Assessment of Dietary Supplementation of Lactobacillus rhamnosus Probiotic on Growth Performance and Disease Resistance in Oreochromis niloticus
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Fish
2.2. Probiotic Treatment
2.3. Growth Performance and Feed Efficiency
2.4. Proximate Analysis
2.5. Serum Analysis
2.5.1. Total Serum Protein Estimation
2.5.2. Hormones Analysis
2.6. Analysis of Macromolecules
2.6.1. Amino Acid Profile
2.6.2. Fatty Acid Profile
2.6.3. Total Carbohydrate Content
2.7. In Vivo Challenge Assay
2.7.1. Colony Forming Unit (CFU) Counting
2.7.2. Hematological Assays
2.7.3. Histopathological Analysis
2.8. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Proximate Analysis and Total Serum Protein Estimation
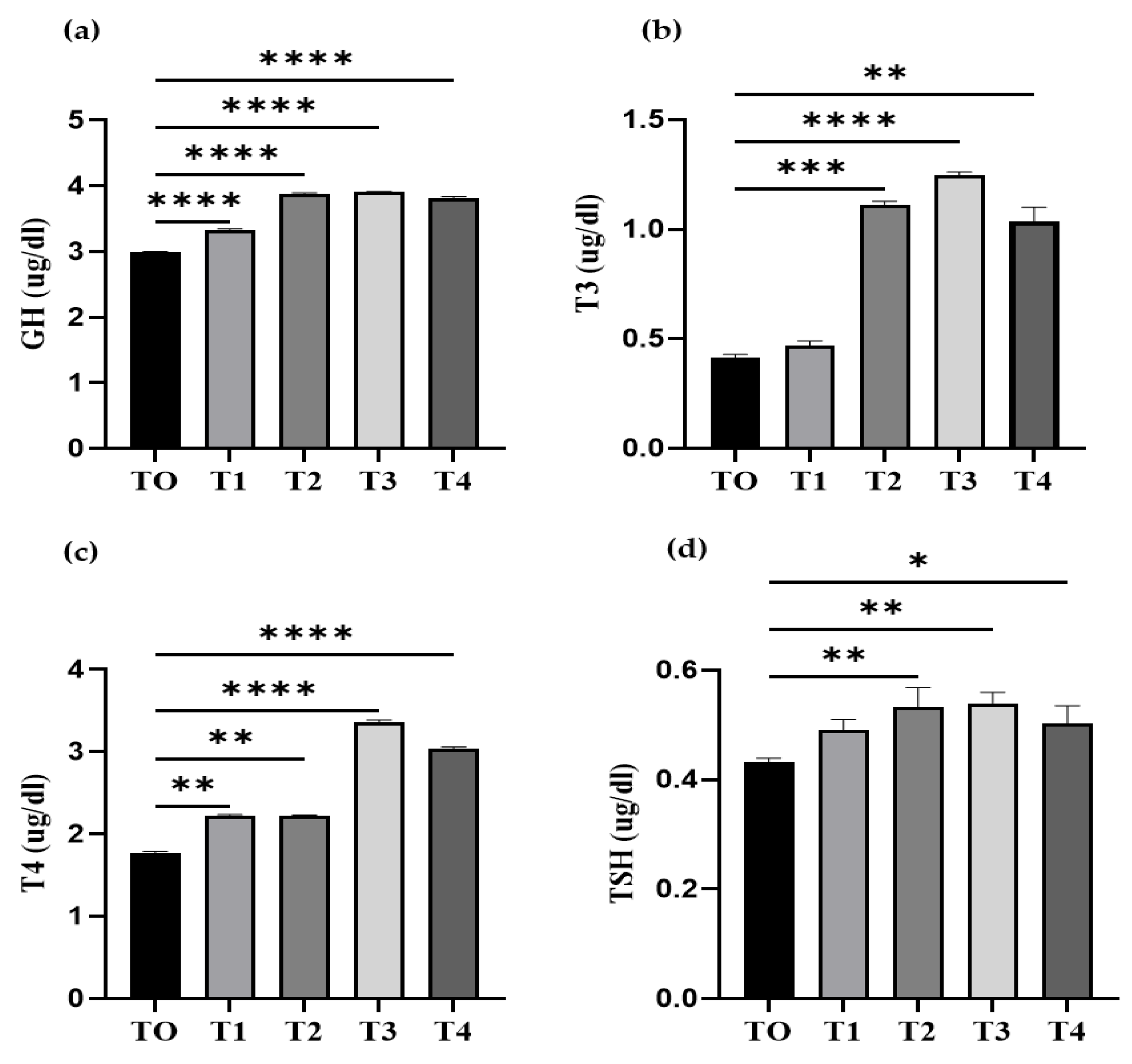
3.3. Quantification of Serum Growth Hormone and Thyroid Hormone
3.4. Analysis of Macromolecules
3.4.1. Amino Acid Profile
3.4.2. Fatty Acid Profile of Fish
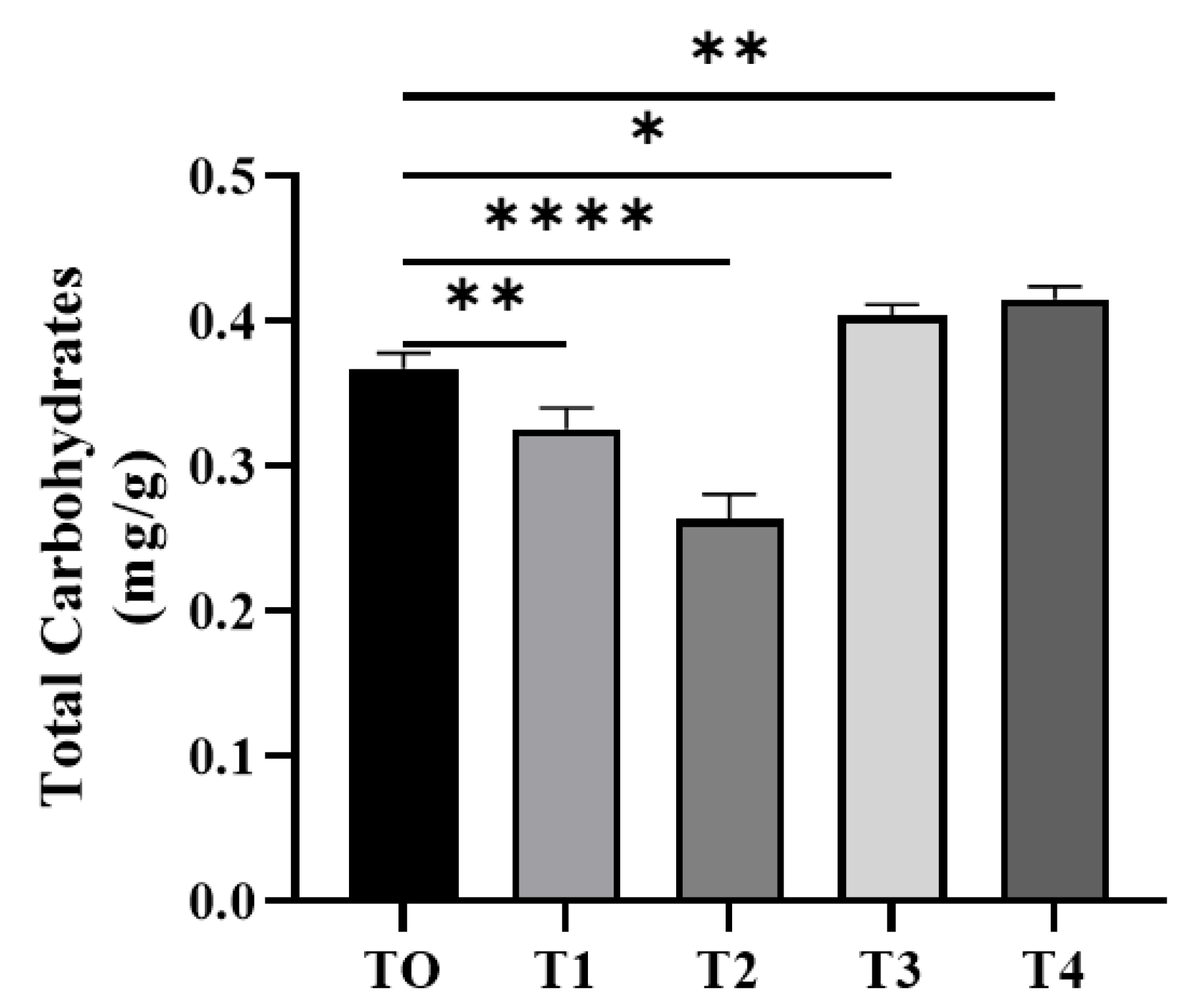
3.4.3. Total Carbohydrate Content
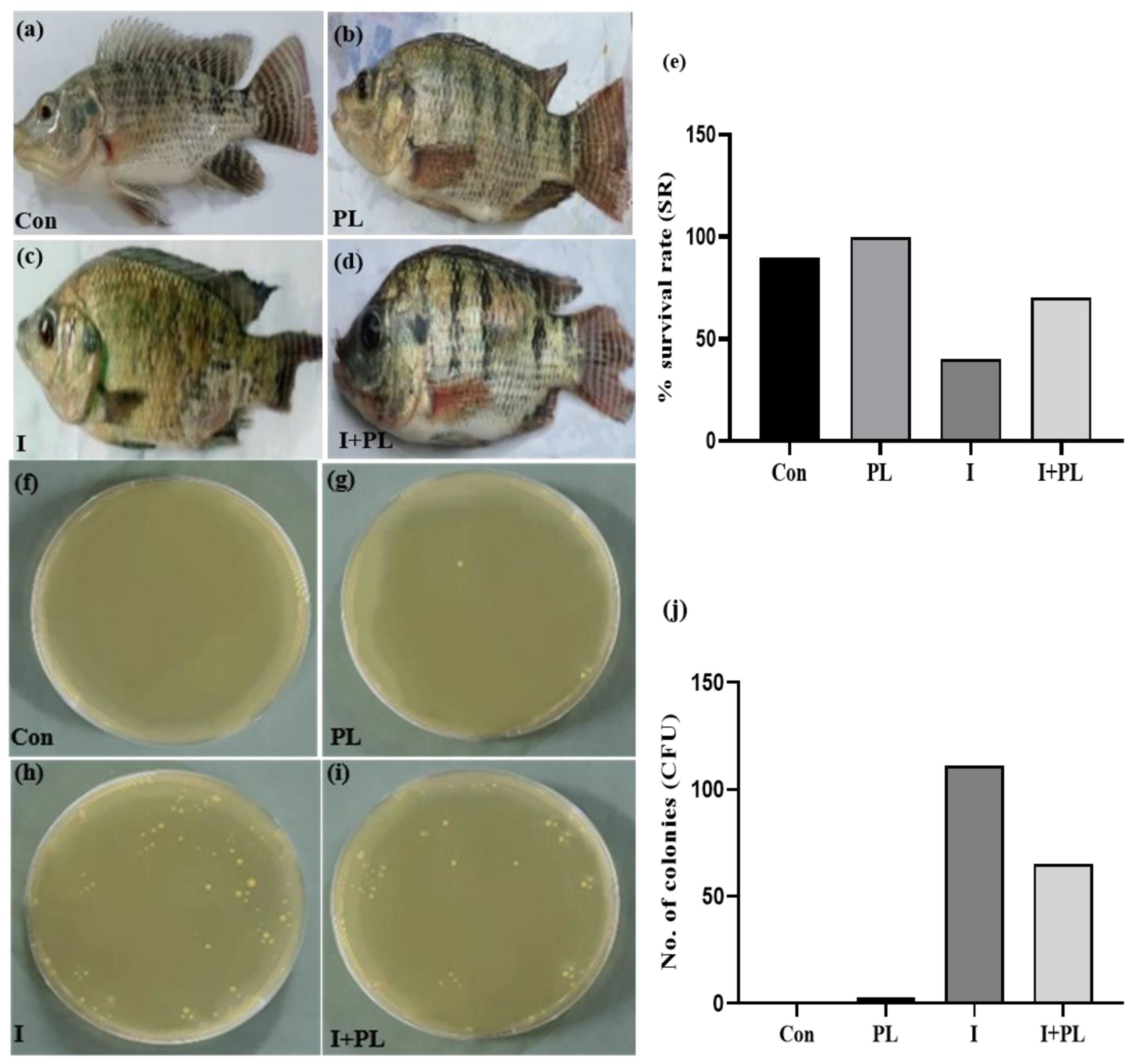
3.5. In Vivo Challenge Assay
3.5.1. Pathological Progression and CFU Analysis
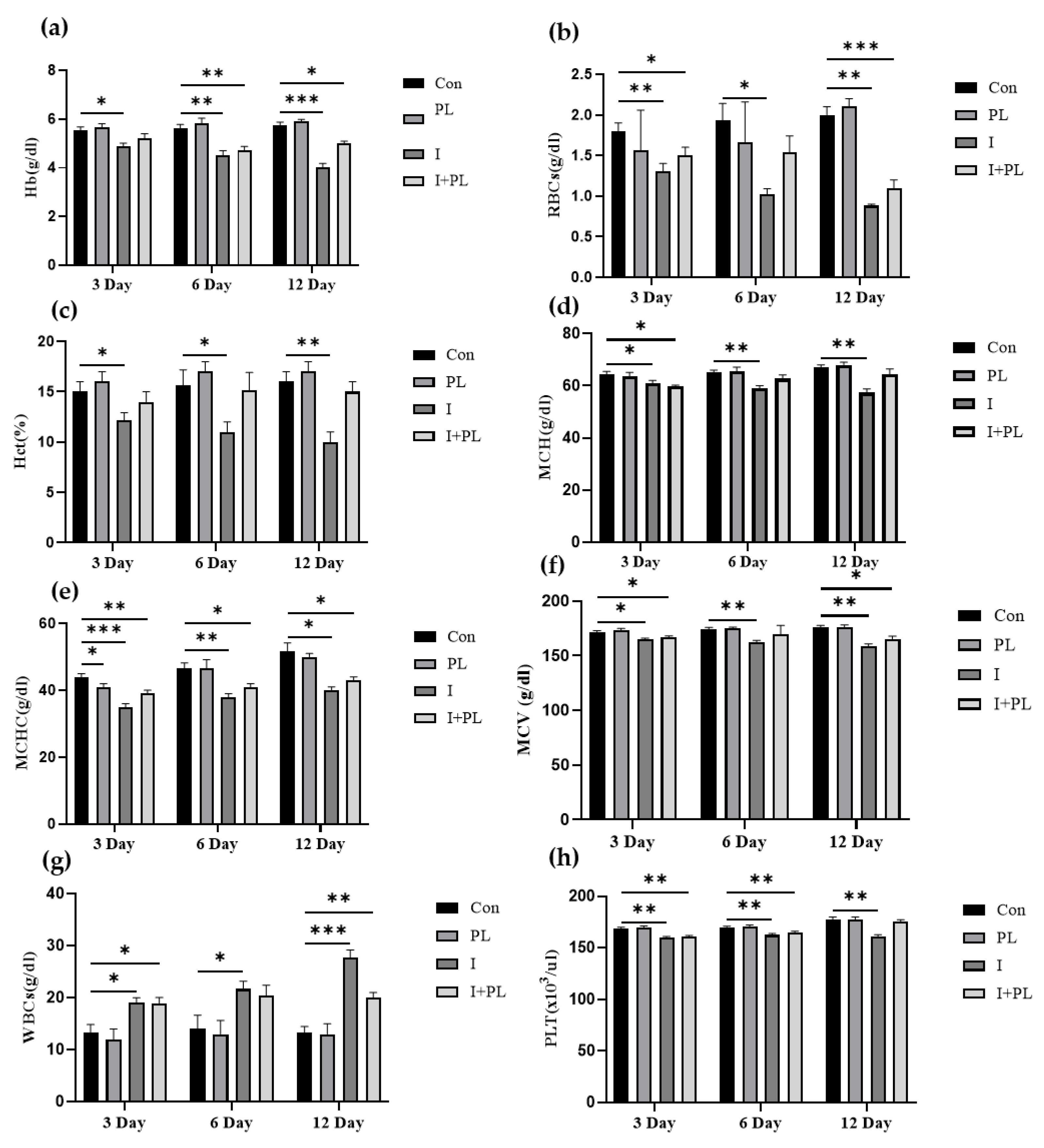
3.5.2. Hematological Indices
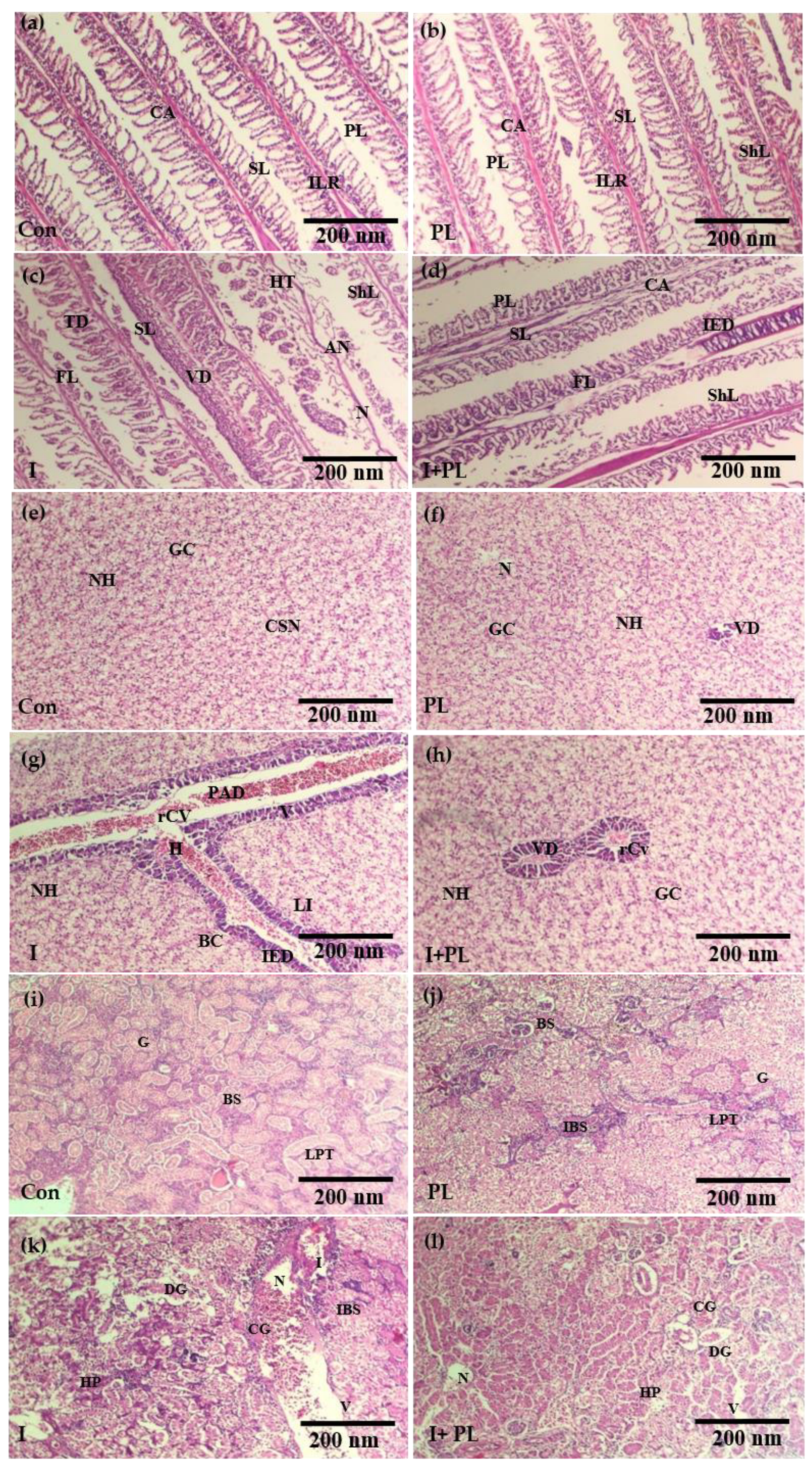
3.5.3. Histopathological Examination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bondad-Reantaso, M.G.; Subasinghe, R.P.; Arthur, J.R.; Ogawa, K.; Chinabut, S.; Adlard, R.; Tan, Z.; Shariff, M. Disease and health management in Asian aquaculture. Vet. Parasitol. 2005, 132, 249–272. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Gil, B.; Roque, A.; Turnbull, J.F. The use and selection of probiotic bacteria for use in the culture of larval aquatic organisms. Aquaculture 2000, 191, 259–270. [Google Scholar] [CrossRef]
- Fernandes, D.C.; Eto, S.F.; Funnicelli, M.I.; Fernandes, C.C.; Charlie-Silva, I.; Belo, M.A.; Pizauro, J.M. Immunoglobulin Y in the diagnosis of Aeromonas hydrophila infection in Nile tilapia (Oreochromis niloticus). Aquaculture 2019, 500, 576–585. [Google Scholar] [CrossRef]
- Ahmadifar, E.; Moghadam, M.S.; Dawood, M.A.O.; Hoseinifar, S.H. Lactobacillus fermentum and/or ferulic acid improved the immune responses, antioxidative defence and resistance against Aeromonas hydrophila in common carp (Cyprinus carpio) fingerlings. Fish Shellfish Immunol. 2019, 94, 916–923. [Google Scholar] [CrossRef]
- Chen, N.; Jiang, J.; Gao, X.; Li, X.; Zhang, Y.; Liu, X.; Yang, H.; Bing, X.; Zhang, X. Histopathological analysis and the immune related gene expression profiles of mandarin fish (Siniperca chuatsi) infected with Aeromonas hydrophila. Fish Shellfish Immunol. 2018, 83, 410–415. [Google Scholar] [CrossRef]
- Nicholson, P.; Monon, N.; Jaemwimol, P.; Tattiyapong, P.; Surachetpong, W. Coinfection of tilapia lake virus and Aeromonas hydrophila synergistically increased mortality and worsened the disease severity in tilapia (Oreochromis spp.). Aquaculture 2020, 520, 734–746. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S.; Abdel-Daim, M.M.; Van-Doan, H. Probiotic application for sustainable aquaculture. Rev. Aquac. 2018, 11, 1–18. [Google Scholar] [CrossRef]
- Akhter, N.; Wu, B.; Memon, A.M.; Mohsin, M. Probiotics and prebiotics associated with aquaculture: A review. Fish Shellfish Immunol. 2015, 45, 733–741. [Google Scholar] [CrossRef]
- Nimrat, S.; Suksawat, S.; Boonthai, T.; Vuthiphandchai, V. Potential Bacillus probiotics enhance bacterial numbers, water quality and growth during early development of white shrimp (Litopenaeus vannamei). Vet. Microbiol. 2012, 159, 443–450. [Google Scholar] [CrossRef]
- Ridha, M.T.; Azad, I.S. Effect of autochthonous and commercial probiotic bacteria on growth, persistence, immunity and disease resistance in juvenile and adult Nile tilapia Oreochromis niloticus. Aquac. Res. 2016, 47, 2757–2767. [Google Scholar] [CrossRef]
- Aly, S.M.; Abd-El-Rahman, A.M.; John, G.; Mohamed, M.F. Characterization of some bacteria isolated from Oreochromis niloticus and their potential use as probiotics. Aquaculture 2008, 277, 1–6. [Google Scholar] [CrossRef]
- Pal, D.; Joardar, S.N.; Roy, B. Immunostimulatory effects of a yeast (Saccharomyces cerevisiae) cell wall feed supplement on rohu (Labeo rohita), an Indian major carp. Isr. J. Aquac.-Bamidgeh. 2007, 59, 175–181. [Google Scholar] [CrossRef]
- Sun, P.; Yin, F.; Tang, B. Effects of acute handling stress on expression of growth-related genes in Pampus argenteus. J. World Aquac. Soc. 2016, 48, 166–179. [Google Scholar] [CrossRef]
- Lim, K.C.; Yusoff, F.M.; Shariff, M.; Kamarudin, M.S. Astaxanthin as feed supplement in aquatic animals. Rev. Aquac. 2018, 10, 738–773. [Google Scholar] [CrossRef]
- Kanwal, Z.; Tayyeb, A. Role of dietary probiotic Ecotec in growth enhancement, thyroid tuning, hematomorphology and resistance to pathogenic challenge in Labeo rohita juveniles. J. Appl. Anim. Res. 2019, 47, 394–402. [Google Scholar] [CrossRef]
- Dorshkind, K.; Horseman, N.D. Anterior pituitary hormones, stress, and immune system homeostasis. Bioessays 2001, 23, 288–294. [Google Scholar] [CrossRef]
- Hrubec, T.C.; Cardinale, J.L.; Smith, S.A. Hematology and plasma chemistryreference intervals for cultured tilapia (Oreochromis hybrid). Vet. Clin. Pathol. 2000, 29, 7–12. [Google Scholar] [CrossRef]
- Adeyemo, O.K. Haematological profile of clariasgariepinus (Burchell, 1822) exposed to lead. Turk. J. Fish. Aquat. Sci. 2007, 7, 163–169. [Google Scholar]
- Sunmonu, T.O.; Oloyede, O. Haematological response of Acan catfish (Clarias gariepinus) and rat to crude oil exposure. J. Haematol. 2007, 4, 1–5. [Google Scholar]
- Nasir, N.A.; Al-Sraji, A.Y.J. Effect of different dietary protein and fats on some biochemical blood parameters in common carp fingerlings (Cyprinus carpio L.) reared in float cages. Asian J. Exp. Biol. Sci. 2013, 4, 293–296. [Google Scholar]
- Barišić, J.; Marijić, V.F.; Mijošek, T.; Čož-Rakovac, R.; Dragun, Z.; Krasnići, N.; Ivanković, D.; Kružlicová, D.; Erk, M. Evaluation of architectural and histopathological biomarkers in the intestine of brown trout (Salmo trutta Linnaeus, 1758) challenged with environmental pollution. Sci. Total. Environ. 2018, 642, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Thophon, S.; Kruatrachue, M.; Upathan, E.S.; Pokethitiyook, P.; Sahaphong, S.; Jarikhuan, S. Histopathological alterations of white seabass, Lates calcarifer, in acute and sub-chronic cadmium exposure. Environ. Pollut. 2003, 121, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Gernhofer, M.; Pawet, M.; Schramm, M.; Müller, E.; Triebskorn, R. Ultrastructural biomarkers as tools to characterize the health status of fish in contaminated streams. J. Aquat. Ecosyst. Stress Recovery 2001, 8, 241–260. [Google Scholar] [CrossRef]
- Caballero, M.J.; Izquierdo, M.S.; Kjorsvik, E.; Fernandez, A.J.; Rosenlund, G. Histological alterations in the liver of sea bream, Sparus aurata L. caused by short- or long-term feeding with vegetable oils. Recovery of normal morphology after feeding fish oil as the sole lipid source. J. Fish Dis. 2004, 27, 531–541. [Google Scholar] [CrossRef]
- Haidera, S.M.; Ashraf, M.; Azmata, H.; Khaliqueb, A.; Javidc, A.; Atiquea, U.; Ziad, M.; Iqbala, J.K.; Akrama, S. Nutritive evaluation of fish acid silage in Labeo rohita fingerlings feed. J. Appl. Anim. Res. 2016, 44, 158–164. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; Association of the Official Analytical Chemists: Washington, DC, USA, 2006. [Google Scholar]
- Palliyeguru, M.W.; Rose, S.P.; Mackenzie, A.M. Effect of dietary protein concentrates on the incidence of subclinical necrotic enteritis and growth performance of broiler chickens. Poult. Sci. 2010, 89, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Lu, H. Determination of Fatty Acids in Rice Oil by Gas Chromatography–Mass Spectrometry (GC–MS) with Geographic and Varietal Discrimination by Supervised Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA). Anal. Lett. 2022, 55, 675–687. [Google Scholar] [CrossRef]
- Pervaiz, S.; Kanwal, Z.; Manzoor, F.; Tayyeb, A.; Akram, Q. Investigations on Blood Physiology, Tissues Histology and Gene Expression Profile of Fusarium oxysporum Challenged Fish. Sains Malays. 2022, 51, 2403–2414. [Google Scholar] [CrossRef]
- Huynh, T.G.; Shiu, Y.L.; Nguyen, T.P.; Truong, Q.P.; Chen, J.C.; Liu, C.H. Current applications, selection, and possible mechanisms of actions of synbiotics in improving the growth and health status in aquaculture: A review. Fish Shellfish Immunol. 2017, 64, 367–382. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Sun, Y.Z.; Wang, A.; Zhou, Z. Probiotics as Means of diseases control in aquaculture, a review of current knowledge and future perspectives. Front. Microbiol. 2018, 9, 24–29. [Google Scholar] [CrossRef]
- Asaduzzaman, M.; Sofia, E.; Shakil, A.; Haque, N.F.; Khan, M.N.A.; Ikeda, D.; Kinoshita, S.; Abol-Munafi, A.B. Host gut-derived probiotic bacteria promote hypertrophic muscle progression and upregulate growth-related gene expression of slow-growing Malaysian Mahseer Tor tambroides. Aquac. Rep. 2018, 9, 37–45. [Google Scholar] [CrossRef]
- Butt, R.L.; Volkoff, H. Gut microbiota and energy homeostasis in fish. Front. Endocrinol. 2019, 10, 9. [Google Scholar] [CrossRef]
- Mary, N.M.; Nanua, N.J.; David, L. A comparative study on growth, composition and sensory quality between farmed and wild Nile Tilapia (Oreochromis niloticus). Net J. Agric. Sci. 2015, 3, 56–61. [Google Scholar]
- El-Haroun, E.R.; Goda, A.M.A.S.; Chowdhury, M.A.K. Effect of dietary probiotic biogen® supplementation as a growth promoter on growth performance and feed utilization of Nile tilapia, Oreochromis niloticus (L.). Aquac. Res. 2006, 37, 1473–1480. [Google Scholar] [CrossRef]
- Lara-Flores, M.; Olvera-Novoa, M.A.; Guzmán-Méndez, B.E.; López-Madrid, W. Use of the bacteria Streptococcus faecium and Lactobacillus acidophilus, and the yeast Saccharomyces cerevisiae as growth promoters in Nile tilapia (Oreochromis niloticus). Aquaculture 2003, 216, 193–201. [Google Scholar] [CrossRef]
- Ringo, E.; Gatesoupe, F.J. Lactic acid bacteria in fish: A Review. Aquaculture 1998, 160, 177–203. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Abdel-Rahman, A.M.; Ismael, N.E.M. Evaluation of commercial live bakers’ yeast, Saccharomyces cerevisiae as a growth and immunity promoter for fry Nile tilapia, Oreochromis niloticus (L.) challenged in situ with Aeromonas hydrophila. Aquaculture 2008, 280, 185–189. [Google Scholar] [CrossRef]
- Azarin, H.; Aramli, M.S.; Imanpour, M.R.; Rajabpour, M. Effect of a probiotic containing Bacillus licheniformis and Bacillus subtilis and ferroin solution on growth performance, body composition and hematological parameters in Kutum (Rutilus frisii kutum) fry. Probiotics Antimicrob. 2015, 7, 31–37. [Google Scholar] [CrossRef]
- Das, A.; Nakhro, K.; Chowdhury, S.; Kamilya, D. Effects of potential probiotic Bacillus amyloliquifaciens FPTB16 on systemic and cutaneous mucosal immune responses and disease resistance of catla (catla catla). Fish Shellfish Immunol. 2013, 35, 1547–1553. [Google Scholar] [CrossRef]
- Reindl, K.M.; Sheridan, M.A. Peripheral regulation of the growth hormone-insulin-like growth factor system in fish and other vertebrates. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2012, 163, 231–245. [Google Scholar] [CrossRef]
- Franz, A.C.; Faass, O.; Köllner, B.; Shved, N.; Link, K.; Casanova, A.; Eppler, E. Endocrine and local IGF-I in the bony fish immune system. Biology 2016, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Devlin, R.; Sakhrania, D.; Tymchuka, W.; Rise, M.; Goha, B. Domestication and growth hormone transgenesis cause similar changes in gene expression in Coho salmon (Oncorhynchus kisutch). Proc. Natl. Acad. Sci. USA 2009, 106, 3047–3052. [Google Scholar] [CrossRef] [PubMed]
- Avella, M.A.; Olivotto, I.; Silvi, S.; Ribecco, C.; Cresci, A.; Palermo, F.; Polzonetti, A.; Carnevali, O. Use of Enterococcus faecium to improve common sole (Solea solea) larviculture. Aquaculture 2011, 315, 384–393. [Google Scholar] [CrossRef]
- Power, D.M.; Llewellyn, L.; Faustino, M.; Nowell, M.A.; Bjornsson, B.T.H.; Einarsdottir, I.E.; Sweeney, G.E. Thyroid hormones in growth and development of fish. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2001, 130, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Adel, M.; Abedian Amiri, A.; Zorriehzahra, J.; Nematolahi, A.; Esteban, M.A. Effects of dietary peppermint (Mentha piperita) on growth performance, chemical body composition and hematological and immune parameters of fry Caspian white fish (Rutilus frisii kutum). Fish Shellfish Immunol. 2015, 45, 841–847. [Google Scholar] [CrossRef]
- Garg, S.K. Effect of oral administration of l-thyroxine (T-4) on growth performance, digestibility, and nutrient retention in Channa punctatus (Bloch) and Heteropneustes fossilis (Bloch). Fish Physiol. Biochem. 2007, 33, 347–358. [Google Scholar] [CrossRef]
- Soldin, O.P.; Chung, S.H.; Colie, C.C. The use of TSH in determining thyroid disease: How does it impact the practice of medicine in pregnancy? J. Thyroid Res. 2013, 2013, 148–157. [Google Scholar] [CrossRef]
- Vancamp, P.; Houbrechts, A.M.; Darras, V.M. Insights from zebrafsh deficiency models to understand the impact of local thyroid hormone regulator action on early development. Gen. Comp. Endocrinol. 2018, 279, 45–52. [Google Scholar] [CrossRef]
- Shkil, F.; Siomava, N.; Voronezhskay, E.; Diogo, R. Effects of hyperthyroidism in the development of the appendicular skeleton and muscles of zebrafish, with notes on evolutionary developmental pathology (EvoDevo-Path). Sci. Rep. 2019, 9, 5413. [Google Scholar] [CrossRef]
- Mat Jais, A.M.; McCulloh, R.; Croft, K. Fatty acid and amino acid composition in haruan as a potential role in wound healing. Gen. Pharmacol. 1994, 25, 947–950. [Google Scholar] [CrossRef]
- Biswas, M.; Islam, M.S.; Das, P.; Das, P.R.; Akter, M. Comparative study on proximate composition and amino acids of probiotics treated and nontreated cage reared monosex tilapia Oreochromis niloticus in Dekar haor, Sunamganj district, Bangladesh. Int. J. Fish. Aquat. Stud. 2018, 6, 431–435. [Google Scholar]
- Witte, M.B.; Thornton, F.J.; Tantry, U.; ABarbul, L. Arginine supplementation enhances diabetic wound healing; involvement of the nitric oxi-de synthase and arginase pathways. Metabolism 2002, 51, 1269–1273. [Google Scholar] [CrossRef]
- Zupan, B.; Jubojevic, D.; Pelic, M.; Cirkovic, M.; Oordevic, O.; Bogut, I.; Res, V.S. Common carp response to the different concentration of linseed oil in diet. Slov. Vet. Res. 2016, 53, 19–28. [Google Scholar]
- Turchini, G.M.; Francis, D.S. Fatty acid metabolism (desaturation, elongation and β-oxidation) in rainbow trout fed fish oil- or linseed oil-based diets. Br. J. Nutr. 2009, 102, 69–81. [Google Scholar] [CrossRef]
- Njinkoue, J.M.; Gouado, I.; Tchoumbougnang, F.; Yanga-Ngueguim, J.H.; Ndinteh, D.T.; Fomogne-Fodjo, C.Y.; Schweigert, F.J. Proximate composition, mineral content and fatty acid profile of two marine fishes from Cameroonian coast: Pseudotolithus typus (Bleeker, 1863) and Pseudotolithus elongatus (Bowdich, 1825). NFS J. 2016, 4, 27–31. [Google Scholar] [CrossRef]
- Aboyadak, I.M.; Ali, N.G.M.; Goda, A.M.A.S.; Aboelgalagel, W.H.; Alnokrashy, A.M.E. Molecular detection of Aeromonas hydrophila as the main cause of outbreak in tilapia farms in Egypt. J. Aquac. Mar. Biol. 2015, 2, 2–5. [Google Scholar] [CrossRef]
- Asaad, T.M.A. Bacteriological Studies on Aeromonas hydrophila in Fish in Kafr El Sheikh Governorate. Ph.D. Thesis, Microbiology Department, Faculty of Veterinary Medicine, Kafrelsheikh University, Kafrelsheikh, Egypt, 2008. [Google Scholar]
- Yiagnisis, M.; Athanassopoulou, F. Bacteria isolated from diseased wild and farmed marine fish in Greece. In Recent Advances in Fish Farms; BoD: Norderstedt, Germany, 2011; Volume 27, pp. 61–69. [Google Scholar]
- Sarker, M.G.A.; Sarker, A.; Chowdhury, M.B.R. Occurrence of aeromonad pathogens in carp fingerling at Mymensingh region of Bangladesh. Bangladesh J. Fish. 1999, 22, 7–12. [Google Scholar] [CrossRef]
- Chowdhury, M.B.R.; Baqui, M.A. Bacterial flora in farmed carp (Labeo rohita) in Bangladesh. In Disease in Asian Aquaculture III; Flegel, T., MacRae, I.H., Eds.; Fish Health Section, Asian Fisheries Society: Manila, Philippines, 1997; pp. 101–105. [Google Scholar]
- Kumar, R.; Mukherjee, S.C.; Prasad, K.P.; Pal, A.K. Evaluation of Bacillus subtilis as a probiotic to Indian major carp Labeo rohita (Ham.). Aquac. Res. 2006, 37, 1215–1221. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Nisha Rani, M.; Balasundaram, C. Hematological and biochemical parameters in common carp, Cyprinus carpio, following herbal treatment for Aeromonas hydrophila infection. J. Aquac. 2003, 221, 41–50. [Google Scholar] [CrossRef]
- Yu, J.; Han, J.; Park, S.W.; Kim, D. Hemato-biochemical and histological changes in Mud loach, Misgurnus mizolepis experimentally infected with Aeromonas sobria. Slov. Vet. Res. 2018, 55, 171–182. [Google Scholar] [CrossRef]
- Scott, A.L.; Rogers, W.A. Haematological effects of prolonged sublethal hypoxia on channel catfish Ictalurus punctatus (Rafinesque). J. Fish Biol. 1981, 18, 591–601. [Google Scholar] [CrossRef]
- AbdAllah, O.A.; Salah, M.A.; Haidy, G.; Abd El-Rahman, L.; Fatma, M.A.; Youssef-Fatma, K. Effect of some immunostimulants on Clinicopathological Findings of African Catfish Clarias gariepinus. Infected with Motile Aeromonas Septicemia. EC Vet. Sci. 2019, 4, 498–510. [Google Scholar]
- Camargo, M.M.P.; Martinez, C.B.R. Histopathology of gills, kidney and liver of a Neotropical fish caged in an urban stream. Neotrop. Ichthyol. 2007, 5, 327–336. [Google Scholar] [CrossRef]
- Palladino, A.; De Felice, E.; Attanasio, C.; Barone, C.M.; Crasto, A.; D’Angelo, L.; Giaquinto, D.; Lambiase, C.; Scocco, P.; Serrapica, F.; et al. A Morphological and Ultrastructural Study of the Anterior Digestive Tract of Adult Nile Tilapia Oreochromis niloticus. Animals 2023, 13, 420. [Google Scholar] [CrossRef]
- Fernandes, M.N.; Mazon, A.F. Environmental pollution and fish gill morphology. J. Environ. Prot. 2003, 8, 203–231. [Google Scholar]
- Hadi, A.A.; Alwan, S.F. Histopathological changes in gills, liver and kidney of fresh water fish, Tilapia zillii, exposed to aluminum. Int. J. Pharm. Life Sci. 2012, 3, 2071–2081. [Google Scholar]
- Okwari, O.O.; Ettarh, R.R.; Akpogomeh, B.A.; Eteng, M.U. Gastric anti-secretory and anti-ulcerogenic effects of Dombeya buettneri in rats. J. Ethnopharmacol. 2000, 71, 315–319. [Google Scholar] [CrossRef]
- El-Barbary, M.I. Some clinical, microbiological and molecular characteristics of Aeromonas hydrophila isolated from various naturally infected fishes. Aquacult. Int. 2010, 18, 943–954. [Google Scholar] [CrossRef]
- Allan, B.J.; Stevenson, R.M.W. Extracellular virulence factors of Aeromonas hydrophila in fish infections. Can. J. Microbiol. 1981, 27, 1114–1122. [Google Scholar] [CrossRef]
- Miyazaki, T.; Kaige, N. A histopathological study on motile aeromonad disease of Crucian carp. Fish Pathol. 1985, 21, 181–185. [Google Scholar] [CrossRef]
- Najiah, M.; Laith, A.R. Aeromonas hydrophila: Antimicrobial susceptibility and histopathology of isolates from diseased catfish, Clarias gariepinus (Burchell). J. Aquacult. Res. Dev. 2013, 5, 215. [Google Scholar] [CrossRef]
| Parameters | Treatments | |||||
|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | T4 | ||
| W | Initial | 20.06 ± 0.089 | 20.02 ± 0.044 | 20.06 ± 0.054 | 20.02 ± 0.044 | 20.06 ± 0.054 |
| 2 Weeks | 21.10 ± 0.787 | 23.02 ± 0.438 * | 23.32 ± 0.455 ** | 23.90 ± 0.430 ** | 23.34 ± 0.482 ** | |
| 4 Weeks | 24.08 ± 0.396 | 25.20 ± 0.495 * | 25.98 ± 0.775 * | 27.26 ± 1.085 ** | 26.50 ± 1.000 * | |
| 6 Weeks | 25.30 ± 0.721 | 28.80 ± 0.908 ** | 30.60 ± 1.140 *** | 33.36 ± 1.270 **** | 31.98 ± 0.746 **** | |
| 8 Weeks | 27.40 ± 0.547 | 30.40 ± 3.362 * | 34.80 ± 1.304 **** | 37.36 ± 1.717 **** | 36.96 ± 0.712 **** | |
| 10 Weeks | 29.00 ± 1.225 | 38.80 ± 1.924 *** | 39.60 ± 1.140 **** | 41.40 ± 1.140 **** | 39.20 ± 1.095 **** | |
| 12 Weeks | 32.00 ± 1.581 | 44.20 ± 1.789 **** | 45.40 ± 1.140 **** | 48.00 ± 1.581 **** | 45.20 ± 0.836 **** | |
| L | Initial | 10.06 ± 0.054 | 10.04 ± 0.054 | 10.08 ± 0.044 | 10.06 ± 0.054 | 10.02 ± 0.044 |
| 2 Weeks | 10.24 ± 0.114 | 10.38 ± 0.044 | 10.42 ± 0.083 | 10.50 ± 0.122 * | 10.42 ± 0.083 | |
| 4 Weeks | 10.72 ± 0.130 | 10.92 ± 0.148 | 11.16 ± 0.219 * | 11.62 ± 0.277 ** | 11.60 ± 0.223 ** | |
| 6 Weeks | 11.32 ± 0.216 | 12.04 ± 0.384 ** | 12.06 ± 0.336 ** | 12.36 ± 0.296 **** | 12.20 ± 0.264 **** | |
| 8 Weeks | 12.20 ± 0.291 | 13.06 ± 0.270 **** | 13.30 ± 0.234 **** | 13.82 ± 0.083 **** | 13.56 ± 0.296 **** | |
| 10 Weeks | 12.72 ± 0.178 | 13.92 ± 0.192 **** | 14.66 ± 0.181 **** | 14.90 ± 0.141 **** | 14.62 ± 0.277 **** | |
| 12 Weeks | 13.18 ± 0.204 | 15.18 ± 0.249 **** | 15.66 ± 0.260 **** | 15.98 ± 0.083 **** | 15.84 ± 0.114 **** | |
| WG | 2 Weeks | 1.100 ± 0.787 | 3.000 ± 0.403 * | 3.260 ± 0.456 ** | 3.880 ± 0.396 ** | 3.300 ± 0.489 ** |
| 4 Weeks | 4.020 ± 0.389 | 5.180 ± 0.521 * | 5.920 ± 0.746 * | 7.240 ± 1.101 ** | 6.460 ± 0.950 * | |
| 6 Weeks | 5.240 ± 0.650 | 8.380 ± 1.359 * | 10.54 ± 1.137 *** | 13.34 ± 1.305 *** | 11.94 ± 0.740 **** | |
| 8 Weeks | 6.940 ± 1.307 | 12.38 ± 2.043 * | 15.30 ± 1.875 *** | 17.78 ± 1.481 *** | 16.52 ± 1.130 **** | |
| 10 Weeks | 8.940 ± 1.307 | 18.78 ± 1.921 *** | 19.54 ± 1.180 **** | 21.38 ± 1.150 **** | 19.16 ± 1.106 **** | |
| 12 Weeks | 11.94 ± 1.599 | 24.18 ± 1.764 **** | 25.34 ± 1.167 **** | 27.98 ± 1.550 **** | 25.16 ± 0.820 **** | |
| LG | 2 Weeks | 0.180 ± 0.109 | 0.340 ± 0.054 | 0.340 ± 0.547 | 0.440 ± 0.167 * | 0.400 ± 0.070 |
| 4 Weeks | 0.660 ± 0.114 | 0.880 ± 0.164 | 1.080 ± 0.216 * | 1.560 ± 0.260 ** | 1.380 ± 0.295 * | |
| 6 Weeks | 1.280 ± 0.204 | 2.000 ± 0.367 * | 1.980 ± 0.334 * | 2.300 ± 0.291 ** | 2.180 ± 0.295 ** | |
| 8 Weeks | 2.140 ± 0.270 | 3.020 ± 0.228 ** | 3.220 ± 0.216 *** | 3.760 ± 0.114 *** | 3.540 ± 0.288 *** | |
| 10 Weeks | 2.660 ± 0.134 | 3.880 ± 0.228 *** | 4.660 ± 0.151 **** | 4.720 ± 0.238 **** | 4.660 ± 0.320 **** | |
| 12 Weeks | 3.120 ± 0.164 | 5.140 ± 0.219 **** | 5.580 ± 0.286 **** | 5.920 ± 0.083 **** | 5.820 ± 0.109 **** | |
| SGR | 2 Weeks | 0.150 ± 0.104 | 0.403 ± 0.054 * | 0.408 ± 0.057 ** | 0.495 ± 0.032 ** | 0.361 ± 0.173 |
| 4 Weeks | 0.265 ± 0.020 | 0.332 ± 0.029 * | 0.373 ± 0.041 * | 0.449 ± 0.054 ** | 0.403 ± 0.050 * | |
| 6 Weeks | 0.225 ± 0.026 | 0.336 ± 0.046 * | 0.406 ± 0.036 *** | 0.492 ± 0.039 **** | 0.450 ± 0.022 **** | |
| 8 Weeks | 0.215 ± 0.033 | 0.361 ± 0.057 * | 0.401 ± 0.029 *** | 0.459 ± 0.028 **** | 0.434 ± 0.022 **** | |
| 10 Weeks | 0.213 ± 0.024 | 0.382 ± 0.028 **** | 0.393 ± 0.018 **** | 0.420 ± 0.016 **** | 0.387 ± 0.016 **** | |
| 12 Weeks | 0.225 ± 0.023 | 0.381 ± 0.019 **** | 0.393 ± 0.012 **** | 0.421 ± 0.015 **** | 0.391 ± 0.008 **** | |
| FCR | 2 Weeks | 4.126 ±1.206 | 2.018 ± 0.303 | 1.856 ± 0.295 | 1.742 ± 0.330 * | 1.842 ± 0.277 |
| 4 Weeks | 2.792 ± 1.059 | 2.340 ± 0.219 | 2.040 ± 0.270 | 1.680 ± 0.238 | 1.860 ± 0.251 | |
| 6 Weeks | 3.478 ± 0.456 | 2.160 ± 0.350 ** | 1.700 ± 0.200 ** | 1.380 ± 0.192 *** | 1.500 ± 0.100 ** | |
| 8 Weeks | 3.578 ± 0.825 | 1.980 ± 0.334 * | 1.600 ± 0.141 * | 1.340 ± 0.114 * | 1.440 ± 0.114 * | |
| 10 Weeks | 3.420 ± 0.575 | 1.580 ± 0.148 ** | 1.520 ± 0.083 ** | 1.400 ± 0.070 ** | 1.540 ± 0.054 ** | |
| 12 Weeks | 3.026 ± 0.448 | 1.500 ± 0.100 ** | 1.400 ± 0.070 ** | 1.320 ± 0.083 ** | 1.420 ± 0.044 ** | |
| PER | 2 Weeks | 3.620 ± 2.639 | 9.960 ± 1.431 * | 10.82 ± 1.527 ** | 12.90 ± 1.319 ** | 29.76 ± 4.371 **** |
| 4 Weeks | 13.34 ± 1.299 | 17.22 ± 1.756 * | 19.72 ± 2.508 * | 24.10 ± 3.673 ** | 22.78 ± 3.627 * | |
| 6 Weeks | 17.42 ± 2.163 | 27.88 ± 4.549 * | 35.08 ± 3.789 *** | 44.42 ± 4.356 *** | 39.78 ± 2.480 **** | |
| 8 Weeks | 22.98 ± 4.214 | 41.22 ± 6.796 ** | 49.84 ± 4.775 *** | 59.22 ± 4.928 **** | 55.06 ± 3.764 **** | |
| 10 Weeks | 29.76 ± 4.371 | 62.58 ± 6.406 *** | 65.10 ± 3.948 **** | 71.22 ± 3.832 **** | 63.82 ± 3.675 **** | |
| 12 Weeks | 39.78 ± 5.320 | 80.56 ± 5.876 **** | 84.44 ± 3.911 **** | 93.22 ± 5.156 **** | 83.84 ± 2.712 **** | |
| CF | 2 Weeks | 1.990 ± 0.731 | 1.636 ± 0.848 | 2.078 ± 0.089 | 2.046 ± 0.040 | 2.058 ± 0.066 |
| 4 Weeks | 1.890 ± 0.022 | 1.910 ± 0.103 | 1.934 ± 0.060 | 1.964 ± 0.047 * | 1.956 ± 0.102 | |
| 6 Weeks | 1.564 ± 0.104 | 1.768 ± 0.092 | 1.906 ± 0.021 ** | 1.868 ± 0.084 ** | 1.802 ± 0.102 * | |
| 8 Weeks | 1.388 ± 0.052 | 1.642 ± 0.207 * | 1.580 ± 0.094 | 1.606 ± 0.102 * | 1.512 ± 0.075 | |
| 10 Weeks | 1.342 ± 0.037 | 1.514 ± 0.072 | 1.528 ± 0.082 * | 1.616 ± 0.100 ** | 1.526 ± 0.068 * | |
| 12 Weeks | 1.310 ± 0.053 | 1.396 ± 0.080 | 1.512 ± 0.132 | 1.540 ± 0.0961 * | 1.544 ± 0.078 ** | |
| Parameters | T0 | T1 | T2 | T3 | T4 |
|---|---|---|---|---|---|
| Moisture (%) | 73.30 ± 0.360 | 74.17 ± 1.258 | 74.73 ± 1.617 | 73.80 ± 0.200 | 74.03 ± 0.450 |
| Ash (%) | 2.057 ± 0.125 | 2.263 ± 0.257 | 2.470 ± 0.036 | 2.537 ± 0.035 * | 2.430 ± 0.130 |
| Fat (%) | 5.133 ± 0.152 | 5.947 ± 0.382 | 6.500 ± 0.264 * | 5.533 ± 0.152 | 6.467 ± 1.002 * |
| Protein (%) | 63.75 ± 2.308 | 71.39 ± 1.451 ** | 70.43 ± 0.684 ** | 68.78 ± 2.335 * | 64.10 ± 2.007 |
| Serum total protein (g/dl) | 2.027 ± 0.064 | 2.130 ± 0.020 ** | 2.240 ± 0.030 *** | 2.823 ± 0.027 **** | 2.643 ± 0.025 **** |
| Amino Acids | Treatments | ||||
|---|---|---|---|---|---|
| EAA (%) | T0 | T1 | T2 | T3 | T4 |
| Cysteine | 0.500 ± 0.010 | 0.563 ± 0.025 | 0.620 ± 0.052 ** | 0.600 ± 0.036 ** | 0.603 ± 0.005 ** |
| Methionine | 1.623 ± 0.020 | 1.657 ± 0.030 | 1.667 ± 0.037 | 1.710 ± 0.020 * | 1.717 ± 0.032 ** |
| Threonine | 2.477 ± 0.453 | 2.253 ± 0.026 | 2.640 ± 0.606 | 2.657 ± 0.617 | 2.337 ± 0.047 |
| Valine | 2.283 ± 0.011 | 2.313 ± 0.033 | 2.350 ± 0.026 | 2.367 ± 0.025 * | 2.350 ± 0.040 |
| Isoleucine | 2.170 ± 0.020 | 2.180 ± 0.010 | 2.177 ± 0.030 | 2.207 ± 0.028 | 2.160 ± 0.036 |
| Leucine | 3.543 ± 0.041 | 3.577 ± 0.021 | 3.653 ± 0.035 ** | 3.667 ± 0.031 ** | 3.620 ± 0.020 * |
| Histidine | 1.340 ± 0.062 | 1.453 ± 0.025 * | 1.430 ± 0.026 | 1.423 ± 0.051 | 1.377 ± 0.032 |
| Lysine | 3.937 ± 0.040 | 3.930 ± 0.017 | 3.943 ± 0.035 | 3.923 ± 0.022 | 3.887 ± 0.011 |
| Arginine | 3.207 ± 0.100 | 3.310 ± 0.015 | 3.260 ± 0.043 | 3.247 ± 0.055 | 3.317 ± 0.045 |
| Non-EAA (%) | T0 | T1 | T2 | T3 | T4 |
| Aspartic Acid | 3.637 ± 0.032 | 3.647 ± 0.049 | 3.683 ± 0.058 | 3.697 ± 0.035 | 3.670 ± 0.020 |
| Serine | 2.287 ± 0.015 | 2.373 ± 0.030 * | 2.557 ± 0.025 **** | 2.520 ± 0.030 **** | 2.490 ± 0.040 **** |
| Glutamic Acid | 7.450 ± 0.040 | 7.570 ± 0.043 ** | 7.707 ± 0.021 **** | 7.687 ± 0.035 **** | 7.657 ± 0.038 *** |
| Glycine | 3.480 ± 0.026 | 3.563 ± 0.030 * | 3.553 ± 0.045 | 3.563 ± 0.031 * | 3.513 ± 0.032 |
| Alanine | 3.163 ± 0.015 | 3.087 ± 0.041 * | 3.220 ± 0.020 | 3.297 ± 0.025 *** | 3.250 ± 0.030 * |
| Phenylalanine | 2.160 ± 0.026 | 2.193 ± 0.011 | 2.190 ± 0.010 | 2.243 ± 0.015 ** | 2.217 ± 0.021 * |
| Tyrosine | 1.263 ± 0.025 | 1.297 ± 0.021 | 1.293 ± 0.040 | 1.320 ± 0.020 | 1.280 ± 0.030 |
| Proline | 2.600 ± 0.050 | 2.633 ± 0.015 | 2.767 ± 0.065 ** | 2.710 ± 0.034 * | 2.620 ± 0.030 |
| Ornithine | 0.156 ± 0.035 | 0.216 ± 0.056 | 0.226 ± 0.032 | 0.176 ± 0.034 | 0.170 ± 0.026 |
| Carbon No. | Fatty Acids | T0 | T1 | T2 | T3 | T4 |
|---|---|---|---|---|---|---|
| C14:0 | Myristic acid | 0.436 ± 0.030 | 0.216 ± 0.006 *** | 0.136 ± 0.021 *** | 0.726 ± 0.025 *** | 0.463 ± 0.020 |
| C16:0 | Palmitic acid | 24.68 ± 0.476 | 31.95 ± 0.832 **** | 38.41 ± 0.364 **** | 28.31 ± 0.305 **** | 31.33 ± 0.115 **** |
| C17:0 | Heptadecanoic acid | 0.913 ± 0.015 | 0.710 ± 0.017 **** | 1.263 ± 0.030 **** | 0.536 ± 0.021 **** | 0.770 ± 0.026 **** |
| C18:0 | Stearic acid | 2.123 ± 0.032 | 0.913 ± 0.035 **** | 1.207 ± 0.040 **** | 0.510 ± 0.026 **** | 1.267 ± 0.025 **** |
| C18:1 | Oleic acid | 35.54 ± 0.134 | 39.53 ± 0.464 **** | 36.08 ± 0.030 **** | 33.03 ± 0.049 **** | 33.19 ± 0.043 **** |
| C18:2 | Linoleic acid | 24.52 ± 0.453 | 19.77 ± 0.091 **** | 12.64 ± 0.106 **** | 16.31 ± 0.337 **** | 19.87 ± 0.585 **** |
| C20:2 | Eicosadionic acid | 1.553 ± 0.045 | 0.560 ± 0.030 **** | 0.783 ± 0.031 **** | 1.330 ± 0.034 **** | - |
| C22:0 | Decosanoic acid | 0.193 ± 0.035 | 0.186 ± 0.023 | - | 0.176 ± 0.040 | 0.126 ± 0.028 |
| C22:6 | Decosahexanoic acid | 0.446 ± 0.015 | 0.276 ± 0.032 **** | 0.376 ± 0.030 * | 0.213 ± 0.025 **** | 0.373 ± 0.028 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noshair, I.; Kanwal, Z.; Jabeen, G.; Arshad, M.; Yunus, F.-U.-N.; Hafeez, R.; Mairaj, R.; Haider, I.; Ahmad, N.; Alomar, S.Y. Assessment of Dietary Supplementation of Lactobacillus rhamnosus Probiotic on Growth Performance and Disease Resistance in Oreochromis niloticus. Microorganisms 2023, 11, 1423. https://doi.org/10.3390/microorganisms11061423
Noshair I, Kanwal Z, Jabeen G, Arshad M, Yunus F-U-N, Hafeez R, Mairaj R, Haider I, Ahmad N, Alomar SY. Assessment of Dietary Supplementation of Lactobacillus rhamnosus Probiotic on Growth Performance and Disease Resistance in Oreochromis niloticus. Microorganisms. 2023; 11(6):1423. https://doi.org/10.3390/microorganisms11061423
Chicago/Turabian StyleNoshair, Iqra, Zakia Kanwal, Ghazala Jabeen, Mateen Arshad, Fakhar-Un-Nisa Yunus, Ramsha Hafeez, Rida Mairaj, Imran Haider, Naushad Ahmad, and Suliman Yousef Alomar. 2023. "Assessment of Dietary Supplementation of Lactobacillus rhamnosus Probiotic on Growth Performance and Disease Resistance in Oreochromis niloticus" Microorganisms 11, no. 6: 1423. https://doi.org/10.3390/microorganisms11061423
APA StyleNoshair, I., Kanwal, Z., Jabeen, G., Arshad, M., Yunus, F.-U.-N., Hafeez, R., Mairaj, R., Haider, I., Ahmad, N., & Alomar, S. Y. (2023). Assessment of Dietary Supplementation of Lactobacillus rhamnosus Probiotic on Growth Performance and Disease Resistance in Oreochromis niloticus. Microorganisms, 11(6), 1423. https://doi.org/10.3390/microorganisms11061423






