Targeted Mutations Produce Divergent Characteristics in Pedigreed Sake Yeast Strains
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Media
2.2. Whole-Genome Sequencing
2.3. Analysis of Structural Changes Predicted Due to Mutations
2.4. Component Analysis of Sake Made in Small-Scale Fermentation Tests
2.5. Fluorescence Staining, Microscopy, and Image Processing
2.6. Morphological Phenotyping of Sake Yeasts
2.6.1. Calculation of the Euclidean Distance in the Degenerated Morphological Space
2.6.2. Principal Component Analysis of the Effects of Genome Editing on Cell Morphology
3. Results
3.1. Isolation of Genome-Edited Sake Yeast Strains in K6, K9, and K10
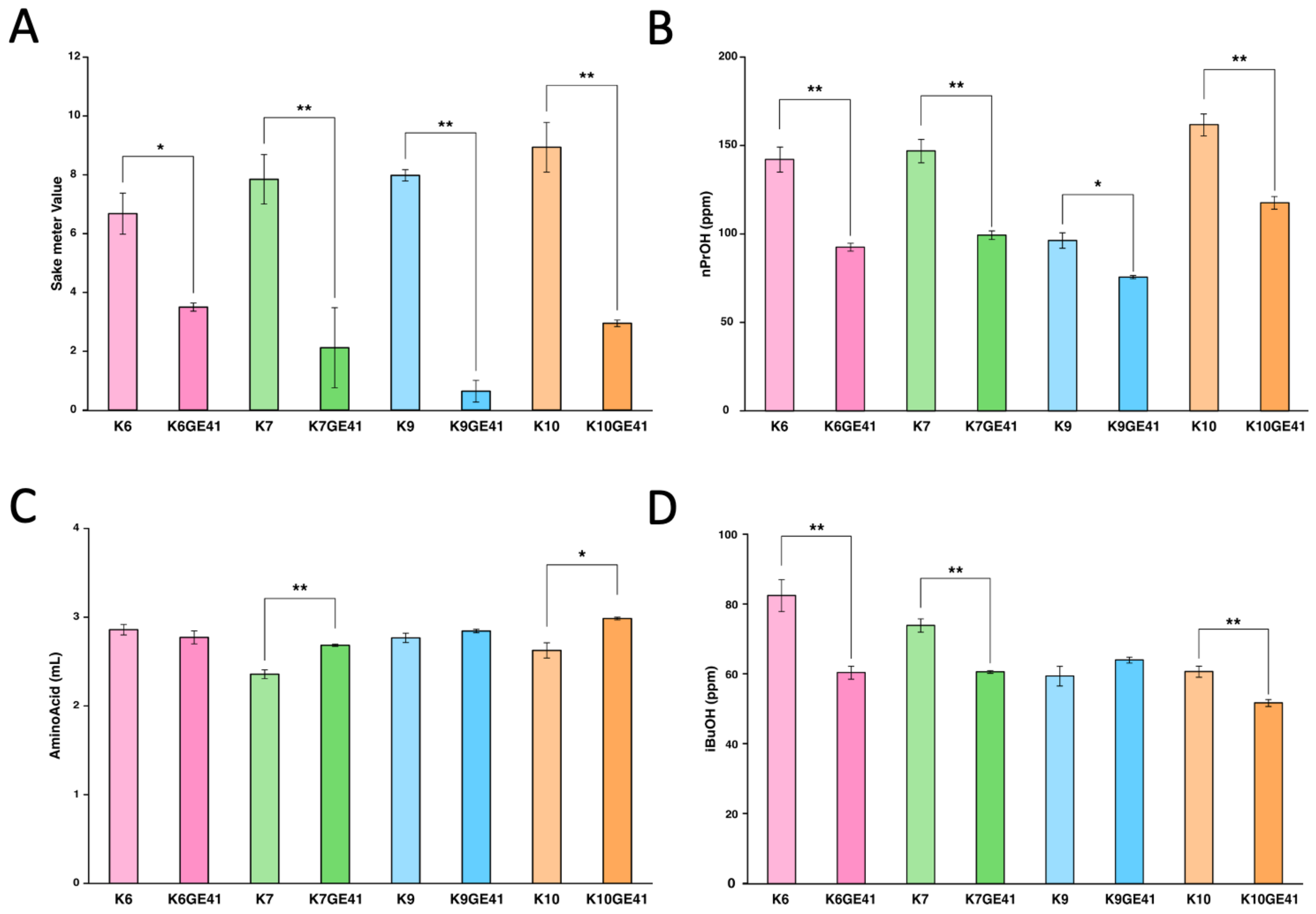
3.2. Excellent Brewing Characteristics of the Genome-Edited Strains
3.3. Component Analysis of Sake Made by the Genome-Edited Strains
3.4. Morphological Analysis of Genome-Edited Sake Yeast Strains
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akiyama, H. Sake: The Essence of 2000 Years of Japanese Wisdom Gained from Brewing Alcoholic Beverages from Rice; Brewing Society of Japan: Tokyo, Japan, 2010; ISBN 978-49-9033-941-8. [Google Scholar]
- Kitagaki, H.; Kitamoto, K. Breeding research on sake yeasts in Japan: History, recent technological advances, and future perspectives. Annu. Rev. Food Sci. Technol. 2013, 4, 215–235. [Google Scholar] [CrossRef] [PubMed]
- Peter, J.; De Chiara, M.; Friedrich, A.; Yue, J.-X.; Pflieger, D.; Bergström, A.; Sigwalt, A.; Barre, B.; Freel, K.; Llored, A.; et al. Genome evolution across 1011 Saccharomyces cerevisiae isolates. Nature 2018, 556, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Shimoi, H.; Sakamoto, K.; Okuda, M.; Atthi, R.; Iwashita, K.; Ito, K. The AWA1 gene is required for the foam-forming phenotype and cell surface hydrophobicity of sake yeast. Appl. Environ. Microbiol. 2002, 68, 2018–2025. [Google Scholar] [CrossRef]
- Ohdate, T.; Omura, F.; Hatanaka, H.; Zhou, Y.; Takagi, M.; Goshima, T.; Akao, T.; Ono, E. MAL73, a novel regulator of maltose fermentation, is functionally impaired by single nucleotide polymorphism in sake brewing yeast. PLoS ONE 2018, 13, e0198744. [Google Scholar] [CrossRef] [PubMed]
- Akao, T. Progress in the genomics and genome-wide study of sake yeast. Biosci. Biotechnol. Biochem. 2019, 83, 1463–1472. [Google Scholar] [CrossRef]
- Ohnuki, S.; Okada, H.; Friedrich, A.; Kanno, Y.; Goshima, T.; Hasuda, H.; Inahashi, M.; Okazaki, N.; Tamura, H.; Nakamura, R.; et al. Phenotypic diagnosis of lineage and differentiation during sake yeast breeding. Genes Genomes Genet. 2017, 7, 2807–2820. [Google Scholar] [CrossRef]
- Ohya, Y.; Kashima, M. History, lineage and phenotypic differentiation of sake yeast. Biosci. Biotechnol. Biochem. 2019, 83, 1442–1448. [Google Scholar] [CrossRef]
- Yoshida, M. Kyoukai Sake Yeast 13. J. Brew. Soc. Jpn. 1985, 80, 601–602. [Google Scholar] [CrossRef]
- Yoshida, K. Kyoukai Sake Yeast 1801. J. Brew. Soc. Jpn. 2006, 101, 910–922. [Google Scholar] [CrossRef]
- Tamura, H.; Okada, H.; Kume, K.; Koyano, T.; Goshima, T.; Nakamura, R.; Akao, T.; Shimoi, H.; Mizunuma, M.; Ohya, Y.; et al. Isolation of a spontaneous cerulenin-resistant sake yeast with both high ethyl caproate-producing ability and normal checkpoint integrity. Biosci. Biotechnol. Biochem. 2015, 79, 1191–1199. [Google Scholar] [CrossRef]
- Kitamoto, K.; Oda, K.; Gomi, K.; Takahashi, K. Genetic engineering of a sake yeast producing no urea by successive disruption of arginase gene. Appl. Environ. Microbiol. 1991, 57, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, K.; Isogai, A.; Watanabe, D.; Fujita, A.; Sudo, S. Involvement of methionine salvage pathway genes of Saccharomyces cerevisiae in the production of precursor compounds of dimethyl trisulfide (DMTS). J. Biosci. Bioeng. 2013, 116, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, E.; Hosokawa, N.; Hata, Y.; Abe, Y.; Suginami, K.; Imayasu, S. Breeding of a sake yeast with improved ethyl caproate productivity. Agric. Biol. Chem. 1991, 55, 2153–2154. [Google Scholar] [CrossRef]
- Chadani, T.; Ohnuki, S.; Isogai, A.; Goshima, T.; Kashima, M.; Ghanegolmohammadi, F.; Nishi, T.; Hirata, D.; Watanabe, D.; Kitamoto, K.; et al. Genome editing to generate sake yeast strains with eight mutations that confer excellent brewing characteristics. Cells 2021, 10, 1299. [Google Scholar] [CrossRef] [PubMed]
- Ryan, O.W.; Skerker, J.M.; Maurer, M.J.; Li, X.; Tsai, J.C.; Poddar, S.; Lee, M.E.; DeLoache, W.; Dueber, J.E.; Arkin, A.P.; et al. Selection of chromosomal DNA libraries using a multiplex CRISPR system. Elife 2014, 3, e03703. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map Format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef]
- Cingolani, A.; Platts, A.; Wang, L.L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, S.; Ruden, D.M. A program for annotating and predicting the effects of single nucleotide poly-morphisms, SnpEff. Fly 2021, 6, 80–92. [Google Scholar] [CrossRef]
- Robinson, J.T.; Thorvaldsdóttir, H.; Winckler, W.; Guttman, M.; Lander, E.S.; Getz, G.; Mesirov, J.P. Integrative genomics viewer. Nat. Biotechnol. 2011, 29, 24–26. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Mirdita, M.; Schütze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold: Making protein folding accessible to all. Nat. Methods 2022, 19, 679–682. [Google Scholar] [CrossRef]
- Frishman, D.; Argos, P. Knowledge-based protein secondary structure assignment. Proteins 1995, 23, 566–579. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Nishida, I.; Ohtake, N.; Hirata, D. Nitrogen fertilization of rice plants before flowering affects sake fermentation and quality. Cereal Chem. 2023, 100, 277–283. [Google Scholar] [CrossRef]
- Nagai, Y.; Suzuki, T.; Hoshino, A.; Kuribayashi, T.; Hara, T.; Joh, T. The simple determination of urea in sake using a commercial assay kit. J. Brew. Soc. Jpn. 2020, 115, 109–114. [Google Scholar]
- Ohya, Y.; Sese, J.; Yukawa, M.; Sano, F.; Nakatani, Y.; Saito, T.L.; Saka, A.; Fukuda, T.; Ishihara, S.; Oka, S.; et al. High-dimensional and large-scale phenotyping of yeast mutants. Proc. Natl. Acad. Sci. USA 2005, 102, 19015–19020. [Google Scholar] [CrossRef] [PubMed]
- Yvert, G.; Ohnuki, S.; Nogami, S.; Imanaga, Y.; Fehrmann, S.; Schacherer, J.; Ohya, Y. Single-cell phenomics reveals intra-species variation of phenotypic noise in yeast. BMC Syst. Biol. 2013, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Deza, M.; Deza, E. Encyclopedia of Distances, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 978-3-642-00234-2. [Google Scholar]
- Yang, M.; Ohnuki, S.; Ohya, Y. Unveiling nonessential gene deletions that confer significant morphological phenotypes beyond natural yeast strains. BMC Genom. 2014, 15, 932. [Google Scholar] [CrossRef]
- Ohnuki, S.; Kobayashi, T.; Ogawa, H.; Kozone, I.; Ueda, J.Y.; Takagi, M.; Shin-Ya, K.; Hirata, D.; Nogami, S.; Ohya, Y. Analysis of the biological activity of a novel 24-membered macrolide JBIR-19 in Saccharomyces cerevisiae by the morphological imaging program CalMorph. FEMS Yeast Res. 2012, 12, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Ohnuki, S.; Kashima, M.; Yamada, T.; Ghanegolmohammadi, F.; Zhou, Y.; Goshima, T.; Maruyama, J.-I.; Kitamoto, K.; Hirata, D.; Akao, T.; et al. Genome editing to generate nonfoam-forming sake yeast strains. Biosci. Biotechnol. Biochem. 2019, 83, 1583–1593. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, G.; Wang, Y.; Kubo, K.; Hirata, E.; Ohnuki, S.; Ohya, Y. Global study of holistic morphological effectors in the budding yeast Saccharomyces cerevisiae. BMC Genom. 2018, 19, 149. [Google Scholar] [CrossRef]
- Deutschbauer, A.M.; Jaramillo, D.F.; Proctor, M.; Kumm, J.; Hillenmeyer, M.E.; Davis, R.W.; Nislow, C.; Giaever, G. Mechanisms of haploinsufficiency revealed by genome-wide profiling in yeast. Genetics 2005, 169, 1915–1925. [Google Scholar] [CrossRef]
- Ohnuki, S.; Ohya, Y. High-dimensional single-cell phenotyping reveals extensive haploinsufficiency. PLoS Biol. 2018, 16, e2005130. [Google Scholar] [CrossRef]
- Dowell, R.D.; Ryan, O.; Jansen, A.; Cheung, D.; Agarwala, S.; Danford, T.; Bernstein, D.A.; Rolfe, P.A.; Heisler, L.E.; Chin, B.; et al. Genotype to phenotype: A complex problem. Science 2010, 328, 469. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Tan, G.; Fink, G.R.; Andrews, B.J.; Boone, C. Complex modifier landscape underlying genetic background effects. Proc. Natl. Acad. Sci. USA 2019, 116, 5045–5054. [Google Scholar] [CrossRef]
- Schacherer, J.; Ruderfer, D.M.; Gresham, D.; Dolinski, K.; Botstein, D.; Kruglyak, L. Genome-wide analysis of nucleotide-level variation in commonly used Saccharomyces cerevisiae strains. PLoS ONE 2007, 2, e322. [Google Scholar] [CrossRef] [PubMed]
- Mozzachiodi, S.; Tattini, L.; Llored, A.; Irizar, A.; Škofljanc, N.; D’Angiolo, M.; De Chiara, M.; Barré, B.P.; Yue, J.X.; Lutazi, A.; et al. Aborting meiosis allows recombination in sterile diploid yeast hybrids. Nat. Commun. 2021, 12, 6564. [Google Scholar] [CrossRef] [PubMed]
- Mozzachiodi, S.; Krogerus, K.; Gibson, B.; Nicolas, A.; Liti, G. Unlocking the functional potential of polyploid yeasts. Nat. Commun. 2022, 13, 2580. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klinkaewboonwong, N.; Ohnuki, S.; Chadani, T.; Nishida, I.; Ushiyama, Y.; Tomiyama, S.; Isogai, A.; Goshima, T.; Ghanegolmohammadi, F.; Nishi, T.; et al. Targeted Mutations Produce Divergent Characteristics in Pedigreed Sake Yeast Strains. Microorganisms 2023, 11, 1274. https://doi.org/10.3390/microorganisms11051274
Klinkaewboonwong N, Ohnuki S, Chadani T, Nishida I, Ushiyama Y, Tomiyama S, Isogai A, Goshima T, Ghanegolmohammadi F, Nishi T, et al. Targeted Mutations Produce Divergent Characteristics in Pedigreed Sake Yeast Strains. Microorganisms. 2023; 11(5):1274. https://doi.org/10.3390/microorganisms11051274
Chicago/Turabian StyleKlinkaewboonwong, Norapat, Shinsuke Ohnuki, Tomoya Chadani, Ikuhisa Nishida, Yuto Ushiyama, Saki Tomiyama, Atsuko Isogai, Tetsuya Goshima, Farzan Ghanegolmohammadi, Tomoyuki Nishi, and et al. 2023. "Targeted Mutations Produce Divergent Characteristics in Pedigreed Sake Yeast Strains" Microorganisms 11, no. 5: 1274. https://doi.org/10.3390/microorganisms11051274
APA StyleKlinkaewboonwong, N., Ohnuki, S., Chadani, T., Nishida, I., Ushiyama, Y., Tomiyama, S., Isogai, A., Goshima, T., Ghanegolmohammadi, F., Nishi, T., Kitamoto, K., Akao, T., Hirata, D., & Ohya, Y. (2023). Targeted Mutations Produce Divergent Characteristics in Pedigreed Sake Yeast Strains. Microorganisms, 11(5), 1274. https://doi.org/10.3390/microorganisms11051274







