Chlamydia trachomatis—An Emerging Old Entity?
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Epidemiology
3.2. Chlamydial Biovars and Serovars
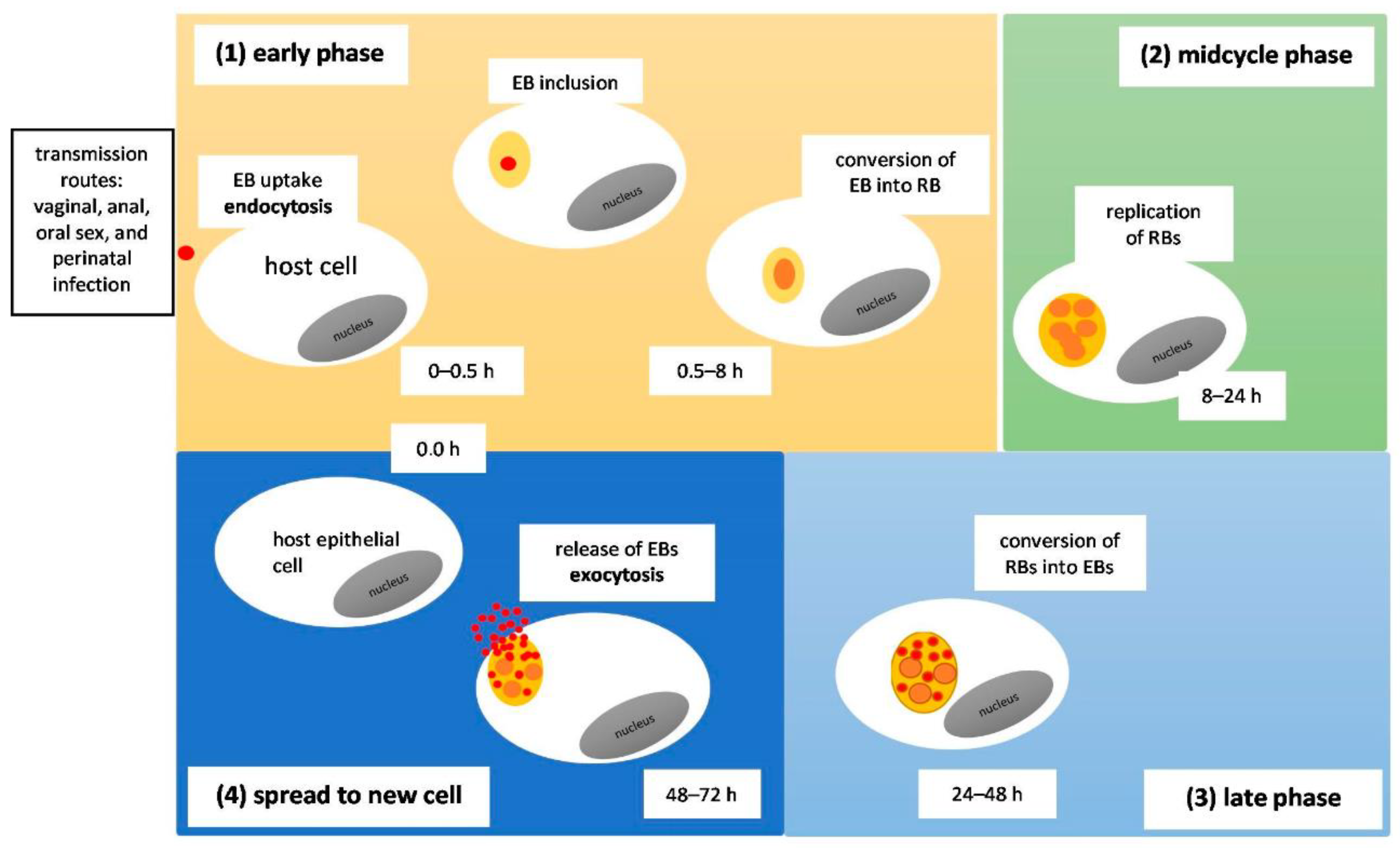
3.3. Modes of Transmission/Pathomechanism
3.4. Virulence Factors
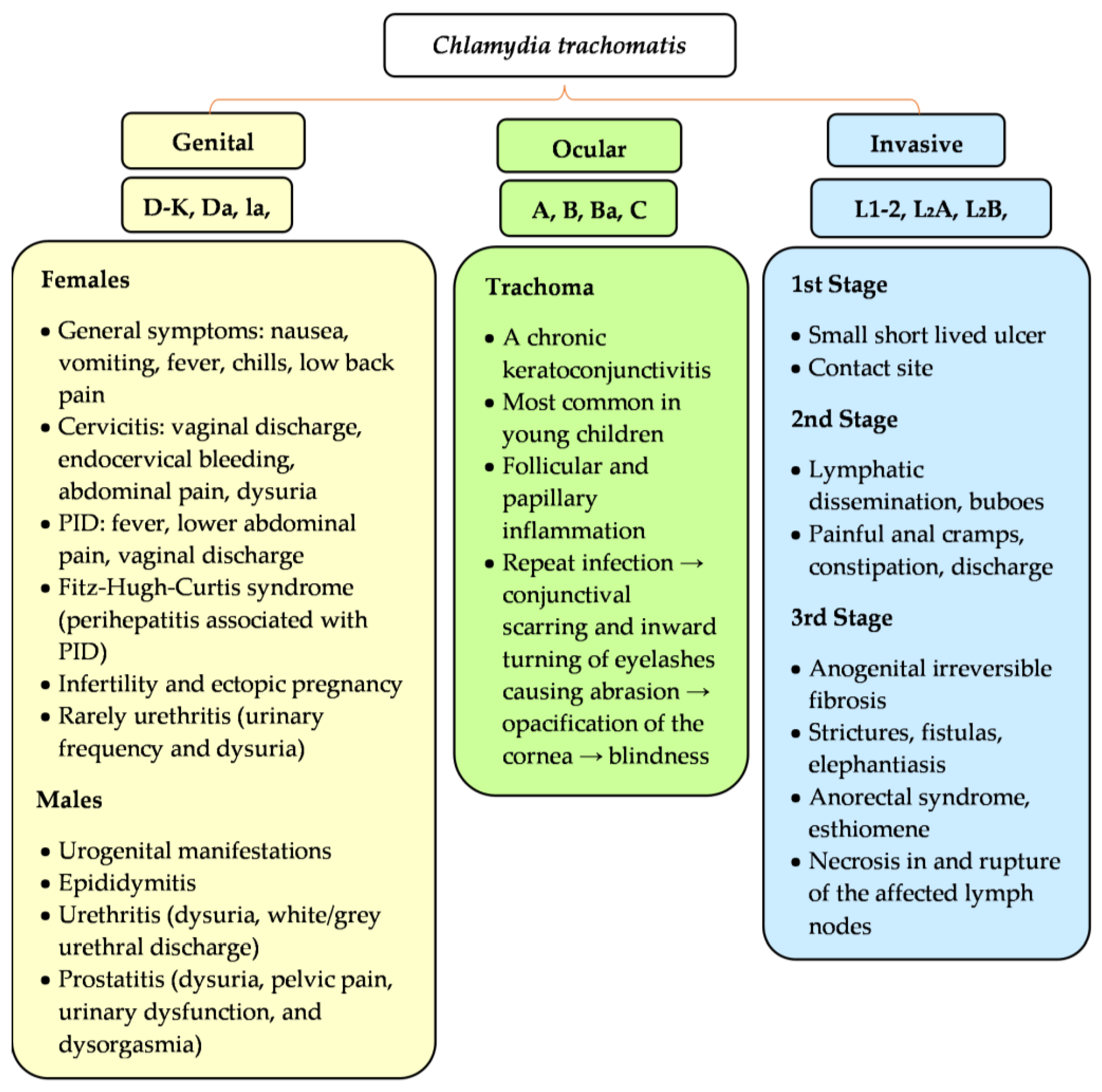
3.5. Clinical Signs and Symptoms
3.5.1. Females
3.5.2. Males
3.5.3. Males and Females
Lymphogranuloma Venereum
Trachoma
3.5.4. Neonates
3.6. Clinical Complications
3.7. Screening for Chlamydia
3.8. Diagnostics/Sampling
3.9. Treatment
3.9.1. Females
3.9.2. Males
3.9.3. Lymphogranuloma venereum
3.9.4. Trachoma
3.9.5. Neonatal Infections
3.9.6. Antibiotic Resistance
4. Discussion
4.1. Prognosis
4.2. Prevention
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Phillips, S.; Quigley, B.L.; Timms, P. Seventy Years of Chlamydia Vaccine Research—Limitations of the Past and Directions for the Future. Front. Microbiol. 2019, 10, 70. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, M.; Sood, S.; Mukherjee, A.; Muralidhar, S.; Bala, M. Genital Chlamydia trachomatis: An update. Indian J. Med. Res. 2013, 138, 303–316. [Google Scholar] [PubMed]
- Mylonas, I. Female genital Chlamydia trachomatis infection: Where are we heading? Arch. Gynecol. Obstet. 2012, 285, 1271–1285. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, M.; Sung, S.; Takov, V. Chlamydia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022; [Updated 18 September 2022]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537286/ (accessed on 18 November 2022).
- Byrne, G.I. Chlamydia trachomatis strains and virulence: Rethinking links to infection prevalence and disease severity. J. Infect. Dis. 2010, 201 (Suppl. S2), S126–S133. [Google Scholar] [CrossRef]
- Zhong, G. Killing me softly: Chlamydial use of proteolysis for evading host defenses. Trends Microbiol. 2009, 17, 467–474. [Google Scholar] [CrossRef]
- Elwell, C.; Mirrashidi, K.; Engel, J. Chlamydia cell biology and pathogenesis. Nat. Rev. Microbiol. 2016, 14, 385–400. [Google Scholar] [CrossRef]
- Meyer, T. Diagnostic Procedures to Detect Chlamydia trachomatis Infections. Microorganisms 2016, 4, 25. [Google Scholar] [CrossRef]
- Hocking, J.S.; Temple-Smith, M.; Guy, R.; Donovan, B.; Braat, S.; Law, M.; Gunn, J.; Regan, D.; Vaisey, A.; Bulfone, L.; et al. Population effectiveness of opportunistic chlamydia testing in primary care in Australia: A cluster-randomised controlled trial. Lancet 2018, 392, 1413–1422. [Google Scholar] [CrossRef]
- Tjahyadi, D.; Ropii, B.; Tjandraprawira, K.D.; Parwati, I.; Djuwantono, T.; Permadi, W.; Li, T. Female urogenital chlamydia: Epidemiology, Chlamydia on pregnancy, current diagnosis, and treatment. Ann. Med. Surg. 2022, 75, 103448. [Google Scholar] [CrossRef]
- O’Connell, C.M.; Ferone, M.E. Chlamydia trachomatis Genital Infections. Microb. Cell 2016, 3, 390–403. [Google Scholar] [CrossRef]
- Newman, L.; Rowley, J.; Hoorn, S.V.; Wijesooriya, N.S.; Unemo, M.; Low, N.; Stevens, G.; Gottlieb, S.; Kiarie, J.; Temmerman, M. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS ONE 2015, 10, e0143304. [Google Scholar] [CrossRef] [PubMed]
- Heijne, J.C.M.; van den Broek, I.V.F.; Bruisten, S.M.; van Bergen, J.E.A.; de Graaf, H.; van Benthem, B.H.B. National prevalence estimates of chlamydia and gonorrhoea in the Netherlands. Sex. Transm. Infect. 2019, 95, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Woodhall, S.C.; Wills, G.S.; Horner, P.J.; Craig, R.; Mindell, J.; Murphy, G.; McClure, M.O.; Soldan, K.; Nardone, A.; Johnson, A.M. Chlamydia trachomatis Pgp3 antibody population seroprevalence before and during an era of widespread opportunistic chlamydia screening in England (1994–2012). PLoS ONE 2017, 12, e0152810. [Google Scholar] [CrossRef]
- van Aar, F.; de Moraes, M.; Morré, S.A.; van Bergen, J.E.; van der Klis, F.R.; Land, J.A.; van der Sande, M.A.; van den Broek, I.V. Chlamydia trachomatis IgG seroprevalence in the general population of the Netherlands in 1996 and in 2007: Differential changes by gender and age. Sex. Transm. Infect. 2014, 90, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Hoenderboom, B.M.; van Benthem, B.H.B.; van Bergen, J.E.A.M.; Dukers-Muijrers, N.H.T.M.; Götz, H.M.; Hoebe, C.J.P.A.; Hogewoning, A.A.; Land, J.A.; van der Sande, M.A.B.; Morré, S.A.; et al. Relation between Chlamydia trachomatis infection and pelvic inflammatory disease, ectopic pregnancy and tubal factor infertility in a Dutch cohort of women previously tested for Chlamydia in a chlamydia screening trial. Sex. Transm. Infect. 2019, 95, 300–306. [Google Scholar] [CrossRef]
- van der Steeg, J.W.; Steures, P.; Eijkemans, M.J.; Habbema, J.D.; Hompes, P.G.; Michgelsen, H.W.; van der Heijden, P.F.; Bossuyt, P.M.; van der Veen, F.; Mol, B.W.; et al. Predictive value of pregnancy history in subfertile couples: Results from a nationwide cohort study in the Netherlands. Fertil. Steril. 2008, 90, 521–527. [Google Scholar] [CrossRef]
- Price, M.J.; Ades, A.; Soldan, K.; Welton, N.J.; Macleod, J.; Simms, I.; DeAngelis, D.; Turner, K.M.; Horner, P.J. The natural history of Chlamydia trachomatis infection in women: A multi-parameter evidence synthesis. Health Technol. Assess. 2016, 20, 1–250. [Google Scholar] [CrossRef] [PubMed]
- Trojian, T.H.; Lishnak, T.S.; Heiman, D. Epididymitis and orchitis: An overview. Am. Fam. Physician 2009, 79, 583–587. [Google Scholar]
- Jordan, S.J.; Toh, E.; Williams, J.A.; Fortenberry, L.; LaPradd, M.L.; Katz, B.P.; Batteiger, B.E.; Nelson, D.E.; Batteiger, T.A. Aetiology and prevalence of mixed-infections and mono-infections in nongonococcal urethritis in men: A case-control study. Sex. Transm. Infect. 2020, 96, 306–311. [Google Scholar] [CrossRef]
- Manhart, L.E.; Gillespie, C.W.; Lowens, M.S.; Khosropour, C.M.; Colombara, D.V.; Golden, M.R.; Hakhu, N.R.; Thomas, K.K.; Hughes, J.P.; Jensen, N.L.; et al. Standard treatment regimens for nongonococcal urethritis have similar but declining cure rates: A randomized controlled trial. Clin. Infect. Dis. 2013, 56, 934–942. [Google Scholar] [CrossRef]
- Bradshaw, C.S.; Tabrizi, S.N.; Read, T.R.H.; Garland, S.M.; Hopkins, C.A.; Moss, L.M.; Fairley, C.K. Etiologies of nongonococcal urethritis: Bacteria, viruses, and the association with orogenital exposure. J. Infect. Dis. 2006, 193, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, G.F.; Muñoz, G.; Sánchez, R.; Henkel, R.; Gallegos-Avila, G.; Díaz-Gutierrez, O.; Vigil, P.; Vásquez, F.; Kortebani, G.; Mazzolli, A.; et al. Update on the impact of Chlamydia trachomatis infection on male fertility. Andrologia 2004, 36, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Moazenchi, M.; Totonchi, M.; Salman Yazdi, R.; Hratian, K.; Mohseni Meybodi, M.A.; Ahmadi Panah, M.; Chehrazi, M.; Mohseni Meybodi, A. The impact of Chlamydia trachomatis infection on sperm parameters and male fertility: A comprehensive study. Int. J. STD AIDS 2018, 29, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Tsuchiya, T.; Yasuda, M.; Yokoi, S.; Nakano, M.; Deguchi, T. Prevalence of genital mycoplasmas and ureaplasmas in men younger than 40 years-of-age with acute epididymitis. Int. J. Urol. 2012, 19, 234–238. [Google Scholar] [CrossRef]
- Satterwhite, C.L.; Torrone, E.; Meites, E.; Dunne, E.F.; Mahajan, R.; Ocfemia, M.C.B.; Su, J.; Xu, F.; Weinstock, H. Sexually Transmitted Infections among US Women and Men: Prevalence and Incidence Estimates, 2008. Sex. Transm. Dis. 2013, 40, 187–193. Available online: https://www.jstor.org/stable/48512402 (accessed on 4 December 2022). [CrossRef]
- Torrone, E.; Papp, J.; Weinstock, H.; Centers for Disease Control and Prevention (CDC). Prevalence of Chlamydia trachomatis genital infection among persons aged 14-39 years—United States, 2007–2012. Morb. Mortal. Wkly. Rep. 2014, 63, 834–838. [Google Scholar]
- Alambo, M.M.; Lake, E.A.; Bitew Workie, S.; Wassie, A.Y. Prevalence of Active Trachoma and Associated Factors in Areka Town, South Ethiopia, 2018. Interdiscip. Perspect. Infect. Dis. 2020, 2020, 8635191. [Google Scholar] [CrossRef]
- Polack, S.; Brooker, S.; Kuper, H.; Mariotti, S.; Mabey, D.; Foster, A. Mapping the global distribution of trachoma. Bull. World Health Organ. 2005, 83, 913–919. [Google Scholar]
- Bourne, R.R.A.; Flaxman, S.R.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; Leasher, J.; Limburg, H.; et al. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e888. [Google Scholar] [CrossRef]
- Wang, S.-P.; Grayston, J. Microimmunofluorescence serology of Chlamydia trachomatis. In Medical Virology; Peterson, E.M., de la Maza, L.M., Eds.; Elsevier Science: New York, NY, USA, 1984; Volume 3. [Google Scholar]
- Stephens, R.S.; Myers, G.; Eppinger, M.; Bavoil, P.M. Divergence without difference: Phylogenetics and taxonomy of Chlamydia resolved. FEMS Immunol. Med. Microbiol. 2009, 55, 115–119. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Chlamydia—CDC Fact Sheet (Detailed). 2021. Available online: https://www.cdc.gov/std/chlamydia/stdfact-chlamydia-detailed.htm (accessed on 7 December 2022).
- Abdelsamed, H.; Peters, J.; Byrne, G.I. Genetic variation in Chlamydia trachomatis and their hosts: Impact on disease severity and tissue tropism. Future Microbiol. 2013, 8, 1129–1146. [Google Scholar] [CrossRef] [PubMed]
- Sam, M.M.; McKay, P.F. Chlamydia trachomatis: Cell biology, immunology and vaccination. Vaccine 2021, 39, 2965–2975. [Google Scholar]
- Bastidas, R.J.; Elwell, C.A.; Engel, J.N.; Valdivia, R.H. Chlamydial intracellular survival strategies. Cold Spring Harb. Perspect. Med. 2013, 3, a010256. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.R.; Ouellette, S.P. Reconceptualizing the chlamydial inclusion as a pathogen-specified parasitic organelle: An expanded role for Inc proteins. Front. Cell. Infect. Microbiol. 2014, 4, 157. [Google Scholar] [CrossRef] [PubMed]
- Mölleken, K.; Schmidt, E.; Hegemann, J.H. Members of the Pmp protein family of Chlamydia pneumoniae mediate adhesion to human cells via short repetitive peptide motifs. Mol. Microbiol. 2010, 78, 1004–1017. [Google Scholar] [CrossRef]
- Casson, N.; Michel, R.; Müller, K.D.; Aubert, J.D.; Greub, G. Protochlamydia naegleriophila as etiologic agent of pneumonia. Emerg. Infect. Dis. 2008, 14, 168–172. [Google Scholar] [CrossRef]
- Choroszy-Król, I.C.-K.; Frej-Mądrzak, M.; Jama-Kmiecik, A.; Bober, T.; Sarowska, J.J. Characteristics of the Chlamydia trachomatis species-immunopathology and infections. Adv. Clin. Exp. Med. 2012, 21, 799–808. [Google Scholar]
- Taylor-Brown, A.; Vaughan, L.; Greub, G.; Timms, P.; Polkinghorne, A. Twenty years of research into Chlamydia-like organisms: A revolution in our understanding of the biology and pathogenicity of members of the phylum Chlamydiae. Pathog. Dis. 2015, 73, 1–15. [Google Scholar] [CrossRef]
- Roger, G.R.; Yeruva, L. Hidden in plain sight: Chlamydial gastrointestinal infection and its relevance to persistence in human genital infection. Infect. Immun. 2014, 82, 1362–1371. [Google Scholar]
- Jewett, T.J.; Miller, N.J.; Dooley, C.A.; Hackstadt, T. The conserved Tarp actin binding domain is important for chlamydial invasion. PLoS Pathog. 2010, 6, e1000997. [Google Scholar] [CrossRef]
- Almeida, F.; Borges, V.; Ferreira, R.; Borrego, M.J.; Gomes, J.P.; Mota, L.J. Polymorphisms in inc proteins and differential expression of inc genes among Chlamydia trachomatis strains correlate with invasiveness and tropism of lymphogranuloma venereum isolates. J. Bacteriol. 2012, 194, 6574–6585. [Google Scholar] [CrossRef] [PubMed]
- Greub, G.; Mege, J.L.; Raoult, D. Parachlamydia acanthamoebae enters and multiplies within human macrophages and induces their apoptosis [corrected]. Infect. Immun. 2003, 71, 5979–5985, Erratum in Infect. Immun. 2004, 72, 621. [Google Scholar] [CrossRef] [PubMed]
- Sixt, B.S.; Kostanjšek, R.; Mustedanagic, A.; Toenshoff, E.R.; Horn, M. Developmental cycle and host interaction of Rhabdochlamydia porcellionis, an intracellular parasite of terrestrial isopods. Environ. Microbiol. 2013, 15, 2980–2993. [Google Scholar] [PubMed]
- Pallen, M.J.; Beatson, S.; Bailey, C.M. Bioinformatics, genomics and evolution of non-flagellar type-III secretion systems: A Darwinian perpective. FEMS Microbiol. Rev. 2005, 29, 201–229. [Google Scholar] [CrossRef] [PubMed]
- Hueck, C.J. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 1998, 62, 379–433. [Google Scholar] [CrossRef]
- Hsia, R.-C.; Pannekoek, Y.; Ingerowski, E.; Bavoil, P.M. Type III secretion genes identify a putative virulence locus of Chlamydia. Mol. Microbiol. 1997, 25, 351–359. [Google Scholar] [CrossRef]
- Betts, H.J.; Wolf, K.; Fields, K.A. Effector protein modulation of host cells: Examples in the Chlamydia spp. arsenal. Curr. Opin. Microbiol. 2009, 12, 81–87. [Google Scholar] [CrossRef]
- Valdivia, R.H. Chlamydia effector proteins and new insights into chlamydial cellular microbiology. Curr. Opin. Microbiol. 2008, 11, 53–59. [Google Scholar] [CrossRef]
- Prantner, D.; Nagarajan, U.M. Role for the chlamydial type III secretion apparatus in host cytokine expression. Infect. Immun. 2009, 77, 76–84. [Google Scholar] [CrossRef]
- Jorgensen, I.; Bednar, M.M.; Amin, V.; Davis, B.K.; Ting, J.P.; McCafferty, D.G.; Valdivia, R.H. The Chlamydia protease CPAF regulates host and bacterial proteins to maintain pathogen vacuole integrity and promote virulence. Cell Host Microbe 2011, 10, 21–32. [Google Scholar] [CrossRef]
- Favaroni, A.; Hegemann, J.H. Chlamydia trachomatis Polymorphic Membrane Proteins (Pmps) Form Functional Homomeric and Heteromeric Oligomers. Front. Microbiol. 2021, 12, 709724. [Google Scholar] [CrossRef] [PubMed]
- Becker, E.; Hegemann, J.H. All subtypes of the Pmp adhesin family are implicated in chlamydial virulence and show species-specific function. Microbiologyopen 2014, 3, 544–556. [Google Scholar] [CrossRef] [PubMed]
- Belland, R.J.; Scidmore, M.A.; Crane, D.D.; Hogan, D.M.; Whitmire, W.; McClarty, G.; Caldwell, H.D. Chlamydia trachomatis cytotoxicity associated with complete and partial cytotoxin genes. Proc. Natl. Acad. Sci. USA 2001, 98, 13984–13989. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Carlson, J.H.; Whitmire, W.M.; Kari, L.; Virtaneva, K.; Sturdevant, D.E.; Watkins, H.; Zhou, B.; Sturdevant, G.L.; Porcella, S.F.; et al. Chlamydia trachomatis plasmid-encoded Pgp4 is a transcriptional regulator of virulence-associated genes. Infect. Immun. 2013, 81, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, Y.M.; Rose, L.A.; Belland, R.J. Developmental expression of non-coding RNAs in Chlamydia trachomatis during normal and persistent growth. Nucleic Acids Res. 2011, 39, 1843–1854. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.S.; Lusher, M.; Storey, C.C.; Clarke, I.N. Plasmid diversity in Chlamydia. Microbiology 1997, 143 Pt 6, 1847–1854. [Google Scholar] [CrossRef]
- Fahr, M.J.; Sriprakash, K.S.; Hatch, T.P. Convergent and overlapping transcripts of the Chlamydia trachomatis 7.5-kb plasmid. Plasmid 1992, 28, 247–257. [Google Scholar] [CrossRef]
- Li, Z.; Chen, D.; Zhong, Y.; Wang, S.; Zhong, G. The chlamydial plasmid-encoded protein pgp3 is secreted into the cytosol of Chlamydia-infected cells. Infect. Immun. 2008, 76, 3415–3428. [Google Scholar] [CrossRef]
- Zhong, G. Chlamydial Plasmid-Dependent Pathogenicity. Trends Microbiol. 2017, 25, 141–152. [Google Scholar] [CrossRef]
- O’Connell, C.M.; Abdel Rahman, Y.M.; Green, E.; Darville, H.K.; Saira, K.; Smith, B.; Darville, T.; Scurlock, A.M.; Meyer, C.R.; Belland, R.J. Toll-like receptor 2 activation by Chlamydia trachomatis is plasmid dependent, and plasmid-responsive chromosomal loci are coordinately regulated in response to glucose limitation by C. trachomatis but not by C. muridarum. Infect. Immun. 2011, 79, 1044–1056. [Google Scholar] [CrossRef]
- Kari, L.; Whitmire, W.M.; Olivares-Zavaleta, N.; Goheen, M.M.; Taylor, L.D.; Carlson, J.H.; Sturdevant, G.L.; Lu, C.; Bakios, L.E.; Randall, L.B.; et al. A live-attenuated chlamydial vaccine protects against trachoma in nonhuman primates. J. Exp. Med. 2011, 208, 2217–2223. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Zavaleta, N.; Whitmire, W.; Gardner, D.; Caldwell, H.D. Immunization with the attenuated plasmidless Chlamydia trachomatis L2(25667R) strain provides partial protection in a murine model of female genitourinary tract infection. Vaccine 2010, 28, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Detels, R.; Green, A.M.; Klausner, J.D.; Katzenstein, D.; Gaydos, C.; Handsfield, H.; Pequegnat, W.; Mayer, K.; Hartwell, T.D.; Quinn, T.C. The incidence and correlates of symptomatic and asymptomatic Chlamydia trachomatis and Neisseria gonorrhoeae infections in selected populations in five countries. Sex. Transm. Dis. 2011, 38, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Margaret, G. Pelvic inflammatory disease. Am. Fam. Physician 2012, 85, 791–796. [Google Scholar]
- Onoh, R.C.; Mgbafuru, C.C.; Onubuogu, S.E.; Ugwuoke, I. Fitz-Hugh-Curtis syndrome: An incidental diagnostic finding in an infertility workup. Niger. J. Clin. Pract. 2016, 19, 834–836. [Google Scholar] [CrossRef]
- Bebear, C.; de Barbeyrac, B. Genital Chlamydia trachomatis infections. Clin. Microbiol. Infect. 2009, 15, 4–10. [Google Scholar] [CrossRef]
- Haggerty, C.L.; Gottlieb, S.L.; Taylor, B.D.; Low, N.; Xu, F.; Ness, R.B. Risk of sequelae after Chlamydia trachomatis genital infection in women. J. Infect. Dis. 2010, 201 (Suppl. S2), S134–S155. [Google Scholar] [CrossRef]
- Hafner, L.M. Pathogenesis of fallopian tube damage caused by Chlamydia trachomatis infections. Contraception 2015, 92, 108–115. [Google Scholar] [CrossRef]
- Steiner, A.Z.; Diamond, M.P.; Legro, R.S.; Schlaff, W.D.; Barnhart, K.T.; Casson, P.R.; Christman, G.M.; Alvero, R.; Hansen, K.R.; Geisler, W.M.; et al. Chlamydia trachomatis immunoglobulin G3 seropositivity is a predictor of reproductive outcomes in infertile women with patent fallopian tubes. Fertil Steril. 2015, 104, 1522–1526. [Google Scholar] [CrossRef]
- Adachi, K.N.; Nielsen-Saines, K.; Klausner, J.D. Chlamydia trachomatis Screening and Treatment in Pregnancy to Reduce Adverse Pregnancy and Neonatal Outcomes: A Review. Front. Public Health. 2021, 9, 531073. [Google Scholar] [CrossRef]
- Kobayashi, S.; Kida, I. Reactive arthritis: Recent advances and clinical manifestations. Intern. Med. 2005, 44, 408–412. [Google Scholar] [CrossRef]
- Cheeti, A.; Chakraborty, R.K.; Ramphul, K. Reactive arthritis. In StatPeals; StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Prashanth, R.; Thandra, K.C.; Limaiem, F. Lymphogranuloma Venereum. In StatPeals; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Mabey, D.; Peeling, R.W. Lymphogranuloma venereum. Sex. Transm. Infect. 2002, 78, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Dal Conte, I.; Mistrangelo, M.; Cariti, C.; Chiriotto, M.; Lucchini, A.; Vigna, M.; Morino, M.; Di Perri, G. Lymphogranuloma venereum: An old, forgotten re-emerging systemic disease. Panminerva Med. 2014, 56, 73–83. [Google Scholar] [PubMed]
- Ceovic, R.; Gulin, S.J. Lymphogranuloma venereum: Diagnostic and treatment challenges. Infect. Drug Resist. 2015, 8, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Handley, B.L.; Roberts, C.H.; Butcher, R. A systematic review of historical and contemporary evidence of trachoma endemicity in the Pacific Islands. PLoS ONE 2018, 13, e0207393. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, B.; Patel, B.C. Trachoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Zikic, A.; Schünemann, H.; Wi, T.; Lincetto, O.; Broutet, N.; Santesso, N. Treatment of Neonatal Chlamydial Conjunctivitis: A Systematic Review and Meta-analysis. J. Pediatric Infect. Dis. Soc. 2018, 7, e107–e115. [Google Scholar] [CrossRef] [PubMed]
- Toni, D. Chlamydia trachomatis infections in neonates and young children. Semin. Pediatr. Infect. Dis. 2005, 16, 235–244. [Google Scholar]
- Carol, P.; Pratt, R.J. Neonatal conjunctivitis and pneumonia due to chlamydia infection. Infant 2006, 2, 16–17. [Google Scholar]
- Zar, H.J. Neonatal chlamydial infections. Pediat. Drugs 2005, 7, 103–110. [Google Scholar] [CrossRef]
- Bialasiewicz, A.A.; Junkenitz, A.; Jahn, G.J. Corneal symptoms in keratoconjunctivitis caused by Chlamydia. Klin. Mon. Fur Augenheilkd. 1985, 187, 36–39. [Google Scholar] [CrossRef]
- Tipple, M.A.; Beem, M.O.; Saxon, E.M. Clinical characteristics of the afebrile pneumonia associated with Chlamydia trachomatis infection in infants less than 6 months of age. Pediatrics 1979, 63, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Safaeian, M.; Quint, K.; Schiffman, M.; Rodriguez, A.C.; Wacholder, S.; Herrero, R.; Hildesheim, A.; Viscidi, R.P.; Quint, W.; Burk, R.D. Chlamydia trachomatis and risk of prevalent and incident cervical premalignancy in a population-based cohort. J. Natl. Cancer Inst. 2010, 102, 1794–1804. [Google Scholar] [CrossRef]
- Horner, P.J.; Boag, F. 2006 UK National Guideline for the Management of Genital Tract Infection with Chlamydia trachomatis; British Association of Sexual Health and HIV (BASHH): London, UK, 2006; Available online: http://www.bashh.org/documents/61/61.pdf (accessed on 22 March 2012).
- Shahmanesh, M.; Moi, H.; Lassau, F.; Janier, M.; IUSTI/WHO. 2009 European guideline on the management of male nongonococcal urethritis. Int. J. STD AIDS 2009, 20, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.D.; Hudson, A.P. The evolving story of chlamydia-induced reactive arthritis. Curr. Opin. Rheumatol. 2010, 22, 424–430. [Google Scholar] [CrossRef]
- Carter, J.D.; Espinoza, L.R.; Inman, R.; Sneed, K.B.; Ricca, L.R.; Vasey, F.B.; Valeriano, J.; Stanich, J.A.; Oszust, C.; Gerard, H.C.; et al. Combination antibiotics as a treatment for chronic chlamydia-induced reactive arthritis: A double-blind, placebo controlled, prospective trial. Arthritis Rheum. 2010, 62, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Laga, M.; Manoka, A.; Kivuvu, M.; Malele, B.; Tuliza, M.; Nzila, N.; Goeman, J.; Behets, F.; Batter, V.; Alary, M.; et al. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: Results from a cohort study. AIDS 1993, 7, 95–102. [Google Scholar] [CrossRef]
- Land, J.; Van Bergen, J.; Morre, S.; Postma, M. Epidemiology of Chlamydia trachomatis infection in women and the cost-effectiveness of screening. Hum. Reprod. Update 2010, 16, 189–204. [Google Scholar] [CrossRef]
- Low, N.; Egger, M.; Sterne, J.A.; Harbord, R.M.; Ibrahim, F.; Lindblom, B.; Herrmann, B. Incidence of severe reproductive tract complications associated with diagnosed genital chlamydial infection: The Uppsala Women’s Cohort Study. Sex. Transm. Infect. 2006, 82, 212–218. [Google Scholar] [CrossRef]
- Bakken, I.J.; Skjeldestad, F.E.; Lydersen, S.; Nordbø, S.A. Births and ectopic pregnancies in a large cohort of women tested for Chlamydia trachomatis. Sex. Transm. Dis. 2007, 34, 739–743. [Google Scholar] [CrossRef]
- Davies, B.; Turner, K.M.; Frølund, M.; Ward, H.; May, M.T.; Rasmussen, S.; Benfield, T.; Westh, H. Risk of reproductive complications following chlamydia testing: A population-based retrospective cohort study in Denmark. Lancet Infect. Dis. 2016, 16, 1057–1064. [Google Scholar] [CrossRef]
- Kavanagh, K.; Wallace, L.A.; Robertson, C.; Wilson, P.; Scoular, A. Estimation of the risk of tubal factor infertility associated with genital chlamydial infection in women: A statistical modelling study. Int. J. Epidemiol. 2013, 42, 493–503. [Google Scholar] [CrossRef] [PubMed]
- US Preventive Services Task Force. Screening for Chlamydia and Gonorrhea: US Preventive Services Task Force Recommendation Statement. JAMA 2021, 326, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Workowski, K.A.; Bachmann, L.H.; Chan, P.A.; Johnston, C.M.; Muzny, C.A.; Park, I.; Reno, H.; Zenilman, J.M.; Bolan, G.A. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm. Rep. 2021, 70, 1–187. [Google Scholar] [CrossRef] [PubMed]
- Papp, J.R.; Schachter, J.; Gaydos, C.A.; van der Pol, B. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae—2014. MMWR Recomm. Rep. 2014, 63, 1–19. [Google Scholar]
- Nwokolo, N.C.; Dragovic, B.; Patel, S.; Tong, C.Y.; Barker, G.; Radcliffe, K. 2015 UK national guideline for the management of infection with Chlamydia trachomatis. Int. J. STD AIDS 2016, 27, 251–267. [Google Scholar] [CrossRef]
- Johansson, M.; Lycke, N. Immunology of the human genital tract. Curr. Opin. Infect. Dis. 2003, 16, 43–49. [Google Scholar] [CrossRef]
- Phalipon, A.; Cardona, A.; Kraehenbul, J.; Edelman, L.; Sansonetti, P.J.; Corthesy, B. Secretory component: A new role in secretory IgA mediated immune exclusion in vivo. Immunity 2002, 17, 107–115. [Google Scholar] [CrossRef]
- Crowley-Nowick, P.A.; Bell, M.; Edwards, R.P.; Mccallister, D.; Gore, H.; Kanbour-Shakir, A.; Mestecky, J.; Partridge, E.E. Normal uterine cervix: Characterization of isolated lymphocyte phenotypes and immunoglobulin secretion. Am. J. Reprod. Immunol. 1995, 34, 241–247. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Kannan, A.; Nunn, K.L.; Murphy, M.A.; Subramani, D.B.; Moench, T.; Cone, R.; Lai, S.K. IgG in cervicovaginal mucus traps HSV and prevents vaginal herpes infections. Mucosal Immunol. 2014, 7, 1036–1044. [Google Scholar] [CrossRef]
- Huang, Y.T.; Wright, A.; Gao, X.; Kulick, L.; Yan, H.; Lamm, M.E. Intraepithelial cell neutralization of HIV-1 replication by IgA. J. Immunol. 2005, 174, 4828–4835. [Google Scholar] [CrossRef]
- Bidgood, S.R.; Tam, J.C.H.; McEwan, W.A.; Mallery, D.L.; James, L.C. Translocalized IgA mediates neutralization and stimulates innate immunity inside infected cells. Proc. Natl. Acad. Sci. USA 2014, 111, 13463–13468. [Google Scholar] [CrossRef] [PubMed]
- Naglak, E.K.; Morrison, S.G.; Morrison, R.P. IFNgamma is required for optimal antibody-mediated immunity against genital Chlamydia infection. Infect. Immun. 2016, 84, 3232–3242. [Google Scholar] [CrossRef]
- Mestecky, J.; Alexander, R.C.; Wei, Q.; Moldoveanu, Z. Methods for evaluation of humoral immune responses in human genital tract secretions. Am. J. Reprod. Immunol. 2011, 65, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Woof, J.M.; Mestecky, J. Mucosal immunoglobulins. Immunol. Rev. 2005, 206, 64–82. [Google Scholar] [CrossRef] [PubMed]
- Wright, P.F. Inductive/effector mechanisms for humoral immunity at mucosal sites. Am. J. Reprod. Immunol. 2011, 65, 248–252. [Google Scholar] [CrossRef]
- Russell, M.W.; Mestecky, J. Humoral immune responses to microbial infections in the genital tract. Microbes Infect. 2002, 4, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A. Antiviral immune responses in the genital tract: Clues for vaccines. Nat. Rev. Immunol. 2010, 10, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Naz, R.K. Female genital tract immunity: Distinct immunological challenges for vaccine development. J. Reprod. Immunol. 2012, 93, 1–8. [Google Scholar] [CrossRef]
- Mestecky, J.; Raska, M.; Novak, J.; Alexander, R.C.; Moldoveanu, Z. Antibody-mediated protection and the mucosal immune system of the genital tract: Relevance to vaccine design. J. Reprod. Immunol. 2010, 85, 81–85. [Google Scholar] [CrossRef]
- Russell, A.N.; Zheng, X.; O’Connell, C.M.; Wiesenfeld, H.C.; Hillier, S.L.; Taylor, B.D.; Picard, M.D.; Flechtner, J.B.; Zhong, W.; Frazer, L.C.; et al. Identification of Chlamydia trachomatis antigens recognized by T Cells from highly exposed women who limit or resist genital tract infection. J. Infect. Dis. 2016, 214, 1884–1892. [Google Scholar] [CrossRef]
- Cohen, C.R.; Koochesfahani, K.M.; Meier, A.S.; Shen, C.; Karunakaran, K.; Ondondo, B.; Kinyari, T.; Mugo, N.R.; Nguti, R.; Brunham, R.C. Immunoepidemiologic profile of Chlamydia trachomatis infection: Importance of heat-shock protein 60 and interferon- gamma. J. Infect. Dis. 2005, 192, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Ness, R.B.; Soper, D.E.; Richter, H.E.; Randall, H.; Peipert, J.F.; Nelson, D.B.; Schubeck, D.; McNeeley, S.G.; Trout, W.; Bass, D.C.; et al. Chlamydia antibodies, chlamydia heat shock protein, and adverse sequelae after pelvic inflammatory disease: The PID evaluation and clinical health (PEACH) study. Sex. Transmit. Dis. 2008, 35, 129–135. [Google Scholar] [CrossRef] [PubMed]
- El Hakim, E.A.; Gordon, U.D.; Akande, V.A. The relationship between serum Chlamydia antibody levels and severity of disease in infertile women with tubal damage. Arch. Gynecol. Obstet. 2010, 281, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Norrby-Teglund, A.; Ihendyane, N.; Kansal, R.; Basma, H.; Kotb, M.; Andersson, J.; Hammarström, L. Relative neutralizing activity in polyspecific IgM, IgA, and IgG preparations against group A streptococcal superantigens. Clin. Infect. Dis. 2000, 31, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Brunham, R.C.; Peeling, R.; Maclean, I.; McDowell, J.; Persson, K.; Osser, S. Postabortal Chlamydia trachomatis salpingitis: Correlating risk with antigen-specific serological responses and with neutralization. J. Infect. Dis. 1987, 155, 749–755. [Google Scholar] [CrossRef]
- Lau, C.Y.; Qureshi, A.K. Azithromycin versus doxycycline for genital chlamydial infections: A meta-analysis of randomized clinical trials. Sex. Transm Dis. 2002, 29, 497–502. [Google Scholar] [CrossRef]
- Wiesenfeld, H.C.; Meyn, L.A.; Darville, T.; Macio, I.S.; Hillier, S.L. A randomized controlled trial of ceftriaxone and doxycycline, with or without metronidazole, for the treatment of acute pelvic inflammatory disease. Clin. Infect. Dis. 2021, 72, 1181–1189. [Google Scholar] [CrossRef]
- Basit, H.; Pop, A.; Malik, A.; Sharma, S. Fitz-Hugh-Curtis Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Pitsouni EIavazzo, C.; Athanasiou, S.; Falagas, M.E. Single-dose azithromycin versus erythromycin or amoxicillin for Chlamydia trachomatis infection during pregnancy: A meta-analysis of randomised controlled trials. Int. J. Antimicrob. Agents 2007, 30, 213–221. [Google Scholar] [CrossRef]
- Kartikeya, M.; Nassar, G.N.; Kaufman, E.J. Neonatal conjunctivitis. In StatPeals; StatPearls Publishing: Treasure Island, FL, USA, 2017. [Google Scholar]
- Gilbert, D.N.; Moellering, R.C.; Eliopoulos, G.M. The Sanford Guide to Antimicrobial Therapy 2010, 40th ed.; Antimicrobial Therapy: Sperryville, VA, USA, 2010. [Google Scholar]
- Katusic, D.; Petricek, I.; Mandic, Z.; Petric, I.; Salopek-Rabatic, J.; Kruzic, V.; Oreskovic, K.; Sikic, J.; Petricek, G. Azithromycin vs. doxycycline in the treatment of inclusion conjunctivitis. Am. J. Ophthalmol. 2003, 135, 447–451. [Google Scholar] [CrossRef]
- Mabey, D.; Fraser-Hurt, N.; Powell, C. Antibiotics for trachoma Cochrane Database. Syst. Rev. 2005, 2, CD001860. [Google Scholar]
- Handsfield, H.H. Lymphogranuloma Venereum Treatment and Terminology. Sex. Transm. Dis. 2018, 45, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Adair, C.D.; Gunter, M.; Stovall, T.G.; McElroy, G.; Veille, J.-C.; Ernest, J.M. Chlamydia in Pregnancy: A Randomized Trial of Azithromycin and Erythromycin. Obstet. Gynecol. 1998, 91, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Kamel, R.M. Management of the infertile couple: An evidence-based protocol. Reprod. Biol. Endocrinol. 2010, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- McLernon, D.J.; Maheshwari, A.; Lee, A.J.; Bhattacharya, S. Cumulative live birth rates after one or more complete cycles of IVF: A population-based study of linked cycle data from 178,898 women. Hum. Reprod. 2016, 31, 572–581. [Google Scholar] [CrossRef]
- Lau, A.; Kong, F.Y.S.; Fairley, C.K.; Templeton, D.J.; Amin, J.; Phillips, S.; Law, M.; Chen, M.Y.; Bradshaw, C.S.; Donovan, B.; et al. Azithromycin or Doxycycline for Asymptomatic Rectal Chlamydia trachomatis. N. Engl. J. Med. 2021, 384, 2418–2427. [Google Scholar] [CrossRef] [PubMed]
- Solomon, A.W.; Zondervan, M.; Kuper, H.; Buchan, J.C.; Mabey, D.C.W.; Foster, A. Trachoma Control a Guide for Programme Managers; World Health Organisation: Geneva, Switzerland, 2006. [Google Scholar]
- World Health Organisation. WHO Report of the 2nd Global Scientific Meeting on Trachoma; World Health Organisation: Geneva, Switzerland, 2003; Volume 44, pp. 447–678. [Google Scholar]
- Sandoz, K.M.; Rockey, D.D. Antibiotic resistance in Chlamydiae. Future Microbiol. 2010, 5, 1427–1442. [Google Scholar] [CrossRef]
- Roberts, M.C. Update on acquired tetracycline resistance genes. FEMS Microbiol. Lett. 2005, 245, 195–203. [Google Scholar] [CrossRef]
- Dugan, J.; Rockey, D.D.; Jones, L.; Andersen, A.A. Tetracycline resistance in Chlamydia suis mediated by genomic islands inserted into the chlamydial inv-like gene. Antimicrob. Agents Chemother. 2004, 48, 3989–3995. [Google Scholar] [CrossRef]
- Suchland, R.J.; Sandoz, K.M.; Jeffrey, B.M.; Stamm, W.E.; Rockey, D.D. Horizontal transfer of tetracycline resistance among Chlamydia spp. in vitro. Antimicrob. Agents Chemother. 2009, 53, 4604–4611. [Google Scholar] [CrossRef]
- Dreses-Werringloer, U.; Padubrin, I.; Köhler, L.; Hudson, A.P. Detection of nucleotide variability in rpoB in both rifampin-sensitive and rifampin-resistant strains of Chlamydia trachomatis. Antimicrob. Agents Chemother. 2003, 47, 2316–2318. [Google Scholar] [CrossRef]
- Kutlin, A.; Kohlhoff, S.; Roblin, P.; Hammerschlag, M.R.; Riska, P. Emergence of resistance to rifampin and rifalazil in Chlamydophila pneumoniae and Chlamydia trachomatis. Antimicrob. Agents Chemother. 2005, 49, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, I.; Salman, H.; Bakker, S.; Farrell, D.; Bébéar, C.M.; Ridgway, G. Serial passage of Chlamydia spp. in sub-inhibitory fluoroquinolone concentrations. J. Antimicrob. Chemother. 2002, 49, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Rupp, J.; Solbach, W.; Gieffers, J. Variation in the mutation frequency determining quinolone resistance in Chlamydia trachomatis serovars L2 and D. J. Antimicrob. Chemother. 2008, 61, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Binet, R.; Maurelli, A.T. The chlamydial functional homolog of KsgA confers kasugamycin sensitivity to Chlamydia trachomatis and impacts bacterial fitness. BMC Microbiol. 2009, 9, 279. [Google Scholar] [CrossRef] [PubMed]
- DeMars, R.; Weinfurter, J. Interstrain gene transfer in Chlamydia trachomatis in vitro: Mechanism and significance. J. Bacteriol. 2008, 190, 1605–1614. [Google Scholar] [CrossRef] [PubMed]
- Sköld, O. Resistance to trimethoprim and sulfonamides. Vet. Res. 2001, 32, 261–273. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, H.-P.; Jiang, Y.; Hou, S.-P.; Liu, Y.-J.; Liu, Q.-Z. Mutations in 23S rRNA and ribosomal protein L4 account for resistance in Chlamydia trachomatis strains selected in vitro by macrolide passage. Andrologia 2010, 42, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre, J.C.; Lépargneur, J.P. Comparative in vitro susceptibility of a tetracycline-resistant Chlamydia trachomatis strain isolated in Toulouse (France). Sex. Transm. Dis. 1998, 25, 350–352. [Google Scholar] [CrossRef]
- Misyurina, O.Y.; Chipitsyna, E.V.; Finashutina, Y.P.; Lazarev, V.N.; Akopian, T.A.; Savicheva, A.M.; Govorun, V.M. Mutations in a 23S rRNA gene of Chlamydia trachomatis associated with resistance to macrolides. Antimicrob. Agents Chemother. 2004, 48, 1347–1349. [Google Scholar] [CrossRef]
- Niemeyer, D.M.; Pucci, M.J.; Thanassi, J.A.; Sharma, V.K.; Archer, G.L. Role of mecA transcriptional regulation in the phenotypic expression of methicillin resistance in Staphylococcus aureus. J. Bacteriol. 1996, 178, 5464–5471. [Google Scholar] [CrossRef]
- Berger-BächiB, B. Expression of resistance to methicillin. Trends Microbiol. 1994, 2, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Wright, H.; Turner, A. Trachoma and poverty: Unnecessary blindness further disadvantages the poorest people in the poorest countries. Clin. Exp. Optom. 2007, 90, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Brunham, R.C.; Pourbohloul, B.; Mak, S.; White, R.; Rekart, M.L. The unexpected impact of a Chlamydia trachomatis infection control program on susceptibility to reinfection. J. Infect. Dis. 2005, 192, 1836–1844. (In Brunham) [Google Scholar] [CrossRef] [PubMed]
- Stephens, R.S.; Sanchez-Pescador, R.; Wagar, E.A.; Inouye, C.; Urdea, M.S. Diversity of Chlamydia trachomatis major outer membrane protein genes. J. Bacteriol. 1987, 169, 3879–3885. [Google Scholar] [CrossRef]
- Fitch, W.M.; Peterson, E.M.; de la Maza, L.M. Phylogenetic analysis of the outer-membrane-protein genes of Chlamydiae, and its implication for vaccine development. Mol. Biol. Evol. 1993, 10, 892–913. [Google Scholar] [CrossRef]
- Tifrea, D.F.; Pal, S.; de la Maza, L.M. A Recombinant Chlamydia trachomatis MOMP Vaccine Elicits Cross-serogroup Protection in Mice Against Vaginal Shedding and Infertility. J. Infect. Dis. 2020, 221, 191–200. [Google Scholar] [CrossRef]
- Unemo, M.; Bradshaw, C.S.; Hocking, J.S.; de Vries, H.J.C.; Francis, S.C.; Mabey, D.; Marrazzo, J.M.; Sonder, G.J.B.; Schwebke, J.R.; Hoornenborg, E.; et al. Sexually transmitted infections: Challenges ahead. Lancet Infect. Dis. 2017, 17, e235–e279. [Google Scholar] [CrossRef]
- US Preventive Services Task Force. Behavioral Counseling Interventions to Prevent Sexually Transmitted Infections: US Preventive Services Task Force Recommendation Statement. JAMA 2020, 324, 674–681. [Google Scholar] [CrossRef]
- Terri, D.C.; Piemonte, J.L. Monogamy as public policy for STD prevention: In theory and in practice. Policy Insights Behav. Brain Sci. 2020, 7, 181–189. [Google Scholar]
- Workowski, K.; Bachmann, L.; Centers for Disease Control and Prevention’s. Sexually Transmitted Diseases Infection Guidelines. Clin. Infect. Dis. 2022, 74 (Suppl. S2), S89–S94. [Google Scholar] [CrossRef]

| Risk Group | Possible Complications | References |
|---|---|---|
| Females |
| [70,71,88] |
| Pregnancy | ↑ risk of pregnancy complications:
| [89] |
| Males |
| [90] |
| Females and Males |
| [91,92,93] |
| Chlamydia trachomatis Infection | |||
|---|---|---|---|
| Chlamydia Infection | Type of Infection | Standard Treatment | Alternative Regimens |
| Genitourinary system [4,100,123,124,125] | Uncomplicated |
|
|
| Complicated (e.g., PID or perihepatitis) |
|
| |
| Pregnancy [4,126] |
|
| |
| Male [4] | Uncomplicated |
|
|
| Complicated |
|
| |
| Chronic ReA [75,91,92] |
|
| |
| Ophthalmia neonatorum or chlamydial neonatal pneumonia [83,85,127] |
|
| |
| Conjunctivitis in adults [128,129] |
|
| |
| Trachoma [83,130] |
|
| |
| Lymphogranuloma Venereum [11,76,84,131] |
|
| |
| Antibiotic Resistance in C. trachomatis Infection | |||
|---|---|---|---|
| Antibiotics | Mechanism of Action | Mechanism of Antibiotic Resistance/Available Data | References |
| Tetracyclines (TET) | Block bacterial protein synthesis by preventing aminoacyl tRNAs from interacting with ribosomes |
| [139] |
| [140] | ||
| Rifamycins (RIF) | Interact with the β-subunit of RNA polymerase to inhibit bacterial transcription |
| [141] |
| [142] | ||
| [143] | ||
| Fluoroquinolones | Inhibit DNA gyrase and DNA topoisomerase IV |
| [144] |
| [145] | ||
| Aminoglycosides | Interfere with translation initiation by interacting with the 30S ribosome |
| [146] |
| [146] | ||
| Sulfonamide and trimethoprim (SFM-TMT) | Interferes with bacterial folate synthesis, which is critical for DNA synthesis, repair and methylation |
| [147] |
| [148] | ||
| Azithromycin (a front-line drug for the treatment of chlamydia infections) | Macrolide, which causes bacterial protein synthesis inhibition |
| [146] |
| [138] | ||
| [149] | ||
| [146] | ||
| Lincomycin | Lincosamide; a bacteriostatic protein synthesis inhibitor, which causes premature dissociation of peptidyl-tRNA from the ribosome | The resistant mutants carried mutations in both 23S rRNA genes | [147] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grygiel-Górniak, B.; Folga, B.A. Chlamydia trachomatis—An Emerging Old Entity? Microorganisms 2023, 11, 1283. https://doi.org/10.3390/microorganisms11051283
Grygiel-Górniak B, Folga BA. Chlamydia trachomatis—An Emerging Old Entity? Microorganisms. 2023; 11(5):1283. https://doi.org/10.3390/microorganisms11051283
Chicago/Turabian StyleGrygiel-Górniak, Bogna, and Barbara Anna Folga. 2023. "Chlamydia trachomatis—An Emerging Old Entity?" Microorganisms 11, no. 5: 1283. https://doi.org/10.3390/microorganisms11051283
APA StyleGrygiel-Górniak, B., & Folga, B. A. (2023). Chlamydia trachomatis—An Emerging Old Entity? Microorganisms, 11(5), 1283. https://doi.org/10.3390/microorganisms11051283







