Evaluation of Legume–Rhizobial Symbiotic Interactions Beyond Nitrogen Fixation That Help the Host Survival and Diversification in Hostile Environments
Abstract
1. Introduction
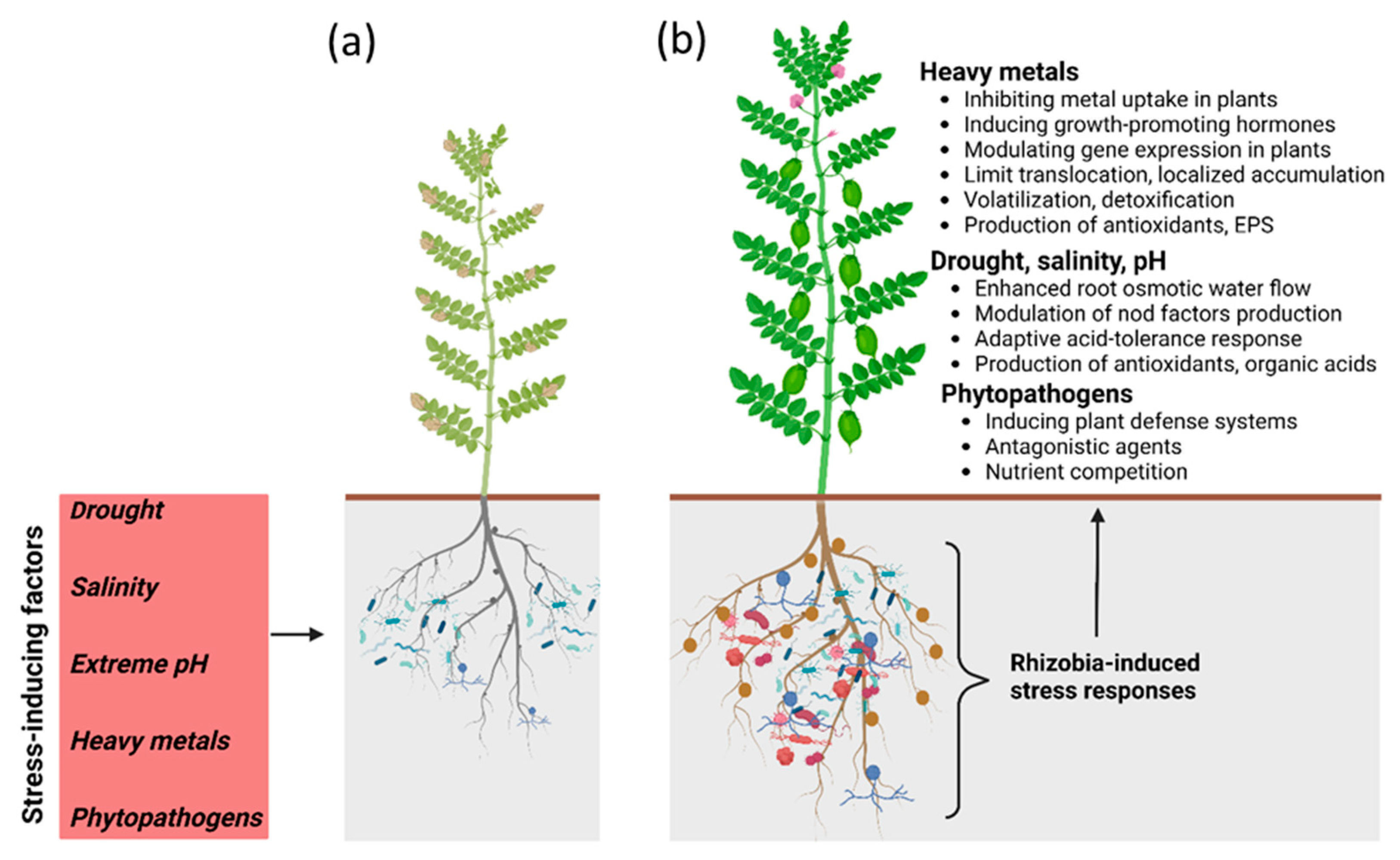
2. Heavy Metal Stress Tolerance
| Symbiotic Rhizobia | Co-Inoculant | Legume Host | Metal | Beneficial Effects on the Plant | Reference |
|---|---|---|---|---|---|
| Bradyrhizobium sp. RM8 | Greengram | Ni | Reduced uptake of Ni and Zn | [44] | |
| Rhizobium sp. RP5 | Pea | Reduced uptake of Ni and Zn | [45] | ||
| Rhizobium TAL–1148 | Bacilus subtilis | Faba bean | Reduced uptake of Ni | [31] | |
| Rhizobium pisi | Ochrobacterium pseudogrignonense | Pongamia pinnata | Ni accumulation in shoots and enhanced antioxidant activities | [46] | |
| Rhizobium pisi PZHK2 | Ochrobacterium pseudo-grignonense PZHK4 | Pongamia pinnata | Enhanced activities of non-enzymatic antioxidants | [47] | |
| Bacilus japonicum CB1809 | Soybean | As | Enhanced production of growth-promoting hormones | [48] | |
| Rizobium sp. VMA301 | Black gram | As accumulation in roots | [24] | ||
| Rhizobium. meliloti Rm5038 | Medicago truncatula | Lowered accumulation of AS in shoots | [33] | ||
| Bacilus japonicum E109 | Azospirillum brasilense Az39 | Soybean | Reduced As translocation to shoots | [49] | |
| Recombinant R. leguminosarum bv. trifolii | Red clover | Alleviated As stress | [42] | ||
| Sinorhizobium medicae | Medicago truncatula | Hg | Alleviated Hg stress | [35] | |
| Bacilus canariense L-7AH | White lupin | Limited mobility of Hg in roots | [34] | ||
| Rhizobium leguminosarum RP 5 | Pea | Cd | Accumulated Cd in roots | [50] | |
| Sinorhizobium meliloti | Medicago sativa | Enhanced absorption in roots | [51] | ||
| Sinorhizobium fredii HH103 | Soybean | Modulated methylation and expression of m6A RNA | [36] | ||
| Rhizobium pusense KG2 | Soybean | Reduced Cd2+ absorption | [41] | ||
| Mesorhizobium strain RC3 | Chickpea | Cr | Reduced Cr uptake | [52] | |
| Rhizobium sp. AS05 | Bacillus sp. AS03 | Horse gram | Reduced shoot translocation | [53] | |
| Rhizobium loti | Lotus purshianus | Cu | Accumulation of Cu | [54] | |
| Sinorhizobium meliloti | Medicago sativa | Increased antioxidant activities | [55] | ||
| Sinorhizobium meliloti | Medicago sativa | Reduced Cu translocation | [37] | ||
| Rhizobium spp. | Phaseolus vulgaris | Al | Production of exopolysaccharides | [39] | |
| Bacilus sp. 750 | Pseudomonas sp., Ochrobactrum cytisi | Lupinus luteus | Pb | Reduced metal accumulation in shoots and roots | [56] |
3. Tolerance to Drought, Salinity, and pH
| Symbiotic Rhizobia | Co-Inoculants | Legume Host | Stress | Beneficial Effects on the Plant | Reference |
|---|---|---|---|---|---|
| Mesorhizobium huakuii strain 7653R | Astragalus sinicus | Drought | Improved N fixation and NH4+ assimilation | [65] | |
| Sinorhizobium medicae or S. meliloti | Medicago truncatula | Enhanced allocation of reserves to osmolytes | [66] | ||
| Sinorhizobium meliloti | Kidney bean, black bean, mung bean, and chickpea | Improved nodule number and photosynthesis | [67] | ||
| Rhizobium meliloti | Medicago sativa | Enhanced antioxidants | [69] | ||
| Sinorhizobium fredii strain SMH12 | Soybean | Improved nodule number and water potentials | [68] | ||
| Rhizobium leguminosarum | Faba bean | Enhanced production of osmoprotectants | [70] | ||
| Rhizobium tropici CIAT 899 | Paenibacillus polymyxa spp. | Phaselus vulgaris | Increased leaf abscisic acid content | [115] | |
| IAA-overproducing Ensifer meliloti 1021 (Ms-RD64) | Medicago sativa | Enhanced production of low-molecular-weight osmolytes | [109] | ||
| Bradyrhizobium sp. SUTN9-2 | Mung bean | Enhanced ACC deaminase activity | [110] | ||
| Rhizobium etli | Phaseolus vulgaris | Overexpressed Trehalose-6-Phosphate Synthase | [111] | ||
| Rhizobium etli | Phaseolus vulgaris | Enhanced expression of Cytochrome cbb(3) oxidases | [112] | ||
| Sinorhizobium meliloti | Alfalfa | Overexpressed cytokinin and antioxidant enzymes | [113] | ||
| Bradyrhizobium RJS9-2 | Stylosanthes guianensis | Salinity | Induced IAA production, enhanced osmoprotectant accumulation | [84] | |
| Rhizobium leguminosarum | Phaseolus vulgaris | Contributed to enhanced root osmotic water flow | [85] | ||
| Rhizobium phaseoli M1, M6, and M9 | Pseudomonas spp. | Mung bean | Expressed ACC deaminase | [116] | |
| Mesorhizobium ciceri IC53 | Bacilus subtilis NUU4 | Cicer arietinum. | Increased proline contents | [117] | |
| Rhizobium meliloti | Medicago sativa | Modulated key plant processes (efficient use of resources, oxidative stress, ion homeostasis) | [87] | ||
| Sinorhizobium meliloti | Overexpressed flavodoxin (Cyanobacterial origin) | [114] | |||
| Rhizobium tropici CIAT899 | Phaseolus vulgaris | pH | Modulated rhizobial nod factors production | [98] | |
| Rhizobium tropici CIAT899 | Phaseolus vulgaris | Induced production of glutathione in beans | [118] | ||
| Sinorhizobium meliloti | Medicago sativa | Adaptive acid-tolerance response | [105] | ||
| Rhizobium spp. | Medicago sativa Longmu 806 | Antioxidants and organic acids production | [108] |
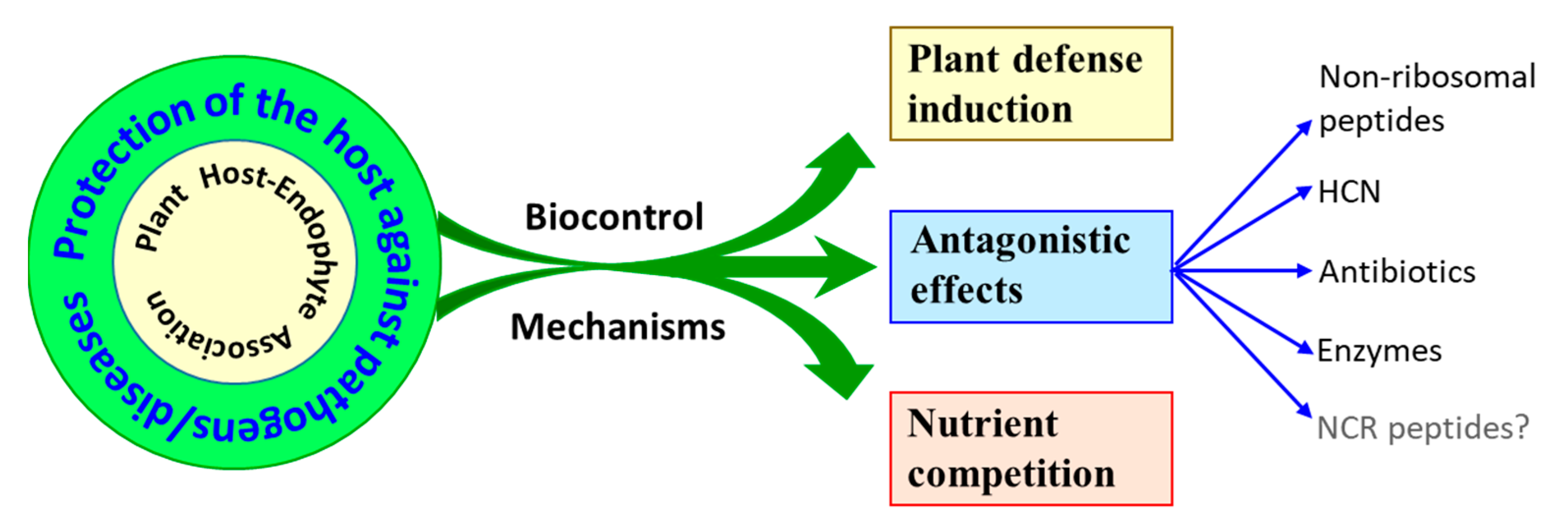
4. Protection against Diseases
5. Role of Soil and Rhizosphere Microbiomes
6. Evolution of Rhizobia for Increased Environmental and Symbiotic Fitness
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Niklas, K.J.; Tiffney, B.H.; Knoll, A.H. Patterns in vascular land plant diversification. Nature 1983, 303, 614–616. [Google Scholar] [CrossRef]
- Friis, E.M.; Crane, P.R.; Pedersen, K.R. Early Flowers and Angiosperm Evolution; Cambridge University Press: Cambridge, UK, 2011; Available online: https://www.cambridge.org/9780521592833 (accessed on 27 March 2023).
- Crepet, W.L.; Niklas, K.J. Darwin’s second ‘abominable mystery’: Why are there so many angiosperm species? Am. J. Bot. 2009, 96, 366–381. [Google Scholar] [CrossRef]
- Joppa, L.N.; Roberts, D.L.; Pimm, S.L. How many species of flowering plants are there? Proc. R. Soc. B Biol. Sci. 2011, 278, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Claramunt, S.; Derryberry, E.P.; Brumfield, R.T.; Remsen, J.V., Jr. Ecological opportunity and diversification in a continental radiation of birds: Climbing adaptations and cladogenesis in the Furnariidae. Am. Nat. 2012, 179, 649–666. [Google Scholar] [CrossRef] [PubMed]
- le Roux, M.M.; Miller, J.T.; Waller, J.; Döring, M.; Bruneau, A. An expert curated global legume checklist improves the accuracy of occurrence, biodiversity and taxonomic data. Sci. Data 2022, 9, 708. [Google Scholar] [CrossRef] [PubMed]
- Linder, H.P. Plant species radiations: Where, when, why? Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 3097–3105. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, W.; Lin, L.; Zhu, X.; Zhu, X.; Li, J.; Chen, Z. Diversification of the phaseoloid legumes: Effects of climate change, range expansion and habit shift. Front. Plant Sci. 2013, 4, 386. [Google Scholar] [CrossRef]
- Roy, S.; Liu, W.; Nandety, R.S.; Crook, A.; Mysore, K.S.; Pislariu, C.I.; Frugoli, J.; Dickstein, R.; Udvardi, M.K. Celebrating 20 years of genetic discoveries in legume nodulation and symbiotic nitrogen fixation. Plant Cell 2020, 32, 15–41. [Google Scholar] [CrossRef]
- Rahimlou, S.; Bahram, M.; Tedersoo, L. Phylogenomics reveals the evolution of root nodulating alpha- and beta-Proteobacteria (rhizobia). Microbiol. Res. 2021, 250, 126788. [Google Scholar] [CrossRef]
- Liu, S.; Jiao, J.; Tian, C.-F. Adaptive Evolution of Rhizobial Symbiosis beyond Horizontal Gene Transfer: From Genome Innovation to Regulation Reconstruction. Genes 2023, 14, 274. [Google Scholar] [CrossRef]
- Quides, K.W.; Weisberg, A.J.; Trinh, J.; Salaheldine, F.; Cardenas, P.; Lee, H.H.; Jariwala, R.; Chang, J.H.; Sachs, J.L. Experimental evolution can enhance benefits of rhizobia to novel legume hosts. Proc. Biol. Sci. 2021, 288, 20210812. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Yang, Y.; Liu, P.; Xu, R.; Rensing, C.; Fu, X.; Liao, H. Genotype and rhizobium inoculation modulate the assembly of soybean rhizobacterial communities. Plant Cell Environ. 2019, 42, 2028–2044. [Google Scholar] [CrossRef] [PubMed]
- Skorupska, A.; Janczarek, M.; Marczak, M.; Mazur, A.; Król, J. Rhizobial exopolysaccharides: Genetic control and symbiotic functions. Microb. Cell Factories 2006, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in soils and groundwater: A review. Appl. Geochem. 2019, 108, 104388. [Google Scholar] [CrossRef]
- Mir, A.R.; Pichtel, J.; Hayat, S. Copper: Uptake, toxicity and tolerance in plants and management of Cu-contaminated soil. Biometals 2021, 34, 737–759. [Google Scholar] [CrossRef]
- Oliveira, H. Chromium as an Environmental Pollutant: Insights on Induced Plant Toxicity. J. Bot. 2012, 2012, 375843. [Google Scholar] [CrossRef]
- Sharma, P.; Dubey, R. Lead Toxicity in Plants. Braz. J. Plant Physiol. 2005, 17, 35–52. [Google Scholar] [CrossRef]
- Farooq, M.A.; Islam, F.; Ali, B.; Najeeb, U.; Mao, B.; Gill, R.A.; Yan, G.; Siddique, K.H.M.; Zhou, W. Arsenic toxicity in plants: Cellular and molecular mechanisms of its transport and metabolism. Environ. Exp. Bot. 2016, 132, 42–52. [Google Scholar] [CrossRef]
- Chaudri, A.M.; McGrath, S.P.; Giller, K.E. Survival of the indigenous population of Rhizobium leguminosarum biovar trifolii in soil spiked with Cd, Zn, Cu and Ni salts. Soil Biol. Biochem. 1992, 24, 625–632. [Google Scholar] [CrossRef]
- Chen, Y.X.; He, Y.F.; Yang, Y.; Yu, Y.L.; Zheng, S.J.; Tian, G.M.; Luo, Y.M.; Wong, M.H. Effect of cadmium on nodulation and N2-fixation of soybean in contaminated soils. Chemosphere 2003, 50, 781–787. [Google Scholar] [CrossRef]
- Chubukova, O.V.; Postrigan’, B.N.; Baimiev, A.K.; Chemeris, A.V. The effect of cadmium on the efficiency of development of legume-rhizobium symbiosis. Biol. Bull. 2015, 42, 458–462. [Google Scholar] [CrossRef]
- Laguerre, G.; Courde, L.; Nouaïm, R.; Lamy, I.; Revellin, C.; Breuil, M.C.; Chaussod, R. Response of rhizobial populations to moderate copper stress applied to an agricultural soil. Microb. Ecol. 2006, 52, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.M.; Gouri, S.S.; De, D.; Das, B.K.; Mondal, K.C.; Pati, B.R. Effect of Arsenic on Nodulation and Nitrogen Fixation of Blackgram (Vigna mungo). Indian J. Microbiol. 2011, 51, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Mondal, N.K.; Das, C.; Datta, J.K. Effect of mercury on seedling growth, nodulation and ultrastructural deformation of Vigna radiata (L) Wilczek. Environ. Monit Assess. 2015, 187, 241. [Google Scholar] [CrossRef] [PubMed]
- Neumann, H.; Bode-Kirchhoff, A.; Madeheim, A.; Wetzel, A. Toxicity testing of heavy metals with the Rhizobium-legume symbiosis: High sensitivity to cadmium and arsenic compounds. Environ. Sci. Pollut. Res. Int. 1998, 5, 28–36. [Google Scholar] [CrossRef]
- Sheirdil, R.A.; Bashir, K.; Hayat, R.; Akhtar, M.S. Effect of cadmium on soybean (Glycine max L.) growth and nitrogen fixation. Afr. J. Biotechnol. 2012, 11, 1886–1891. [Google Scholar] [CrossRef]
- Talano, M.A.; Cejas, R.B.; González, P.S.; Agostini, E. Arsenic effect on the model crop symbiosis Bradyrhizobium-soybean. Plant Physiol. Biochem. 2013, 63, 8–14. [Google Scholar] [CrossRef]
- Mengoni, A.; Schat, H.; Vangronsveld, J. Plants as extreme environments? Ni-resistant bacteria and Ni-hyperaccumulators of serpentine flora. Plant Soil 2010, 331, 5–16. [Google Scholar] [CrossRef]
- Fagorzi, C.; Checcucci, A.; diCenzo, G.C.; Debiec-Andrzejewska, K.; Dziewit, L.; Pini, F.; Mengoni, A. Harnessing Rhizobia to Improve Heavy-Metal Phytoremediation by Legumes. Genes 2018, 9, 542. [Google Scholar] [CrossRef]
- Elbagory, M.; El-Nahrawy, S.; Omara, A.E. Synergistic Interaction between Symbiotic N(2) Fixing Bacteria and Bacillus strains to Improve Growth, Physiological Parameters, Antioxidant Enzymes and Ni Accumulation in Faba Bean Plants (Vicia faba) under Nickel Stress. Plants 2022, 11, 1812. [Google Scholar] [CrossRef]
- Wani, P.A.; Khan, M.S. Nickel detoxification and plant growth promotion by multi metal resistant plant growth promoting Rhizobium species RL9. Bull. Environ. Contam. Toxicol. 2013, 91, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Yang, P.; Zeng, Y.; Li, C.; Jian, N.; Wang, R.; Huang, S.; Yang, R.; Wei, L.; Zhao, H.; et al. Rhizobium symbiosis modulates the accumulation of arsenic in Medicago truncatula via nitrogen and NRT3.1-like genes regulated by ABA and linalool. J. Hazard. Mater. 2021, 415, 125611. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, M.A.; Ruiz-Díez, B.; Fajardo, S.; López-Berdonces, M.A.; Higueras, P.L.; Fernández-Pascual, M. Lupinus albus plants acquire mercury tolerance when inoculated with an Hg-resistant Bradyrhizobium strain. Plant Physiol. Biochem. 2013, 73, 168–175. [Google Scholar] [CrossRef]
- Arregui, G.; Hipólito, P.; Pallol, B.; Lara-Dampier, V.; García-Rodríguez, D.; Varela, H.P.; Tavakoli Zaniani, P.; Balomenos, D.; Paape, T.; Coba de la Peña, T.; et al. Mercury-Tolerant Ensifer medicae Strains Display High Mercuric Reductase Activity and a Protective Effect on Nitrogen Fixation in Medicago truncatula Nodules Under Mercury Stress. Front. Plant Sci. 2020, 11, 560768. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wang, J.; Zhang, Y.; Kong, Y.; Dong, H.; Feng, X.; Li, T.; Zhou, C.; Yu, J.; Xin, D.; et al. Changes in the m6A RNA methylome accompany the promotion of soybean root growth by rhizobia under cadmium stress. J. Hazard. Mater. 2022, 441, 129843. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.Q.; Yan, X.W.; Wei, G.H.; Zhang, J.H.; Fang, L.C. Rhizobium inoculation enhances copper tolerance by affecting copper uptake and regulating the ascorbate-glutathione cycle and phytochelatin biosynthesis-related gene expression in Medicago sativa seedlings. Ecotoxicol. Env. Saf. 2018, 162, 312–323. [Google Scholar] [CrossRef]
- Lu, M.; Jiao, S.; Gao, E.; Song, X.; Li, Z.; Hao, X.; Rensing, C.; Wei, G. Transcriptome Response to Heavy Metals in Sinorhizobium meliloti CCNWSX0020 Reveals New Metal Resistance Determinants That Also Promote Bioremediation by Medicago lupulina in Metal-Contaminated Soil. Appl. Environ. Microbiol. 2017, 83, e01244-17. [Google Scholar] [CrossRef]
- Avelar Ferreira, P.A.; Bomfeti, C.A.; Lima Soares, B.; de Souza Moreira, F.M. Efficient nitrogen-fixing Rhizobium strains isolated from amazonian soils are highly tolerant to acidity and aluminium. World J. Microbiol. Biotechnol. 2012, 28, 1947–1959. [Google Scholar] [CrossRef]
- Wekesa, C.; Muoma, J.O.; Reichelt, M.; Asudi, G.O.; Furch, A.C.U.; Oelmüller, R. The Cell Membrane of a Novel Rhizobium phaseoli Strain Is the Crucial Target for Aluminium Toxicity and Tolerance. Cells 2022, 11, 873. [Google Scholar] [CrossRef]
- Li, Y.; Yu, X.; Cui, Y.; Tu, W.; Shen, T.; Yan, M.; Wei, Y.; Chen, X.; Wang, Q.; Chen, Q.; et al. The potential of cadmium ion-immobilized Rhizobium pusense KG2 to prevent soybean root from absorbing cadmium in cadmium-contaminated soil. J. Appl. Microbiol. 2019, 126, 919–930. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Y.; Cao, T.; Chen, J.; Rosen, B.P.; Zhao, F.J. Arsenic methylation by a genetically engineered Rhizobium-legume symbiont. Plant Soil 2017, 416, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Nies, A.; Nies, D.H.; Silver, S. Nucleotide sequence and expression of a plasmid-encoded chromate resistance determinant from Alcaligenes eutrophus. J. Biol. Chem. 1990, 265, 5648–5653. [Google Scholar] [CrossRef] [PubMed]
- Wani, P.A.; Khan, M.S.; Zaidi, A. Effect of metal tolerant plant growth promoting Bradyrhizobium sp. (vigna) on growth, symbiosis, seed yield and metal uptake by greengram plants. Chemosphere 2007, 70, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Wani, P.A.; Khan, M.S.; Zaidi, A. Effect of metal-tolerant plant growth-promoting Rhizobium on the performance of pea grown in metal-amended soil. Arch. Environ. Contam. Toxicol. 2008, 55, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, M.; Hussain, S.; Cheng, X.; Cui, Y.; Liu, H.; Chen, Q.; Ma, M.; Gu, Y.; Zhao, K.; Xiang, Q.; et al. Synergistic anti-oxidative effects of Pongamia pinnata against nickel mediated by Rhizobium pisi and Ochrobacterium pseudogrignonense. Ecotoxicol. Environ. Saf. 2021, 217, 112244. [Google Scholar] [CrossRef]
- Yu, X.; Shoaib, M.; Cheng, X.; Cui, Y.; Hussain, S.; Yan, J.; Zhou, J.; Chen, Q.; Gu, Y.; Zou, L.; et al. Role of rhizobia in promoting non-enzymatic antioxidants to mitigate nitrogen-deficiency and nickel stresses in Pongamia pinnata. Ecotoxicol. Environ. Saf. 2022, 241, 113789. [Google Scholar] [CrossRef]
- Reichman, S.M. The potential use of the legume–rhizobium symbiosis for the remediation of arsenic contaminated sites. Soil Biol. Biochem. 2007, 39, 2587–2593. [Google Scholar] [CrossRef]
- Armendariz, A.L.; Talano, M.A.; Olmos Nicotra, M.F.; Escudero, L.; Breser, M.L.; Porporatto, C.; Agostini, E. Impact of double inoculation with Bradyrhizobium japonicum E109 and Azospirillum brasilense Az39 on soybean plants grown under arsenic stress. Plant Physiol. Biochem. 2019, 138, 26–35. [Google Scholar] [CrossRef]
- Wani, P.A.; Khan, M.S.; Zaidi, A. Effects of heavy metal toxicity on growth, symbiosis, seed yield and metal uptake in pea grown in metal amended soil. Bull Environ. Contam. Toxicol. 2008, 81, 152–158. [Google Scholar] [CrossRef]
- Ghnaya, T.; Mnassri, M.; Ghabriche, R.; Wali, M.; Poschenrieder, C.; Lutts, S.; Abdelly, C. Nodulation by Sinorhizobium meliloti originated from a mining soil alleviates Cd toxicity and increases Cd-phytoextraction in Medicago sativa L. Front. Plant Sci. 2015, 6, 863. [Google Scholar] [CrossRef]
- Wani, P.A.; Khan, M.S.; Zaidi, A. Chromium-reducing and plant growth-promoting Mesorhizobium improves chickpea growth in chromium-amended soil. Biotechnol. Lett. 2008, 30, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Dhali, S.; Pradhan, M.; Sahoo, R.K.; Mohanty, S.; Pradhan, C. Alleviating Cr(VI) stress in horse gram (Macrotyloma uniflorum Var. Madhu) by native Cr-tolerant nodule endophytes isolated from contaminated site of Sukinda. Environ. Sci. Pollut. Res. Int. 2021, 28, 31717–31730. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Lin, S.L. Copper tolerance and copper uptake of Lotus purshianus (Benth.) Clem. & Clem. and its symbiotic Rhizobium loti derived from a copper mine waste population. New Phytol. 1990, 116, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Mei, Y.; Wang, Q.; Wang, Y.; Li, Q.; Hong, M.; Hu, S.; Li, S.; Fang, L. Rhizobium Inoculation Enhances the Resistance of Alfalfa and Microbial Characteristics in Copper-Contaminated Soil. Front. Microbiol. 2021, 12, 781831. [Google Scholar] [CrossRef]
- Dary, M.; Chamber-Perez, M.A.; Palomares, A.J.; Pajuelo, E. In situ phytostabilisation of heavy metal polluted soils using Lupinus luteus inoculated with metal resistant plant-growth promoting rhizobacteria. J. Hazard. Mater. 2010, 177, 323–330. [Google Scholar] [CrossRef]
- Marino, D.; Frendo, P.; Ladrera, R.; Zabalza, A.; Puppo, A.; Arrese-Igor, C.; Gonzalez, E.M. Nitrogen fixation control under drought stress. Localized or systemic? Plant Physiol. 2007, 143, 1968–1974. [Google Scholar] [CrossRef]
- McCulloch, L.A.; Piotto, D.; Porder, S. Drought and soil nutrients effects on symbiotic nitrogen fixation in seedlings from eight Neotropical legume species. Biotropica 2021, 53, 703–713. [Google Scholar] [CrossRef]
- Serraj, R.; Sinclair, T.R.; Purcell, L.C. Symbiotic N2 fixation response to drought. J. Exp. Bot. 1999, 50, 143–155. [Google Scholar] [CrossRef]
- Streeter, J.G. Effects of drought on nitrogen fixation in soybean root nodules. Plant Cell Environ. 2003, 26, 1199–1204. [Google Scholar] [CrossRef]
- Cruz de Carvalho, M.H. Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Drought Stress Effects on Growth, ROS Markers, Compatible Solutes, Phenolics, Flavonoids, and Antioxidant Activity in Amaranthus tricolor. Appl. Biochem. Biotechnol. 2018, 186, 999–1016. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Chan, Z. ROS Regulation During Abiotic Stress Responses in Crop Plants. Front. Plant Sci. 2015, 6, 1092. [Google Scholar] [CrossRef]
- Cerezini, P.; Kuwano, B.H.; Grunvald, A.K.; Hungria, M.; Nogueira, M.A. Soybean tolerance to drought depends on the associated Bradyrhizobium strain. Braz. J. Microbiol. 2020, 51, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, Z.; Shi, H. Rhizobium Symbiosis Leads to Increased Drought Tolerance in Chinese Milk Vetch (Astragalus sinicus L.). Agronomy 2022, 12, 725. [Google Scholar] [CrossRef]
- Staudinger, C.; Mehmeti-Tershani, V.; Gil-Quintana, E.; Gonzalez, E.M.; Hofhansl, F.; Bachmann, G.; Wienkoop, S. Evidence for a rhizobia-induced drought stress response strategy in Medicago truncatula. J. Proteom. 2016, 136, 202–213. [Google Scholar] [CrossRef]
- Siddiqui, Z.S.; Ali, F.; Uddin, Z. Sustainable effect of a symbiotic nitrogen-fixing bacterium Sinorhizobium meliloti on nodulation and photosynthetic traits of four leguminous plants under low moisture stress environment. Lett. Appl. Microbiol. 2021, 72, 714–724. [Google Scholar] [CrossRef]
- Kibido, T.; Kunert, K.; Makgopa, M.; Greve, M.; Vorster, J. Improvement of rhizobium-soybean symbiosis and nitrogen fixation under drought. Food Energy Secur. 2020, 9, e177. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, P.; Li, B.; Hu, T. Effect of nodules on dehydration response in alfalfa (Medicago sativa L.). Environ. Exp. Bot. 2013, 86, 29–34. [Google Scholar] [CrossRef]
- Amine-Khodja, I.R.; Boscari, A.; Riah, N.; Kechid, M.; Maougal, R.T.; Belbekri, N.; Djekoun, A. Impact of Two Strains of Rhizobium leguminosarum on the Adaptation to Terminal Water Deficit of Two Cultivars Vicia faba. Plants 2022, 11, 515. [Google Scholar] [CrossRef]
- Yadav, A.; Singh, R.P.; Singh, A.L.; Singh, M. Identification of genes involved in phosphate solubilization and drought stress tolerance in chickpea symbiont Mesorhizobium ciceri Ca181. Arch. Microbiol. 2021, 203, 1167–1174. [Google Scholar] [CrossRef]
- Corwin, D.L. Climate change impacts on soil salinity in agricultural areas. Eur. J. Soil Sci. 2020, 72, 842–862. [Google Scholar] [CrossRef]
- Tedeschi, A.; Dell’Aquila, R. Effects of irrigation with saline waters, at different concentrations, on soil physical and chemical characteristics. Agric. Water Manag. 2005, 77, 308–322. [Google Scholar] [CrossRef]
- Wang, Q.; Huo, Z.; Zhang, L.; Wang, J.; Zhao, Y. Impact of saline water irrigation on water use efficiency and soil salt accumulation for spring maize in arid regions of China. Agric. Water Manag. 2016, 163, 125–138. [Google Scholar] [CrossRef]
- Gul, Z.; Tang, Z.-H.; Arif, M.; Ye, Z. An Insight into Abiotic Stress and Influx Tolerance Mechanisms in Plants to Cope in Saline Environments. Biology 2022, 11, 597. [Google Scholar] [CrossRef]
- Chakraborty, S.; Harris, J.M. At the Crossroads of Salinity and Rhizobium-Legume Symbiosis. Mol. Plant Microbe Interact. 2022, 35, 540–553. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.K.; Vyas, S.R. Salt and pH tolerance of Rhizobia. Folia Microbiol. 1973, 18, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Dardanelli, M.S.; Manyani, H.; González-Barroso, S.; Rodríguez-Carvajal, M.A.; Gil-Serrano, A.M.; Espuny, M.R.; López-Baena, F.J.; Bellogín, R.A.; Megías, M.; Ollero, F.J. Effect of the presence of the plant growth promoting rhizobacterium (PGPR) Chryseobacterium balustinum Aur9 and salt stress in the pattern of flavonoids exuded by soybean roots. Plant Soil 2009, 328, 483–493. [Google Scholar] [CrossRef]
- Zahran, H.H.; Sprent, J.I. Effects of sodium chloride and polyethylene glycol on root-hair infection and nodulation of Vicia faba L. plants by Rhizobium leguminosarum. Planta 1986, 167, 303–309. [Google Scholar] [CrossRef]
- Tu, J.C. Effect of salinity on rhizobium-root-hair interaction, nodulation and growth of soybean. Can. J. Plant Sci. 1981, 61, 231–239. [Google Scholar] [CrossRef]
- Borucki, W.; Sujkowska, M. The effects of sodium chloride-salinity upon growth, nodulation, and root nodule structure of pea (Pisum sativum L.) plants. Acta Physiol. Plant. 2007, 30, 293–301. [Google Scholar] [CrossRef]
- L’Taief, B.; Sifi, B.; Zaman-Allah, M.; Drevon, J.J.; Lachaâl, M. Effect of salinity on root-nodule conductance to the oxygen diffusion in the Cicer arietinum-Mesorhizobium ciceri symbiosis. J. Plant Physiol. 2007, 164, 1028–1036. [Google Scholar] [CrossRef] [PubMed]
- Aridhi, F.; Sghaier, H.; Gaitanaros, A.; Khadri, A.; Aschi-Smiti, S.; Brouquisse, R. Nitric oxide production is involved in maintaining energy state in Alfalfa (Medicago sativa L.) nodulated roots under both salinity and flooding. Planta 2020, 252, 22. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Zhang, J.; Huan, H.; Bai, C.; Chen, Z.; Liu, G. High Salt Tolerance of a Bradyrhizobium Strain and Its Promotion of the Growth of Stylosanthes guianensis. Int. J. Mol. Sci. 2017, 18, 1625. [Google Scholar] [CrossRef] [PubMed]
- Franzini, V.I.; Azcón, R.; Ruiz-Lozano, J.M.; Aroca, R. Rhizobial symbiosis modifies root hydraulic properties in bean plants under non-stressed and salinity-stressed conditions. Planta 2019, 249, 1207–1215. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, P.; Zhou, Y.; Hu, T.; Zhang, P.; Wu, Y. A proteomic approach to understand the impact of nodulation on salinity stress response in alfalfa (Medicago sativa L.). Plant Biol. 2022, 24, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Montaño, F.; del Cerro, P.; Jiménez-Guerrero, I.; López-Baena, F.J.; Cubo, M.T.; Hungria, M.; Megías, M.; Ollero, F.J. RNA-seq analysis of the Rhizobium tropici CIAT 899 transcriptome shows similarities in the activation patterns of symbiotic genes in the presence of apigenin and salt. BMC Genom. 2016, 17, 198. [Google Scholar] [CrossRef] [PubMed]
- López-Bucio, J.; Guevara-Garcia, A.; Ramírez-Rodríguez, V.; Nieto Jacobo, M.; de la Fuente Martínez, J.; Herrera-Estrella, L. Agriculture for Marginal Lands: Transgenic Plants Towards the Third Millennium. Dev. Plant Genet. Breed. 2000, 5, 159–165. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Lin, M.H.; Gresshoff, P.M. Regulation of legume nodulation by acidic growth conditions. Plant Signal. Behav. 2013, 8, e23426. [Google Scholar] [CrossRef]
- Cooper, J.E. Nodulation of Legumes by Rhizobia in Acid Soils. In Developments in Soil Science; Vančura, V., Kunc, F., Eds.; Elsevier: Amsterdam, The Netherlands, 1989; Volume 18, pp. 57–61. [Google Scholar]
- Hungria, M.; Stacey, G. Molecular signals exchanged between host plants and rhizobia: Basic aspects and potential application in agriculture. Soil Biol. Biochem. 1997, 29, 819–830. [Google Scholar] [CrossRef]
- Miransari, M.; Balakrishnan, P.; Smith, D.; Mackenzie, A.F.; Bahrami, H.A.; Malakouti, M.J.; Rejali, F. Overcoming the Stressful Effect of Low pH on Soybean Root Hair Curling using Lipochitooligosacharides. Commun. Soil Sci. Plant Anal. 2006, 37, 1103–1110. [Google Scholar] [CrossRef]
- Richardson, A.E.; Simpson, R.J.; Djordjevic, M.A.; Rolfe, B.G. Expression of Nodulation Genes in Rhizobium leguminosarum biovar trifolii is Affected by Low pH and by Ca and Al Ions. Appl Environ. Microbiol 1988, 54, 2541–2548. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.C.; Aguilar, J.V.; Souza, L.A.; Justino, G.C.; Aguiar, L.F.; Camargos, L.S. pH effects on nodulation and biological nitrogen fixation in Calopogonium mucunoides. Braz. J. Bot. 2016, 39, 1015–1020. [Google Scholar] [CrossRef]
- Han, Q.; Ma, Q.; Chen, Y.; Tian, B.; Xu, L.; Bai, Y.; Chen, W.; Li, X. Variation in rhizosphere microbial communities and its association with the symbiotic efficiency of rhizobia in soybean. ISME J. 2020, 14, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Checcucci, A.; Marchetti, M. The Rhizosphere Talk Show: The Rhizobia on Stage. Front. Agron. 2020, 2, 591494. [Google Scholar] [CrossRef]
- Mehboob, I.; Naveed, M.; Zahir, Z.A.; Sessitsch, A. Potential of Rhizosphere Bacteria for Improving Rhizobium-Legume Symbiosis. In Plant Microbe Symbiosis: Fundamentals and Advances; Arora, N., Ed.; Springer: New Delhi, India, 2013. [Google Scholar] [CrossRef]
- Moron, B.; Soria-Diaz, M.E.; Ault, J.; Verroios, G.; Noreen, S.; Rodriguez-Navarro, D.N.; Gil-Serrano, A.; Thomas-Oates, J.; Megias, M.; Sousa, C. Low pH changes the profile of nodulation factors produced by Rhizobium tropici CIAT899. Chem. Biol. 2005, 12, 1029–1040. [Google Scholar] [CrossRef]
- Kiss, E.; Huguet, T.; Poinsot, V.; Batut, J. The typA gene is required for stress adaptation as well as for symbiosis of Sinorhizobium meliloti 1021 with certain Medicago truncatula lines. Mol. Plant Microbe. Interact. 2004, 17, 235–244. [Google Scholar] [CrossRef]
- Martini, M.C.; Vacca, C.; Torres Tejerizo, G.A.; Draghi, W.O.; Pistorio, M.; Lozano, M.J.; Lagares, A.; del Papa, M.F. ubiF is involved in acid stress tolerance and symbiotic competitiveness in Rhizobium favelukesii LPU83. Braz. J. Microbiol. 2022, 53, 1633–1643. [Google Scholar] [CrossRef]
- Reeve, W.G.; Bräu, L.; Castelli, J.; Garau, G.; Sohlenkamp, C.; Geiger, O.; Dilworth, M.J.; Glenn, A.R.; Howieson, J.G.; Tiwari, R.P. The Sinorhizobium medicae WSM419 lpiA gene is transcriptionally activated by FsrR and required to enhance survival in lethal acid conditions. Microbiology 2006, 152, 3049–3059. [Google Scholar] [CrossRef]
- Tiwari, S.; Sarangi, B.K.; Thul, S.T. Identification of arsenic resistant endophytic bacteria from Pteris vittata roots and characterization for arsenic remediation application. J. Environ. Manag. 2016, 180, 359–365. [Google Scholar] [CrossRef]
- Martinez, E.; Pardo, M.A.; Palacios, R.; Miguel, A.C. Reiteration of Nitrogen Fixation Gene Sequences and Specificity of Rhizobium in Nodulation and Nitrogen Fixation in Phaseolus vulgaris. Microbiology 1985, 131, 1779–1786. [Google Scholar] [CrossRef][Green Version]
- Riccillo, P.M.; Muglia, C.I.; de Bruijn, F.J.; Roe, A.J.; Booth, I.R.; Aguilar, O.M. Glutathione is involved in environmental stress responses in Rhizobium tropici, including acid tolerance. J. Bacteriol. 2000, 182, 1748–1753. [Google Scholar] [CrossRef] [PubMed]
- Draghi, W.O.; del Papa, M.F.; Pistorio, M.; Lozano, M.; de Los Angeles Giusti, M.; Torres Tejerizo, G.A.; Jofré, E.; Boiardi, J.L.; Lagares, A. Cultural conditions required for the induction of an adaptive acid-tolerance response (ATR) in Sinorhizobium meliloti and the question as to whether or not the ATR helps rhizobia improve their symbiosis with alfalfa at low pH. FEMS Microbiol. Lett. 2010, 302, 123–130. [Google Scholar] [CrossRef]
- Soto, M.J.; Dillewijn, P.; Martínez-Abarca, F.; Jiménez-Zurdo, J.I.; Toro, N. Attachment to plant roots and nod gene expression are not affected by pH or calcium in the acid-tolerant alfalfa-nodulating bacteria Rhizobium sp. LPU83. FEMS Microbiol. Ecol. 2004, 48, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Nautiyal, C.S. Effects of salt and pH stress on temperature-tolerant Rhizobium sp. NBRI330 nodulating Prosopis juliflora. Curr. Microbiol. 2000, 40, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Sun, N.; Dong, L.; Cai, H. Enhanced alkali tolerance of rhizobia-inoculated alfalfa correlates with altered proteins and metabolic processes as well as decreased oxidative damage. Plant Physiol. Biochem. 2021, 159, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Defez, R.; Andreozzi, A.; Dickinson, M.; Charlton, A.; Tadini, L.; Pesaresi, P.; Bianco, C. Improved Drought Stress Response in Alfalfa Plants Nodulated by an IAA Over-producing Rhizobium Strain. Front. Microbiol. 2017, 8, 2466. [Google Scholar] [CrossRef]
- Sarapat, S.; Songwattana, P.; Longtonglang, A.; Umnajkitikorn, K.; Girdthai, T.; Tittabutr, P.; Boonkerd, N.; Teaumroong, N. Effects of Increased 1-Aminocyclopropane-1-Carboxylate (ACC) Deaminase Activity in Bradyrhizobium sp. SUTN9-2 on Mung Bean Symbiosis under Water Deficit Conditions. Microbes Environ. 2020, 35, ME20024. [Google Scholar] [CrossRef]
- Suárez, R.; Wong, A.; Ramírez, M.; Barraza, A.; Orozco Mdel, C.; Cevallos, M.A.; Lara, M.; Hernández, G.; Iturriaga, G. Improvement of drought tolerance and grain yield in common bean by overexpressing trehalose-6-phosphate synthase in rhizobia. Mol. Plant Microbe Interact. 2008, 21, 958–966. [Google Scholar] [CrossRef]
- Talbi, C.; Sanchez, C.; Hidalgo-Garcia, A.; Gonzalez, E.M.; Arrese-Igor, C.; Girard, L.; Bedmar, E.J.; Delgado, M.J. Enhanced expression of Rhizobium etli cbb(3) oxidase improves drought tolerance of common bean symbiotic nitrogen fixation. J. Exp. Bot. 2012, 63, 5035–5043. [Google Scholar] [CrossRef]
- Xu, J.; Li, X.L.; Luo, L. Effects of engineered Sinorhizobium meliloti on cytokinin synthesis and tolerance of alfalfa to extreme drought stress. Appl. Environ. Microbiol. 2012, 78, 8056–8061. [Google Scholar] [CrossRef]
- Redondo, F.J.; Coba de la Peña, T.; Lucas, M.M.; Pueyo, J.J. Alfalfa nodules elicited by a flavodoxin-overexpressing Ensifer meliloti strain display nitrogen-fixing activity with enhanced tolerance to salinity stress. Planta 2012, 236, 1687–1700. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, M.V.B.; Burity, H.A.; Martínez, C.R.; Chanway, C.P. Alleviation of drought stress in the common bean (Phaseolus vulgaris L.) by co-inoculation with Paenibacillus polymyxa and Rhizobium tropici. Appl. Soil Ecol. 2008, 40, 182–188. [Google Scholar] [CrossRef]
- Ahmad, M.; Zahir, Z.A.; Asghar, H.N.; Asghar, M. Inducing salt tolerance in mung bean through coinoculation with rhizobia and plant-growth-promoting rhizobacteria containing 1-aminocyclopropane-1-carboxylate deaminase. Can. J. Microbiol. 2011, 57, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Egamberdieva, D.; Wirth, S.J.; Shurigin, V.V.; Hashem, A.; Abd Allah, E.F. Endophytic Bacteria Improve Plant Growth, Symbiotic Performance of Chickpea (Cicer arietinum L.) and Induce Suppression of Root Rot Caused by Fusarium solani under Salt Stress. Front. Microbiol. 2017, 8, 1887. [Google Scholar] [CrossRef] [PubMed]
- Vinuesa, P.; Neumann-Silkow, F.; Pacios-Bras, C.; Spaink, H.P.; Martínez-Romero, E.; Werner, D. Genetic analysis of a pH-regulated operon from Rhizobium tropici CIAT899 involved in acid tolerance and nodulation competitiveness. Mol. Plant Microbe Interact. 2003, 16, 159–168. [Google Scholar] [CrossRef]
- Dini-Andreote, F. Endophytes: The Second Layer of Plant Defense. Trends Plant Sci. 2020, 25, 319–322. [Google Scholar] [CrossRef]
- Goyal, R.K.; Mattoo, A.K. Multitasking antimicrobial peptides in plant development and host defense against biotic/abiotic stress. Plant Sci. 2014, 228, 135–149. [Google Scholar] [CrossRef]
- Balint-Kurti, P. The plant hypersensitive response: Concepts, control and consequences. Mol. Plant Pathol. 2019, 20, 1163–1178. [Google Scholar] [CrossRef]
- Carrión, V.J.; Perez-Jaramillo, J.; Cordovez, V.; Tracanna, V.; de Hollander, M.; Ruiz-Buck, D.; Mendes, L.W.; van Ijcken, W.F.J.; Gomez-Exposito, R.; Elsayed, S.S.; et al. Pathogen-induced activation of disease-suppressive functions in the endophytic root microbiome. Science 2019, 366, 606–612. [Google Scholar] [CrossRef]
- Chandra, S.; Choure, K.; Dubey, R.C.; Maheshwari, D. Rhizosphere competent Mesorhizobiumloti MP6 induces root hair curling, inhibits Sclerotinia sclerotiorum and enhances growth of Indian mustard (Brassica campestris). Braz. J. Microbiol. 2007, 38, 124–130. [Google Scholar] [CrossRef]
- Ganesan, S.; Kuppusamy, R.; Sekar, R. Integrated management of stem rot disease (Sclerotium rolfsii) of groundnut (Arachis hypogaea L.) using Rhizobium and Trichoderma harzianum (ITCC-4572). Turk. J. Agric. For. 2007, 31, 103–108. [Google Scholar]
- Parveen, G.; Noreen, R.; Shafique, H.A.; Sultana, V.; Ehteshamul-Haque, S.; Athar, M. Role of Rhizobia in Suppressing the Root Diseases of Soybean Under Soil Amendment. Planta Daninha 2019, v37, e019172336. [Google Scholar] [CrossRef]
- Özkoç, İ.; Deliveli, M.H. In Vitro Inhibition of the Mycelial Growth of Some Root Rot Fungi by Rhizobium leguminosarum Biovar phaseoli Isolates. Turk. J. Biol. 2001, 25, 435–445. [Google Scholar]
- Ehteshamul-Haque, S.; Ghaffar, A. Use of Rhizobia in the Control of Root Rot Diseases of Sunflower, Okra, Soybean and Mungbean. J. Phytopathol. 1993, 138, 157–163. [Google Scholar] [CrossRef]
- Das, K.; Prasanna, R.; Saxena, A.K. Rhizobia: A potential biocontrol agent for soilborne fungal pathogens. Folia Microbiol. 2017, 62, 425–435. [Google Scholar] [CrossRef]
- Jack, C.N.; Wozniak, K.J.; Porter, S.S.; Friesen, M.L. Rhizobia protect their legume hosts against soil-borne microbial antagonists in a host-genotype-dependent manner. Rhizosphere 2019, 9, 47–55. [Google Scholar] [CrossRef]
- Díaz-Valle, A.; López-Calleja, A.C.; Alvarez-Venegas, R. Enhancement of Pathogen Resistance in Common Bean Plants by Inoculation With Rhizobium etli. Front. Plant Sci. 2019, 10, 1317. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Mishra, A.K.; Dileep Kumar, B.S. Induction of systemic resistance against fusarial wilt in pigeon pea through interaction of plant growth promoting rhizobacteria and rhizobia. Soil Biol. Biochem. 2008, 40, 452–461. [Google Scholar] [CrossRef]
- Osdaghi, E.; Shams-Bakhsh, M.; Alizadeh, A.; Lak, M.R.; Maleki, H.H. Induction of resistance in common bean by Rhizobium leguminosarum bv. phaseoli and decrease of common bacterial blight. Phytopathol. Mediterr. 2011, 50, 45–54. [Google Scholar]
- Elbadry, M.; Taha, R.M.; Eldougdoug, K.A.; Gamal-Eldin, H. Induction of systemic resistance in faba bean (Vicia faba L.) to bean yellow mosaic potyvirus (BYMV) via seed bacterization with plant growth promoting. J. Plant Dis. Prot. 2006, 113, 247–251. [Google Scholar] [CrossRef]
- Hasky-Guenther, K.; Hoffmann-Hergarten, S.; Sikora, R.A. Resistance against the potato cyst nematode Globodera pallida systemically induced by the rhizobacteria Agrobacterium radiobacter (G12) and Bacillus sphaericus (B43). Fundam. Appl. Nematol. 1998, 21, 511–517. [Google Scholar]
- Shafee, T.M.; Lay, F.T.; Phan, T.K.; Anderson, M.A.; Hulett, M.D. Convergent evolution of defensin sequence, structure and function. Cell Mol. Life Sci. 2017, 74, 663–682. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Velivelli, S.L.S.; Shah, D.M. Antifungal Potency and Modes of Action of a Novel Olive Tree Defensin Against Closely Related Ascomycete Fungal Pathogens. Mol. Plant Microbe Interact. 2019, 32, 1649–1664. [Google Scholar] [CrossRef]
- Kim, M.; Chen, Y.; Xi, J.; Waters, C.; Chen, R.; Wang, D. An antimicrobial peptide essential for bacterial survival in the nitrogen-fixing symbiosis. Proc. Natl. Acad. Sci. USA 2015, 112, 15238–15243. [Google Scholar] [CrossRef] [PubMed]
- Maróti, G.; Downie, J.A.; Kondorosi, É. Plant cysteine-rich peptides that inhibit pathogen growth and control rhizobial differentiation in legume nodules. Curr. Opin. Plant Biol. 2015, 26, 57–63. [Google Scholar] [CrossRef]
- Hakim, S.; Naqqash, T.; Nawaz, M.S.; Laraib, I.; Siddique, M.J.; Zia, R.; Mirza, M.S.; Imran, A. Rhizosphere Engineering with Plant Growth-Promoting Microorganisms for Agriculture and Ecological Sustainability. Front. Sustain. Food Syst. 2021, 5, 617157. [Google Scholar] [CrossRef]
- Tian, T.; Reverdy, A.; She, Q.; Sun, B.; Chai, Y. The role of rhizodeposits in shaping rhizomicrobiome. Environ. Microbiol. Rep. 2020, 12, 160–172. [Google Scholar] [CrossRef]
- Herren, C.M.; McMahon, K.D. Keystone taxa predict compositional change in microbial communities. Environ. Microbiol. 2018, 20, 2207–2217. [Google Scholar] [CrossRef]
- Banerjee, S.; Schlaeppi, K.; van der Heijden, M.G.A. Keystone taxa as drivers of microbiome structure and functioning. Nat. Rev. Microbiol. 2018, 16, 567–576. [Google Scholar] [CrossRef]
- Xu, H.; Yang, Y.; Tian, Y.; Xu, R.; Zhong, Y.; Liao, H. Rhizobium Inoculation Drives the Shifting of Rhizosphere Fungal Community in a Host Genotype Dependent Manner. Front. Microbiol. 2019, 10, 3135. [Google Scholar] [CrossRef]
- Robleto, E.A.; Borneman, J.; Triplett, E.W. Effects of bacterial antibiotic production on rhizosphere microbial communities from a culture-independent perspective. Appl. Environ. Microbiol. 1998, 64, 5020–5022. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Xiao, X.; Guo, Y.; Zhang, J.; Wang, E.; Chen, W.; Lin, Y.; Wei, G. Enhanced phytoremdiation of Robinia pseudoacacia in heavy metal-contaminated soils with rhizobia and the associated bacterial community structure and function. Chemosphere 2018, 197, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, B.; Chen, W.; Schlaeppi, K.; Erb, M.; Stirling, E.; Hu, L.; Wang, E.; Zhang, Y.; Zhao, K.; et al. Rhizobium Symbiotic Capacity Shapes Root-Associated Microbiomes in Soybean. Front. Microbiol. 2021, 12, 709012. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, H.; Wang, Y.; Wang, M.; Xie, X.; Chang, H.; Wang, L.; Qu, J.; Sun, K.; He, W.; et al. Mycorrhizal symbiosis modulates the rhizosphere microbiota to promote rhizobia-legume symbiosis. Mol. Plant 2021, 14, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Dhali, S.; Acharya, S.; Pradhan, M.; Patra, D.K.; Pradhan, C. Synergistic effect of Bacillus and Rhizobium on cytological and photosynthetic performance of Macrotyloma uniflorum (Lam.) Verdc. Grown in Cr (VI) contaminated soil. Plant Physiol. Biochem. 2022, 190, 62–69. [Google Scholar] [CrossRef]
- Zayed, A.; Lytle, C.M.; Qian, J.-H.; Terry, N. Chromium accumulation, translocation and chemical speciation in vegetable crops. Planta 1998, 206, 293–299. [Google Scholar] [CrossRef]
- Goyal, R.; Schmidt, A.; Hynes, M. Molecular Biology in the Improvement of Biological Nitrogen Fixation by Rhizobia and Extending the Scope to Cereals. Microorganisms 2021, 9, 125. [Google Scholar] [CrossRef]
- Koskey, G.; Mburu, S.W.; Njeru, E.M.; Kimiti, J.M.; Ombori, O.; Maingi, J.M. Potential of Native Rhizobia in Enhancing Nitrogen Fixation and Yields of Climbing Beans (Phaseolus vulgaris L.) in Contrasting Environments of Eastern Kenya. Front. Plant Sci. 2017, 8, 443. [Google Scholar] [CrossRef]
- Ouma, E.W.; Asango, A.M.; Maingi, J.; Njeru, E.M. Elucidating the Potential of Native Rhizobial Isolates to Improve Biological Nitrogen Fixation and Growth of Common Bean and Soybean in Smallholder Farming Systems of Kenya. Int. J. Agron. 2016, 2016, 4569241. [Google Scholar] [CrossRef]
- Goyal, R.; Mattoo, A.; Schmidt, A. Rhizobial–Host Interactions and Symbiotic Nitrogen Fixation in Legume Crops toward Agriculture Sustainability. Front. Microbiol. 2021, 12, 669404. [Google Scholar] [CrossRef]
- Coba de la Peña, T.; Fedorova, E.; Pueyo, J.J.; Lucas, M.M. The Symbiosome: Legume and Rhizobia Co-evolution toward a Nitrogen-Fixing Organelle? Front. Plant Sci. 2018, 8, 2229. [Google Scholar] [CrossRef]
- Lavin, M.; Herendeen, P.S.; Wojciechowski, M.F. Evolutionary rates analysis of Leguminosae implicates a rapid diversification of lineages during the tertiary. Syst. Biol. 2005, 54, 575–594. [Google Scholar] [CrossRef] [PubMed]
- Provorov, N.A.; Andronov, E.E. Evolution of root nodule bacteria: Reconstruction of the speciation processes resulting from genomic rearrangements in a symbiotic system. Microbiology 2016, 85, 131–139. [Google Scholar] [CrossRef]
- de Sousa, B.F.S.; Castellane, T.C.L.; Tighilt, L.; Lemos, E.G.d.M.; Rey, L. Rhizobial Exopolysaccharides and Type VI Secretion Systems: A Promising Way to Improve Nitrogen Acquisition by Legumes. Front. Agron. 2021, 3, 661468. [Google Scholar] [CrossRef]
- Jiao, Y.S.; Liu, Y.H.; Yan, H.; Wang, E.T.; Tian, C.F.; Chen, W.X.; Guo, B.L.; Chen, W.F. Rhizobial Diversity and Nodulation Characteristics of the Extremely Promiscuous Legume Sophora flavescens. Mol. Plant Microbe Interact. 2015, 28, 1338–1352. [Google Scholar] [CrossRef]
- Roche, P.; Maillet, F.; Plazanet, C.; Debellé, F.; Ferro, M.; Truchet, G.; Promé, J.C.; Dénarié, J. The common nodABC genes of Rhizobium meliloti are host-range determinants. Proc. Natl. Acad. Sci. USA 1996, 93, 15305–15310. [Google Scholar] [CrossRef]
- Hacker, J.; Carniel, E. Ecological fitness, genomic islands and bacterial pathogenicity. EMBO Rep. 2001, 2, 376–381. [Google Scholar] [CrossRef]
- Haritha, A.; Sagar, K.P.; Tiwari, A.; Kiranmayi, P.; Rodrigue, A.; Mohan, P.M.; Singh, S.S. MrdH, a novel metal resistance determinant of Pseudomonas putida KT2440, is flanked by metal-inducible mobile genetic elements. J. Bacteriol. 2009, 191, 5976–5987. [Google Scholar] [CrossRef]
- Kamal, S.M.; Simpson, D.J.; Wang, Z.; Gänzle, M.; Römling, U. Horizontal Transmission of Stress Resistance Genes Shape the Ecology of Beta- and Gamma-Proteobacteria. Front. Microbiol. 2021, 12, 696522. [Google Scholar] [CrossRef]
- Haimlich, S.; Fridman, Y.; Khandal, H.; Savaldi-Goldstein, S.; Levy, A. Widespread horizontal gene transfer between plants and their microbiota. bioRxiv 2022. [CrossRef]
- Li, M.; Chen, Q.; Wu, C.; Li, Y.; Wang, S.; Chen, X.; Qiu, B.; Li, Y.; Mao, D.; Lin, H.; et al. A Novel Module Promotes Horizontal Gene Transfer in Azorhizobium caulinodans ORS571. Genes 2022, 13, 1895. [Google Scholar] [CrossRef] [PubMed]
- Durrant, M.G.; Li, M.M.; Siranosian, B.A.; Montgomery, S.B.; Bhatt, A.S. A Bioinformatic Analysis of Integrative Mobile Genetic Elements Highlights Their Role in Bacterial Adaptation. Cell Host Microbe 2020, 27, 140–153.e149. [Google Scholar] [CrossRef] [PubMed]
- Forero, L.E.; Kulmatiski, A.; Grenzer, J.; Norton, J.M. Plant-soil feedbacks help explain biodiversity-productivity relationships. Commun. Biol. 2021, 4, 789. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goyal, R.K.; Habtewold, J.Z. Evaluation of Legume–Rhizobial Symbiotic Interactions Beyond Nitrogen Fixation That Help the Host Survival and Diversification in Hostile Environments. Microorganisms 2023, 11, 1454. https://doi.org/10.3390/microorganisms11061454
Goyal RK, Habtewold JZ. Evaluation of Legume–Rhizobial Symbiotic Interactions Beyond Nitrogen Fixation That Help the Host Survival and Diversification in Hostile Environments. Microorganisms. 2023; 11(6):1454. https://doi.org/10.3390/microorganisms11061454
Chicago/Turabian StyleGoyal, Ravinder K., and Jemaneh Z. Habtewold. 2023. "Evaluation of Legume–Rhizobial Symbiotic Interactions Beyond Nitrogen Fixation That Help the Host Survival and Diversification in Hostile Environments" Microorganisms 11, no. 6: 1454. https://doi.org/10.3390/microorganisms11061454
APA StyleGoyal, R. K., & Habtewold, J. Z. (2023). Evaluation of Legume–Rhizobial Symbiotic Interactions Beyond Nitrogen Fixation That Help the Host Survival and Diversification in Hostile Environments. Microorganisms, 11(6), 1454. https://doi.org/10.3390/microorganisms11061454





