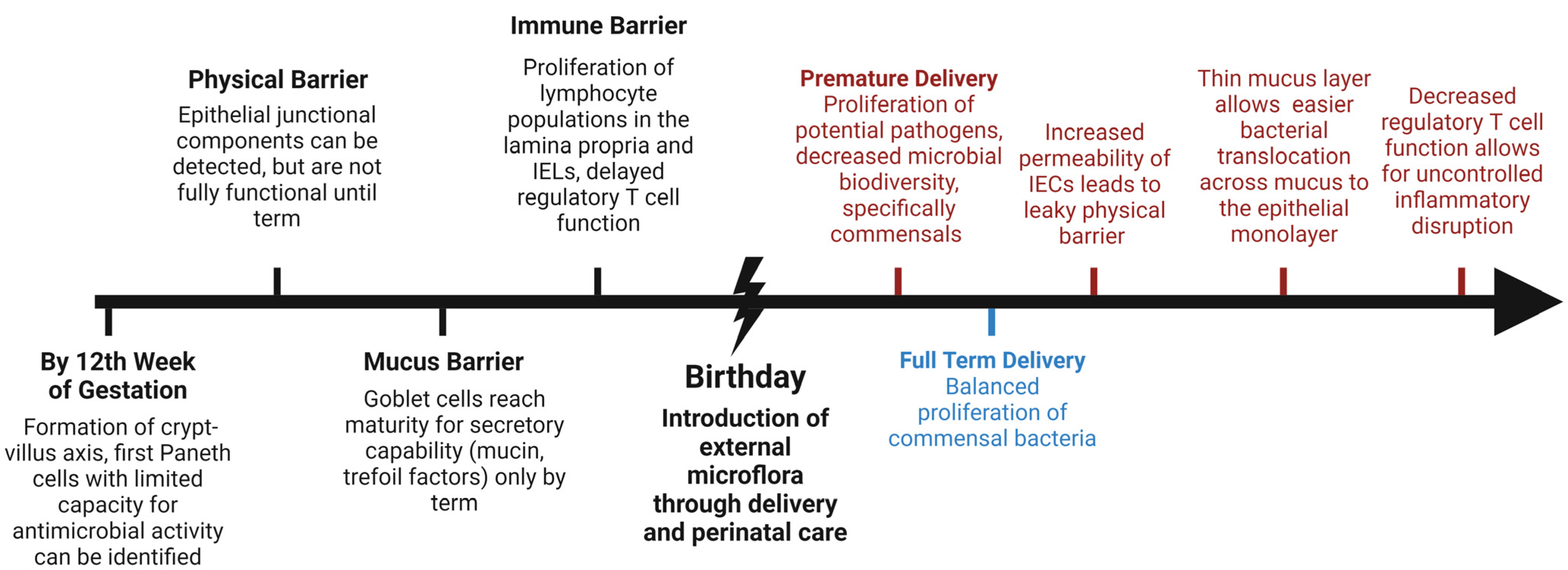
1. Introduction
2. Development and Composition of the Intestinal Barrier
3. The Mucus Barrier
4. The Intestinal Epithelial Barrier
5. The Immune Barrier: Lamina Propria, Intraepithelial Lymphocytes (IEL), and Peyer’s Patches
6. Microbiome of the Maturing Gut
7. Breast Milk and Biotics
7.1. Breast Milk
7.2. Biotics
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Halpern, M.D.; Denning, P.W. The role of intestinal epithelial barrier function in the development of NEC. Tissue Barriers 2015, 3, e1000707. [Google Scholar] [CrossRef]
- Hunter, C.J.; De Plaen, I.G. Inflammatory signaling in NEC: Role of NF-κB, cytokines and other inflammatory mediators. Pathophysiology 2014, 21, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Hartz, L.E.; Bradshaw, W.; Brandon, D.H. Potential NICU Environmental Influences on the Neonate’s Microbiome: A Systematic Review. Adv. Neonatal. Care 2015, 15, 324–335. [Google Scholar] [CrossRef]
- D’Agata, A.L.; Wu, J.; Welandawe, M.K.V.; Dutra, S.V.O.; Kane, B.; Groer, M.W. Effects of early life NICU stress on the developing gut microbiome. Dev. Psychobiol. 2019, 61, 650–660. [Google Scholar] [CrossRef]
- Denning, N.-L.; Prince, J.M. Neonatal intestinal dysbiosis in necrotizing enterocolitis. Mol. Med. 2018, 24, 4. [Google Scholar] [CrossRef]
- Hanson, L.A.; Winberg, J. Breast milk and defence against infection in the newborn. Arch. Dis. Child. 1972, 47, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Cacho, N.T.; Lawrence, R.M. Innate Immunity and Breast Milk. Front. Immunol. 2017, 8, 584. [Google Scholar] [CrossRef] [PubMed]
- Townsend, C.M.; Beauchamp, R.D.; Evers, B.M.; Mattox, K.L. Sabiston Textbook of Surgery, 20th ed.; Elsevier–Health Sciences Division: Philadelphia, PA, USA, 2016. [Google Scholar]
- Barreto, L.B.; Rattes, I.C.; Costa, A.V.; Gama, P. Paneth cells and their multiple functions. Cell Biol. Int. 2022, 46, 701–710. [Google Scholar] [CrossRef]
- Simons, B.D.; Clevers, H. Stem cell self-renewal in intestinal crypt. Exp. Cell Res. 2011, 317, 2719–2724. [Google Scholar] [CrossRef]
- Barker, N.; van Es, J.H.; Kuipers, J.; Kujala, P.; van den Born, M.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; Peters, P.J.; et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007, 449, 1003–1007. [Google Scholar] [CrossRef]
- Demers-Mathieu, V. The immature intestinal epithelial cells in preterm infants play a role in the necrotizing enterocolitis pathogenesis: A review. Health Sci. Rev. 2022, 4, 100033. [Google Scholar] [CrossRef]
- Hodzic, Z.; Bolock, A.M.; Good, M. The Role of Mucosal Immunity in the Pathogenesis of Necrotizing Enterocolitis. Front. Pediatr. 2017, 5, 40. [Google Scholar] [CrossRef]
- Kandasamy, J.; Huda, S.; Ambalavanan, N.; Jilling, T. Inflammatory signals that regulate intestinal epithelial renewal, differentiation, migration and cell death: Implications for necrotizing enterocolitis. Pathophysiology 2014, 21, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Howitt, M.R.; Lavoie, S.; Michaud, M.; Blum, A.M.; Tran, S.V.; Weinstock, J.V.; Gallini, C.A.; Redding, K.; Margolskee, R.F.; Osborne, L.C.; et al. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science 2016, 351, 1329–1333. [Google Scholar] [CrossRef] [PubMed]
- Gerbe, F.; van Es, J.H.; Makrini, L.; Brulin, B.; Mellitzer, G.; Robine, S.; Romagnolo, B.; Shroyer, N.F.; Bourgaux, J.-F.; Pignodel, C.; et al. Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J. Cell Biol. 2011, 192, 767–780. [Google Scholar] [CrossRef]
- Lock, J.Y.; Carlson, T.L.; Yu, Y.; Lu, J.; Claud, E.C.; Carrier, R.L. Impact of Developmental Age, Necrotizing Enterocolitis Associated Stress, and Oral Therapeutic Intervention on Mucus Barrier Properties. Sci. Rep. 2020, 10, 6692. [Google Scholar] [CrossRef] [PubMed]
- Henderickx, J.G.E.; Zwittink, R.D.; Renes, I.B.; van Lingen, R.A.; van Zoeren-Grobben, D.; Jebbink, L.J.G.; Boeren, S.; van Elburg, R.M.; Knol, J.; Belzer, C. Maturation of the preterm gastrointestinal tract can be defined by host and microbial markers for digestion and barrier defense. Sci. Rep. 2021, 11, 12808. [Google Scholar] [CrossRef]
- Martens, E.C.; Neumann, M.; Desai, M.S. Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier. Nat. Rev. Microbiol. 2018, 16, 457–470. [Google Scholar] [CrossRef]
- Fang, J.; Wang, H.; Zhou, Y.; Zhang, H.; Zhou, H.; Zhang, X. Slimy partners: The mucus barrier and gut microbiome in ulcerative colitis. Exp. Mol. Med. 2021, 53, 772–787. [Google Scholar] [CrossRef]
- Glover, J.S.; Ticer, T.D.; Engevik, M.A. Characterizing the mucin-degrading capacity of the human gut microbiota. Sci. Rep. 2022, 12, 8456. [Google Scholar] [CrossRef]
- Carlson, T.L.; Lock, J.Y.; Carrier, R.L. Engineering the Mucus Barrier. Annu. Rev. Biomed. Eng. 2018, 20, 197–220. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yu, M. Role of Goblet Cells in Intestinal Barrier and Mucosal Immunity. J. Inflamm. Res. 2021, 14, 3171–3183. [Google Scholar] [CrossRef]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232. [Google Scholar] [CrossRef]
- Van der Sluis, M.; De Koning, B.A.E.; De Bruijn, A.C.J.M.; Velcich, A.; Meijerink, J.P.P.; Van Goudoever, J.B.; Büller, H.A.; Dekker, J.; Van Seuningen, I.; Renes, I.B.; et al. Muc2-Deficient Mice Spontaneously Develop Colitis, Indicating That MUC2 Is Critical for Colonic Protection. Gastroenterology 2006, 131, 117–129. [Google Scholar] [CrossRef]
- Meldrum, O.W.; Yakubov, G.E.; Bonilla, M.R.; Deshmukh, O.; McGuckin, M.A.; Gidley, M.J. Mucin gel assembly is controlled by a collective action of non-mucin proteins, disulfide bridges, Ca2+-mediated links, and hydrogen bonding. Sci. Rep. 2018, 8, 5802. [Google Scholar] [CrossRef] [PubMed]
- Godl, K.; Johansson, M.E.V.; Lidell, M.E.; Mörgelin, M.; Karlsson, H.; Olson, F.J.; Gum, J.R., Jr.; Kim, Y.S.; Hansson, G.C. The N Terminus of the MUC2 Mucin Forms Trimers That Are Held Together within a Trypsin-resistant Core Fragment*. J. Biol. Chem. 2002, 277, 47248–47256. [Google Scholar] [CrossRef] [PubMed]
- McGuckin, M.A.; Lindén, S.K.; Sutton, P.; Florin, T.H. Mucin dynamics and enteric pathogens. Nat. Rev. Microbiol. 2011, 9, 265–278. [Google Scholar] [CrossRef]
- Birchenough, G.M.H.; Dalgakiran, F.; Witcomb, L.A.; Johansson, M.E.V.; McCarthy, A.J.; Hansson, G.C.; Taylor, P.W. Postnatal development of the small intestinal mucosa drives age-dependent, regio-selective susceptibility to Escherichia coli K1 infection. Sci. Rep. 2017, 7, 83. [Google Scholar] [CrossRef]
- Ren, S.; Hui, Y.; Obelitz-Ryom, K.; Brandt, A.B.; Kot, W.; Nielsen, D.S.; Thymann, T.; Sangild, P.T.; Nguyen, D.N. Neonatal gut and immune maturation is determined more by postnatal age than by postconceptional age in moderately preterm pigs. Am. J. Physiol.-Gastrointest. Liver Physiol. 2018, 315, G855–G867. [Google Scholar] [CrossRef]
- Liu, D.; Xu, Y.; Feng, J.; Yu, J.; Huang, J.; Li, Z. Mucins and Tight Junctions are Severely Altered in Necrotizing Enterocolitis Neonates. Am. J. Perinatol. 2020, 38, 1174–1180. [Google Scholar] [CrossRef]
- Hoffmann, W. Trefoil factors. Cell. Mol. Life Sci. 2005, 62, 2932–2938. [Google Scholar] [CrossRef]
- Bossenmeyer-Pourié, C.; Kannan, R.; Ribieras, S.; Wendling, C.; Stoll, I.; Thim, L.; Tomasetto, C.; Rio, M.C. The trefoil factor 1 participates in gastrointestinal cell differentiation by delaying G1-S phase transition and reducing apoptosis. J. Cell Biol. 2002, 157, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Renes, I.B.; Verburg, M.; Van Nispen, D.J.; Taminiau, J.A.; Büller, H.A.; Dekker, J.; Einerhand, A.W. Epithelial proliferation, cell death, and gene expression in experimental colitis: Alterations in carbonic anhydrase I, mucin MUC2, and trefoil factor 3 expression. Int. J. Color. Dis. 2002, 17, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Kim, D.; Ha, Y.; Cho, K.D.; Lee, B.H.; Seo, I.W.; Kim, S.H.; Chae, C. Expression of Mucins and Trefoil Factor Family Protein-1 in the Colon of Pigs Naturally Infected with Salmonella typhimurium. J. Comp. Pathol. 2009, 140, 38–42. [Google Scholar] [CrossRef]
- Olli, K.E.; Rapp, C.; O’Connell, L.; Collins, C.B.; McNamee, E.N.; Jensen, O.; Jedlicka, P.; Allison, K.C.; Goldberg, M.S.; Gerich, M.E.; et al. Muc5ac Expression Protects the Colonic Barrier in Experimental Colitis. Inflamm. Bowel Dis. 2020, 26, 1353–1367. [Google Scholar] [CrossRef] [PubMed]
- Odenwald, M.A.; Turner, J.R. The intestinal epithelial barrier: A therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 9–21. [Google Scholar] [CrossRef]
- Shen, L.; Weber, C.R.; Raleigh, D.R.; Yu, D.; Turner, J.R. Tight junction pore and leak pathways: A dynamic duo. Annu. Rev. Physiol. 2011, 73, 283–309. [Google Scholar] [CrossRef]
- Shawki, A.; McCole, D.F. Mechanisms of Intestinal Epithelial Barrier Dysfunction by Adherent-Invasive Escherichia coli. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 41–50. [Google Scholar] [CrossRef]
- Hackam, D.J.; Good, M.; Sodhi, C.P. Mechanisms of gut barrier failure in the pathogenesis of necrotizing enterocolitis: Toll-like receptors throw the switch. Semin. Pediatr. Surg. 2013, 22, 76–82. [Google Scholar] [CrossRef]
- Ares, G.; Wood, D.R.; Yuan, C.Y.; Hunter, C.J. Necrotizing Enterocolitis Induces Changes in Intestinal Epithelial Tight Junctions. J. Am. Coll. Surg. 2017, 225, e136–e137. [Google Scholar] [CrossRef]
- Hunter, C.J.; Singamsetty, V.K.; Chokshi, N.K.; Boyle, P.; Camerini, V.; Grishin, A.V.; Upperman, J.S.; Ford, H.R.; Prasadarao, N.V. Enterobacter sakazakii Enhances Epithelial Cell Injury by Inducing Apoptosis in a Rat Model of Necrotizing Enterocolitis. J. Infect. Dis. 2008, 198, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Ayabe, T.; Satchell, D.P.; Wilson, C.L.; Parks, W.C.; Selsted, M.E.; Ouellette, A.J. Secretion of microbicidal α-defensins by intestinal Paneth cells in response to bacteria. Nat. Immunol. 2000, 1, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Lokken-Toyli, K.L.; de Steenhuijsen Piters, W.A.A.; Zangari, T.; Martel, R.; Kuipers, K.; Shopsin, B.; Loomis, C.; Bogaert, D.; Weiser, J.N. Decreased production of epithelial-derived antimicrobial molecules at mucosal barriers during early life. Mucosal Immunol. 2021, 14, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, H.B.; da Mota, H.C.; Coutinho, V.B.; Robalinho, T.I.; Furtado, A.F.; Walker, E.; King, G.; Mahida, Y.R.; Sewell, H.F.; Wakelin, D. Absence of lysozyme (muramidase) in the intestinal Paneth cells of newborn infants with necrotising enterocolitis. J. Clin. Pathol. 1998, 51, 512. [Google Scholar] [CrossRef] [PubMed]
- McElroy, S.J.; Underwood, M.A.; Sherman, M.P. Paneth Cells and Necrotizing Enterocolitis: A Novel Hypothesis for Disease Pathogenesis. Neonatology 2013, 103, 10–20. [Google Scholar] [CrossRef]
- Zhang, C.; Sherman, M.P.; Prince, L.S.; Bader, D.; Weitkamp, J.-H.; Slaughter, J.C.; McElroy, S.J. Paneth cell ablation in the presence of Klebsiella pneumoniae induces necrotizing enterocolitis (NEC)-like injury in the small intestine of immature mice. Dis. Model. Mech. 2012, 5, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Leaphart, C.L.; Cavallo, J.; Gribar, S.C.; Cetin, S.; Li, J.; Branca, M.F.; Dubowski, T.D.; Sodhi, C.P.; Hackam, D.J. A Critical Role for TLR4 in the Pathogenesis of Necrotizing Enterocolitis by Modulating Intestinal Injury and Repair1. J. Immunol. 2007, 179, 4808–4820. [Google Scholar] [CrossRef]
- Heida, F.H.; Beyduz, G.; Bulthuis, M.L.C.; Kooi, E.M.W.; Bos, A.F.; Timmer, A.; Hulscher, J.B.F. Paneth cells in the developing gut: When do they arise and when are they immune competent? Pediatr. Res. 2016, 80, 306–310. [Google Scholar] [CrossRef]
- Brandtzaeg, P.; Kiyono, H.; Pabst, R.; Russell, M.W. Terminology: Nomenclature of mucosa-associated lymphoid tissue. Mucosal Immunol. 2008, 1, 31–37. [Google Scholar] [CrossRef]
- Maheshwari, A. Immunologic and Hematological Abnormalities in Necrotizing Enterocolitis. Clin. Perinatol. 2015, 42, 567–585. [Google Scholar] [CrossRef]
- Savilahti, E.; Järvenpää, A.-L.; Räihä, N.C.R. Serum Immunoglobulins in Preterm Infants: Comparison of Human Milk and Formula Feeding. Pediatrics 1983, 72, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Weitkamp, J.-H.; Koyama, T.; Rock, M.T.; Correa, H.; Goettel, J.A.; Matta, P.; Oswald-Richter, K.; Rosen, M.J.; Engelhardt, B.G.; Moore, D.J.; et al. Necrotising enterocolitis is characterised by disrupted immune regulation and diminished mucosal regulatory (FOXP3)/effector (CD4, CD8) T cell ratios. Gut 2013, 62, 73. [Google Scholar] [CrossRef] [PubMed]
- Weitkamp, J.-H.; Rosen, M.J.; Zhao, Z.; Koyama, T.; Geem, D.; Denning, T.L.; Rock, M.T.; Moore, D.J.; Halpern, M.D.; Matta, P.; et al. Small Intestinal Intraepithelial TCRγδ+ T Lymphocytes Are Present in the Premature Intestine but Selectively Reduced in Surgical Necrotizing Enterocolitis. PLoS ONE 2014, 9, e99042. [Google Scholar] [CrossRef] [PubMed]
- Neutra, M.R.; Mantis, N.J.; Kraehenbuhl, J.-P. Collaboration of epithelial cells with organized mucosal lymphoid tissues. Nat. Immunol. 2001, 2, 1004–1009. [Google Scholar] [CrossRef]
- Heel, K.A.; McCauley, R.D.; Papadimitriou, J.M.; Hall, J.C. REVIEW: Peyer’s patches. J. Gastroenterol. Hepatol. 1997, 12, 122–136. [Google Scholar] [CrossRef]
- Cornes, J.S. Number, size, and distribution of Peyer’s patches in the human small intestine: Part I The development of Peyer’s patches. Gut 1965, 6, 225–229. [Google Scholar] [CrossRef]
- Hunter, C.J.; Upperman, J.S.; Ford, H.R.; Camerini, V. Understanding the Susceptibility of the Premature Infant to Necrotizing Enterocolitis (NEC). Pediatr. Res. 2008, 63, 117–123. [Google Scholar] [CrossRef]
- Ho, N.T.; Li, F.; Lee-Sarwar, K.A.; Tun, H.M.; Brown, B.P.; Pannaraj, P.S.; Bender, J.M.; Azad, M.B.; Thompson, A.L.; Weiss, S.T.; et al. Meta-analysis of effects of exclusive breastfeeding on infant gut microbiota across populations. Nat. Commun. 2018, 9, 4169. [Google Scholar] [CrossRef]
- Dogra, S.K.; Chung, C.K.; Wang, D.; Sakwinska, O.; Colombo Mottaz, S.; Sprenger, N. Nurturing the Early Life Gut Microbiome and Immune Maturation for Long Term Health. Microorganisms 2021, 9, 2110. [Google Scholar] [CrossRef]
- Bittinger, K.; Zhao, C.; Li, Y.; Ford, E.; Friedman, E.S.; Ni, J.; Kulkarni, C.V.; Cai, J.; Tian, Y.; Liu, Q.; et al. Bacterial colonization reprograms the neonatal gut metabolome. Nat. Microbiol. 2020, 5, 838–847. [Google Scholar] [CrossRef]
- Saturio, S.; Nogacka, A.M.; Alvarado-Jasso, G.M.; Salazar, N.; de Los Reyes-Gavilán, C.G.; Gueimonde, M.; Arboleya, S. Role of Bifidobacteria on Infant Health. Microorganisms 2021, 9, 2415. [Google Scholar] [CrossRef] [PubMed]
- Arboleya, S.; Binetti, A.; Salazar, N.; Fernández, N.; Solís, G.; Hernandez-Barranco, A.; Margolles, A.; de los Reyes-Gavilán, C.G.; Gueimonde, M. Establishment and development of intestinal microbiota in preterm neonates. FEMS Microbiol. Ecol. 2012, 79, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Dahlgren, A.F.; Pan, A.; Lam, V.; Gouthro, K.C.; Simpson, P.M.; Salzman, N.H.; Nghiem-Rao, T.H. Longitudinal changes in the gut microbiome of infants on total parenteral nutrition. Pediatr. Res. 2019, 86, 107–114. [Google Scholar] [CrossRef]
- Rina, P.; Zeng, Y.; Ying, J.; Qu, Y.; Mu, D. Association of initial empirical antibiotic therapy with increased risk of necrotizing enterocolitis. Eur. J. Pediatr. 2020, 179, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Saleem, B.; Okogbule-Wonodi, A.C.; Fasano, A.; Magder, L.S.; Ravel, J.; Kapoor, S.; Viscardi, R.M. Intestinal Barrier Maturation in Very Low Birthweight Infants: Relationship to Feeding and Antibiotic Exposure. J. Pediatr. 2017, 183, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Chaaban, H.; Patel, M.M.; Burge, K.; Eckert, J.V.; Lupu, C.; Keshari, R.S.; Silasi, R.; Regmi, G.; Trammell, M.; Dyer, D.; et al. Early Antibiotic Exposure Alters Intestinal Development and Increases Susceptibility to Necrotizing Enterocolitis: A Mechanistic Study. Microorganisms 2022, 10, 519. [Google Scholar] [CrossRef] [PubMed]
- Dollings, M.C.; Brown, L. An Integrated Review of Intestinal Microbiota in the Very Premature Infant. Neonatal Netw. 2016, 35, 204–216. [Google Scholar] [CrossRef]
- He, Y.; Du, W.; Xiao, S.; Zeng, B.; She, X.; Liu, D.; Du, H.; Li, L.; Li, F.; Ai, Q.; et al. Colonization of fecal microbiota from patients with neonatal necrotizing enterocolitis exacerbates intestinal injury in germfree mice subjected to necrotizing enterocolitis-induction protocol via alterations in butyrate and regulatory T cells. J. Transl. Med. 2021, 19, 510. [Google Scholar] [CrossRef]
- Fu, X.; Li, S.; Jiang, Y.; Hu, X.; Wu, H. Necrotizing Enterocolitis and Intestinal Microbiota: The Timing of Disease and Combined Effects of Multiple Species. Front. Pediatr. 2021, 9, 657349. [Google Scholar] [CrossRef]
- Yan, Q.Q.; Condell, O.; Power, K.; Butler, F.; Tall, B.D.; Fanning, S. Cronobacter species (formerly known as Enterobacter sakazakii) in powdered infant formula: A review of our current understanding of the biology of this bacterium. J. Appl. Microbiol. 2012, 113, 1–15. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Y.; Lin, R.; Meng, X.; Zhao, W.; Shen, W.; Fan, H. Cronobacter sakazakii induces necrotizing enterocolitis by regulating NLRP3 inflammasome expression via TLR4. J. Med. Microbiol. 2020, 69, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Akkerman, R.; Kong, C.; Walvoort, M.T.C.; de Vos, P. More than sugar in the milk: Human milk oligosaccharides as essential bioactive molecules in breast milk and current insight in beneficial effects. Crit. Rev. Food Sci. Nutr. 2021, 61, 1184–1200. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.-T.; Nanthakumar, N.N.; Newburg, D.S. The Human Milk Oligosaccharide 2′-Fucosyllactose Quenches Campylobacter jejuni–Induced Inflammation in Human Epithelial Cells HEp-2 and HT-29 and in Mouse Intestinal Mucosa. J. Nutr. 2016, 146, 1980–1990. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, M.; Guo, H.; Yan, J.; Liu, F.; Chen, J.; Li, Y.; Ren, F. Human Milk Oligosaccharides Protect against Necrotizing Enterocolitis by Inhibiting Intestinal Damage via Increasing the Proliferation of Crypt Cells. Mol. Nutr. Food Res. 2019, 63, 1900262. [Google Scholar] [CrossRef]
- Roux, M.E.; McWilliams, M.; Phillips-Quagliata, J.M.; Weisz-Carrington, P.; Lamm, M.E. Origin of IgA-secreting plasma cells in the mammary gland. J. Exp. Med. 1977, 146, 1311–1322. [Google Scholar] [CrossRef]
- Gopalakrishna, K.P.; Macadangdang, B.R.; Rogers, M.B.; Tometich, J.T.; Firek, B.A.; Baker, R.; Ji, J.; Burr, A.H.P.; Ma, C.; Good, M.; et al. Maternal IgA protects against the development of necrotizing enterocolitis in preterm infants. Nat. Med. 2019, 25, 1110–1115. [Google Scholar] [CrossRef]
- Kordy, K.; Gaufin, T.; Mwangi, M.; Li, F.; Cerini, C.; Lee, D.J.; Adisetiyo, H.; Woodward, C.; Pannaraj, P.S.; Tobin, N.H.; et al. Contributions to human breast milk microbiome and enteromammary transfer of Bifidobacterium breve. PLoS ONE 2020, 15, e0219633. [Google Scholar] [CrossRef]
- McGuire, M.K.; McGuire, M.A. Got bacteria? The astounding, yet not-so-surprising, microbiome of human milk. Curr. Opin. Biotechnol. 2017, 44, 63–68. [Google Scholar] [CrossRef]
- Moossavi, S.; Sepehri, S.; Robertson, B.; Bode, L.; Goruk, S.; Field, C.J.; Lix, L.M.; de Souza, R.J.; Becker, A.B.; Mandhane, P.J.; et al. Composition and Variation of the Human Milk Microbiota Are Influenced by Maternal and Early-Life Factors. Cell Host Microbe 2019, 25, 324–335. [Google Scholar] [CrossRef]
- Di Simone, N.; Santamaria Ortiz, A.; Specchia, M.; Tersigni, C.; Villa, P.; Gasbarrini, A.; Scambia, G.; D’Ippolito, S. Recent Insights on the Maternal Microbiota: Impact on Pregnancy Outcomes. Front. Immunol. 2020, 11, 528202. [Google Scholar] [CrossRef] [PubMed]
- Arboleya, S.; Suárez, M.; Fernández, N.; Mantecón, L.; Solís, G.; Gueimonde, M.; de los Reyes-Gavilán, C.G. C-section and the Neonatal Gut Microbiome Acquisition: Consequences for Future Health. Ann. Nutr. Metab. 2018, 73 (Suppl. S3), 17–23. [Google Scholar] [CrossRef]
- Chu, D.M.; Antony, K.M.; Ma, J.; Prince, A.L.; Showalter, L.; Moller, M.; Aagaard, K.M. The early infant gut microbiome varies in association with a maternal high-fat diet. Genome Med. 2016, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Maher, S.E.; O’Brien, E.C.; Moore, R.L.; Byrne, D.F.; Geraghty, A.A.; Saldova, R.; Murphy, E.F.; Van Sinderen, D.; Cotter, P.D.; McAuliffe, F.M. The association between the maternal diet and the maternal and infant gut microbiome: A systematic review. Br. J. Nutr. 2023, 129, 1491–1499. [Google Scholar] [CrossRef]
- Murphy, K.; Ross, R.P.; Ryan, C.A.; Dempsey, E.M.; Stanton, C. Probiotics, Prebiotics, and Synbiotics for the Prevention of Necrotizing Enterocolitis. Front. Nutr. 2021, 8, 667188. [Google Scholar] [CrossRef] [PubMed]
- György, P.; Norris, R.F.; Rose, C.S. Bifidus factor. I. A variant of Lactobacillus bifidus requiring a special growth factor. Arch. Biochem. Biophys. 1954, 48, 193–201. [Google Scholar] [CrossRef]
- Srinivasjois, R.; Rao, S.; Patole, S. Prebiotic supplementation in preterm neonates: Updated systematic review and meta-analysis of randomised controlled trials. Clin. Nutr. 2013, 32, 958–965. [Google Scholar] [CrossRef]
- Deshmukh, M.; Patole, S. Prophylactic Probiotic Supplementation for Preterm Neonates—A Systematic Review and Meta-Analysis of Nonrandomized Studies. Adv. Nutr. 2021, 12, 1411–1423. [Google Scholar] [CrossRef]
- Chi, C.; Buys, N.; Li, C.; Sun, J.; Yin, C. Effects of prebiotics on sepsis, necrotizing enterocolitis, mortality, feeding intolerance, time to full enteral feeding, length of hospital stay, and stool frequency in preterm infants: A meta-analysis. Eur. J. Clin. Nutr. 2019, 73, 657–670. [Google Scholar] [CrossRef]
- Holscher, H.D.; Czerkies, L.A.; Cekola, P.; Litov, R.; Benbow, M.; Santema, S.; Alexander, D.D.; Perez, V.; Sun, S.; Saavedra, J.M.; et al. Bifidobacterium lactis Bb12 Enhances Intestinal Antibody Response in Formula-Fed Infants. J. Parenter. Enter. Nutr. 2012, 36, 106S–117S. [Google Scholar] [CrossRef]
- O’Brien, C.E.; Meier, A.K.; Cernioglo, K.; Mitchell, R.D.; Casaburi, G.; Frese, S.A.; Henrick, B.M.; Underwood, M.A.; Smilowitz, J.T. Early probiotic supplementation with B. infantis in breastfed infants leads to persistent colonization at 1 year. Pediatr. Res. 2022, 91, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Underwood, M.A.; Arriola, J.; Gerber, C.W.; Kaveti, A.; Kalanetra, K.M.; Kananurak, A.; Bevins, C.L.; Mills, D.A.; Dvorak, B. Bifidobacterium longum subsp. infantis in experimental necrotizing enterocolitis: Alterations in inflammation, innate immune response, and the microbiota. Pediatr. Res. 2014, 76, 326–333. [Google Scholar] [CrossRef]
- Lin, P.W.; Nasr, T.R.; Berardinelli, A.J.; Kumar, A.; Neish, A.S. The probiotic Lactobacillus GG may augment intestinal host defense by regulating apoptosis and promoting cytoprotective responses in the developing murine gut. Pediatr. Res. 2008, 64, 511–516. [Google Scholar] [CrossRef]
- Blackwood, B.P.; Yuan, C.Y.; Wood, D.R.; Nicolas, J.D.; Grothaus, J.S.; Hunter, C.J. Probiotic Lactobacillus Species Strengthen Intestinal Barrier Function and Tight Junction Integrity in Experimental Necrotizing Enterocolitis. J. Probiotics Health 2017, 5, 1. [Google Scholar] [CrossRef]
- Pell, L.G.; Loutet, M.G.; Roth, D.E.; Sherman, P.M. Arguments against routine administration of probiotics for NEC prevention. Curr. Opin. Pediatr. 2019, 31, 195–201. [Google Scholar] [CrossRef] [PubMed]
- García-Montero, C.; Fraile-Martinez, O.; Rodriguez-Martín, S.; Saz, J.V.; Rodriguez, R.A.; Moreno, J.M.P.; Labarta, J.R.; García-Honduvilla, N.; Alvarez-Mon, M.; Bravo, C.; et al. The Use of Prebiotics from Pregnancy and Its Complications: Health for Mother and Offspring— A Narrative Review. Foods 2023, 12, 1148. [Google Scholar] [PubMed]
- Yu, Y.; Lu, J.; Oliphant, K.; Gupta, N.; Claud, K.; Lu, L. Maternal administration of probiotics promotes gut development in mouse offsprings. PLoS ONE 2020, 15, e0237182. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golubkova, A.; Hunter, C.J. Development of the Neonatal Intestinal Barrier, Microbiome, and Susceptibility to NEC. Microorganisms 2023, 11, 1247. https://doi.org/10.3390/microorganisms11051247
Golubkova A, Hunter CJ. Development of the Neonatal Intestinal Barrier, Microbiome, and Susceptibility to NEC. Microorganisms. 2023; 11(5):1247. https://doi.org/10.3390/microorganisms11051247
Chicago/Turabian StyleGolubkova, Alena, and Catherine J. Hunter. 2023. "Development of the Neonatal Intestinal Barrier, Microbiome, and Susceptibility to NEC" Microorganisms 11, no. 5: 1247. https://doi.org/10.3390/microorganisms11051247
APA StyleGolubkova, A., & Hunter, C. J. (2023). Development of the Neonatal Intestinal Barrier, Microbiome, and Susceptibility to NEC. Microorganisms, 11(5), 1247. https://doi.org/10.3390/microorganisms11051247




