Current Insights in Fungal Importance—A Comprehensive Review
Abstract
:1. Introduction
2. Significance of Fungi in Different Sectors
2.1. Beneficial Roles
2.1.1. Medicine/Health
2.1.2. Fungi in Agriculture
2.1.3. Industry
Metabolites Produced by Fungi with Industrial Applications
| Enzymes | Fungal Species | Non-Conventional Growth Substrates | Applications | References | ||
|---|---|---|---|---|---|---|
| Amylases | A. niger A. oryzae A. fumigatus Aspergillus flavus; A. awamori; A. kawachii; Penicillium brunneum; Penicillium expansum; P. roqueforti; P. camemberti; Helminthosporium oxysporum; Penicillium frequestans P. chrysogenum Penicillium fellutanum | Coconut oil cake; groundnut oil cake; sesame oil cake; olive oil cake; wheat bran; corncob leaf; rye straw; wheat straw; banana waste; residues obtained from rice husking; cassava peels; yam peels; pomegranate peel; molasses |
| [161,162,179,180,181,182,183,184,185,186] | ||
| Proteases | A. flavus; Aspergillus ochraceus Conidiobolus coronatus; Rhizomucor miehei; Endothia parasitica; Mucor circinelloides; Mucor pusillus; P. camemberti; P. citrinum; Penicillium griseoroseum; Penicillium restrictum; P. roqueforti; A. flavus; A. oryzae; A. niger; R. oryzae; T. reesei; Trichoderma harzianum | Wheat and rice bran, soybean meal; oil seed cake |
| [49,163,187,188,189,190,191,192,193,194,195] | ||
| Pectinase | A. niger A. flavus A. sojae A. terreus Alternaria citri Claviceps purpurea Fusarium moniliforme Botrytis cinerea A. kawakii Thermoascus aurantiacus Acrophialophora nainiana Aspergillus japonicus | Wheat bran; rice husk and bran; papaya peel; mango peel; sugarcane bagasse; sunflower head; grape and strawberry pomace |
| [166,196,197,198,199,200,201,202,203,204] | ||
| Galactosidases | α-Galactosidases | Mortierella vinaceae Tricholoma matsutake A. niger A. oryzae A. fumigatus | Soybean meal and wheat bran red gram plant waste; soy flour |
| [101,102] | |
| β-galactosidase | A. niger A oryzae A. flavus Aspergillus uvarum P. brevicompactum F. oxysporum | Lemon peel, pineapple peel, musk melon peel, banana peel, musambi peel, pomegranate peel, orange peel; soybean residue, okara, soymilk; wheat straw, rice straw, and peanut pod |
| [168,205,206,207,208,209] | ||
| Chitinases | Thermomyces lanuginosus; T. viride; T. harzianum; A. nidulans, A. fumigatus, P. chrysogenum | Wheat bran; rice bran; chitin flakes; waste products obtained from crabs, shrimps and prawn |
| [171,210,211,212,213,214] | ||
| Lipases | Mucor circinelloides Penicillium aurantiogriseum Rhizopus rhizopodiformis Rhizomucor pusillus Rhizopus oligosporus P. restrictum Penicillium simplicissimum Aspergillus carneus Penicillium verrucossum P. chrysogenum A. awamori A. terreus Fusarium solani | Soya bean oil; olive oil cake; babassu oil cake Almond meal; mustard oil cake, sunflower oil; soybean bran; rice bran oil; olive mill wastewater |
| [215,216,217,218,219,220,221,222,223,224] | ||
| Lignocellulolytic enzymes | Cellulase | A. niger T. reesei Aspergillus heteromorphus A. fumigatus R. oryzae | Wheat straw and bran; maize straw; banana peel Coir waste; grass; sugarcane bagasse; corn cob residue |
| [225,226,227,228,229,230] | |
| Ligninanses | Laccases | Aspergillus niveus Rhizoctonia solani B. cinerea Myceliophthora thermophila Pycnoporus cinnabarinus Trametes villosa Coriolopsis gallica Coprinopsis cinerea | Wheat bran, rice husk, mango peel, orange peel, groundnut husk and saw dusk |
| [175,231,232,233,234,235,236,237,238] | |
| Peroxidases | Phanerochaete chrysosporium; A. sclerotiorum Cladosporium cladosporioides M. racemosus Neurospora discreta | Cocopeat, sugarcane bagasse |
| [239,240,241] | ||
2.1.4. Fungi and the Environment
2.1.5. Research
2.2. Damaging Effects
2.2.1. Etiological Agents of Diseases in Plants, Animals and Humans
2.2.2. Mycotoxin Production
Mycotoxins Biosynthetic Pathways—Mechanisms and Genetic Background
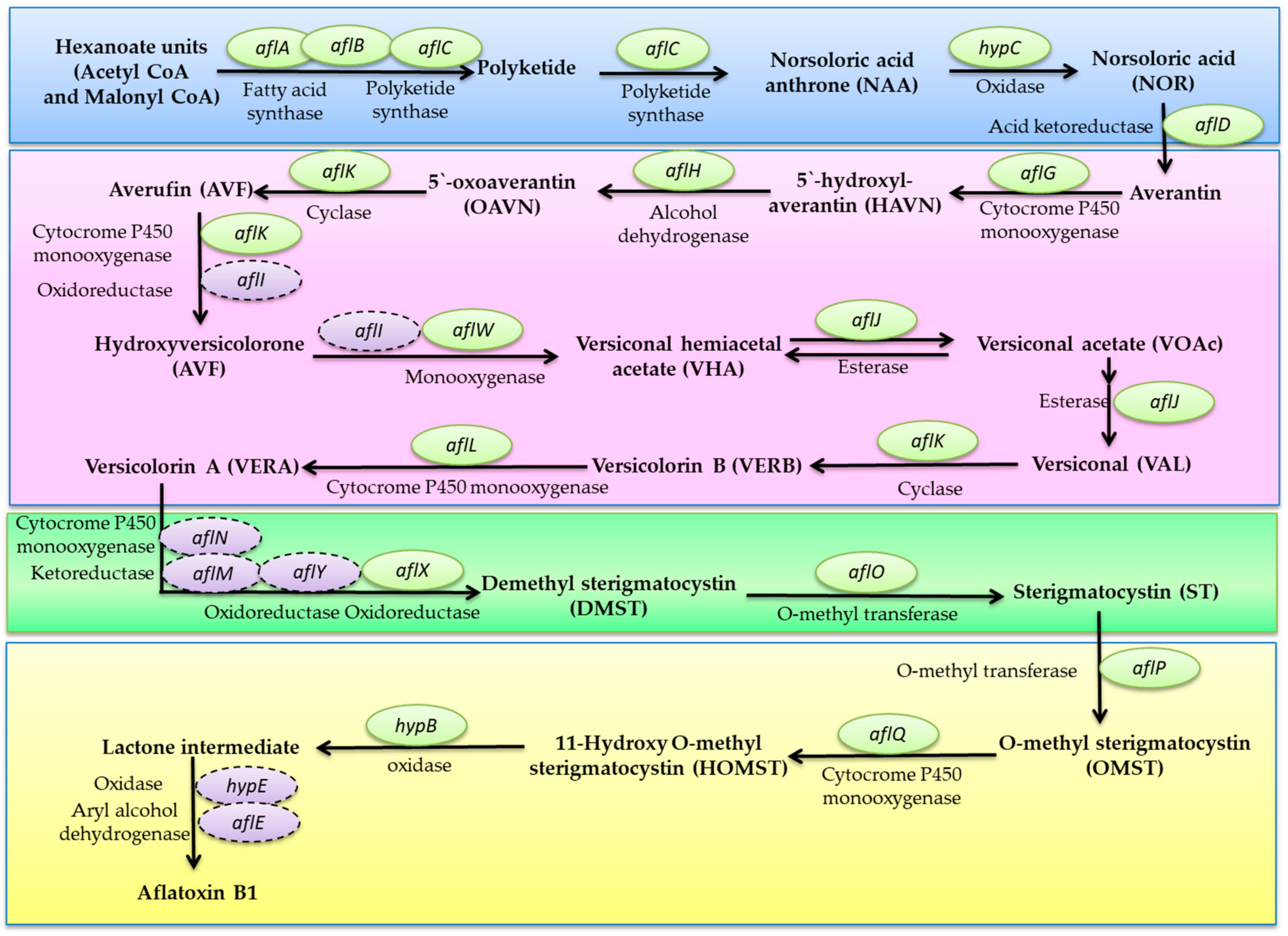
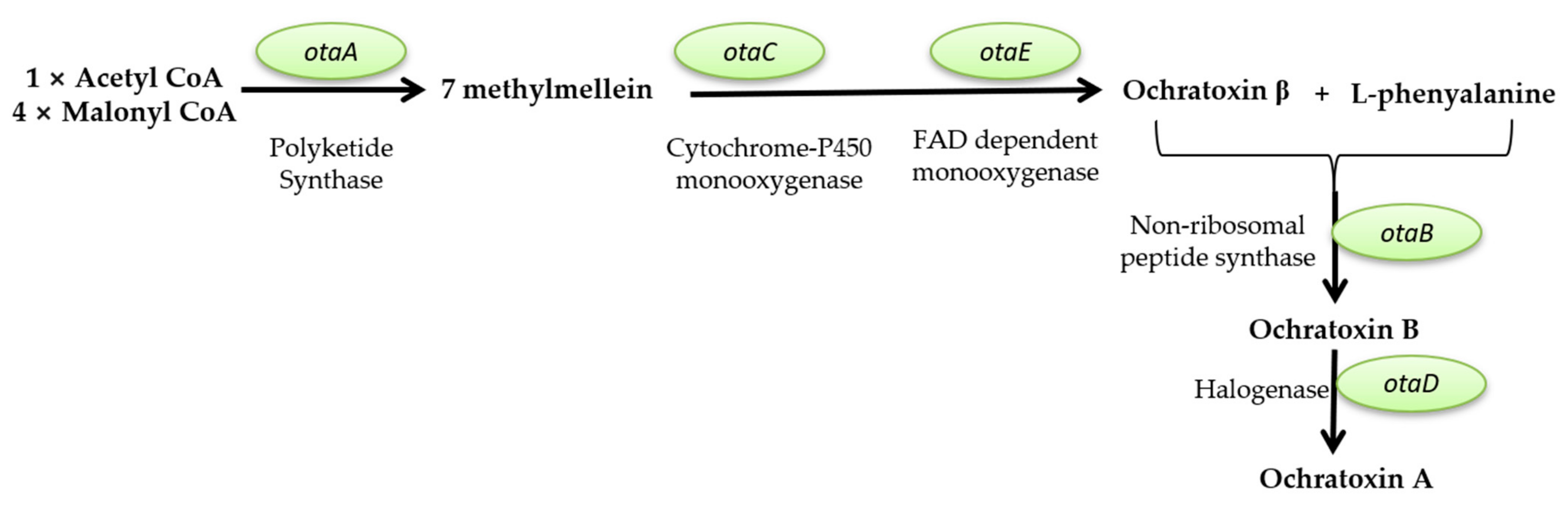
Health Implications of Mycotoxins Exposure
2.2.3. Deteriogenic Agents
The Most Frequently Encountered Cultural Heritage Objects and Their Deterioration by Fungi
- Stone Objects
- Wooden Objects
- Paintings
- Textiles
- Paper and Paper-Based Materials
- Parchments
| Deteriogenic Agents/Group | Genus | Substrate | Alteration Types | References |
|---|---|---|---|---|
| Ascomycota Phyllum | Acrodictys spp. | stone artifacts | [401] | |
| Dothideomycetes class | Aureobasidium spp. | black patina, black spots, biofilm formation, discolorations, stone erosion, and disintegration | [402,403,404] | |
| Capnobotryella spp. | black spots, crater shaped lesions, superficial deposit, and biofilm formation | [403,405] | ||
| Coniosporium spp. | black patina, black spots, pitting, exfoliation, superficial deposit, and biofilm formation | [403,406] | ||
| Phoma spp. | black spots, black patinas, exfoliation, pitting, superficial deposit, biofilm formation | [404,407,408] | ||
| Alternaria spp. | black spots, black patina, biofilm formation, black crusts | |||
| Cladosporium spp. | black spots, patinas, pitting, biofilm formation, erosion, discoloration, disintegration | |||
| Epicoccum spp. | black spots, black patinas, superficial deposit, biofilm formation with salt efflorescence | |||
| Eurotiomycetes class | Exophiala spp. | black patina, black spots, detachment of discolorations, visible damage | [389,406] | |
| Knuffia spp. | stone | black spots, patinas, pitting, discolorations, visible damage | [389,405,406,409] | |
| Lithophyla spp. | black patina, black spots | [245,402] | ||
| Dothideomycetes class | Chaetomium spp., Aureobasidium spp., Epicoccum spp., Cladosporium spp. | wall paintings | brown discolorations, degrade protein binders of the painted layer, which results in the lifting and separation of the painted layer from the support | [86,410,411] |
| Eurotiomycetes class | Penicillium spp., Aspergillus spp. | primary fresco deteriogens, damage of wall paintings due to its intensely sporulation degree | [86,412] | |
| Zygomycetes class | Mucor spp., Rhizopus spp., Actinomucor spp. | surface contaminants | [413,414] | |
| Basidiomycetes class | Coprinus spp. | contamination | [413] | |
| Dothideomycetes and Eurotiomycetes classes | A. alternata, A. flavus, A. niger, A. versicolor, Aureobasidium pullulans, Chaetomium globosum, C. cladosporoides, Eurotium chevalieri, and P. chrysogenum | canvas oil paintings | the detachment of the paint layer from the support, the loss of material due to the excretion of metabolites, esthetic changes of materials, biofilm formation, chromatic alteration of the painted surfaces and detachment of the support | [89,393,410] |
| Zygomycetes class | Cunninghamella spp., Mucor spp., Rhizopus spp. Phycomyces spp. | dust deposits | [398] | |
| Basidiomycetes class | Puccinia spp. | contaminants | [415] | |
| Basidiomycetes class | Bjerkandera spp., Donkioporia spp., Fomes spp., Irpex spp., Phanerochaete spp., Pholiota spp., Pleurotus spp., Trametes spp. | wooden | white rot fungi complete depolymerization and degradation of lignin, cellulose, and hemicellulose components | [86,416] |
| Eurotiomycetes class Sordariomycetes class | Aspergillus spp., Fusarium spp., | [86] | ||
| Basidiomycetes class | Antrodia spp., Coniophora spp., Coriolellus spp., Gloeophyllum spp., Paxillus spp., Poria spp., Postia spp., Serpula (Merulius) lacrymans | brown rot fungi cellulose and hemicellulose decomposition and lignin degradation is restricted to methoxyl group demethylation | [86,417] | |
| Dothideomycetes class | Alternaria spp., Stemphylium spp., | soft rot fungi cellulose and hemicellulose decomposition and lignin degradation is restricted to methoxyl group demethylation, leading to discoloration and cracking pattern | [86,392,417] | |
| Sordariomycetes class | Chaetomium spp., Daldinia spp., Humicola spp., Xylaria spp. | |||
| Eurotiomycetes Sordariomycetes and Dothideomycetes classes | Penicillium spp., Aspergillus spp., Eurotium spp., Myxotrichum spp. Trichoderma spp., Chaetomium spp., Acremonium spp., Paecilomyces spp., Stachybotrys spp., Myrothecium spp., Cladosporium spp., Bipolaris spp., Aureobasidium spp., Alternaria spp., Epicoccum spp. | paper and paper-based materials | pigments and organic acid production, brown to red spots (foxing) | [86,418,419,420,421,422] |
| Basidiomycetes classTritirachiomycetes classes | Bjerkandera spp., Tritirachium spp. | |||
| Zygomycetes class | Rhizopus arrhyzus | |||
| Eurotiomycetes and Sordariomycetes classes | Aspergillus spp., Penicillium spp., Microsporum spp., Trichophyton spp., Chaetomium spp., Fusarium spp., | textiles | wool fibers degradation | [86,423] |
| Zygomycetes class | Rhizopus spp. | wool fibers degradation | [423] | |
| Eurotiomycetes class | Chaetomium globosum | silk deterioration, causing cracks and gaps in fibroin fibers | [424] | |
| Dothideomycetes and Eurotiomycetes classes | Alternaria spp., Aureobasidium spp., Cladosporium spp., Epicoccum spp., Penicillium spp. | parchment | [86,425,426] | |
| Ascomycota phyllum | Diploospora rosea | the detachment of large parts of the artwork’s preparative layer and the overlying illumination. | [427] |
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bonfante, P.; Venice, F.; Lanfranco, L. The mycobiota: Fungi take their place between plants and bacteria. Curr. Opin. Microbiol. 2019, 49, 18–25. [Google Scholar] [CrossRef]
- Wösten, H.A.B. Filamentous fungi for the production of enzymes, chemicals and materials. Curr. Opin. Biotechnol. 2019, 59, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Nash, A.K.; Auchtung, T.A.; Wong, M.C. The gut mycobiome of the human microbiome project healthy cohort. Microbiome 2017, 5, 153. [Google Scholar] [CrossRef] [PubMed]
- Hüttner, S.; Johansson, A.; Gonçalves Teixeira, P.; Achterberg, P.; Nair, R.B. Recent advances in the intellectual property landscape of filamentous fungi. Fungal Biol. Biotechnol. 2020, 7, 16. [Google Scholar] [CrossRef]
- Mei, Y.Z.; Zhu, Y.L.; Huang, P.W.; Yang, Q.; Dai, C.C. Strategies for gene disruption and expression in filamentous fungi. Appl. Microbiol. Biotechnol. 2019, 103, 6041–6059. [Google Scholar] [CrossRef]
- Jones, M.P.; Huynh, T.; Dekiwadia, C.D.; Daver, F.; John, S. Mycelium composites: A review of engineering characteristics and growth kinetics. J. Bionanosci. 2017, 11, 241–257. [Google Scholar] [CrossRef]
- Appels, F.V.W.; Dijksterhuis, J.; Lukasiewicz, C.E. Hydrophobin gene deletion and environmental growth conditions impact mechanical properties of mycelium by affecting the density of the material. Sci. Rep. 2018, 8, 4703. [Google Scholar] [CrossRef]
- Pombeiro-Sponchiado, S.R.; Sousa, G.S.; Andrade, J.C.R.; Lisboa, H.F.; Gonçalves, R.C.R. Production of melanin pigment by fungi and its biotechnological applications. In Melanin; IntechOpen: London, UK, 2017. [Google Scholar]
- Poorniammal, R.; Prabhu, S.; Dufossé, L.; Kannan, J. Safety evaluation of fungal pigments for food applications. J. Fungi 2021, 7, 692. [Google Scholar] [CrossRef] [PubMed]
- Iram, W.; Anjum, T. Production enhancement of cyclosporin “A” by Aspergillus terreus through mutation. Afr. J. Biotechnol. 2015, 11, 1736–1743. [Google Scholar] [CrossRef]
- Karwehl, S.; Stadler, M. Exploitation of fungal biodiversity for discovery of novel antibiotics. In How to Overcome the Antibiotic Crisis. Current Topics in Microbiology and Immunology; Stadler, M., Dersch, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; Volume 398, pp. 303–338. [Google Scholar]
- Smith, S.E.; Read, D. The symbionts forming arbuscular mycorrhizas. In Mycorrhizal Symbiosis; Smith, S.E., Read, D., Eds.; Academic Press: Cambridge, MA, USA, 2008; pp. 13–41. [Google Scholar]
- Şesan, T.E.; Oancea, F. Trichoderma viride Pers.—Experimental model for biological and biotechnological investigations of mycromyceta with importance in obtaining plant protection bioproducts. J. Plant Dev. 2010, 17, 49–62. [Google Scholar]
- Şesan, T.E.; Oancea, F.; Toma, C.; Matei, G.-M.; Matei, S.M.; Chira, F.; Chira, D.; Fodor, E.; Mocan, C.; Ene, M.A.; et al. Approaches to the study of mycorrhizas in Romania. Ed. Univ. Buc. 2010, 51, 75–85. [Google Scholar] [CrossRef]
- Galindo-Solís, J.M.; Fernández, F.J. Endophytic fungal terpenoids: Natural role and bioactivities. Microorganisms 2022, 10, 339. [Google Scholar] [CrossRef]
- Kaur, C.; Mishra, Y.; Mishra, V.; Saraogi, G.K.; Tambuwala, M.M. Recent advancement and biomedical applications of fungal metabolites. In New and Future Developments in Microbial Biotechnology and Bioengineering; Singh, J., Gehlot, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 47–67. [Google Scholar]
- Fodor, E.; Şesan, T.E. Fitopatogeni în Ecosistemele Forestiere [Phytopathogens in Forest Ecosystems]; Editura Universitatii din Bucyresti: Bucharest, Romania, 2014; p. 648. [Google Scholar]
- Kim, J.-S.; Yoon, S.-J.; Park, Y.-J.; Kim, S.-Y.; Ryu, C.-M. Crossing the kingdom border: Human diseases caused by plant pathogens. Environ. Microbiol. 2020, 22, 2485–2495. [Google Scholar] [CrossRef]
- Stajich, J.E. Fungal genomes and insights into the evolution of the kingdom. Microbiol. Spectr. 2017, 5, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, D.; Singh, S.; Kumar, M.; Kumar, V.; Datta, S.; Dhanjal, D.S. Fungal biotechnology: Role and aspects. In Fungi and their Role in Sustainable Development: Current Perspectives; Gehlot, P., Singh, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 91–103. [Google Scholar]
- Adrio, J.L.; Demain, A.L. Fungal biotechnology. Int. Microbiol. 2003, 6, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Cerimi, K.; Akkaya, K.C.; Pohl, C.; Schmidt, B.; Neubauer, P. Fungi as Source for new bio-based materials: A patent review. Fungal Biol. Biotechnol. 2019, 6, 17. [Google Scholar] [CrossRef]
- Meyer, V.; Basenko, E.Y.; Benz, J.P.; Braus, G.H.; Caddick, M.X.; Csukai, M.; de Vries, R.P.; Endy, D.; Frisvad, J.C.; Gunde-Cimerman, N.; et al. Growing a circular economy with fungal biotechnology: A white paper. Fungal Biol. Biotechnol. 2020, 7, 5. [Google Scholar] [CrossRef]
- Jakubczyk, D.; Dussart, F. Selected fungal natural products with antimicrobial properties. Molecules 2020, 25, 911. [Google Scholar] [CrossRef]
- Rani, A.; Saini, K.C.; Bast, F.; Varjani, S.; Mehariya, S.; Bhatia, S.K.; Sharma, N.; Funk, C. A Review on microbial products and their perspective application as antimicrobial agents. Biomolecules 2021, 11, 1860. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, L.; Madhusudana, S.N.; Ravi, V.; Desai, A. Mycophenolic acid inhibits replication of japanese encephalitis virus. Chemotherapy 2011, 57, 56–61. [Google Scholar] [CrossRef]
- Demain, A.; Martens, E. Production of valuable compounds by molds and yeasts. J. Antibiot. 2017, 70, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.L. The penicillins: A review and update. J. Midwifery Womens Health 2002, 47, 426–434. [Google Scholar] [CrossRef]
- Tipper, D.J. Mode of action of beta-lactam antibiotics. Pharmacol. Ther. 1985, 27, 1–35. [Google Scholar] [CrossRef]
- Moldenhauer, G.; Salnikov, A.V.; Lüttgau, S.; Herr, I.; Anderl, J.; Faulstich, H. Therapeutic potential of amanitin-conjugated anti-epithelial cell adhesion molecule monoclonal antibody against pancreatic carcinoma. J. Natl. Cancer Inst. 2012, 104, 622–634. [Google Scholar] [CrossRef]
- Koiso, Y.; Li, Y.; Iwasaki, S.; Hanaka, K.; Kobayashi, T.; Sonoda, R.; Fujita, Y.; Yaegashi, H.; Sato, Z. Ustiloxins, antimitotic cyclic peptides from false smut balls on rice panicles caused by Ustilaginoidea virens. J. Antibiot. 1994, 47, 765–773. [Google Scholar] [CrossRef]
- Anke, H.; Laatsch, H. Cyclic peptides and depsipeptides from Fungi. In Physiology and Genetics. The Mycota (A Comprehensive Treatise on Fungi as Experimental Systems for Basic and Applied Research); Esser, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 15, pp. 331–335. [Google Scholar]
- Umeyama, A.; Takahashi, K.; Grudniewska, A.; Shimizu, M.; Hayashi, S.; Kato, M.; Okamoto, Y.; Suenaga, M.; Ban, S.; Kumada, T.; et al. In vitro antitrypanosomal activity of the cyclodepsipeptides, cardinalisamides A-C, from the insect pathogenic fungus Cordyceps cardinalis NBRC 103832. J. Antibiot. 2014, 67, 163–166. [Google Scholar] [CrossRef]
- Haritakun, R.; Sappan, M.; Suvannakad, R.; Tasanathai, K.; Isaka, M. An antimycobacterial cyclodepsipeptide from the entomopathogenic fungus Ophiocordyceps communis BCC 16475. J. Nat. Prod. 2010, 73, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, S.; Mizuno, S.; Ishigami, H.; Yamakawa, Y.; Kawagishi, H.; Ushimaru, T. New rapid screening method for anti-aging compounds using budding yeast and identification of beauveriolide I as a potent active compound. Biosci. Biotechnol. Biochem. 2012, 76, 1226–1228. [Google Scholar] [CrossRef] [PubMed]
- Arnold, D.; Scott, P.; McGuire, P.; Harwig, J.; Nera, E. Acute toxicity studies on roquefortine and pr toxin, metabolites of the Penicillium roqueforti in the mouse. Food Cosmet. Toxicol. 1978, 16, 369–371. [Google Scholar] [CrossRef]
- Eriksen, G.S.; Jäderlund, K.H.; Moldes-Anaya, A.; Schönheit, J.; Bernhoft, A.; Jæger, G.; Rundberget, T.; Skaar, I. Poisoning of dogs with tremorgenic Penicillium toxins. Med. Mycol. 2010, 48, 188–196. [Google Scholar] [CrossRef]
- Pahl, B.H.L.; Kraub, B.; Schulze-osthoff, K.; Decker, T.; Traenckner, E.B.; Myersfl, C.; Parksfl, T.; Warring, P.; Miihlbacher, I.I.A.; Czernilofiky, A. The immunosuppressive fungal metabolite gliotoxin specifically inhibits transcription factor NF-KB. J. Exp. Med. 1996, 183, 1829–1840. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.J.; Ghosh, S.; Okoli, I.; Mylonakis, E. Antifungal activity of microbial secondary metabolites. PLoS ONE 2011, 6, e25321. [Google Scholar] [CrossRef]
- Aris, P.; Wei, Y.; Mohamadzadeh, M.; Xia, X. Griseofulvin: An updated overview of old and current knowledge. Molecules 2022, 18, 7034. [Google Scholar] [CrossRef]
- Riley, R.T.; Showker, J.L. The mechanism of patulin’s cytotoxicity and the antioxidant activity of indole tetramic acids. Toxicol. Appl. Pharm. 1991, 109, 108–126. [Google Scholar] [CrossRef] [PubMed]
- Sumbu, Z.L.; Thomart, P.; Bechet, J. Action of patulin on yeast. Appl. Environ. Microbiol. 1983, 45, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Huang, Y.; Zhan, H.; Bhatt, P.; Chen, S. Species specificity and mechanism of action of strobilurins. Dechema Monogr. 1993, 129, 27–38. [Google Scholar]
- Miethbauer, S.; Gaube, F.; Mollmann, U.; Dahse, H.M.; Schimidtke, M.; Gareis, M.; Pickhardt, M.; Liebermann, B. Antimicrobial, antiproliferative, cytotoxic, and tau inhibitory activity of rubellins and caeruleoramularin produced by the phytopathogenic fungus Ramularia collo-cygni. Planta Med. 2009, 75, 1523–1525. [Google Scholar] [CrossRef]
- Schueffler, A.; Anke, T. Fungal natural products in research and development. Nat. Prod. Rep. 2014, 31, 1425–1448. [Google Scholar] [CrossRef]
- Denning, D. Echinocandins: A new class of antifungal. J. Antimicrob. Chemother 2002, 49, 889–891. [Google Scholar] [CrossRef]
- Houšť, J.; Spížek, J.; Havlíček, V. Antifungal drugs. Metabolites 2020, 10, 106. [Google Scholar] [CrossRef]
- Bouz, G.; Doležal, M. Advances in antifungal drug development: An up-to-date mini review. Pharmaceuticals 2021, 14, 1312. [Google Scholar] [CrossRef] [PubMed]
- El-Khonezy, M.I.; Elgammal, E.W.; Ahmed, E.F.; Abd-Elaziz, A.M. Detergent stable thiol-dependant alkaline protease produced from the endophytic fungus Aspergillus ochraceus BT21: Purification and kinetics. Biocatal. Agric. Biotechnol. 2021, 35, 102046. [Google Scholar] [CrossRef]
- Strobel, G.; Daisy, B. Bioprospecting for microbial endophytes and their natural products. Microbiol. Mol. Biol. Rev. 2003, 67, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Strobel, G.A.; Miller, R.V.; Martinez-Miller, C.; Condron, M.M.; Teplow, D.B.; Hess, W.M. Cryptocandin, a potent antimycotic from the endophytic fungus Cryptosporiopsis cf. quercina. Microbiology 1999, 145, 1919–1926. [Google Scholar] [CrossRef]
- Endo, M.; Takesako, K.; Kato, I.; Yamaguchi, H. Fungicidal action of aureobasidin A, a cyclic depsipeptide antifungal antibiotic, against Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 1997, 41, 672–676. [Google Scholar] [CrossRef]
- Tan, H.W.; Tay, S.T. The inhibitory effects of aureobasidin A on Candida planktonic and biofilm cells. Mycoses 2013, 56, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, E.K.S.; Roy, K.; Chatterjee, S.; Deshmukh, S.K.; Ganguli, B.N.; Fehlhaber, H.W.; Kogler, H. Arthrichitin. A new cell wall active metabolite from Arthrinium phaeospermum. J. Org. Chem. 1996, 61, 6591–6593. [Google Scholar] [CrossRef]
- King, A.M. Aspergillomarasmine A overcomes metallo-β-lactamase antibiotic resistance. Nature 2014, 510, 503–506. [Google Scholar] [CrossRef]
- Borel, J.F.; Feurer, C.; Gabler, H.U.; Stahelin, H. Biological effects of cyclosporine A: A new anti- lymphocytic agent. Agents Action 1976, 6, 468–475. [Google Scholar] [CrossRef]
- Marik, T.; Tyagi, C.; Balázs, D.; Urbán, P.; Szepesi, Á.; Bakacsy, L.; Endre, G.; Rakk, D.; Szekeres, A.; Andersson, M.A. Structural diversity and bioactivities of peptaibol compounds from the Longibrachiatum clade of the filamentous fungal genus Trichoderma. Front. Microbiol. 2019, 10, 1434. [Google Scholar]
- Mygind, P.H.; Fischer, R.L.; Schnorr, K.M.; Hansen, M.T.; Sönksen, C.P.; Ludvigsen, S.; Raventós, D.; Buskov, S.; Christensen, B.; De Maria, L.; et al. Plectasin is a peptide antibiotic with therapeutic potential from a saprophytic fungus. Nature 2005, 437, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Shima, A.; Fukushima, K.; Arai, T.; Terada, H. Dual Inhibitory effects of the peptide antibiotics leucinostatins on oxidative phosphorylation in mitochondria. Cell Struct. Funct. 1990, 15, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Iwatsuki, M.; Kinoshita, Y.; Niitsuma, M.; Hashida, J.; Mori, M.; Ishiyama, A.; Namatame, M.; Nishihara-Tsukashima, A.; Nonaka, K.; Masuma, R.; et al. Antitrypanosomal peptaibiotics, trichosporins B-VIIa and B-VIIb, produced by Trichoderma polysporum FKI-4452. J. Antibiot. 2010, 63, 331–333. [Google Scholar] [CrossRef] [PubMed]
- Shiomi, K.; Yamada, H.; Omura, S. In vitro and in vivo antitrypanosomal activities of three peptide antibiotics: Leucinostatin A and B, alamethicin I and tsushimycin. J. Antibiot. 2009, 62, 303–308. [Google Scholar] [CrossRef]
- Pelaez, F.; Cabello, A.; Platas, G.; Díez, M.T.; Del Val, A.G.; Basilio, A.; Martán, I.; Vicente, F.; Bills, G.F.; Giacobbe, R.A. The discovery of enfumafungin, a novel antifungal compound produced by an endophytic Hormonema species biological activity and taxonomy of the producing organisms. Syst. Appl. Microbiol. 2000, 23, 333–343. [Google Scholar] [CrossRef]
- Vicente, M.F.; Cabello, A.; Platas, G.; Basilio, A.; Díez, M.T.; Dreikorn, S.; Giacobbe, R.A.; Onishi, J.C.; Meinz, M.; Kurtz, M.B. Antimicrobial activity of ergokonin A from Trichoderma longibrachiatum. J. Appl. Microbiol. 2001, 91, 806–813. [Google Scholar] [CrossRef]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef]
- Bills, G.F.; Platas, G.; Overy, D.P.; Collado, J.; Fillola, A.; Jiménez, M.R.; Martín, J.; Val, A.G.; Francisca Vicente, J.R.T.; Peláez, F.; et al. Discovery of the parnafungins, antifungal metabolites that inhibit MRNA polyadenylation, from the Fusarium larvarum complex and other hypocrealean fungi. Mycologia 2009, 101, 449–472. [Google Scholar] [CrossRef]
- Manzoni, M.; Rollini, M. Biosynthesis and biotechnological production of statins by filamentous fungi and application of these cholesterol-lowering drugs. Appl. Microbiol. Biotechnol. 2002, 58, 555–564. [Google Scholar] [CrossRef]
- McLean, K.J.; Hans, M.; Meijrink, B.; Scheppingen, W.B.; Vollebregt, A.; Tee, K.L.; Laan, J.-M.; Leys, D.; Munro, A.W.; Berg, M.A. Single-step fermentative production of the cholesterol lowering drug pravastatin via reprogramming of Penicillium chrysogenum. Proc. Natl. Acad. Sci. USA 2015, 112, 2847–2852. [Google Scholar] [CrossRef]
- Zhang, C.-L.; Zheng, B.-Q.; Lao, J.-P.; Mao, L.-J.; Chen, S.-Y.; Kubicek, C.P.; Lin, F.-C. Clavatol and patulin formation as the antagonistic principle of Aspergillus clavatonanicus, an endophytic fungus of Taxus mairei. Appl. Microbiol. Biotechnol. 2008, 78, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Riko, R.; Nakamura, H.; Shindo, K. Studies on pyranonigrins–isolation of pyranonigrin E and biosynthetic studies on pyranonigrin A. J. Antibiot. 2014, 67, 179–181. [Google Scholar] [CrossRef]
- Şesan, T.E. Studiul biologic al speciilor de ciuperci antagoniste faţă de unii patogeni cu produc micoze la plante [Biological study of fungi species antagonistic towards some phytopathogens]. ICEBiol 1986, 1, 89. [Google Scholar]
- Şesan, T.E. Trichoderma spp. Applications in Agriculture and Horticulture; Editura Universitatii din Bucuresti: Bucharest, Romania, 2017; p. 380. ISBN 978-606-16-0900-0. [Google Scholar]
- Şesan, T.E. Sustainable management of gray mold (Botrytis spp.) of horticultural crops. Adv. Plant Dis. Manag. Res. Signpost 2003, 37, 121–152. [Google Scholar]
- Hermosa, R.; Rubio, B.; Cardoza, R.; Nicolas, C.; Monte, E.; Gutierrez, S. The Contribution of Trichoderma to balancing the costs of plant growth and defense. Int. Microbiol. 2013, 16, 69–80. [Google Scholar] [CrossRef]
- Lorito, M.; Woo, S.L.; Harman, G.E.; Monte, E. Translational research on Trichoderma: From omics to the field. Annu. Rev. Phytopathol. 2010, 48, 395–418. [Google Scholar] [CrossRef]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El Hamss, H.; Belabess, Z.; Barka, E.A. Biological control of plant pathogens: A global perspective. Microorganisms 2022, 10, 596. [Google Scholar] [CrossRef] [PubMed]
- Şesan, T.E. Ciuperci cu importanţă practică în combaterea biologică a micozelor plantelor [Microfungi with practical importance in biocontrol of plant mycoses]. Red Prop. Tehnol. Agric. 1986, 1, 56. [Google Scholar]
- Şesan, T.E.; Enache, E.; Iacomi, B.M.; Oprea, M.; Oancea, F.; Iacomi, C. In vitro antifungal activity of some plant extracts against Fusarium oxysporum in the blackcurrant crop (Ribes Nigrum L.). Acta Sci. Pol. Hortorum. Cultus 2017, 16, 163–172. [Google Scholar] [CrossRef]
- Şesan, T.E.; Oancea, A.O.; Ştefan, L.; Mănoiu, V.S.; Ghiurea, M.; Răuţ, I.; Constantinescu-Aruxandei, D.; Toma, A.; Savin, S.; Bira, A.F.; et al. Effect of foliar treatment with a Trichoderma plant biostimulant consortium on Passiflora caerulea L. yield and quality. Microorganisms 2020, 8, 123. [Google Scholar] [CrossRef]
- Monte, E. Understanding Trichoderma: Between biotechnology and microbial ecology. Int. Microbiol. 2001, 4, 1–4. [Google Scholar] [CrossRef]
- Feng, M.; Zhang, Y.; Coates, B.S.; Du, Q.; Gao, Y.; Li, L.; Yuan, H.; Sun, W.; Chang, X.; Zhou, S.; et al. Assessment of Beauveria bassiana for the biological control of corn borer, Ostrinia furnacalis, in sweet maize by irrigation application. BioControl 2023, 68, 49–60. [Google Scholar] [CrossRef]
- Javed, K.; Javed, H.; Mukhtar, T.; Qiu, D. Efficacy of Beauveria Bassiana and Verticillium Lecanii for the management of whitefly and aphid. Pak. J. Agric. Sci. 2019, 56, 669–674. [Google Scholar]
- Yuvaraj, M.; Ramasamy, M. Chapter 7 Role of Fungi in agriculture. In Biostimulants in Plant Sciences; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Poveda, J.; Abril-Urias, P.; Escobar, C. Biological control of plant-parasitic nematodes by filamentous fungi inducers of resistance: Trichoderma, mycorrhizal and endophytic fungi. Front. Microbiol. 2020, 11, 992. [Google Scholar] [CrossRef]
- Șesan, T.E.; Crișan, A. Cercetări de biologie asupra ciupercii Coniothyrium minitans Campbell—Specie hiperparazită nou semnalată în România [Researches on the biology of C. minitans Campbell—Fungal hyperparasitic species newly recorded in Romania]. St. Cerc. Biol. Biol. Veget. 1988, 40, 71–77. [Google Scholar]
- Şesan, T.E.; Csép, N. Investigations on Coniothyrium minitans and Trichoderma spp. to control diseases of industrial crops caused by Sclerotinia sclerotiorum. IOBC Wprs Bull. 1995, 18, 26–33. [Google Scholar]
- Şesan, T.E.; Tănase, C. Fungi cu Importanţă în Agricultură, Medicină şi Patrimoniu [Fungi with Importance in Agriculture, Medicine and Patrimony; Editura Universitatii din Bucuresti: Bucharest, Romania, 2009; p. 305. [Google Scholar]
- Chang, S.T.; Hayes, W.A.P. Biology and Cultivation Edible Mushrooms; Academic Press: London, UK, 1978. [Google Scholar]
- Amara, A.A.; El-Baky, N.A. Fungi as a source of edible proteins and animal feed. J. Fungi 2023, 9, 73. [Google Scholar] [CrossRef]
- Scott, R. Cheesemaking Practice, 2nd ed.; Elsevier Applied Science Publishers: London, UK, 1986. [Google Scholar]
- Ropars, J.; Didiot, E.; Vega, R.C.; Bennetot, B.; Coton, M.; Poirier, E.; Coton, E.; Snirc, A.; Le Prieur, S.; Giraud, T. Domestication of the emblematic white cheese-making fungus Penicillium camemberti and its diversification into two varieties. Curr. Biol. 2020, 30, 4441–4453. [Google Scholar] [CrossRef]
- Ropars, J.; Cruaud, C.; Lacoste, S.; Dupont, J. A taxonomic and ecological overview of cheese fungi. Int. J. Food Microbiol. 2012, 155, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Pederson, C.S. Microbiology of Food Fermentations; AVI Publishing Co. Inc.: Westport, CN, USA, 1971. [Google Scholar]
- Laranjo, M.; Elias, M.; Fraqueza, M.J. The use of starter cultures in traditional meat products. J. Food Qual. 2017, 2017, 9546026. [Google Scholar] [CrossRef]
- Whittaker, J.; Johnson, R.; Finnigan, T.; Avery, S.; Dyer, P. The biotechnology of Quorn mycoprotein: Past, present and future challenges. In Grand Challenges in Fungal Biotechnology; Grand Challenges in Biology and Biotechnology; Nevalainen, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 59–79. [Google Scholar]
- Wiebe, M.G. QuornTM myco-protein—Overview of a successful fungal product. Mycologist 2004, 18, 17–20. [Google Scholar] [CrossRef]
- Nelson, P.E.; Desjardins, A.E.; Plattner, R.D. Fumonisins, mycotoxins produced by Fusarium species: Biology, chemistry and significance. Annu. Rev. Phytopathol. 1993, 31, 233–252. [Google Scholar] [CrossRef] [PubMed]
- Machida, M.; Yamada, O.; Gomi, K. Genomics of Aspergillus oryzae: Learning from the history of koji mold and exploration of its future. DNA Res. 2018, 15, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Kitamoto, K. Molecular biology of the koji molds. In Advances in Applied Microbiology; Laskin, A.I., Bennett, J.W., Gadd, G.M., Eds.; Academic Press: Cambridge, MA, USA, 2002; pp. 129–153. [Google Scholar]
- Fournier, E.; Gladieux, P.; Giraud, T. The ‘Dr Jekyll and Mr Hyde Fungus’: Noble rot versus gray mold symptoms of Botrytis cinerea on grapes. Evol. Appl. 2013, 6, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Dupont, J.; Dequin, S.; Giraud, T.; Le Tacon, F.; Marsit, S.; Ropars, J.; Richard, F.; Selosse, M.A. Fungi as a source of food. Microbiol. Spectr. 2017, 5, 1063–1085. [Google Scholar] [CrossRef]
- Antranikian, G.; Streit, W.R. Microorganisms harbor keys to a circular bioeconomy making them useful tools in fighting plastic pollution and rising CO2 levels. Extremophiles 2022, 26, 10. [Google Scholar] [CrossRef]
- O’Connor, K.E. Microbiology challenges and opportunities in the circular economy. Microbiol. Read. 2021, 167, 001026. [Google Scholar] [CrossRef] [PubMed]
- Copetti, M.V. Fungi as industrial producers of food ingredients. Curr. Opin. Food Sci. 2019, 25, 52–56. [Google Scholar] [CrossRef]
- Mores, S.; Souza Vandenberghe, L.; Irineudo Magalhães Júnior, A.; Carvalho, J.C.; Mello, A.F.; Pandey, A.; Soccol, C.R. Citric acid bioproduction and downstream processing: Status, opportunities, and challenges. Bioresour. Technol. 2021, 320, 124426. [Google Scholar] [CrossRef]
- Show, P.L.; Oladele, K.O.; Siew, Q.Y.; Aziz Zakry, F.A.; Lan, J.C.-W.; Ling, T.C. Overview of citric acid production from Aspergillus niger. Front. Life Sci. 2015, 8, 271–283. [Google Scholar] [CrossRef]
- Behera, B.C. Citric acid from Aspergillus niger: A comprehensive overview. Crit. Rev. Microbiol. 2020, 46, 727–749. [Google Scholar] [CrossRef]
- Ma, C.; Gerhard, E.; Lu, D.; Yang, J. Citrate chemistry and biology for biomaterials design. Biomaterials 2018, 178, 383–400. [Google Scholar] [CrossRef]
- Tran, R.T.; Yang, J.; Ameer, G.A. Citrate-based biomaterials and their applications in regenerative engineering. Annu. Rev. Mater. Res. 2015, 45, 277–310. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Meneguzzo, F.; Delisi, R.; Pagliaro, M. Citric acid: Emerging applications of key biotechnology industrial product. Chem. Cent. J. 2017, 11, 22. [Google Scholar] [CrossRef]
- Nangare, S.; Vispute, Y.; Tade, R.; Dugam, S.; Patil, P. Pharmaceutical applications of citric acid. Future J. Pharm. Sci. 2021, 7, 54. [Google Scholar] [CrossRef]
- Berovic, M.; Legisa, M. Citric acid production. In Biotechnology Annual Review; El-Gewely, M.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 13, pp. 303–343. [Google Scholar]
- Kareem, S.O.; Akpan, I.; Alebiowu, O.O. Production of citric acid by Aspergillus niger using pineapple waste. Malays. J. Microbiol. 2010, 6, 161–165. [Google Scholar] [CrossRef]
- Dienye, B.N.; Ahaotu, I.; Agwa, O.K.; Odu, N.N. Citric acid production potential of Aspergillus niger using Chrysophyllum albidum peel. Adv. Biosci. Biotechnol. 2018, 9, 190–203. [Google Scholar] [CrossRef]
- Kareem, S.; Rahman, R. Utilization of banana peels for citric acid production by Aspergillus niger. Agric. Biol. J. North Am. 2013, 4, 384–387. [Google Scholar] [CrossRef]
- De Oliveira, P.Z.; de Souza Vandenberghe, L.P.; Rodrigues, C.; de Melo Pereira, G.V.; Soccol, C.R. Exploring cocoa pod husks as a potential substrate for citric acid production by solid-state fermentation using Aspergillus niger mutant strain. Process. Biochem. 2022, 113, 107–112. [Google Scholar] [CrossRef]
- Ramachandra, Y.L.; Narayanamurthy, G.; Jois, S.; Chavan, A.; Satwadi, P.R. Production of citric acid in basal coffee husk medium by Aspergillus niger under solid state fermentation. Adv. Biol. Res. 2013, 7, 234–240. [Google Scholar]
- Betiku, E.; Adesina, O.A. Statistical approach to the optimization of citric acid production using filamentous fungus Aspergillus niger grown on sweet potato starch hydrolyzate. Biomass Bioenergy 2013, 55, 350–354. [Google Scholar] [CrossRef]
- Ramachandran, S.; Fontanille, P.; Pandey, A.; Larroche, C. Gluconic acid: Properties, applications and microbial production. Food Technol. Biotechnol. 2006, 44, 185–195. [Google Scholar]
- Sharma, A.A.; Gomashe, D.A.; Bawane, H. Gluconic acid production by Aspergillus niger from banana must. J. Innov. Sci. 2015, 2, 2394. [Google Scholar]
- Elhousni, L.; Galai, M.; ElKamraoui, F.Z.; Dkhireche, N.; Touhami, M.E.; Chebabe, D.; Sfaira, M.; Zarrouk, A. Study of sodium gluconate and cetyltrimethyl ammonium bromide as inhibitor for copper in moroccan industrial cooling water systems. J. Mater. Environ. Sci. 2016, 7, 2513–2525. [Google Scholar]
- Rabie, A.I.; El-Din, H.A. Sodium gluconate as a new environmentally friendly iron controlling agent for HP/HT acidizing treatments. In Proceedings of the SPE Middle East Oil & Gas Show and Conference, Manama, Bahrain, 8 March 2015; OnePetro: Richardson, TX, USA, 2015. [Google Scholar]
- García-Padilla, S.; Duarte-Vázquez, M.A.; Gonzalez-Romero, K.E.; del Caamaño, M.C.; Rosado, J.L. Effectiveness of intra-articular injections of sodium bicarbonate and calcium gluconate in the treatment of osteoarthritis of the knee: A randomized double-blind clinical trial. BMC Musculoskelet. Disord. 2015, 16, 114. [Google Scholar] [CrossRef]
- Miller, H.J.; Hu, J.; Valentine, J.K.; Gable, P.S. Efficacy and tolerability of intravenous ferric gluconate in the treatment of iron deficiency anemia in patients without kidney disease. Arch. Intern. Med. 2007, 167, 1327–1328. [Google Scholar] [CrossRef]
- Silva, R.F.; Álvarez, M.E.; Ríos, D.L.; López, C.; Carmona, J.U.; Rezende, C.M. Evaluation of the effect of calcium gluconate and bovine thrombin on the temporal release of transforming growth factor beta 1 and platelet-derived growth factor isoform BB from feline platelet concentrates. BMC Vet. Res. 2012, 8, 212. [Google Scholar] [CrossRef]
- Ajala, E.O.; Ajala, M.A.; Ogunniyi, D.S.; Sunmonu, M.O. Kinetics of gluconic acid production and cell growth in a batch bioreactor by Aspergillus niger using breadfruit hydrolysate. J. Food Process. Eng. 2017, 40, e12461. [Google Scholar] [CrossRef]
- Singh, O.V.; Singh, R.P. Bioconversion of grape must into modulated gluconic acid production by Aspergillus niger ORS-4·410. J. Appl. Microbiol. 2006, 100, 1114–1122. [Google Scholar] [CrossRef]
- Ikeda, Y.; Park, E.Y.; Okuda, N. Bioconversion of waste office paper to gluconic acid in a turbine blade reactor by the filamentous fungus Aspergillus niger. Bioresour. Technol. 2006, 97, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, R.; Chatterjee, S.; Chatterjee, B.P.; Banerjee, P.C.; Guha, A.K. Production of gluconic acid from whey by free and immobilized Aspergillus niger. Int. Dairy J. 2005, 15, 299–303. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Bao, J. High titer gluconic acid fermentation by Aspergillus niger from dry dilute acid pretreated corn stover without detoxification. Bioresour. Technol. 2016, 203, 211–219. [Google Scholar] [CrossRef]
- Katsuya Tooyama, T.M. Simultaneous saccharification of corn starch in gluconic acid production by Aspergillus niger immobilized on nonwoven fabric in a pressurized reactor. Microb. Biochem. Technol. 2013, 5, 1000106. [Google Scholar] [CrossRef]
- Sharma, A.; Vivekanand, V.; Singh, R.P. Solid-state fermentation for gluconic acid production from sugarcane molasses by Aspergillus niger ARNU-4 employing tea waste as the novel solid support. Bioresour. Technol. 2008, 99, 3444–3450. [Google Scholar] [CrossRef]
- Huang, J.; Huang, L.; Lin, J.; Xu, Z.; Cen, P. Organic chemicals from bioprocesses in China. Adv. Biochem. Eng. Biotechnol. 2010, 122, 43–71. [Google Scholar] [CrossRef]
- Hajian, H.; Yusoff, W.M.W. Itaconic acid production by microorganisms: A review. Curr. Res. J. Biol. Sci. 2015, 7, 37–42. [Google Scholar] [CrossRef]
- Teleky, B.-E.; Vodnar, D.C. Recent advances in biotechnological itaconic acid production, and application for a sustainable approach. Polymers 2021, 13, 3574. [Google Scholar] [CrossRef] [PubMed]
- Bellasio, M.; Mattanovich, D.; Sauer, M.; Marx, H. Organic acids from lignocellulose: Candida lignohabitans as a new microbial cell factory. J. Ind. Microbiol. Biotechnol. 2015, 42, 681–691. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, G.; Zhang, J.; Balan, V.; Bao, J. Itaconic acid fermentation using activated charcoal-treated corn stover hydrolysate and process evaluation based on aspen plus model. Biomass Conv. Bioref. 2020, 10, 463–470. [Google Scholar] [CrossRef]
- Pedroso, G.B.; Montipó, S.; Mario, D.A.N.; Alves, S.H.; Martins, A.F. Building block itaconic acid from left-over biomass. Biomass Conv. Bioref. 2017, 7, 23–35. [Google Scholar] [CrossRef]
- Gnanasekaran, R.; Dhandapani, B.; Gopinath, K.P.; Iyyappan, J. Synthesis of itaconic acid from agricultural waste using novel Aspergillus niveus. Prep. Biochem. Biotechnol. 2018, 48, 605–609. [Google Scholar] [CrossRef]
- Regestein, L.; Klement, T.; Grande, P.; Kreyenschulte, D.; Heyman, B.; Maßmann, T.; Eggert, A.; Sengpiel, R.; Wang, Y.; Wierckx, N.; et al. From beech wood to itaconic acid: Case study on biorefinery process integration. Biotechnol. Biofuels 2018, 11, 279. [Google Scholar] [CrossRef] [PubMed]
- Tippkötter, N.; Duwe, A.-M.; Wiesen, S.; Sieker, T.; Ulber, R. Enzymatic hydrolysis of beech wood lignocellulose at high solid contents and its utilization as substrate for the production of biobutanol and dicarboxylic acids. Bioresour. Technol. 2014, 167, 447–455. [Google Scholar] [CrossRef]
- Reddy, C.S.K.; Singh, R.P. Enhanced Production of itaconic acid from corn starch and market refuse fruits by genetically manipulated Aspergillus terreus SKR10. Bioresour. Technol. 2002, 85, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Castillo Martinez, F.A.; Balciunas, E.M.; Salgado, J.M.; Domínguez González, J.M.; Converti, A.; Oliveira, R.P. de S. Lactic acid properties, applications and production: A review. Trends Food Sci. Technol. 2013, 30, 70–83. [Google Scholar] [CrossRef]
- Aung, S.P.S.; Shein, H.H.H.; Aye, K.N.; Nwe, N. Environment-friendly biopolymers for food packaging: Starch, protein, and poly-lactic acid (PLA). In Bio-Based Materials for Food Packaging: Green and Sustainable Advanced Packaging Materials; Ahmed, S., Ed.; Springer: Singapore, 2018; pp. 173–195. ISBN 9789811319099. [Google Scholar]
- Tawakkal, I.S.M.A.; Cran, M.J.; Miltz, J.; Bigger, S.W. A review of Poly(Lactic Acid)-based materials for antimicrobial packaging. J. Food Sci. 2014, 79, R1477–R1490. [Google Scholar] [CrossRef] [PubMed]
- Blasi, P. Poly(Lactic Acid)/Poly(Lactic-Co-Glycolic Acid)-Based Microparticles: An Overview. J. Pharm. Investig. 2019, 49, 337–346. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Jin, B.; Kelly, J.M. Production of lactic acid from renewable materials by Rhizopus fungi. Biochem. Eng. J. 2007, 35, 251–263. [Google Scholar] [CrossRef]
- Huang, L.P.; Jin, B.; Lant, P.; Zhou, J. Biotechnological production of lactic acid integrated with potato wastewater treatment by Rhizopus arrhizus. J. Chem. Technol. Biotechnol. 2003, 78, 899–906. [Google Scholar] [CrossRef]
- Kumar, R.; Shivakumar, S. Production of L-lactic acid from starch and food waste by amylolytic Rhizopus oryzae MTCC 8784. Int. J. ChemTech Res. 2014, 6, 527–537. [Google Scholar]
- Ruengruglikit, C.; Hang, Y.D. L(+)-lactic acid production from corncobs by Rhizopus oryzae NRRL-395. LWT Food Sci. Technol. 2003, 36, 573–575. [Google Scholar] [CrossRef]
- Shahri, S.Z.; Vahabzadeh, F.; Mogharei, A. Lactic acid production by loofah-immobilized Rhizopus oryzae through one-step fermentation process using starch substrate. Bioprocess Biosyst. Eng 2020, 43, 333–345. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Xue, Y.; Zhang, T.-A.; Li, Y.; Hu, J.; Tsang, Y.F.; Zhang, H.; Gao, M.-T. Influence of rice straw-derived dissolved organic matter on lactic acid fermentation by Rhizopus oryzae. J. Biosci. Bioeng. 2018, 125, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Oonkhanond, B.; Jonglertjunya, W.; Srimarut, N.; Bunpachart, P.; Tantinukul, S.; Nasongkla, N.; Sakdaronnarong, C. Lactic acid production from sugarcane bagasse by an integrated system of lignocellulose fractionation, saccharification, fermentation, and ex-situ nanofiltration. J. Environ. Chem. Eng. 2017, 5, 2533–2541. [Google Scholar] [CrossRef]
- Vially, G.; Marchal, R.; Guilbert, N. L(+) Lactate Production from Carbohydrates and Lignocellulosic Materials by Rhizopus Oryzae UMIP 4.77. World J. Microbiol. Biotechnol. 2010, 26, 607–614. [Google Scholar] [CrossRef]
- Ilica, R.-A.; Kloetzer, L.; Galaction, A.-I.; Caşcaval, D. Fumaric Acid: Production and Separation. Biotechnol. Lett. 2019, 41, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Dörsam, S.; Fesseler, J.; Gorte, O.; Hahn, T.; Zibek, S.; Syldatk, C.; Ochsenreither, K. Sustainable Carbon Sources for Microbial Organic Acid Production with Filamentous Fungi. Biotechnol. Biofuels 2017, 10, 242. [Google Scholar] [CrossRef] [PubMed]
- Iyyappan, J.; Baskar, G.; Bharathiraja, B.; Saravanathamizhan, R. Malic Acid Production from Biodiesel Derived Crude Glycerol Using Morphologically Controlled Aspergillus niger in Batch Fermentation. Bioresour. Technol. 2018, 269, 393–399. [Google Scholar] [CrossRef]
- Zou, X.; Zhou, Y.; Yang, S.-T. Production of Polymalic Acid and Malic Acid by Aureobasidium pullulans Fermentation and Acid Hydrolysis. Biotechnol. Bioeng. 2013, 110, 2105–2113. [Google Scholar] [CrossRef]
- Das, R.K.; Brar, S.K.; Verma, M. A fermentative approach towards optimizing directed biosynthesis of fumaric acid by Rhizopus oryzae 1526 utilizing apple industry waste biomass. Fungal Biol. 2015, 119, 1279–1290. [Google Scholar] [CrossRef]
- Fan, T.; Liu, X.; Zhao, R.; Zhang, Y.; Liu, H.; Wang, Z.; Wang, F.; Nie, K.; Deng, L. Hydrolysis of food waste by hot water extraction and subsequent Rhizopus fermentation to fumaric acid. J. Environ. Manag. 2020, 270, 110954. [Google Scholar] [CrossRef]
- Liu, H.; Ma, J.; Wang, M.; Wang, W.; Deng, L.; Nie, K.; Yue, X.; Wang, F.; Tan, T. Food waste fermentation to fumaric acid by Rhizopus arrhizus RH7-13. Appl. Biochem. Biotechnol. 2016, 180, 1524–1533. [Google Scholar] [CrossRef] [PubMed]
- De Souza, P.M. Application of microbial -amylase in industry—A review. Braz. J. Microbiol. 2010, 41, 850–861. [Google Scholar] [CrossRef]
- Saranraj, P.; Stella, D. Fungal Amylase—A Review. Int. J. Microbiol. Res. 2013, 4, 203–211. [Google Scholar]
- De Souza, P.M.; Bittencourt, M.L.d.A.; Caprara, C.C.; de Freitas, M.; de Almeida, R.P.C.; Silveira, D.; Fonseca, Y.M.; Ferreira Filho, E.X.; Pessoa Junior, A.; Magalhães, P.O. A biotechnology perspective of fungal proteases. Braz. J. Microbiol. 2015, 46, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Merheb, C.W.; Cabral, H.; Gomes, E.; Da-Silva, R. Partial characterization of protease from a thermophilic fungus, Thermoascus aurantiacus, and its hydrolytic activity on bovine casein. Food Chem. 2007, 104, 127–131. [Google Scholar] [CrossRef]
- Ghareib, M.; Fawzi, E.M.; Aldossary, N.A. Thermostable alkaline protease from thermomyces lanuginosus: Optimization, purification and characterization. Ann. Microbiol. 2014, 64, 859–867. [Google Scholar] [CrossRef]
- Pedrolli, D.B.; Monteiro, A.C.; Gomes, E.; Carmona, E.C. Pectin and pectinases: Production, characterization and industrial application of microbial pectinolytic enzymes. Open Biotechnol. J. 2009, 3, 9–18. [Google Scholar] [CrossRef]
- Katrolia, P.; Jia, H.; Yan, Q.; Song, S.; Jiang, Z.; Xu, H. Characterization of a protease-resistant α-Galactosidase from the thermophilic fungus Rhizomucor miehei and its application in removal of raffinose family oligosaccharides. Bioresour. Technol. 2012, 110, 578–586. [Google Scholar] [CrossRef]
- Balu, V.; Muthusamy, P.; Gopalakrishnan, V.K. Screening and optimization of β-galactosidase from fungal strains by using agro residues. World J. Pharm. Pharm. Sci. 2014, 3, 1809–1821. [Google Scholar]
- Saqib, S.; Akram, A.; Halim, S.A.; Tassaduq, R. Sources of β-galactosidase and its applications in food industry. 3 Biotech 2017, 7, 79. [Google Scholar] [CrossRef]
- Singh, R.V.; Sambyal, K. β-galactosidase as an industrial enzyme: Production and potential. Chem. Pap. 2023, 77, 11–31. [Google Scholar] [CrossRef]
- Hartl, L.; Zach, S.; Seidl-Seiboth, V. Fungal chitinases: Diversity, mechanistic properties and biotechnological potential. Appl. Microbiol. Biotechnol. 2012, 93, 533–543. [Google Scholar] [CrossRef]
- El-Gendi, H.; Saleh, A.K.; Badierah, R.; Redwan, E.M.; El-Maradny, Y.A.; El-Fakharany, E.M. A comprehensive insight into fungal enzymes: Structure, classification, and their role in mankind’s challenges. J. Fungi 2022, 8, 23. [Google Scholar] [CrossRef]
- Mehta, A.; Bodh, U.; Gupta, R. Fungal lipases: A review. J. Biotech Res. 2017, 8, 58–77. [Google Scholar]
- Shu, Z.-Y.; Yan, Y.-J.; Yang, J.-K.; Xu, L. Aspergillus niger lipase: Gene cloning, over-expression in Escherichia coli and in vitro refolding. Biotechnol. Lett. 2007, 29, 1875–1879. [Google Scholar] [CrossRef] [PubMed]
- Viswanath, B.; Rajesh, B.; Janardhan, A.; Kumar, A.P.; Narasimha, G. Fungal laccases and their applications in bioremediation. Enzym. Res. 2014, 2014, 163242. [Google Scholar] [CrossRef]
- Senthivelan, T.; Kanagaraj, J.; Panda, R.C. Recent trends in fungal laccase for various industrial applications: An eco-friendly approach—A review. Biotechnol. Bioprocess Eng. 2016, 21, 19–38. [Google Scholar] [CrossRef]
- Sajith, S.; Priji, P.; Sreedevi, S.; Benjamin, S. An overview on fungal cellulases with an industrial perspective. J. Nutr. Food Sci. 2016, 6, 461. [Google Scholar]
- Payne, C.M.; Knott, B.C.; Mayes, H.B.; Hansson, H.; Himmel, M.E.; Sandgren, M.; Ståhlberg, J.; Beckham, G.T. Fungal cellulases. Chem. Rev. 2015, 115, 1308–1448. [Google Scholar] [CrossRef]
- Adeniran, A.H.; Abiose, S.H. Amylolytic potentiality of fungi isolated from some nigerian agricultural wastes. Afr. J. Biotechnol. 2009, 8, 59910. [Google Scholar]
- Anto, H.; Trivedi, U.B.; Patel, K.C. Glucoamylase production by solid-state fermentation using rice flake manufacturing waste products as substrate. Bioresour. Technol. 2006, 97, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- Baeyens, J.; Kang, Q.; Appels, L.; Dewil, R.; Lv, Y.; Tan, T. Challenges and opportunities in improving the production of bio-ethanol. Prog. Energy Combust. Sci. 2015, 47, 60–88. [Google Scholar] [CrossRef]
- Farooq, M.A.; Ali, S.; Hassan, A.; Tahir, H.M.; Mumtaz, S.; Mumtaz, S. Biosynthesis and industrial applications of α-amylase: A review. Arch. Microbiol. 2021, 203, 1281–1292. [Google Scholar] [CrossRef]
- Ghorai, S.; Banik, S.P.; Verma, D.; Chowdhury, S.; Mukherjee, S.; Khowala, S. Fungal biotechnology in food and feed processing. Food Res. Int. 2009, 42, 577–587. [Google Scholar] [CrossRef]
- Rana, N.; Walia, A.; Gaur, A. α-amylases from microbial sources and its potential applications in various industries. Natl. Acad. Sci. Lett. 2013, 36, 9–17. [Google Scholar] [CrossRef]
- Sakthi, S.S.; Kanchana, D.; Saranraj, P.; Usharani, G. Evaluation of amylase activity of the amylolytic fungi Aspergillus niger using cassava as substrate. Int. J. Appl. Microbiol. Sci. 2012, 1, 24–34. [Google Scholar]
- Singh, S.; Singh, S.; Bali, V.; Sharma, L.; Mangla, J. Production of fungal amylases using cheap, readily available agriresidues, for potential application in textile industry. BioMed Res. Int. 2014, 2014, e215748. [Google Scholar] [CrossRef]
- Bah, C.S.F.; Bekhit, A.E.-D.A.; McConnell, M.A.; Carne, A. Generation of bioactive peptide hydrolysates from cattle plasma using plant and fungal proteases. Food Chem. 2016, 213, 98–107. [Google Scholar] [CrossRef]
- Brandelli, A.; Daroit, D.J.; Riffel, A. Biochemical features of microbial keratinases and their production and applications. Appl. Microbiol. Biotechnol. 2010, 85, 1735–1750. [Google Scholar] [CrossRef]
- Chutmanop, J.; Chuichulcherm, S.; Chisti, Y.; Srinophakun, P. Protease production by Aspergillus oryzae in solid-state fermentation using agroindustrial substrates. J. Chem. Technol. Biotechnol. 2008, 83, 1012–1018. [Google Scholar] [CrossRef]
- Ha, M.; Bekhit, A.E.-D.; Carne, A.; Hopkins, D.L. Comparison of the proteolytic activities of new commercially available bacterial and fungal proteases toward meat proteins. J. Food Sci. 2013, 78, C170–C177. [Google Scholar] [CrossRef] [PubMed]
- Kranthi, V.S.; Rao, D.M.; Jaganmohan, P. Production of protease by Aspergillus flavus through solid state fermentation using different oil seed cakes. Int. J. Microbiol. Res. 2012, 3, 12–15. [Google Scholar]
- Kumar, S.; Sharma, N.S.; Saharan, M.R.; Singh, R. Extracellular acid protease from Rhizopus oryzae: Purification and characterization. Process. Biochem. 2005, 40, 1701–1705. [Google Scholar] [CrossRef]
- Novelli, P.K.; Barros, M.M.; Fleuri, L.F. Novel inexpensive fungi proteases: Production by solid state fermentation and characterization. Food Chem. 2016, 198, 119–124. [Google Scholar] [CrossRef]
- Savitha, S.; Sadhasivam, S.; Swaminathan, K.; Lin, F.H. Fungal protease: Production, purification and compatibility with laundry detergents and their wash performance. J. Taiwan Inst. Chem. Eng. 2011, 42, 298–304. [Google Scholar] [CrossRef]
- Zanutto-Elgui, M.R.; Vieira, J.C.S.; do Prado, D.Z.; Buzalaf, M.A.R.; Padilha, P.d.M.; Elgui de Oliveira, D.; Fleuri, L.F. Production of milk peptides with antimicrobial and antioxidant properties through fungal proteases. Food Chem. 2019, 278, 823–831. [Google Scholar] [CrossRef]
- Anand, G.; Yadav, S.; Yadav, D. Production, purification and biochemical characterization of an exo-polygalacturonase from Aspergillus niger MTCC 478 suitable for clarification of orange juice. 3 Biotech 2017, 7, 122. [Google Scholar] [CrossRef]
- Demir, H.; Tarı, C. Valorization of wheat bran for the production of polygalacturonase in SSF of Aspergillus sojae. Ind. Crops Prod. 2014, 54, 302–309. [Google Scholar] [CrossRef]
- Nikolić, M.V.; Mojovic, L. Hydrolysis of apple pectin by the coordinated activity of pectic enzymes. Food Chem. 2007, 101, 1580–1584. [Google Scholar] [CrossRef]
- Patidar, M.K.; Nighojkar, S.; Kumar, A.; Nighojkar, A. Pectinolytic enzymes-solid state fermentation, assay methods and applications in fruit juice industries: A review. 3 Biotech 2018, 8, 199. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Rathore, M.; Sharma, M. Microbial pectinase: Sources, characterization and applications. Rev. Environ. Sci. Biotechnol. 2013, 12, 45–60. [Google Scholar] [CrossRef]
- Ortiz, G.E.; Ponce-Mora, M.C.; Noseda, D.G.; Cazabat, G.; Saravalli, C.; López, M.C.; Gil, G.P.; Blasco, M.; Albertó, E.O. Pectinase production by Aspergillus giganteus in solid-state fermentation: Optimization, scale-up, biochemical characterization and its application in olive-oil extraction. J. Ind. Microbiol. Biotechnol. 2017, 44, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Tai, E.-S.; Hsieh, P.-C.; Sheu, S.-C. Effect of polygalacturonase and feruloyl esterase from Aspergillus tubingensis on demucilage and quality of coffee beans. Process. Biochem. 2014, 49, 1274–1280. [Google Scholar] [CrossRef]
- Barman, S.; Sit, N.; Badwaik, L.S.; Deka, S.C. Pectinase Production by Aspergillus niger using banana (Musa balbisiana) peel as substrate and its effect on clarification of banana juice. J. Food Sci. Technol. 2015, 52, 3579–3589. [Google Scholar] [CrossRef] [PubMed]
- Sethi, B.K.; Nanda, P.K.; Sahoo, S. Enhanced production of pectinase by Aspergillus terreus NCFT 4269.10 Using Banana Peels as Substrate. 3 Biotech 2016, 6, 36. [Google Scholar] [CrossRef]
- Geng, X.; Tian, G.; Zhao, Y.; Zhao, L.; Wang, H.; Ng, T.B. A fungal α-galactosidase from Tricholoma matsutake with broad substrate specificity and good hydrolytic activity on raffinose family oligosaccharides. Molecules 2015, 20, 13550–13562. [Google Scholar] [CrossRef]
- Bhatia, S.; Singh, A.; Batra, N.; Singh, J. Microbial production and biotechnological applications of α-galactosidase. Int. J. Biol. Macromol. 2020, 150, 1294–1313. [Google Scholar] [CrossRef]
- Martarello, R.D.; Cunha, L.; Cardoso, S.L.; de Freitas, M.M.; Silveira, D.; Fonseca-Bazzo, Y.M.; Homem-de-Mello, M.; Filho, E.X.F.; Magalhães, P.O. Optimization and partial purification of beta-galactosidase production by Aspergillus niger isolated from brazilian soils using soybean residue. AMB Express 2019, 9, 81. [Google Scholar] [CrossRef]
- Kazemi, S.; Khayati, G.; Faezi-Ghasemi, M. β-galactosidase production by Aspergillus niger ATCC 9142 using inexpensive substrates in solid-state fermentation: Optimization by orthogonal arrays design. Iran. Biomed. J. 2016, 20, 287–294. [Google Scholar] [CrossRef]
- Silvério, S.C.; Macedo, E.A.; Teixeira, J.A.; Rodrigues, L.R. New β-galactosidase producers with potential for prebiotic synthesis. Bioresour. Technol. 2018, 250, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Dario Rafael, O.-H.; Luis Fernándo, Z.-G.; Abraham, P.-T.; Pedro Alberto, V.-L.; Guadalupe, G.-S.; Pablo, P.J. Production of chitosan-oligosaccharides by the chitin-hydrolytic system of Trichoderma harzianum and their antimicrobial and anticancer effects. Carbohydr. Res. 2019, 486, 107836. [Google Scholar] [CrossRef]
- Abu-Tahon, M.A.; Isaac, G.S. Anticancer and antifungal efficiencies of purified chitinase produced from Trichoderma viride under submerged fermentation. J. Gen. Appl. Microbiol. 2020, 66, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Puri, A.K.; Govender, A.; Wang, Z.; Singh, S.; Permaul, K. The multi-chitinolytic enzyme system of the compost-dwelling thermophilic fungus Thermomyces lanuginosus. Process. Biochem. 2015, 50, 237–244. [Google Scholar] [CrossRef]
- Nampoothiri, K.M.; Baiju, T.V.; Sandhya, C.; Sabu, A.; Szakacs, G.; Pandey, A. Process optimization for antifungal chitinase production by Trichoderma harzianum. Process. Biochem. 2004, 39, 1583–1590. [Google Scholar] [CrossRef]
- Sudhakar, P.; Nagarajan, P. Production of chitinase by solid state fermentation from rice bran. Int. J. Environ. Sci. Dev. 2010, 1, 435–440. [Google Scholar] [CrossRef]
- Marchut-Mikolajczyk, O.; Kwapisz, E.; Wieczorek, D.; Antczak, T. Biodegradation of diesel oil hydrocarbons enhanced with Mucor circinelloides enzyme preparation. Int. Biodeterior. Biodegrad. 2015, 104, 142–148. [Google Scholar] [CrossRef]
- Wang, X.; Lu, D.; Jönsson, L.J.; Hong, F. Preparation of a PET-hydrolyzing lipase from Aspergillus oryzae by the addition of Bis(2-hydroxyethyl) terephthalate to the culture medium and enzymatic modification of PET fabrics. Eng. Life Sci. 2008, 8, 268–276. [Google Scholar] [CrossRef]
- Romdhane, I.B.-B.; Fendri, A.; Gargouri, Y.; Gargouri, A.; Belghith, H. A novel thermoactive and alkaline lipase from Talaromyces thermophilus fungus for use in laundry detergents. Biochem. Eng. J. 2010, 53, 112–120. [Google Scholar] [CrossRef]
- Colla, L.M.; Primaz, A.L.; Benedetti, S.; Loss, R.A.; de Lima, M.; Reinehr, C.O.; Bertolin, T.E.; Costa, J.A.V. Surface response methodology for the optimization of lipase production under submerged fermentation by filamentous fungi. Braz. J. Microbiol. 2016, 47, 461–467. [Google Scholar] [CrossRef]
- Aguieiras, E.C.G.; Cavalcanti-Oliveira, E.D.; Freire, D.M.G. Current status and new developments of biodiesel production using fungal lipases. Fuel 2015, 159, 52–67. [Google Scholar] [CrossRef]
- Lima, V.M.G.; Krieger, N.; Sarquis, M.I.M.; Mitchell, D.A.; Ramos, L.P.; Fontana, J.D. Effect of nitrogen and carbon sources on lipase production by Penicillium aurantiogriseum. Food Technol. Biotechnol. 2003, 41, 105–110. [Google Scholar]
- Gombert, A.K.; Pinto, A.L.; Castilho, L.R.; Freire, D.M.G. Lipase production by Penicillium restrictum in solid-state fermentation using babassu oil cake as substrate. Process. Biochem. 1999, 35, 85–90. [Google Scholar] [CrossRef]
- Gutarra, M.L.E.; Godoy, M.G.; Castilho, L.R.; Freire, D.M.G. Inoculum strategies for Penicillium simplicissimum lipase production by solid-state fermentation using a residue from the babassu oil industry. J. Chem. Technol. Biotechnol. 2007, 82, 313–318. [Google Scholar] [CrossRef]
- Ruela, H.S.; Sutili, F.K.; Leal, I.C.R.; Carvalho, N.M.F.; Miranda, L.S.M.; de Souza, R.O.M.A. Lipase-catalyzed synthesis of secondary glucose esters under continuous flow conditions. Eur. J. Lipid Sci. Technol. 2013, 115, 464–467. [Google Scholar] [CrossRef]
- Sethi, B.K.; Nanda, P.K.; Sahoo, S. Characterization of biotechnologically relevant extracellular lipase produced by Aspergillus Terreus NCFT 4269.10. Braz. J. Microbiol. 2016, 47, 143–149. [Google Scholar] [CrossRef]
- Wang, H.; Kaur, G.; Pensupa, N.; Uisan, K.; Du, C.; Yang, X.; Lin, C.S.K. Textile waste valorization using submerged filamentous fungal fermentation. Process. Saf. Environ. Prot. 2018, 118, 143–151. [Google Scholar] [CrossRef]
- Hu, Y.; Du, C.; Leu, S.-Y.; Jing, H.; Li, X.; Lin, C.S.K. Valorisation of textile waste by fungal solid state fermentation: An example of circular waste-based biorefinery. Resour. Conserv. Recycl. 2018, 129, 27–35. [Google Scholar] [CrossRef]
- Chen, Y.; Wan, J.; Zhang, X.; Ma, Y.; Wang, Y. Effect of beating on recycled properties of unbleached eucalyptus cellulose fiber. Carbohydr. Polym. 2012, 87, 730–736. [Google Scholar] [CrossRef]
- Kuhad, R.C.; Gupta, R.; Singh, A. Microbial cellulases and their industrial applications. Enzym. Res. 2011, 2011, 280696. [Google Scholar] [CrossRef]
- Mrudula, S.; Murugammal, R. Production of cellulase by Aspergillus niger under submerged and solid state fermentation using coir waste as a substrate. Braz. J. Microbiol. 2011, 42, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, S.; Mattoo, R.L.; Nehvi, F.A. Isolation, characterization and molecular weight determination of cellulase from Trichoderma viride. Afr. J. Biotechnol. 2013, 12, 4503–4511. [Google Scholar] [CrossRef]
- Garcia, L.F.; Lacerda, M.F.A.R.; Thomaz, D.V.; de Souza Golveia, J.C.; das Pereira, M.G.C.; de Souza Gil, E.; Schimidt, F.; Santiago, M.F. Optimization of laccase-alginate-chitosan-based matrix toward 17 α-ethinylestradiol removal. Prep. Biochem. Biotechnol. 2019, 49, 375–383. [Google Scholar] [CrossRef]
- Garcia-Ubasart, J.; Esteban, A.; Vila, C.; Roncero, M.B.; Colom, J.F.; Vidal, T. Enzymatic treatments of pulp using laccase and hydrophobic compounds. Bioresour. Technol. 2011, 102, 2799–2803. [Google Scholar] [CrossRef] [PubMed]
- Vantamuri, A.B.; Kaliwal, B.B. Purification and characterization of laccase from Marasmius species BBKAV79 and effective decolorization of selected textile dyes. 3 Biotech. 2016, 6, 189. [Google Scholar] [CrossRef]
- Xu, L.; Sun, K.; Wang, F.; Zhao, L.; Hu, J.; Ma, H.; Ding, Z. Laccase production by Trametes versicolor in solid-state fermentation using tea residues as substrate and its application in dye decolorization. J. Environ. Manag. 2020, 270, 110904. [Google Scholar] [CrossRef] [PubMed]
- Renzetti, S.; Courtin, C.M.; Delcour, J.A.; Arendt, E.K. Oxidative and proteolytic enzyme preparations as promising improvers for oat bread formulations: Rheological, biochemical and microstructural background. Food Chem. 2010, 119, 1465–1473. [Google Scholar] [CrossRef]
- De Peixoto-Nogueira, S.C.; Betini, J.H.A.; Michelin, M.; de Carvalho, C.C.; Lucca, A.L.; Vici, A.C.; de Jorge, J.A.M. Laccase production by Aspergillus niveus on SSF using wheat bran as alternative carbon source and its synergistic effect on pulp biobleaching using a mix of laccase/xylanase from the same microorganism. J. Biochem. Technol. 2015, 6, 929–937. [Google Scholar]
- Omeje, K.O.; Nnolim, N.E.; Ezema, B.O.; Ozioko, J.N.; Eze, S.O.O. Synthetic dyes decolorization potential of agroindustrial waste-derived thermo-active laccase from Aspergillus species. Biocatal. Agric. Biotechnol. 2020, 29, 101800. [Google Scholar] [CrossRef]
- Singh Arora, D.; Kumar Sharma, R. Ligninolytic fungal laccases and their biotechnological applications. Appl. Biochem. Biotechnol. 2010, 160, 1760–1788. [Google Scholar] [CrossRef]
- Falade, A.O.; Nwodo, U.U.; Iweriebor, B.C.; Green, E.; Mabinya, L.V.; Okoh, A.I. Lignin peroxidase functionalities and prospective applications. MicrobiologyOpen 2017, 6, e00394. [Google Scholar] [CrossRef] [PubMed]
- Bonugli-Santos, R.C.; Durrant, L.R.; Da Silva, M.; Sette, L.D. Production of laccase, manganese peroxidase and lignin peroxidase by brazilian marine-derived fungi. Enzym. Microb. Technol. 2010, 46, 32–37. [Google Scholar] [CrossRef]
- Pamidipati, S.; Ahmed, A. Degradation of lignin in agricultural residues by locally isolated fungus Neurospora discreta. Appl. Biochem. Biotechnol. 2017, 181, 1561–1572. [Google Scholar] [CrossRef] [PubMed]
- Akilandeswari, P.; Pradeep, B.V. Exploration of industrially important pigments from soil fungi. Appl. Microbiol. Biotechnol. 2016, 100, 1631–1643. [Google Scholar] [CrossRef] [PubMed]
- Lopes, F.C.; Tichota, D.M.; Pereira, J.Q.; Segalin, J.; de Oliveira Rios, A.; Brandelli, A. Pigment production by filamentous fungi on agro-industrial byproducts: An eco-friendly alternative. Appl. Biochem. Biotechnol. 2013, 171, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Räisänen, R. Fungal colorants in applications—Focus on Cortinarius Species. Color. Technol. 2019, 135, 22–31. [Google Scholar] [CrossRef]
- Meruvu, H.; dos Santos, J.C. Colors of life: A review on fungal pigments. Crit. Rev. Biotechnol. 2021, 41, 1153–1177. [Google Scholar] [CrossRef]
- Chen, W.; Chen, R.; Liu, Q.; He, Y.; He, K.; Ding, X.; Kang, L.; Guo, X.; Xie, N.; Zhou, Y.; et al. Orange, red, yellow: Biosynthesis of azaphilone pigments in Monascus fungi. Chem. Sci. 2017, 8, 4917–4925. [Google Scholar] [CrossRef]
- Rapoport, A.; Guzhova, I.; Bernetti, L.; Buzzini, P.; Kieliszek, M.; Kot, A.M. Carotenoids and some other pigments from fungi and yeasts. Metabolites 2021, 11, 92. [Google Scholar] [CrossRef]
- Nanou, K.; Roukas, T. Waste cooking oil: A new substrate for carotene production by Blakeslea trispora in submerged fermentation. Bioresour. Technol. 2016, 203, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Roukas, T.; Varzakakou, M.; Kotzekidou, P. From cheese whey to carotenes by Blakeslea trispora in a bubble column reactor. Appl. Biochem. Biotechnol. 2015, 175, 182–193. [Google Scholar] [CrossRef]
- Klempová, T.; Slaný, O.; Šišmiš, M.; Marcinčák, S.; Čertík, M. Dual production of polyunsaturated fatty acids and beta-carotene with Mucor wosnessenskii by the process of solid-state fermentation using agro-industrial waste. J. Biotechnol. 2020, 311, 1–11. [Google Scholar] [CrossRef]
- Bayram, S. A comparative characterization study between fungal and bacterial eumelanin pigments. Indian J. Microbiol. 2022, 62, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Meng, X.; Mo, C.; Wei, X.; Ma, A. Melanin of Fungi: From Classification to Application. World J. Microbiol. Biotechnol. 2022, 38, 228. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Rusyn, I.; Dmytruk, O.V.; Onyeaka, H.; Gryzenhout, M.; Gafforov, Y. Filamentous fungi for sustainable remediation of pharmaceutical compounds, heavy metal and oil hydrocarbons. Front. Bioeng. Biotechnol. 2023, 11, 1106973. [Google Scholar] [CrossRef] [PubMed]
- Dusengemungu, L.; Kasali, G.; Gwanama, C.; Ouma, K.O. Recent advances in biosorption of copper and Cobalt by filamentous fungi. Front. Microbiol. 2020, 11, 582016. [Google Scholar] [CrossRef]
- Asemoloye, M.D.; Tosi, S.; Daccò, C.; Wang, X.; Xu, S.; Marchisio, M.A.; Gao, W.; Jonathan, S.G.; Pecoraro, L. Hydrocarbon degradation and enzyme activities of Aspergillus oryzae and Mucor irregularis isolated from nigerian crude oil-polluted sites. Microorganisms 2020, 8, 1912. [Google Scholar] [CrossRef]
- Milala, M.; Shugaba, A.; Gidado, A.; Ene, A.C.; Wafar, J.A. Studies on the use of agricultural wastes for cellulase enzyme production by Aspegillus niger. Res. J. Agric. Biol. Sci. 2005, 1, 325–328. [Google Scholar]
- Zheng, W.; Zheng, Q.; Xue, Y.; Hu, J.; Gao, M.T. Influence of rice straw polyphenols on cellulase production by Trichoderma Reesei. J. Biosci. Bioeng. 2017, 123, 731–738. [Google Scholar] [CrossRef]
- Vasić, K.; Knez, Ž.; Leitgeb, M. Bioethanol production by enzymatic hydrolysis from different lignocellulosic sources. Molecules 2021, 26, 753. [Google Scholar] [CrossRef]
- Mnkandla, M.; Otomo, P. Effectiveness of mycofiltration for removal of contaminants from water: A systematic review protocol. Environ. Evid. 2021, 10, 17. [Google Scholar] [CrossRef]
- Taylor, A.; Flatt, A.; Beutel, M.W.; Wolff, M.; Brownson, K.; Stamets, P. Removal of Escherichia coli from synthetic stormwater using mycofiltration. Ecol. Eng. 2015, 78, 79–86. [Google Scholar] [CrossRef]
- Van Der Aa Kühle, A.; Jespersen, L. The taxonomic position of Saccharomyces boulardii as evaluated by sequence analysis of the D1/D2 domain of 26S RDNA, the ITS1-5.8S RDNA-ITS2 region and the mitochondrial cytochrome-c oxidase II gene. Syst. Appl. Microbiol. 2003, 26, 564–571. [Google Scholar] [CrossRef]
- Aramayo, R.; Selker, E.U. Neurospora crassa, a model system for epigenetics research. Cold Spring Harb. Perspect. Biol. 2013, 5, a017921. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elsalam, K.A. Special issue: Fungal nanotechnology. J. Fungi 2021, 7, 583. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.M.; Morrissey, J.P. Kluyveromyces marxianus: A yeast emerging from its sister’s shadow. Fungal Biol. Rev. 2010, 24, 17–26. [Google Scholar] [CrossRef]
- Bilal, M.; Ji, L.; Xu, Y.; Xu, S.; Lin, Y.; Iqbal, H.M.N.; Cheng, H. Bioprospecting Kluyveromyces marxianus as a robust host for industrial biotechnology. Front. Bioeng. Biotechnol. 2022, 10, 851768. [Google Scholar] [CrossRef]
- Coelho, M.A.Z.; Amaral, P.F.F.; Belo, I. Yarrowia lipolytica: An Industrial Workhorse; Formatex Research Center: Badajoz, Spain, 2010. [Google Scholar]
- Park, Y.-K.; Ledesma-Amaro, R. What makes Yarrowia lipolytica well suited for industry? Trends Biotechnol. 2023, 41, 242–254. [Google Scholar] [CrossRef]
- Barone, G.D.; Emmerstorfer-Augustin, A.; Biundo, A.; Pisano, I.; Coccetti, P.; Mapelli, V.; Camattari, A. Industrial production of proteins with Pichia pastoris (Komagataella phaffii). Biomolecules 2023, 13, 441. [Google Scholar] [CrossRef]
- Nazarov, P.A.; Baleev, D.N.; Ivanova, M.I.; Sokolova, L.M.; Karakozova, M.V. Infectious plant diseases: Etiology, current status, problems and prospects in plant protection. Acta Nat. 2020, 12, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiang, N.; Liu, J.; Liu, W.; Wang, G.-L. The role of effectors and host immunity in plant-necrotrophic fungal interactions. Virulence 2014, 5, 722–732. [Google Scholar] [CrossRef]
- Zeilinger, S.; Gupta, V.K.; Dahms, T.E.S.; Silva, R.N.; Singh, H.B.; Upadhyay, R.S.; Gomes, E.V.; Tsui, C.K.-M.; Nayak, S.C. Friends or foes? emerging insights from fungal interactions with plants. FEMS Microbiol. Rev. 2016, 40, 182–207. [Google Scholar] [CrossRef] [PubMed]
- Şesan, T.E.; Enache, E.; Iacomi, B.M.; Oprea, M.; Oancea, F.; Iacomi, C. Antifungal activity of some plant extracts against Botrytis cinerea Pers. in the blackcurrant crop (Ribes nigrum L.). Acta Sci. Pol. Hortorum Cultus 2015, 14, 29–43. [Google Scholar]
- Doehlemann, G.; Ökmen, B.; Zhu, W.; Sharon, A. Plant pathogenic fungi. Microbiol. Spectr. 2017, 5, 703–726. [Google Scholar] [CrossRef]
- Hassan, E.A.; Mostafa, Y.S.; Alamri, S.; Hashem, M.; Nafady, N.A. Biosafe management of Botrytis grey mold of strawberry fruit by novel bioagents. Plants 2021, 10, 2737. [Google Scholar] [CrossRef] [PubMed]
- Bi, K.; Liang, Y.; Mengiste, T.; Sharon, A. Killing softly: A roadmap of Botrytis cinerea pathogenicity. Trends Plant. Sci. 2023, 28, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Pei, T.; Jiang, J.; Yang, H.; Zhang, H.; Li, J.; Xu, X. Understanding the mechanisms of resistance to tomato leaf mold: A review. Hortic. Plant. J. 2022, 8, 667–675. [Google Scholar] [CrossRef]
- Skamnioti, P.; Gurr, S.J. Against the grain: Safeguarding rice from rice blast disease. Trends Biotechnol. 2009, 27, 141–150. [Google Scholar] [CrossRef]
- Doehlemann, G.; Wahl, R.; Vranes, M.; de Vries, R.P.; Kämper, J.; Kahmann, R. Establishment of compatibility in the Ustilago maydis/maize pathosystem. J. Plant. Physiol. 2008, 165, 29–40. [Google Scholar] [CrossRef]
- Ponomarenko, A.; Goodwin, S.B.; Kema, G.H.J. Septoria tritici blotch (STB) of wheat. In Septoria Tritici Blotch (STB) Wheat; APSnet: St. Paul, MN, USA, 2011. [Google Scholar]
- Orton, E.S.; Deller, S.; Brown, J.K.M. Mycosphaerella graminicola: From genomics to disease control. Mol. Plant Pathol. 2011, 12, 413–424. [Google Scholar] [CrossRef]
- Șesan, T.E.; Enache, E.; Iacomi, B.M.; Oprea, M.; Oancea, F.; Iacomi, C. Antifungal activity of some plant extracts against Alternaria alternata (Fr.) Keissl. in the blackcurrant crop (Ribes nigrum L.). Acta Sci. Pol. Hortorum. Cultus 2016, 15, 57–68. [Google Scholar]
- Pryor, B.M.; Michailides, T.J. Morphological, pathogenic, and molecular characterization of Alternaria isolates associated with Alternaria late blight of pistachio. Phytopathology 2002, 92, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Halaby, T.; Boots, H.; Vermeulen, A.; van der Ven, A.; Beguin, H.; van Hooff, H.; Jacobs, J. Phaeohyphomycosis caused by Alternaria infectoria in a renal transplant recipient. J. Clin. Microbiol. 2001, 39, 1952–1955. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Denis, M.; Sutton, D.A.; Martin-Vicente, A.; Cano-Lira, J.F.; Wiederhold, N.; Guarro, J.; Gené, J. Cladosporium species recovered from clinical samples in the United States. J. Clin. Microbiol. 2015, 53, 2990–3000. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Luo, H.-B.; Hu, J.-Q.; Hong, H.-H. Pulmonary Cladosporium infection coexisting with subcutaneous Corynespora cassiicola infection in a patient: A case report. World J. Clin. Cases 2022, 10, 3490–3495. [Google Scholar] [CrossRef] [PubMed]
- Solomon, P.S.; Oliver, R.P. The nitrogen content of the tomato leaf apoplast increases during infection by Cladosporium fulvum. Planta 2001, 213, 241–249. [Google Scholar] [CrossRef]
- Kim, J.-Y. Human fungal pathogens: Why should we learn? J. Microbiol. 2016, 54, 145–148. [Google Scholar] [CrossRef]
- Ijaz, N.; Tanveer, W.; Qureshi, A. Review on common fungal diseases of humans. Pak. Res. J. Biol. Sci. 2021, 1, 14–22. [Google Scholar]
- Robert, V.A.; Casadevall, A. Vertebrate endothermy restricts most fungi as potential pathogens. J. Infect. Dis. 2009, 200, 1623–1626. [Google Scholar] [CrossRef]
- Berker, D. Fungal nail disease. N. Engl. J. Med. 2009, 60, 2108–2116. [Google Scholar] [CrossRef]
- Brakhage, A. Systemic fungal infections caused by Aspergillus species: Epidemiology, infection process and virulence determinants. Curr. Drug Targets 2005, 6, 875–886. [Google Scholar] [CrossRef]
- Sherman, R.G.; Prusinski, L.; Ravenel, M.C.; Joralmon, R.A. Oral candidiasis. Quintessence Int. 2002, 33, 455–459. [Google Scholar]
- Ibrahim, A.S.; Edwards, J.E., Jr.; Bryant, R.; Spellberg, B. Economic burden of mucormycosis in the United States: Can a vaccine be cost-effective? Med. Mycol. 2009, 47, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.K.; Kang, J.I.; Bajpai, V.K.; Kim, K.; Lee, H.; Sonwal, S.; Simal-Gandara, J.; Xiao, J.; Ali, S.; Huh, Y.S.; et al. Mycotoxins in food and feed: Toxicity, preventive challenges, and advanced detection techniques for associated diseases. Crit. Rev. Food Sci. Nutr. 2022, 62, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Balendres, M.A.O.; Karlovsky, P.; Cumagun, C.J.R. Mycotoxigenic fungi and mycotoxins in agricultural crop commodities in the Philippines: A review. Foods 2019, 8, 249. [Google Scholar] [CrossRef] [PubMed]
- Daou, R.; Joubrane, K.; Maroun, R.G.; Khabbaz, L.R.; Ismail, A.; Khoury, A.E.; Daou, R.; Joubrane, K.; Maroun, R.G.; Khabbaz, L.R.; et al. Mycotoxins: Factors influencing production and control strategies. AIMS Agric. Food 2021, 6, 416–447. [Google Scholar] [CrossRef]
- Elkenany, R.M.; Awad, A. Types of mycotoxins and different approaches used for their detection in foodstuffs. Mansoura Vet. Med. J. 2021, 22, 25–32. [Google Scholar] [CrossRef]
- Tola, M.; Kebede, B. Occurrence, importance and control of mycotoxins: A review. Cogent Food Agric. 2016, 2, 1191103. [Google Scholar] [CrossRef]
- Tabuc, C.; Marin, D.; Guerre, P.; Sesan, T.; Bailly, J.D. Molds and mycotoxin content of cereals in southeastern Romania. J. Food Prot. 2009, 72, 662–665. [Google Scholar] [CrossRef]
- Liao, J.; He, Z.; Xia, Y.; Lei, Y.; Liao, B. A review on biosynthesis and genetic regulation of aflatoxin production by major Aspergillus fungi. Oil Crop. Sci. 2020, 5, 166–173. [Google Scholar] [CrossRef]
- Caceres, I.; Al Khoury, A.; El Khoury, R.; Lorber, S.; Oswald, P.I.; El Khoury, A.; Atoui, A.; Puel, O.; Bailly, J.-D. Aflatoxin biosynthesis and genetic regulation: A review. Toxins 2020, 12, 150. [Google Scholar] [CrossRef]
- Roze, L.V.; Hong, S.-Y.; Linz, J.E. Aflatoxin biosynthesis: Current frontiers. Annu. Rev. Food Sci. Technol. 2013, 4, 293–311. [Google Scholar] [CrossRef]
- Yu, J. Current understanding on aflatoxin biosynthesis and future perspective in reducing aflatoxin contamination. Toxins 2012, 4, 1024–1057. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, K.C.; Li, P.; Scharfenstein, L.; Chang, P.-K. HypC, the anthrone oxidase involved in aflatoxin biosynthesis. Appl. Environ. Microbiol. 2010, 76, 3374–3377. [Google Scholar] [CrossRef]
- Khan, R.; Ghazali, F.M.; Mahyudin, N.A.; Samsudin, N.I.P. Aflatoxin biosynthesis, genetic regulation, toxicity, and control strategies: A review. J. Fungi 2021, 7, 606. [Google Scholar] [CrossRef]
- Sakuno, E.; Wen, Y.; Hatabayashi, H.; Arai, H.; Aoki, C.; Yabe, K.; Nakajima, H. Aspergillus parasiticus cyclase catalyzes two dehydration steps in aflatoxin biosynthesis. Appl. Environ. Microbiol. 2005, 71, 2999–3006. [Google Scholar] [CrossRef] [PubMed]
- Yabe, K.; Nakajima, H. Enzyme reactions and genes in aflatoxin biosynthesis. Appl. Microbiol. Biotechnol. 2004, 64, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Henry, K.M.; Townsend, C.A. Ordering the reductive and cytochrome P450 oxidative steps in demethylsterigmatocystin formation yields general insights into the biosynthesis of aflatoxin and related fungal metabolites. J. Am. Chem. Soc. 2005, 127, 3724–3733. [Google Scholar] [CrossRef]
- Yu, J.; Woloshuk, C.P.; Bhatnagar, D.; Cleveland, T.E. Cloning and characterization of AvfA and OmtB genes involved in aflatoxin biosynthesis in three Aspergillus species. Gene 2000, 248, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, Z.; Wang, Y.; Li, Y.; Zhu, J.; Hu, R.; Yang, Y.; Liu, M. Quantitative proteomic analysis for high- and low-aflatoxin-yield Aspergillus flavus strains isolated from natural environments. Front. Microbiol. 2021, 12, 741875. [Google Scholar] [CrossRef] [PubMed]
- Scherm, B.; Palomba, M.; Serra, D.; Marcello, A.; Migheli, Q. Detection of transcripts of the aflatoxin genes AflD, AflO, and AflP by reverse transcription-polymerase chain reaction allows differentiation of aflatoxin-producing and non-producing isolates of Aspergillus flavus and Aspergillus Parasit. Int. J. Food Microbiol. 2005, 98, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, K.C. Predicted roles of the uncharacterized clustered genes in aflatoxin biosynthesis. Toxins 2009, 1, 37–58. [Google Scholar] [CrossRef]
- Zeng, H.; Hatabayashi, H.; Nakagawa, H.; Cai, J.; Suzuki, R.; Sakuno, E.; Tanaka, T.; Ito, Y.; Ehrlich, K.C.; Nakajima, H.; et al. Conversion of 11-hydroxy-O-methylsterigmatocystin to aflatoxin G1 in Aspergillus parasiticus. Appl. Microbiol. Biotechnol. 2011, 90, 635–650. [Google Scholar] [CrossRef] [PubMed]
- Caceres, I.; El Khoury, R.; Bailly, S.; Oswald, I.P.; Puel, O.; Bailly, J.-D. Piperine inhibits aflatoxin B1 production in Aspergillus flavus by modulating fungal oxidative stress response. Fungal Genet. Biol. 2017, 107, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, J.E.; Payne, G.A. Overexpression of AflR Leads to upregulation of pathway gene transcription and increased aflatoxin production in Aspergillus flavus. Appl. Environ. Microbiol. 1997, 63, 3995–4000. [Google Scholar] [CrossRef]
- Chang, P.-K. The Aspergillus parasiticus protein AFLJ interacts with the aflatoxin pathway-specific regulator AFLR. Mol. Genet. Genomics 2003, 268, 711–719. [Google Scholar] [CrossRef]
- Bhatnagar, D.; Cary, J.W.; Ehrlich, K.; Yu, J.; Cleveland, T.E. Understanding the genetics of regulation of aflatoxin production and Aspergillus flavus development. Mycopathologia 2006, 162, 155–166. [Google Scholar] [CrossRef]
- Kolawole, O.; Meneely, J.; Petchkongkaew, A.; Elliott, C. A Review of mycotoxin biosynthetic pathways: Associated genes and their expressions under the influence of climatic factors. Fungal Biol. Rev. 2021, 37, 8–26. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Liu, F.; Wang, Q.; Selvaraj, J.N.; Xing, F.; Zhao, Y.; Liu, Y. Ochratoxin A producing fungi, biosynthetic pathway and regulatory mechanisms. Toxins 2016, 8, 83. [Google Scholar] [CrossRef]
- Adeyeye, S.A.O. Fungal mycotoxins in foods: A review. Cogent Food Agric. 2016, 2, 1213127. [Google Scholar] [CrossRef]
- Davolos, D.; Pietrangeli, B. A molecular and bioinformatic study on the ochratoxin A (OTA)-producing Aspergillus affinis (section Circumdati). Mycotoxin Res. 2014, 30, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Gil-Serna, J.; Patiño, B.; Cortes, L.; Gonzalez-Jaen, M.T.; Vazquez, C. Aspergillus steynii and Aspergillus westerdijkiae as potential risk of OTA contamination in food products in warm climates. Food Microbiol. 2015, 46, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.J.; Tang, D.; Zhou, Y.Q.; Sun, B.D.; Li, X.J.; Wang, L.Z.; Gao, W.W. Identification of ochratoxin A producing fungi associated with fresh and dry liquorice. PLoS ONE 2013, 8, e78285. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Heydt, M.; Graf, E.; Stoll, D.; Geisen, R. The biosynthesis of Ochratoxin A by Penicillium as one mechanism for adaptation to NaCl rich foods. Food Microbiol. 2012, 29, 233–241. [Google Scholar] [CrossRef]
- Gallo, A.; Ferrara, M.; Perrone, G. Recent advances on the molecular aspects of ochratoxin A biosynthesis. Curr. Opin. Food Sci. 2017, 17, 49–56. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Wu, F.; Liu, F.; Wang, Q.; Zhang, X.; Selvaraj, J.N.; Zhao, Y.; Xing, F.; Yin, W.-B.; et al. A Consensus ochratoxin A biosynthetic pathway: Insights from the genome sequence of Aspergillus ochraceus and a comparative genomic analysis. Appl. Environ. Microbiol. 2018, 84, e01009-18. [Google Scholar] [CrossRef]
- Ferrara, M.; Gallo, A.; Perrone, G.; Magistà, D.; Baker, S.E. Comparative genomic analysis of ochratoxin A biosynthetic cluster in producing fungi: New evidence of a cyclase gene involvement. Front. Microbiol. 2020, 11, e01009-18. [Google Scholar] [CrossRef]
- Visentin, I.; Montis, V.; Döll, K.; Alabouvette, C.; Tamietti, G.; Karlovsky, P.; Cardinale, F. Transcription of genes in the biosynthetic pathway for fumonisin mycotoxins is epigenetically and differentially regulated in the fungal maize pathogen Fusarium verticillioides. Eukaryot. Cell 2012, 11, 252–259. [Google Scholar] [CrossRef]
- Ahangarkani, F.; Rouhi, S.; Gholamour Azizi, I. A review on incidence and toxicity of fumonisins. Toxin Rev. 2014, 33, 95–100. [Google Scholar] [CrossRef]
- Alexander, N.J.; Proctor, R.H.; McCormick, S.P. Genes, gene clusters, and biosynthesis of trichothecenes and fumonisins in Fusarium. Toxin Rev. 2009, 28, 198–215. [Google Scholar] [CrossRef]
- Yi, H.; Bojja, R.S.; Fu, J.; Du, L. Direct evidence for the function of FUM13 in 3-ketoreduction of mycotoxin fumonisins in Fusarium verticillioides. J. Agric. Food Chem. 2005, 53, 5456–5460. [Google Scholar] [CrossRef] [PubMed]
- Lia, Y.; Lou, L.; Cerny, R.L.; Butchko, R.A.E.; Proctor, R.H.; Shen, Y.; Du, L. Tricarballylic ester formation during biosynthesis of fumonisin mycotoxins in Fusarium verticillioides. Mycology 2013, 4, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.W.; Butchko, R.A.E.; Busman, M.; Proctor, R.H. The Fusarium verticillioides FUM gene cluster encodes a Zn(II)2Cys6 protein that affects FUM gene expression and fumonisin production. Eukaryot. Cell 2007, 6, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Janevska, S.; Ferling, I.; Jojić, K.; Rautschek, J.; Hoefgen, S.; Proctor, R.H.; Hillmann, F.; Valiante, V. Self-protection against the sphingolipid biosynthesis inhibitor fumonisin B1 is conferred by a FUM cluster-encoded ceramide synthase. mBio 2020, 11, e00455-20. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Chen, X.; Gao, J.; Zhao, Y.; Liu, L.; Hou, Y.; Wang, L.; Huang, S. Effects of disruption of five FUM genes on fumonisin biosynthesis and pathogenicity in Fusarium proliferatum. Toxins 2019, 11, 327. [Google Scholar] [CrossRef] [PubMed]
- Huffman, J.; Gerber, R.; Du, L. Recent advancements in the biosynthetic mechanisms for polyketide-derived mycotoxins. Biopolymers 2010, 93, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; He, D.; Olaniran, A.O.; Mokoena, M.P.; Xu, J.; Shi, J. Occurrence, toxicity, production and detection of Fusarium mycotoxin: A review. Food Prod. Process. Nutr. 2019, 1, 6. [Google Scholar] [CrossRef]
- McCormick, S.P.; Stanley, A.M.; Stover, N.A.; Alexander, N.J. Trichothecenes: From simple to complex mycotoxins. Toxins 2011, 3, 802–814. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Kistler, H.C.; Ma, Z. Fusarium graminearum trichothecene mycotoxins: Biosynthesis, regulation, and management. Annu. Rev. Phytopathol. 2019, 57, 15–39. [Google Scholar] [CrossRef]
- Nahle, S.; El Khoury, A.; Atoui, A. Current status on the molecular biology of zearalenone: Its biosynthesis and molecular detection of zearalenone producing Fusarium species. Eur. J. Plant. Pathol. 2021, 159, 247–258. [Google Scholar] [CrossRef]
- Lysøe, E.; Klemsdal, S.S.; Bone, K.R.; Frandsen, R.J.N.; Johansen, T.; Thrane, U.; Giese, H. The PKS4 gene of Fusarium graminearum is essential for zearalenone production. Appl. Environ. Microbiol. 2006, 72, 3924–3932. [Google Scholar] [CrossRef]
- Borzekowski, A.; Drewitz, T.; Keller, J.; Pfeifer, D.; Kunte, H.-J.; Koch, M.; Rohn, S.; Maul, R. Biosynthesis and characterization of zearalenone-14-Sulfate, zearalenone-14-glucoside and zearalenone-16-glucoside using common fungal strains. Toxins 2018, 10, 104. [Google Scholar] [CrossRef]
- Vidal, A.; Ouhibi, S.; Ghali, R.; Hedhili, A.; De Saeger, S.; De Boevre, M. The mycotoxin patulin: An updated short review on occurrence, toxicity and analytical challenges. Food Chem. Toxicol. 2019, 129, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Frisvad, J.C.; Smedsgaard, J.; Larsen, T.O.; Samson, R.A. Mycotoxins, drugs and other extrolites produced by species in Penicillium subgenus Penicillium. Stud. Mycol. 2004, 49, e41. [Google Scholar]
- Puel, O.; Galtier, P.; Oswald, I.P. Biosynthesis and toxicological effects of patulin. Toxins 2010, 2, 613–631. [Google Scholar] [CrossRef]
- Li, B.; Chen, Y.; Zhang, Z.; Qin, G.; Chen, T.; Tian, S. Molecular basis and regulation of pathogenicity and patulin biosynthesis in Penicillium expansum. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3416–3438. [Google Scholar] [CrossRef]
- Saleh, I.; Goktepe, I. The characteristics, occurrence, and toxicological effects of patulin. Food Chem. Toxicol. 2019, 129, 301–311. [Google Scholar] [CrossRef]
- Artigot, M.P.; Loiseau, N.; Laffitte, J.; Mas-Reguieg, L.; Tadrist, S.; Oswald, I.P.; Puel, O. Molecular cloning and functional characterization of two CYP619 cytochrome P450s involved in biosynthesis of patulin in Aspergillus clavatus. Microbiology 2009, 155, 1738–1747. [Google Scholar] [CrossRef]
- Li, B.; Chen, Y.; Zong, Y.; Shang, Y.; Zhang, Z.; Xu, X.; Wang, X.; Long, M.; Tian, S. Dissection of patulin biosynthesis, spatial control and regulation mechanism in Penicillium expansum. Environ. Microbiol. 2019, 21, 1124–1139. [Google Scholar] [CrossRef]
- Pickova, D.; Ostry, V.; Toman, J.; Malir, F. Aflatoxins: History, significant milestones, recent data on their toxicity and ways to mitigation. Toxins 2021, 13, 399. [Google Scholar] [CrossRef] [PubMed]
- Omotayo, O.P.; Omotayo, A.O.; Mwanza, M.; Babalola, O.O. Prevalence of mycotoxins and their consequences on human health. Toxicol. Res. 2019, 35, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Claeys, L.; Romano, C.; De Ruyck, K.; Wilson, H.; Fervers, B.; Korenjak, M.; Zavadil, J.; Gunter, M.J.; De Saeger, S.; De Boevre, M.; et al. Mycotoxin exposure and human cancer risk: A systematic review of epidemiological studies. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1449–1464. [Google Scholar] [CrossRef] [PubMed]
- Ahmed Adam, M.A.; Tabana, Y.M.; Musa, K.B.; Sandai, D.A. Effects of different mycotoxins on humans, cell genome and their involvement in cancer (review). Oncol. Rep. 2017, 37, 1321–1336. [Google Scholar] [CrossRef]
- Moradi, S.; Azari, H.; Jafari Anarkooli, I.; Qasemi-Panahi, B.; Elhami, S.; Forouharmehr, A. Effect of aflatoxin B1 on BRCA1 and BRCA2 genes expression under in vitro cultured cell line of normal human mammary epithelial cells (HMEC). J. Police Med. 2015, 3, 211–220. [Google Scholar] [CrossRef]
- Kouadio, J.H.; Mobio, T.A.; Baudrimont, I.; Moukha, S.; Dano, S.D.; Creppy, E.E. Comparative study of cytotoxicity and oxidative stress induced by deoxynivalenol, zearalenone or fumonisin B1 in human intestinal cell line Caco-2. Toxicology 2005, 213, 56–65. [Google Scholar] [CrossRef]
- Mohsenzadeh, M.S.; Hedayati, N.; Riahi-Zanjani, B.; Karimi, G. Immunosuppression following dietary aflatoxin B1 exposure: A review of the existing evidence. Toxin Rev. 2016, 35, 121–127. [Google Scholar] [CrossRef]
- Qian, G.; Tang, L.; Guo, X.; Wang, F.; Massey, M.E.; Su, J.; Guo, T.L.; Williams, J.H.; Phillips, T.D.; Wang, J.-S. Aflatoxin B1 modulates the expression of phenotypic markers and cytokines by splenic lymphocytes of male F344 Rats. J. Appl. Toxicol. 2014, 34, 241–249. [Google Scholar] [CrossRef]
- Valtchev, I.; Koynarski, T.; Sotirov, L.; Nikolov, Y.; Petkov, P. Effect of aflatoxin B1 on moulard duck’s natural immunity. Pak. Vet. J. 2015, 35, 67–70. [Google Scholar]
- Bui-Klimke, T.R.; Wu, F. Ochratoxin A and human health risk: A review of the evidence. Crit. Rev. Food Sci. Nutr. 2015, 55, 1860–1869. [Google Scholar] [CrossRef]
- Limonciel, A.; Jennings, P. A review of the evidence that ochratoxin A is an Nrf2 inhibitor: Implications for nephrotoxicity and renal carcinogenicity. Toxins 2014, 6, 371–379. [Google Scholar] [CrossRef]
- Malir, F.; Ostry, V.; Pfohl-Leszkowicz, A.; Malir, J.; Toman, J. Ochratoxin A: 50 years of research. Toxins 2016, 8, 191. [Google Scholar] [CrossRef] [PubMed]
- Paradells, S.; Rocamonde, B.; Llinares, C.; Herranz-Pérez, V.; Jimenez, M.; Garcia-Verdugo, J.M.; Zipancic, I.; Soria, J.M.; Garcia-Esparza, M.A. Neurotoxic effects of ochratoxin A on the subventricular zone of adult mouse brain. J. Appl. Toxicol. 2015, 35, 737–751. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lim, W.; You, S.; Song, G. Ochratoxin A exerts neurotoxicity in human astrocytes through mitochondria-dependent apoptosis and intracellular calcium overload. Toxicol. Lett. 2019, 313, 42–49. [Google Scholar] [CrossRef]
- Stockmann-Juvala, H.; Savolainen, K. A review of the toxic effects and mechanisms of action of fumonisin B1. Hum. Exp. Toxicol. 2008, 27, 799–809. [Google Scholar] [CrossRef]
- Summerell, B.A.; Leslie, J.F. Fifty years of Fusarium: How could nine species have ever been enough? Fungal Divers 2011, 50, 135–144. [Google Scholar] [CrossRef]
- Voss, K.A.; Riley, R.T. Fumonisin toxicity and mechanism of action: Overview and current perspectives. Food Saf. 2013, 1, 2013006. [Google Scholar] [CrossRef]
- Ahamed, S.; Foster, J.S.; Bukovsky, A.; Wimalasena, J. Signal Transduction through the Ras/Erk pathway is essential for the mycoestrogen zearalenone-induced cell-cycle progression in MCF-7 Cells. Mol. Carcinog. 2001, 30, 88–98. [Google Scholar] [CrossRef]
- Koraichi, F.; Videmann, B.; Mazallon, M.; Benahmed, M.; Prouillac, C.; Lecoeur, S. Zearalenone exposure modulates the expression of ABC transporters and nuclear receptors in pregnant rats and fetal liver. Toxicol. Lett. 2012, 211, 246–256. [Google Scholar] [CrossRef]
- Pistol, G.C.; Braicu, C.; Motiu, M.; Gras, M.A.; Marin, D.E.; Stancu, M.; Calin, L.; Israel-Roming, F.; Berindan-Neagoe, I.; Taranu, I. Zearalenone mycotoxin affects immune mediators, MAPK signalling molecules, nuclear receptors and genome-wide gene expression in pig spleen. PLoS ONE 2015, 10, e0127503. [Google Scholar] [CrossRef]
- Zhang, G.-L.; Feng, Y.-L.; Song, J.-L.; Zhou, X.-S. Zearalenone: A mycotoxin with different toxic effect in domestic and laboratory animals’ granulosa cells. Front. Genet. 2018, 9, 667. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, Z.; Yin, S.; Shan, A.; Gao, R.; Qu, Z.; Liu, M.; Nie, S. Toxic effects of maternal zearalenone exposure on uterine capacity and fetal development in gestation rats. Reprod. Sci. 2014, 21, 743–753. [Google Scholar] [CrossRef]
- Koçkaya, E.; Selmanoğlu, G.; Ozsoy, N.; Gül, N. Evaluation of patulin toxicity in the thymus of growing male rats. Arch. Ind. Hyg. Toxicol. 2009, 60, 411–418. [Google Scholar] [CrossRef]
- Lupescu, A.; Jilani, K.; Zbidah, M.; Lang, F. Patulin-induced suicidal erythrocyte death. Cell. Physiol. Biochem. 2013, 32, 291–299. [Google Scholar] [CrossRef]
- Selmanoğlu, G. Evaluation of the reproductive toxicity of patulin in growing male rats. Food Chem. Toxicol. 2006, 44, 2019–2024. [Google Scholar] [CrossRef]
- Berthiller, F.; Krska, R.; Domig, K.J.; Kneifel, W.; Juge, N.; Schuhmacher, R.; Adam, G. Hydrolytic fate of deoxynivalenol-3-glucoside during digestion. Toxicol. Lett. 2011, 206, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.; Priest, E.; Naglik, J.R.; Richardson, J.P. Fungal toxins and host immune responses. Front. Microbiol. 2021, 12, 643639. [Google Scholar] [CrossRef]
- Fang, H.; Zhi, Y.; Yu, Z.; Lynch, R.A.; Jia, X. The embryonic toxicity evaluation of deoxynivalenol (DON) by murine embryonic stem cell test and human embryonic stem cell test models. Food Control 2018, 86, 234–240. [Google Scholar] [CrossRef]
- Islam, Z.; Gray, J.S.; Pestka, J.J. P38 Mitogen-activated protein kinase mediates IL-8 induction by the ribotoxin deoxynivalenol in human monocytes. Toxicol. Appl. Pharmacol. 2006, 213, 235–244. [Google Scholar] [CrossRef]
- Gerez, J.R.; Pinton, P.; Callu, P.; Grosjean, F.; Oswald, I.P.; Bracarense, A.P.F.L. Deoxynivalenol alone or in combination with nivalenol and zearalenone induce systemic histological changes in pigs. Exp. Toxicol. Pathol. 2015, 67, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, H.; Nakagawa, H. Differences in the toxicities of trichothecene mycotoxins, deoxynivalenol and nivalenol, in cultured cells. Jpn. Agric. Res. Q. JARQ 2014, 48, 393–397. [Google Scholar] [CrossRef]
- Fang, H.; Wu, Y.; Guo, J.; Rong, J.; Ma, L.; Zhao, Z.; Zuo, D.; Peng, S. T-2 toxin induces apoptosis in differentiated murine embryonic stem cells through reactive oxygen species-mediated mitochondrial pathway. Apoptosis 2012, 17, 895–907. [Google Scholar] [CrossRef]
- Janik, E.; Niemcewicz, M.; Podogrocki, M.; Ceremuga, M.; Stela, M.; Bijak, M. T-2 Toxin—The most toxic trichothecene mycotoxin: Metabolism, toxicity, and decontamination strategies. Molecules 2021, 26, 6868. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Z.; Beier, R.C.; Shen, J.; De Smet, D.; De Saeger, S.; Zhang, S. T-2 Toxin, a trichothecene mycotoxin: Review of toxicity, metabolism, and analytical methods. J. Agric. Food Chem. 2011, 59, 3441–3453. [Google Scholar] [CrossRef] [PubMed]
- Madheswaran, R.; Balachandran, C.; Murali Manohar, B. Influence of dietary culture material containing aflatoxin and T2 toxin on certain serum biochemical constituents in japanese quail. Mycopathologia 2004, 158, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Weidner, M.; Lenczyk, M.; Schwerdt, G.; Gekle, M.; Humpf, H.-U. Neurotoxic potential and cellular uptake of T-2 toxin in human astrocytes in primary culture. Chem. Res. Toxicol. 2013, 26, 347–355. [Google Scholar] [CrossRef]
- Indrie, L.; Oana, D.; Ilies, M.; Ilies, D.C.; Lincu, A.; Ilies, A.; Baias, S.; Herman, G.V.; Onet, A.; Costea, M.; et al. Indoor air quality of museums and conservation of textiles art works. Case study: Salacea Museum House, Romania. Ind. Text. 2019, 70, 88–93. [Google Scholar] [CrossRef]
- Zhang, G.X.; Gong, C.J.; Gu, J.G.; Katayama, Y.; Someya, T.; Gu, J.D. Biochemical reactions and mechanisms involved in the biodeterioration of stone world cultural heritage under the tropical climate conditions. Int. Biodeterior. Biodegrad. 2019, 143, 104723. [Google Scholar] [CrossRef]
- Savković, Ž.; Stupar, M.; Unković, N.; Knežević, A.; Vukojević, J.; Ljaljević Grbić, M. Fungal deterioration of cultural heritage objects. In Biodegradation; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Owczarek-Kościelniak, M.; Krzewicka, B.; Piątek, J.; Kołodziejczyk, Ł.M.; Kapusta, P. Is there a link between the biological colonization of the gravestone and its deterioration? Int. Biodeterior. Biodegrad. 2020, 148, 104879. [Google Scholar] [CrossRef]
- Scheerer, S.; Ortega-Morales, O.C.G. Microbial deterioration of stone monuments—An updated overview. Adv. Appl. Microbiol. 2009, 66, 97–139. [Google Scholar] [PubMed]
- Piñar, G.; Garcia-Valles, M.; Gimeno-Torrente, D.; Fernandez-Turiel, J.L.; Ettenauer, J.; Sterflinger, K. Microscopic, chemical, and molecular-biological investigation of the decayed medieval stained window glasses of two catalonian churches. Int. Biodeterior. Biodegrad. 2013, 84, 388–400. [Google Scholar] [CrossRef]
- Kim, Y.S.; Singh, A.P. Wood as cultural heritage material and its deterioration by biotic and abiotic agents. In Secondary Xylem Biology: Origins, Functions, and Applications; Kim, Y.S., Funada, R., Singh, A.P., Eds.; Academic Press: London, UK, 2016; pp. 233–257, Chapter 12. [Google Scholar] [CrossRef]
- Koestler, R.J.; Koestler, V.R.; Charola, A.E.; Nieto-Fernandez, F.E. Art, Biology, and Conservation: Biodeterioration of Works of Art; The Metropolitan Museum of Art: New York, NY, USA, 2010; ISBN 978-1-58839-107-0. [Google Scholar]
- Schmidt, O. Chapter 6 Wood Discoloration. In Wood and Tree Fungi; Springer: Berlin/Heidelberg, Germany, 2006; p. 120. [Google Scholar]
- Alfieri, P.V.; Correa, M.V. Fungi observation in deteriorated wooden heritage: Analysis using different imaging techniques. Rev. Argent. Microbiol. 2017, 49, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Poyatos, F.; Morales, F.; Nicholson, A.W.; Giordano, A. Physiology of biodeterioration on canvas paintings. J. Cell Physiol. 2018, 233, 2741–2751. [Google Scholar] [CrossRef] [PubMed]
- Caselli, E.; Pancaldi, S.; Baldisserotto, C.; Petrucci, F.; Impallaria, A.; Volpe, L.; D’Accolti, M.; Soffritti, I.; Coccagna, M.; Sassu, G.; et al. Characterization of Biodegradation in a 17th century easel painting and potential for a biological approach. PLoS ONE 2018, 13, e0207630. [Google Scholar] [CrossRef]
- Santos, A.; Cerrada, A.; García, S.; Andrés, M.S.; Abrusci, C.; Marquina, D. Application of molecular techniques to the elucidation of the microbial community structure of antique paintings. Microb. Ecol. 2009, 58, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Szostak-Kotowa, J. Biodeterioration of textiles. Int. Biodeterior. Biodegrad. 2004, 53, 165–170. [Google Scholar] [CrossRef]
- Pinzari, F.; Colaizzi, P.; Maggi, O.; Persiani, A.M.; Schütz, R.; Rabin, I. Fungal bioleaching of mineral components in a twentieth-century illuminated parchment. Anal. Bioanal. Chem. 2012, 402, 1541–1550. [Google Scholar] [CrossRef]
- Pinna, D.; Salvadori, O. Stone and Related Materials. In Plant Biology for Cultural Heritage; The Getty Conservation Institute: Los Angeles, CA, USA, 2008; pp. 128–144. [Google Scholar]
- Isola, D.; Zucconi, L.; Onofri, S.; Caneva, G.; Hoog, G.S.; Selbmann, L. Extremotolerant rock inhabiting black fungi from italian monumental sites. Fungal Divers. 2016, 76, 75–96. [Google Scholar]
- Sterflinger, K. Fungi: Their role in deterioration of cultural heritage. Fungal Biol. Rev. 2010, 24, 47–55. [Google Scholar] [CrossRef]
- Sazanova, K.V.; Zelenskaya, M.S.; Vlasov, A.D.; Bobir, S.Y.; Yakkonen, K.L.; Vlasov, D.Y. Microorganisms in superficial deposits on the stone monuments in Saint Petersburg. Microorganisms 2022, 10, 316. [Google Scholar] [CrossRef]
- Sert, H.B.; Sümbül, H.; Sterflinger, K. Microcolonial fungi from antique marbles in Perge/Side/Termessos (Antalya/Turkey). Antonie Van Leeuwenhoek J. Microb. 2007, 91, 217–227. [Google Scholar] [CrossRef]
- Sterflinger, K.; Prillinger, H. Molecular taxonomy and biodiversity of rock fungal communities in an urban environment (Vienna, Austria). Antonie Van Leeuwenhoek J. Microb. 2001, 80, 275–286. [Google Scholar] [CrossRef]
- Ricca, M.; Urzì, C.E.; Rovella, N.; Sardella, A.; Bonazza, A.; Ruffolo, S.A.; Leo, F.; Randazzo, L.; Arcudi, A.; Russa, M.F. Multidisciplinary approach to characterize archaeological materials and status of conservation of the roman thermae of Reggio Calabria site (Calabria, South Italy). Appl. Sci. 2020, 10, 5106. [Google Scholar] [CrossRef]
- Ruffolo, S.A.; Leo, F.; Ricca, M.; Arcudi, A.; Silvestri, C.; Bruno, L.; Urzì, C.; Russa, M.F. Medium-term in situ experiment by using organic biocides and titanium dioxide for the mitigation of microbial colonization on stone surfaces. Int. Biodeterior. Biodegrad. 2017, 123, 17–26. [Google Scholar]
- Sterflinger, K.; Baere, R.; Hoog, G.S.; Wachter, R.; Krumbein, W.E.; Haase, G. Coniosporium perforans and C. apollinis, two new rock-inhabiting fungi isolated from marble in the sanctuary of delos (Cyclades, Greece). Antonie Van Leeuwenhoek J. Microb. 1997, 72, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Ruga, L.; Orlandi, F.; Romano, B.; Fornaciari, M. The assessment of fungal bioaerosols in the crypt of St. Peter in Perugia (Italy). Int. Biodeterior. Biodegrad. 2015, 98, 121–130. [Google Scholar]
- Pangallo, D.; Bučková, M.; Kraková, L.; Puškárová, A.; Šaková, N.; Grivalský, T.; Chovanová, K.; Zemánková, M. Biodeterioration of epoxy resin: A microbial survey through culture-independent and culture-dependent approaches. Environ. Microbiol. 2014, 17, 462–479. [Google Scholar] [CrossRef]
- Nevalainen, A.; Täubel, M.; Hyvärinen, A. Indoor fungi: Companions and contaminants. Indoor Air 2015, 25, 125–156. [Google Scholar]
- Ciferri, O. Microbial degradation of paintings. Appl. Environ. Microbiol. 1999, 65, 879–885. [Google Scholar] [CrossRef]
- Ma, W.; Wu, F.; Tian, T.; He, D.; Zhang, Q.; Gu, J.-D.; Duan, Y.; Ma, D.; Wang, W.; Feng, H. Fungal diversity and its contribution to the biodeterioration of mural paintings in two 1700-year-old tombs of China. Int. Biodeterior. Biodegrad 2020, 152, 104972. [Google Scholar]
- López-Miras, M.D.M.; Martín-Sánchez, I.; Yebra-Rodríguez, Á.; Romero-Noguera, J.; Bolívar-Galiano, F.; Ettenauer, J.; Sterflinger, K.; Piñar, G. Contribution of the microbial communities detected on an oil painting on canvas to its biodeterioration. PLoS ONE 2013, 8, e80198. [Google Scholar] [CrossRef]
- Tiano, P. Biodegradation of cultural heritage: Decay mechanisms and control methods. In Proceedings of the 9th ARIADNE Workshop “Historic Material and Their Diagnostic” ARCCHIP, Prague, Czech Republic, 22–28 April 2002. [Google Scholar]
- Pyzik, A.; Ciuchcinski, K.; Dziurzynski, M.; Dziewit, L. The bad and the good-microorganisms in cultural heritage environments—An update on biodeterioration and biotreatment approaches. Materials 2021, 14, 177. [Google Scholar] [CrossRef]
- Arai, H. Foxing caused by fungi: Twenty-five years of study. Int. Biodeterior. Biodegrad. 2000, 46, 181–188. [Google Scholar] [CrossRef]
- Corte, A.M.; Ferroni, A.; Salvo, V.S. Isolation of fungal species from test samples and maps damaged by foxing, and correlation between these species and the environment. Int. Biodeterior. Biodegrad. 2003, 51, 167–173. [Google Scholar] [CrossRef]
- Koul, B.; Upadhyay, H. Fungi-Mediated Biodeterioration of Household Materials, Libraries, Cultural Heritage and Its Control. In Fungi and Their Role in Sustainable Development: Current Perspectives; Gehlot, P., Singh, J., Eds.; Springer: Singapore, 2018; pp. 597–615. [Google Scholar]
- Nitiu, D.S.; Mallo, A.C.; Saparrat, M.C.N. Fungal melanins that deteriorate paper cultural heritage: An overview. Mycologia 2020, 112, 859–870. [Google Scholar] [CrossRef]
- Pinheiro, A.C.; Sequeira, S.O.; Macedo, M.F. Fungi in archives, libraries, and museums: A review on paper conservation and human health. Crit. Rev. Microbiol. 2019, 45, 686–700. [Google Scholar] [CrossRef] [PubMed]
- Kavkler, K.; Demšar, A. Impact of fungi on contemporary and accelerated aged wool fibres. Polym. Degrad. Stab. 2012, 97, 786–792. [Google Scholar] [CrossRef]
- Ljaljević Grbić, M.; Unković, N.; Stupar, M.; Vukojević, J.; Nedeljković, T. Implementation of ATP biolumenescence method in the study of the fungal deterioration of textile artefacts. Fibres Text. East. Eur. 2014, 22, 132–136. [Google Scholar]
- Troiano, F.; Polo, A.; Villa, F.; Cappitelli, F. Assessing the microbiological risk to stored sixteenth century parchment manuscripts: A holistic approach based on molecular and environmental studies. Biofouling 2014, 30, 299–311. [Google Scholar] [CrossRef]
- Paiva de Carvalho, H.; Mesquita, N.; Trovão, J.; Peixoto da Silva, J.; Rosa, B.; Martins, R.; Bandeira, A.M.L.; Portugal, A. Diversity of fungal species in ancient parchments collections of the archive of the University of Coimbra. Int. Biodeterior. Biodegrad. 2016, 108, 57–66. [Google Scholar] [CrossRef]
- Tanney, J.B.; Nguyen, H.D.T.; Pinzari, F.; Seifert, K.A. A century later: Rediscovery, culturing and phylogenetic analysis of Diploöspora rosea, a rare onygenalean hyphomycete. Antonie van Leeuwenhoek 2015, 108, 1023–1035. [Google Scholar] [CrossRef]
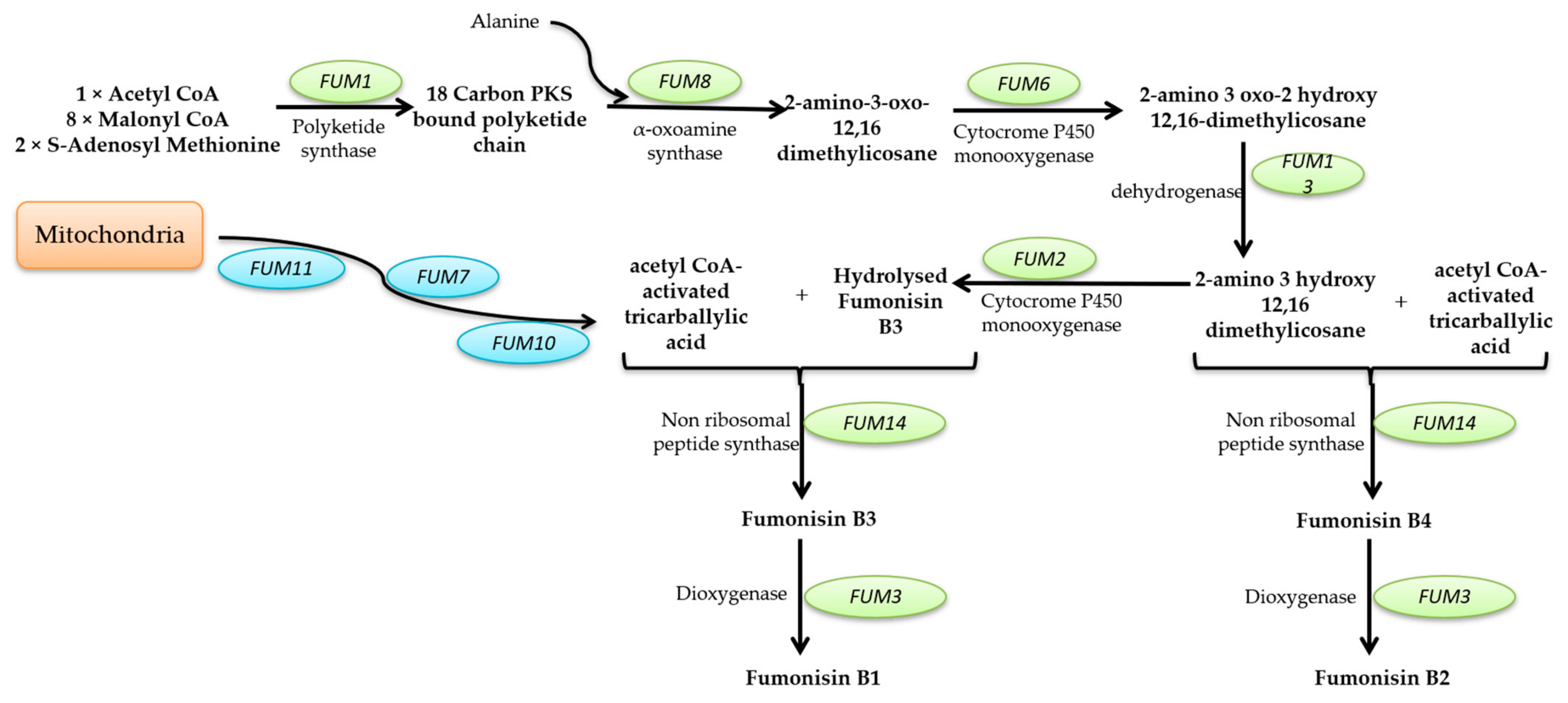
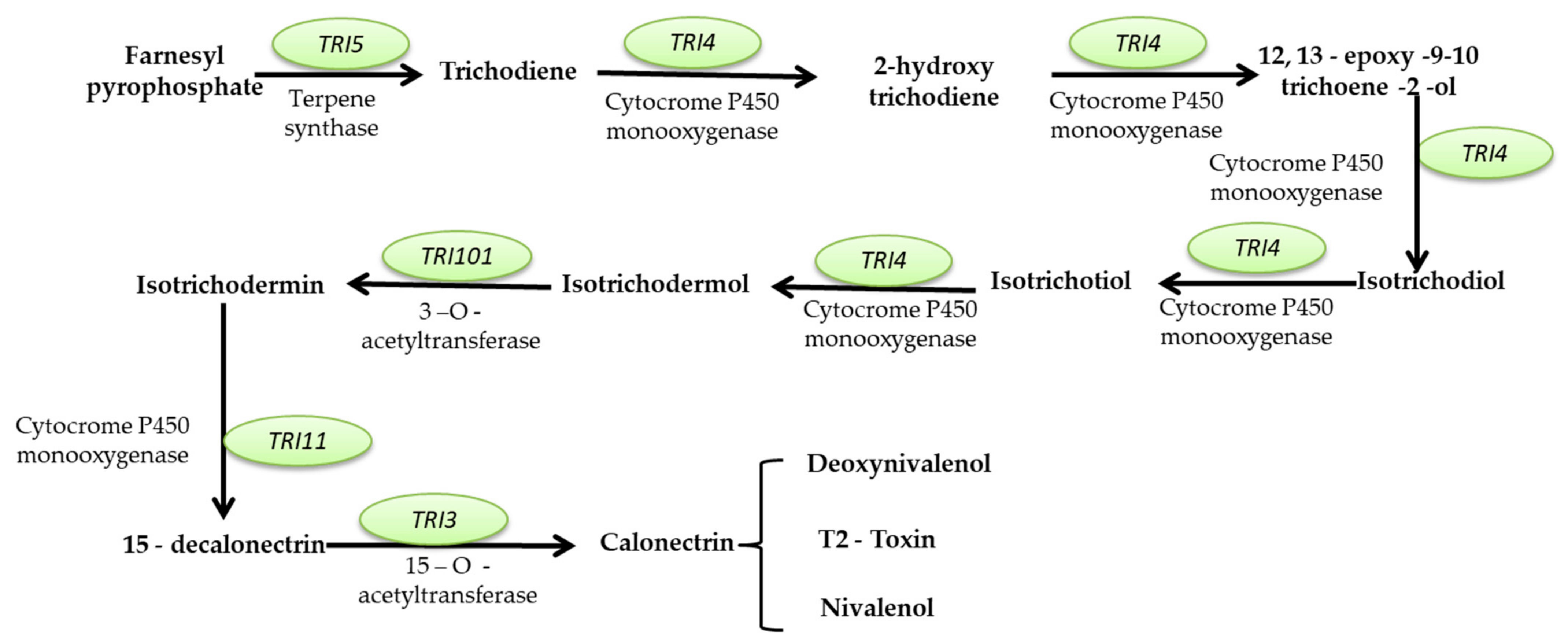
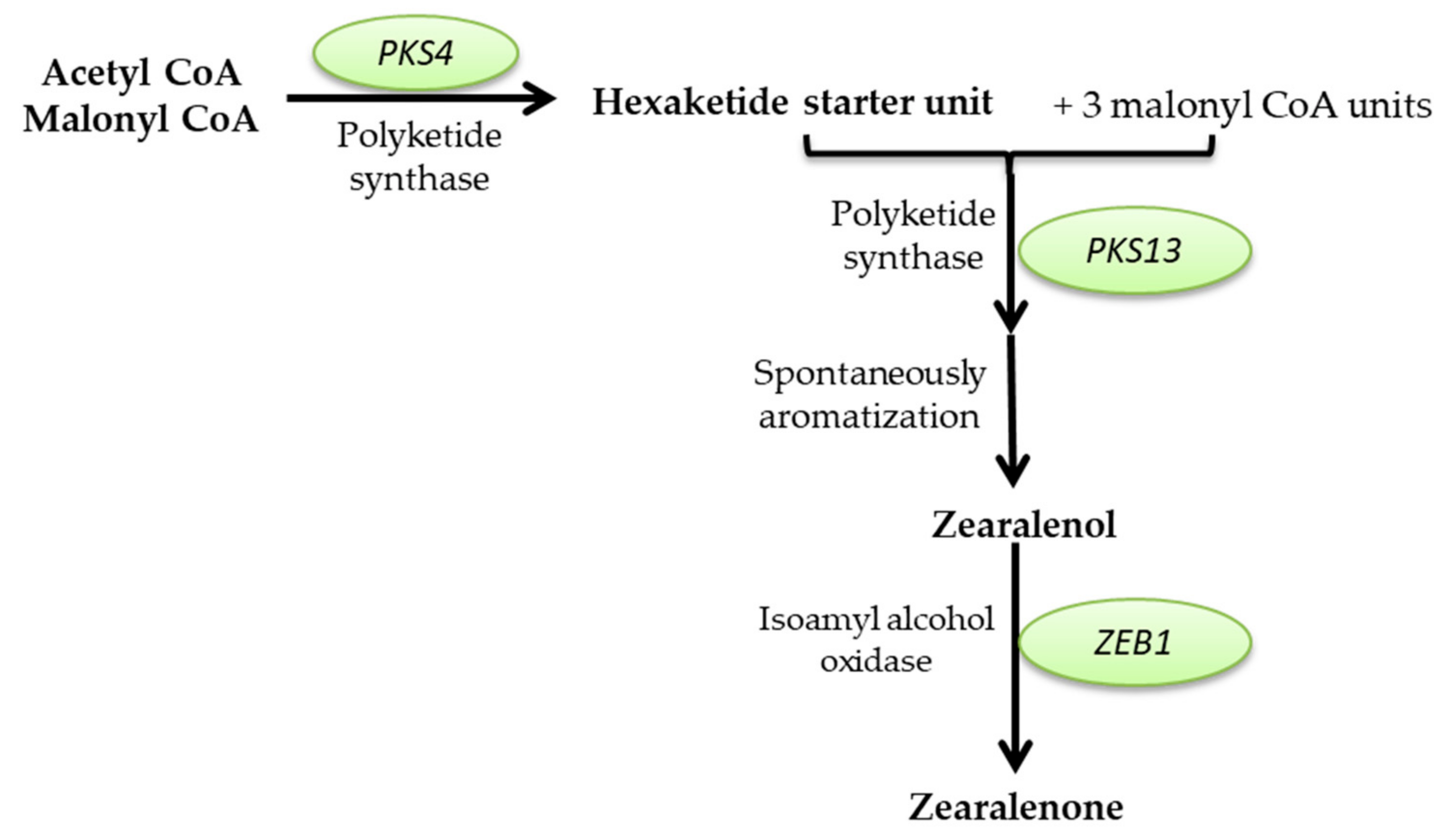

| Antimicrobial Agents | Producer | Active Against | Mode of Action | References |
|---|---|---|---|---|
| Non-ribosomal peptides | ||||
| Mycophenolic acid | Penicillium brevicompactum | several species | antibacterial, antifungal, antiviral, antitumor, antipsoriasis and immunosuppressive, anti-angiogenic activities | [26] |
| Penicillin | Penicillium notatum | Staphylococcus aureus | [27] | |
| Penicillin G | P. rubens | Streptococcus, Staphylococcus, Enterococcus, Clostrodium and Treponema spp. | inhibit the peptidoglycan synthesis | [28,29] |
| Cephalosporin C | A. chrysogenum | broad spectrum antibiotic | [2] | |
| Ribosomal peptides | ||||
| Amatoxin Phallotoxin | Amanita spp. | anticancer drugs | RNA polymerase II inhibitors | [30] |
| Ustilotoxin | Ustilaginoidea virens | cytotoxicity against different anticancer cell lines | anti-mitotic activity | [31] |
| Depsipeptides Beauvericin (A–H) Beauveriolide | Fusarium, Alternaria, Calonectria, Cochliobolus, Cordyceps cardinalis, Ophiocordyceps communis Cordyceps militaris | antimicrobial and insecticidal activity anti-aging activity against S. cerevisiae | [32,33,34,35] | |
| Piperazines | ||||
| Roquefortine C | Penicillium roqueforti | acute toxicity in mice and dogs | [36,37] | |
| Gliotoxin | Aspergillus fumigatus | antifungal activity against Candida albicans and Aspergillus spp. | inhibit the activation of lymphocyte B and T | [38,39] |
| Polyketides | ||||
| Griseofulvin | Penicillium griseofulvum | dermatophytes fungal infections in humans and animals;non-fungal inflammatory diseases; cardiovascular, antitumor and antiviral applications; | inhibit fungal cell mitosis and nuclear acid synthesis | [40] |
| Patulin | Aspergillus clavatus | mycotoxin, fungistatic activity against Rhizoctonia solani, S. cerevisiae, Didymella bryoniae, Botrytis cinerea, Fusarium oxysporum, clerotium rolfsii, Pithium ultinum | destabilization of of the plasma membrane integrity, blockage in rRNA, tRNA, and mRNA synthesis | [41,42] |
| Strobilurins | Strobilurus tenacellus, Oudemansiella mucida | antifungal activity | inhibit the transfer of electrons between complexes II and III of the electron transport chain in the mitochondria, resulting in impaired cell respiration and ATP synthesis | [43] |
| Uredinorubellin derivatives | Torrubiella spp. | antibacterial activity against S. aureus strains | [34] | |
| Rubellins antraquinones | Ramularia collo-cygni | antiproliferative, cytotoxic, aggregation inhibitory and antimicrobial activity against B. subtilis, S. aureus, S. aureus MRSA, Enterococcus faecalis clinical and reference strains | phytotoxic activity | [44] |
| Viriditoxin | Penicillium radicum | antimicrobial activity against S. aureus MRSA | inhibiting FtsZ, the bacterial tubulin | [45] |
| Lindgomycin | Lindgomycetaceae family | antimicrobial (against Gram-positive and C. albicans strains) and antiviral activity | [24] | |
| Lipopeptides | ||||
| Echinocandin B | Aspergilllus nidulans | inhibiting β (1,3)-glucan synthase | [46] | |
| Pneumocandin B0 | Glarea lozoyensis | antifungal activity against C. albicans and Pneumocystis carinii | inhibiting β (1,3)-glucan synthase | |
| Caspofungin | G. lozoyensis | blocking cell wall biosynthesis by inhibiting β (1,3)-D-glucan synthase | [47] | |
| Micafungin | Coleophoma empetri | |||
| Anidulafungin | A. nidulans | |||
| Mulundocandin | Aspergillus sydowii | Aspergillus niger, C. albicans, Candida non-albicans | [48] | |
| Anidulafungin | A. nidulans | Candida parapsilosis, Candida guilliermondii Aspergillus spp. Fusarium spp. | ||
| Rezafungin | Candida spp., Aspergillus spp., Pneumocystis murina | [48,49] | ||
| Cryptocandin | Cryptosporiopsis quercina | antifungal activity against Tricophyton rubrum, Sclerotinia sclerotiorum, Botrytis cinerea | [50,51] | |
| Lipodepsipeptide | ||||
| Aureobasidin A | Aureobasidium pullulans | fungicidal activity against Candida spp., C. neoformans, Blastomyces dermatitidis, and Histoplasma capsulatum | noncompetitive inhibition of the inositol phosphorylceramide synthase | [52,53] |
| Nucleosidic peptide | ||||
| Arthrichitin FR-90403 | Arthrinium phaeospermum and Kernia spp. | C. albicans | chitin synthase inhibitors | [54] |
| Other peptides | ||||
| Aspergillomarasmine | Aspergillus versicolor | Gram-negative rods | inhibit the NDM-1 and VIM-2 metallo-β-lactamases | [55] |
| Cyclosporin A | Tolypocladium nivenum | immunosuppressive, anti- coronaviruses activity | [56] | |
| Peptaibols | Trichoderma reesei | antimicrobial activity against Alternaria alternata, Phoma cucurbitaceum, Fusarium spp., A. fumigatus | [57] | |
| Plectasin | Pseudoplectania nigrella | Streptococcus pneumoniae | [58] | |
| Leucinostatin A | Purpureocillium lilacinum | antifungal activity against Candida spp. (including C. albicans, Candida krusei, Candida tropicalis, and C. guilliermondii); antitrypanosomal and antitumoral activities | [59,60,61] | |
| Terpene derivated metabolites | ||||
| Enfumafungin Ergokonin | Hormonema spp. Trichoderma spp. | antimicrobial activity against Bacillus subtilis, Cryptococcus neoformans, C. albicans, A. fumigatus | glucan synthesis inhibitors | [62,63] |
| Antifungal metabolites | ||||
| Parnafungin | Fusarium lavarum | inhibits inhibit mRNA polyadenylation in Candida albicans and pathogenic fungi | [64,65] | |
| Other pharmaceutical agents | ||||
| Lovastatin | Aspergillus terreus | hypercholesterolemia treatment | [2] | |
| Mevastatin | Penicillium citrinum | [66] | ||
| Pravastatin | Penicillium chrysogenum | [67] | ||
| Other bioactive compounds | ||||
| Clavatol | A. clavatus, Aspergillus clavatonanicus | fungistatic activiy against C. albicans, A. niger, F. oxysporum, Rhizoctonia solani, Pythium ultimum, Didymella bryoniae, B. cinerea | [68] | |
| Pyranonigrins A, B, C, D, E, S | A. niger | [69] | ||
| Pyranonigrins A and F | Penicillium brocae | Antimicrobial activity against Gram-positive and Gram-negative strains | [25] | |
| Mycotoxins | Source | Effect on Human/Animal Health | References | |
|---|---|---|---|---|
| Aflatoxins | B1 B2 G1 G2 | Maize, peanuts, copra, corn, coffee beans, rice, sorghum, soybean |
| [353,356,357,358] |
| Ochratoxins | A | barley, wheat, coffee beans, citrus, grape, beer, fruits, soybean, cereals; dried fruits; breast milk of exposed mothers; smoked and salted dried fish; cheese |
| [359,360,361,362,363] |
| Fumonisins | Maize; rice, wheat, sorghum; barley, oats |
| [337,364,365,366] | |
| Zearalenone | Maize; wheat; barley; oats; grains; animal feed |
| [367,368,369,370,371] | |
| Patulin | Fruits; fruit juices, cheese, wheat |
| [372,373,374] | |
| Trichothecenes | Deoxynivalenol | Maize; wheat; barley; oats; grains; animal feed |
| [337,375,376,377,378] |
| Nivalenol |
| [337,379,380] | ||
| T-2 toxin and HT-2 toxin | Maize, oat, barley, wheat, rice, soybean |
| [337,381,382,383,384,385] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corbu, V.M.; Gheorghe-Barbu, I.; Dumbravă, A.Ș.; Vrâncianu, C.O.; Șesan, T.E. Current Insights in Fungal Importance—A Comprehensive Review. Microorganisms 2023, 11, 1384. https://doi.org/10.3390/microorganisms11061384
Corbu VM, Gheorghe-Barbu I, Dumbravă AȘ, Vrâncianu CO, Șesan TE. Current Insights in Fungal Importance—A Comprehensive Review. Microorganisms. 2023; 11(6):1384. https://doi.org/10.3390/microorganisms11061384
Chicago/Turabian StyleCorbu, Viorica Maria, Irina Gheorghe-Barbu, Andreea Ștefania Dumbravă, Corneliu Ovidiu Vrâncianu, and Tatiana Eugenia Șesan. 2023. "Current Insights in Fungal Importance—A Comprehensive Review" Microorganisms 11, no. 6: 1384. https://doi.org/10.3390/microorganisms11061384
APA StyleCorbu, V. M., Gheorghe-Barbu, I., Dumbravă, A. Ș., Vrâncianu, C. O., & Șesan, T. E. (2023). Current Insights in Fungal Importance—A Comprehensive Review. Microorganisms, 11(6), 1384. https://doi.org/10.3390/microorganisms11061384








