Glucose Transport in Escherichia coli: From Basics to Transport Engineering
Abstract
1. Introduction
2. The Cellular Membrane in Escherichia coli and Solute Transport by Outer Membrane Porins (OMP)
3. The Cytoplasmic Membrane Transport System: Glucose Transport against a Gradient Concentration Mechanism
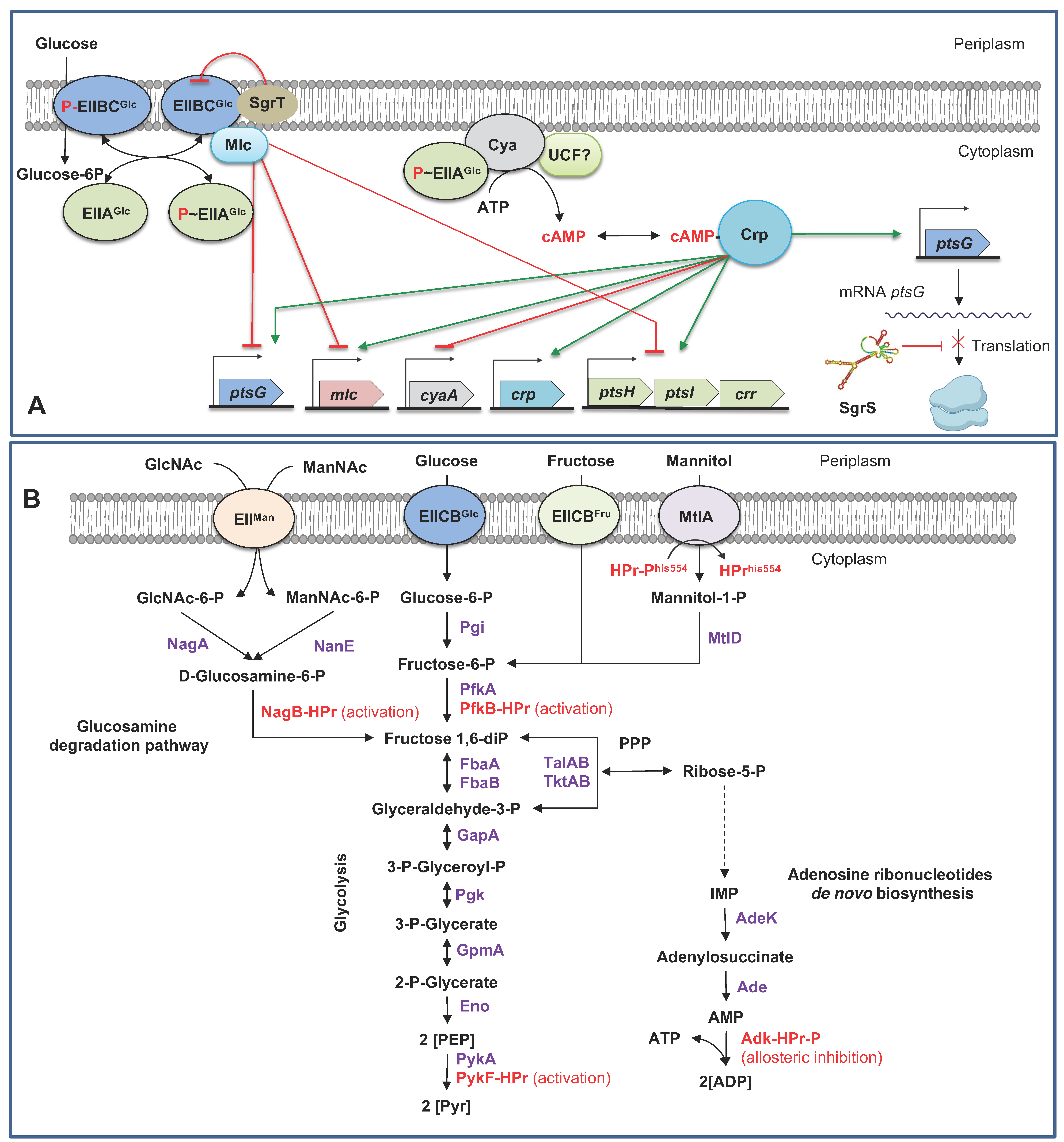
3.1. The Phosphoenolpyruvate (PEP):Glucose Phosphotransferase System (PTS Glucose)
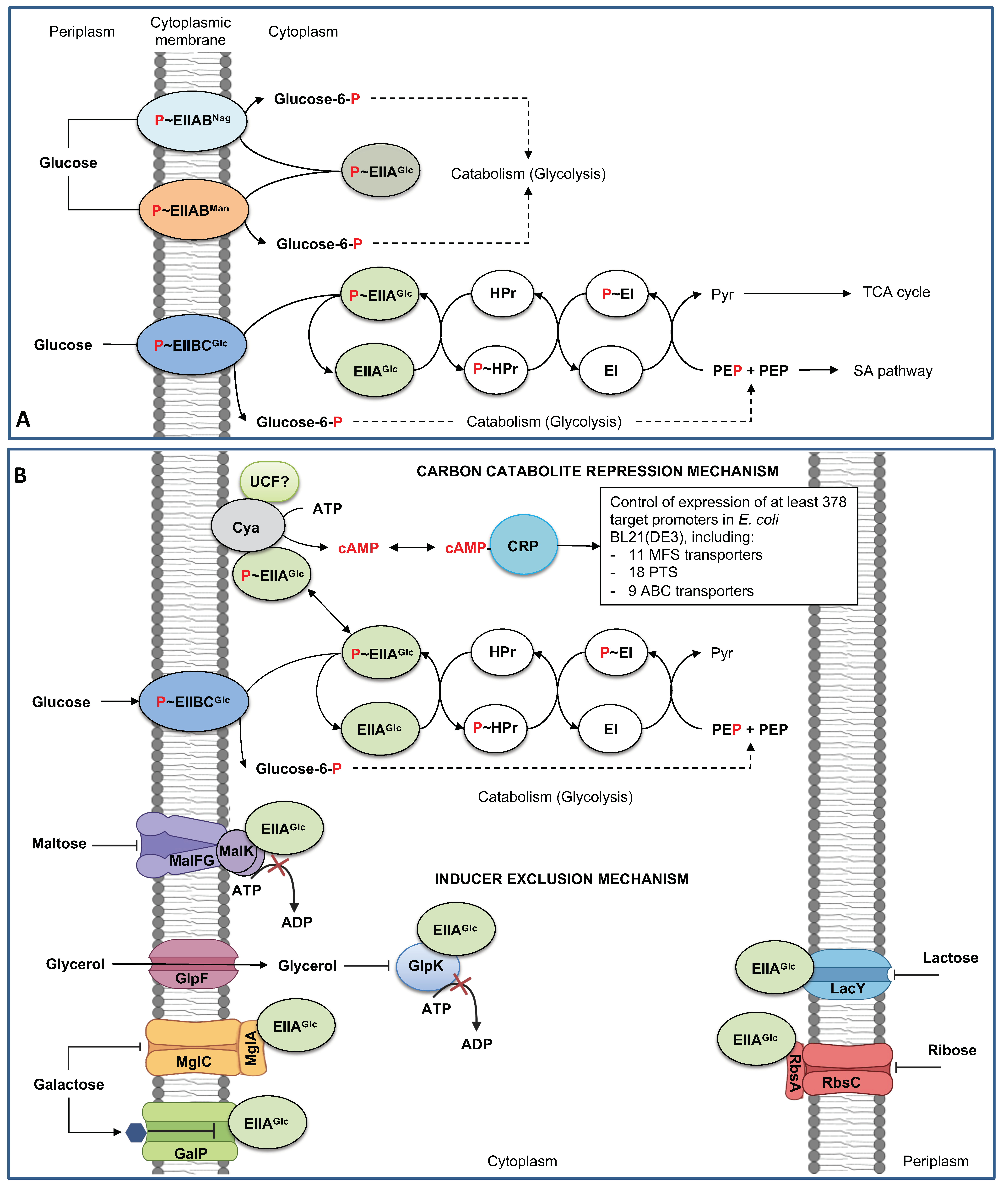
3.1.1. Control of Carbon Metabolism in E. coli by PTS Glucose by CCR and Inducer Exclusion Mechanisms: The Role of EIICDGlc and EIIAGlc
3.1.2. Regulatory Mechanisms of the PTS Proteins EI and Hpr
3.2. Primary and Secondary Active Transporters Can Transport Glucose in E. coli
3.2.1. The ATP-Dependent Cassette (ABC) Transporters
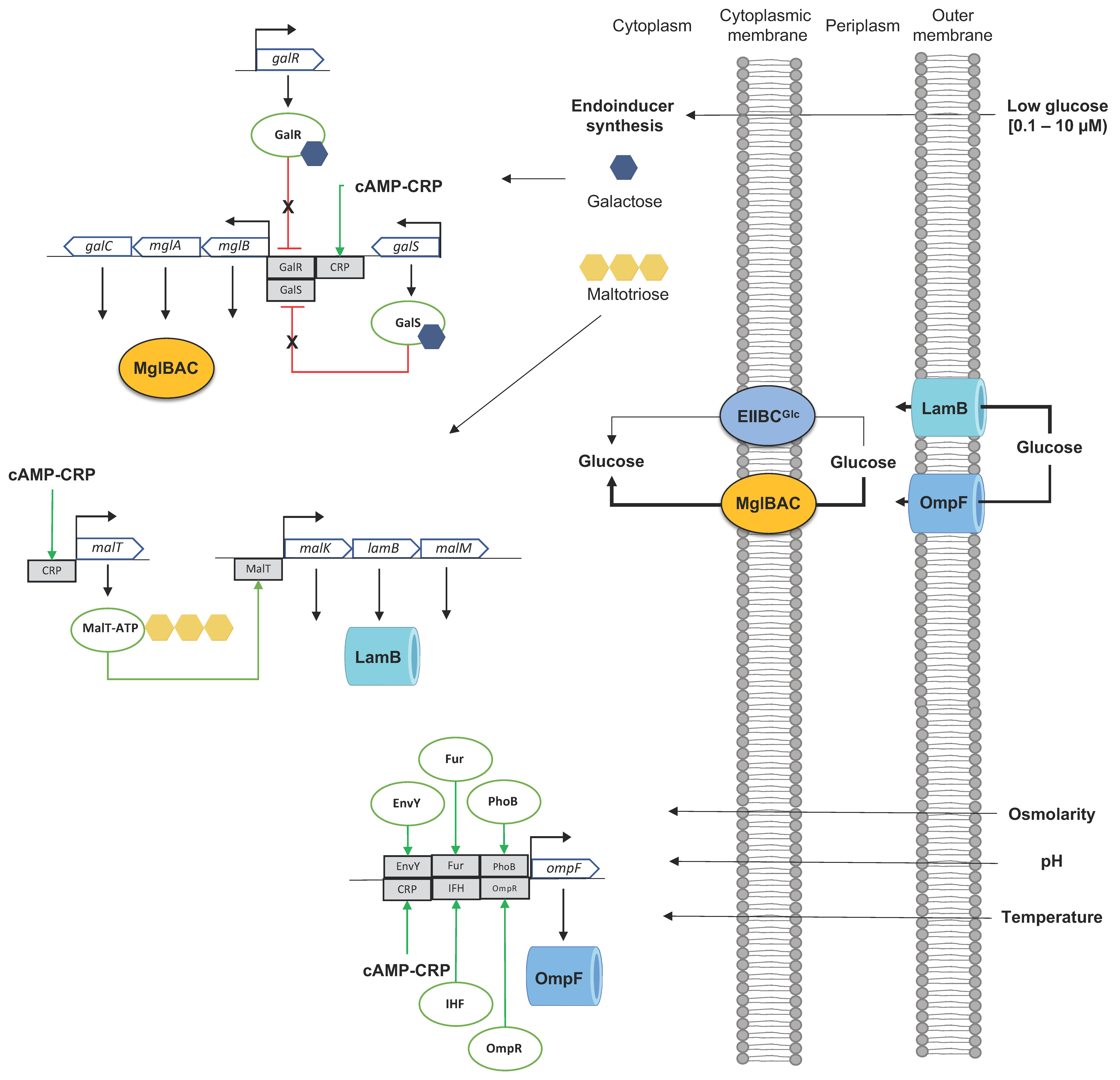
3.2.2. The D-Galactose/Methyl-β-D-Galactoside ABC Transporter MglBAC
3.2.3. The D-Galactose/H+ Symporter GalP
4. Dynamics of Glucose Transport in E. coli under Sugar-Limiting Conditions
5. Transport Engineering or Improving Sugar Uptake Capabilities for Metabolic Engineering Purposes
| Derivative Mutant and (Parental Strain) | Mutation | Alternative Glucose Transport System | Resultant Phenotype or Metabolic Engineering Applications | References |
|---|---|---|---|---|
| SP1.1 pSC6.090B (RB791 derivative) | ptsHIcrr KO | Glf from Zymomonas mobilis. | Cloning of Glk from Z. mobilis improved glucose phosphorylation. Genetic background for SA overproduction: 87 g/L of SA in 36% mol SA/mol Glc yield in the final derivative. | [72] |
| PB12 (JM101) | ptsHIcrr KO | GalP was selected as the leading glucose transporter during an ALE experiment. Selection of MglBAC during the early ALE experiment as primary glucose transporter. | Abolition of CCR mechanism. Increased glycolytic fluxes of 93.1% compared to the parental strain. Higher Glk activity. Genetic background for SA overproduction: SA titer of 42–60 g/L in 42% mol SA/mol Glc yield. | [71,74,77,84] |
| YL104H (MG1655) | ptsH KO | Not described | Abolition of CCR mechanism. PTS glucose mutant for succinate production in cultures with glucose: xylose mixtures under anaerobic conditions. Succinic acid production of 511.11 mM and 1.01 g/L/h. | [81] |
| SB2/pPckA (MG1655) | ptsH KO | Glf from Z. mobilis | Resultant PtsG− GlfZ. mobilis mutant increased succinate yield by 489. 65 X compared to the parental strain. | [85] |
| STG8 (E. coli W KCTC1039) | ΔptsG, ΔmalE, ΔmglB, ΔGalP | Upregulation of remaining functional PTS sugar systems and ABC transporters. | Delayed glucose consumption, extended lag phase, low or no acetate production. Upregulation of PTS systems: Trehalose, glutitol/sorbitol, Mannose/fructose/ sorbose/D-GalNAc, UDP-GlcNAc, N-acetylmuramic acid. Upregulation of ABC systems: Arabinose, glycerol- 3-P, ribose, xylose, gluconate, hexuronate. Derivative STG8 strain increased the yield for EGFP to 132%, GABA titer to 130% with increased specific yield of 176%, increased lycopene yield of 90% | [86] |
| CFT5 (E. coli ATCC31882) | ptsG KO by replacing with galactose permease/glucokinase | Transport of glucose by GalP. | Abolition of the CCR mechanism, simultaneous use of glucose and xylose as carbon sources, independence of glycolysis and PPP from TCA. The heterologous Dahms pathway channeled xylose into TCA, glucose transported by GalP channeled to cis, cis-muconic acid production. | [87] |
| Several derivatives from W3110 | WG, ptsG KO; WGM, manX KO; WGMC, mglBAC KO; WHIC, ptsHIcrr and mglBAC KO | Glucose transport by alternative systems: WG: IICDMan, MglBAC; WGM: MglBAC; WGMX, Unknown; WHIC, Unknown. Differential upregulation of other PTS:sugar systems, non-PTS sugar transporters, and catabolic proteins for several sugars. | Mutants with reduced μ values compared to W3110, lower acetate production. Increased transcription in genes of alternative sugar transport and metabolism, energy generation, and amino acid biosynthesis in WG derivative compared to W3110: Upregulated PTS systems: D-GalNAc, fructose, galactitol, mannose, mannitol, glucitol/sorbitol, UDP-GlcNAc, trehalose. Upregulated non-PTS transporters: maltose, ribose, galactose/glucose, arabinose, inositol, 2-D-3-deoxygalactose, fuculose, 5-keto-4-deoxy-D-glucarate and 2-keto-3-deoxy-D-glucarate, tagatose, maltose and maltodextrin, ManNAc, 2-methylisocitrate, glucuronate altronate, mannonate, 5-dehydro-4-deoxy-D-glucuronate. | [88] |
6. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, D.; Prabowo, C.P.S.; Eun, H.; Park, S.Y.; Cho, I.J.; Jiao, S.; Lee, S.Y. Escherichia coli as a platform microbial host for systems metabolic engineering. Essays Biochem. 2021, 65, 225–246. [Google Scholar] [CrossRef]
- McElwain, L.; Phair, K.; Kealey, C.; Brady, D. Current trends in biopharmaceuticals production in Escherichia coli. Biotechnol. Lett. 2022, 44, 917–931. [Google Scholar] [CrossRef] [PubMed]
- Martínez, K.; de Anda, R.; Hernández, G.; Escalante, A.; Gosset, G.; Ramírez, O.T.; Bolívar, F.G. Coutilization of glucose and glycerol enhances the production of aromatic compounds in an Escherichia coli strain lacking the phosphoenolpyruvate: Carbohydrate phosphotransferase system. Microb. Cell Factories 2008, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Bren, A.; Park, J.O.; Towbin, B.D.; Dekel, E.; Rabinowitz, J.D.; Alon, U. Glucose becomes one of the worst carbon sources for E. coli on poor nitrogen sources due to suboptimal levels of cAMP. Sci. Rep. 2016, 6, 24834. [Google Scholar] [CrossRef]
- Alva, A.; Sabido-Ramos, A.; Escalante, A.; Bolívar, F. New Insights into transport capability of sugars and its impact on growth from novel mutants of Escherichia coli. Appl. Microbiol. Biotechnol. 2020, 104, 1463–1479. [Google Scholar] [CrossRef]
- Jeckelmann, J.-M.; Erni, B. Transporters of glucose and other carbohydrates in Bacteria. Pflug. Arch. Eur. J. Physiol. 2020, 472, 1129–1153. [Google Scholar] [CrossRef]
- Jahreis, K.; Pimentel-Schmitt, E.F.; Brückner, R.; Titgemeyer, F. Ins and outs of glucose transport systems in Eubacteria. FEMS Microbiol. Rev. 2008, 32, 891–907. [Google Scholar] [CrossRef]
- Dean, D.A.; Reizer, J.; Nikaido, H.; Saier, M.H. Regulation of the maltose transport system of Escherichia coli by the glucose-specific enzyme iii of the phosphoenolpyruvate-sugar phosphotransferase system. characterization of inducer exclusion-resistant mutants and reconstitution of inducer exclusion in proteoliposomes. J. Biol. Chem. 1990, 265, 21005–21010. [Google Scholar] [CrossRef]
- Görke, B.; Stülke, J. Carbon catabolite repression in bacteria: Many ways to make the most out of nutrients. Nat. Rev. Microbiol. 2008, 6, 613–624. [Google Scholar] [CrossRef]
- Carmona, S.B.; Moreno, F.; Bolívar, F.; Gosset, G.; Escalante, A. Inactivation of the PTS as a strategy to engineer the production of aromatic metabolites in Escherichia coli. J. Mol. Microbiol. Biotechnol. 2015, 25, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Keseler, I.M.; Gama-Castro, S.; Mackie, A.; Billington, R.; Bonavides-Martínez, C.; Caspi, R.; Kothari, A.; Krummenacker, M.; Midford, P.E.; Muñiz-Rascado, L.; et al. The EcoCyc Database in 2021. Front. Microbiol. 2021, 12, 711077. [Google Scholar] [CrossRef] [PubMed]
- Karp, P.D.; Billington, R.; Caspi, R.; Fulcher, C.A.; Latendresse, M.; Kothari, A.; Keseler, I.M.; Krummenacker, M.; Midford, P.E.; Ong, Q.; et al. The BioCyc collection of microbial genomes and metabolic pathways. Brief. Bioinform. 2017, 20, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Paalme, T.; Elken, R.; Kahru, A.; Vanatalu, K.; Vilu, R. The growth rate control in Escherichia coli at near to maximum growth rates: The A-stat approach. Antonie Leeuwenhoek 1997, 71, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, H. Porins and specific channels of bacterial outer membranes. Mol. Microbiol. 1992, 6, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Winterhalter, M.; Pagès, J.-M. Outer membrane porins. In Bacterial Cell Walls and Membranes; Kuhn, A., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 79–123. ISBN 978-3-030-18768-2. [Google Scholar]
- Saier, M.H.; Reddy, V.S.; Moreno-Hagelsieb, G.; Hendargo, K.J.; Zhang, Y.; Iddamsetty, V.; Lam, K.J.K.; Tian, N.; Russum, S.; Wang, J.; et al. The Transporter Classification Database (TCDB): 2021 Update. Nucleic Acids Res. 2020, 49, D461–D467. [Google Scholar] [CrossRef]
- Jeckelmann, J.-M.; Erni, B. Carbohydrate transport by group translocation: The Bacterial phosphoenolpyruvate: Sugar phosphotransferase system. In Bacterial Cell Walls and Membranes; Kuhn, A., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 223–274. ISBN 978-3-030-18768-2. [Google Scholar]
- Deutscher, J.; Francke, C.; Postma, P.W. How Phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol. Mol. Biol. Rev. 2006, 70, 939–1031. [Google Scholar] [CrossRef]
- Somavanshi, R.; Ghosh, B.; Sourjik, V. Sugar influx sensing by the phosphotransferase system of Escherichia coli. PLoS Biol. 2016, 14, e2000074. [Google Scholar] [CrossRef]
- Steinsiek, S.; Bettenbrock, K. Glucose transport in Escherichia coli mutant strains with defects in sugar transport systems. J. Bacteriol. 2012, 194, 5897–5908. [Google Scholar] [CrossRef]
- Shimada, T.; Fujita, N.; Yamamoto, K.; Ishihama, A. Novel roles of cAMP receptor protein (CRP) in regulation of transport and metabolism of carbon sources. PLoS ONE 2011, 6, e20081. [Google Scholar] [CrossRef]
- Tierrafría, V.H.; Rioualen, C.; Salgado, H.; Lara, P.; Gama-Castro, S.; Lally, P.; Gómez-Romero, L.; Peña-Loredo, P.; López-Almazo, A.G.; Alarcón-Carranza, G.; et al. RegulonDB 11.0: Comprehensive high-throughput datasets on transcriptional regulation in Escherichia coli K-12. Microb. Genom. 2022, 8, mgen000833. [Google Scholar] [CrossRef]
- Escalante, A.; Salinas Cervantes, A.; Gosset, G.; Bolívar, F. Current knowledge of the Escherichia coli phosphoenolpyruvate–carbohydrate phosphotransferase system: Peculiarities of regulation and impact on growth and product formation. Appl. Microbiol. Biotechnol. 2012, 94, 1483–1494. [Google Scholar] [CrossRef]
- Wang, J.; Ma, W.; Wang, X. Insights into the structure of Escherichia coli outer membrane as the target for engineering microbial cell factories. Microb. Cell Factories 2021, 20, 73. [Google Scholar] [CrossRef]
- Cronan, J.E.; Gennis, R.B.; Maloy, S.R. Cytoplasmic membrane. In Escherichia coli and Salmonella Typhimurium. Cellular and Molecular Biology; American Society for Microbiology: Washington, DC, USA, 1987; Volume 1, pp. 31–55. [Google Scholar]
- Nikaido, H.; Vaara, M. Outer membrane. In Escherichia coli and Salmonella Typhimurium. Cellular and Molecular Biology; American Society for Microbiology: Washington, DC, USA, 1987; Volume 1, pp. 7–22. ISBN 0-914826-89-1. [Google Scholar]
- Sperandeo, P.; Martorana, A.M.; Polissi, A. Lipopolysaccharide biosynthesis and transport to the outer membrane of gram-negative bacteria. Subcell. Biochem. 2019, 92, 9–37. [Google Scholar] [CrossRef]
- Onyeabor, M.; Martinez, R.; Kurgan, G.; Wang, X. Engineering transport systems for microbial production. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 111, pp. 33–87. ISBN 978-0-12-820705-5. [Google Scholar]
- Nikaido, H.; Vaara, M. Molecular basis of bacterial outer membrane permeability. Microbiol. Rev. 1985, 49, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Betton, J.M.; Michel, V.; Hofnung, M.; Charbit, A. Identification of a cryptic porin gene in the Escherichia coli genome: Expression and insertion of a monomeric form of the protein into the outer membrane. Mol. Microbiol. 1997, 26, 1141–1143. [Google Scholar] [PubMed]
- Ferenci, T. Selectivity in solute transport: Binding sites and channel structure in maltoporin and other bacterial sugar transport proteins. Bioessays 1989, 10, 1–7. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, T.; Wu, H. The Transport and mediation mechanisms of the common sugars in Escherichia coli. Biotechnol. Adv. 2014, 32, 905–919. [Google Scholar] [CrossRef]
- Ferenci, T. Adaptation to life at micromolar nutrient levels: The regulation of Escherichia coli glucose transport by endoinduction and cAMP. FEMS Microbiol. Rev. 1996, 18, 301–317. [Google Scholar] [CrossRef]
- Ferenci, T. Hungry Bacteria—Definition and properties of a nutritional state. Environ. Microbiol. 2001, 3, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Lux, R.; Munasinghe, V.R.N.; Castellano, F.; Lengeler, J.W.; Corrie, J.E.T.; Khan, S. Elucidation of a PTS–carbohydrate chemotactic signal pathway in Escherichia coli using a time-resolved behavioral assay. Mol. Biol. Cell 1999, 10, 1133–1146. [Google Scholar] [CrossRef] [PubMed]
- Neumann, S.; Grosse, K.; Sourjik, V. Chemotactic signaling via carbohydrate phosphotransferase systems in Escherichia coli. Proc. Natl. Acad. Sci. USA 2012, 109, 12159–12164. [Google Scholar] [CrossRef]
- Lengeler, J.W. PTS 50: Past, present and future, or diauxie revisited. Microb. Physiol. 2015, 25, 79–93. [Google Scholar] [CrossRef]
- Lengeler, J.W.; Jahreis, K. Bacterial PEP-dependent carbohydrate: Phosphotransferase systems couple sensing and global control mechanisms. In Contributions to Microbiology; Collin, M., Schuch, R., Eds.; KARGER: Basel, Switzerland, 2009; Volume 16, pp. 65–87. ISBN 978-3-8055-9132-4. [Google Scholar]
- Jeckelmann, J.-M.; Harder, D.; Mari, S.A.; Meury, M.; Ucurum, Z.; Müller, D.J.; Erni, B.; Fotiadis, D. Structure and function of the glucose PTS transporter from Escherichia coli. J. Struct. Biol. 2011, 176, 395–403. [Google Scholar] [CrossRef]
- Kotrba, P.; Inui, M.; Yukawa, H. The ptsI Gene encoding Enzyme I of the phosphotransferase system of Corynebacterium glutamicum. Biochem. Biophys. Res. Commun. 2001, 289, 1307–1313. [Google Scholar] [CrossRef]
- Francke, C.; Postma, P.W.; Westerhoff, H.V.; Blom, J.G.; Peletier, M.A. Why the phosphotransferase system of Escherichia coli escapes diffusion limitation. Biophys. J. 2003, 85, 612–622. [Google Scholar] [CrossRef]
- Huber, R.E.; Lytton, J.; Fung, E.B. Efflux of Fl-galactosidase products from Escherichia coli. J. Bacteriol. 1980, 141, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Plumbridge, J. Expression of ptsG, the gene for the major glucose PTS transporter in Escherichia coli, is repressed by Mlc and induced by growth on glucose. Mol. Microbiol. 1998, 29, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Negrete, A.; Ng, W.-I.; Shiloach, J. Glucose uptake regulation in E. coli by the small RNA SgrS: Comparative analysis of E. coli K-12 (JM109 and MG1655) and E. coli B (BL21). Microb. Cell Factories 2010, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Vanderpool, C.K.; Gottesman, S. Involvement of a novel transcriptional activator and small RNA in post-transcriptional regulation of the glucose phosphoenolpyruvate phosphotransferase system: RNA regulator of phosphosugar stress response. Mol. Microbiol. 2004, 54, 1076–1089. [Google Scholar] [CrossRef]
- Rodionova, I.A.; Zhang, Z.; Mehla, J.; Goodacre, N.; Babu, M.; Emili, A.; Uetz, P.; Saier, M.H. The phosphocarrier protein HPr of the bacterial phosphotransferase system globally regulates energy metabolism by directly interacting with multiple enzymes in Escherichia coli. J. Biol. Chem. 2017, 292, 14250–14257. [Google Scholar] [CrossRef]
- Choe, M.; Park, Y.-H.; Lee, C.-R.; Kim, Y.-R.; Seok, Y.-J. The general PTS component HPr determines the preference for glucose over mannitol. Sci. Rep. 2017, 7, 43431. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, R.; Bernhart, S.H.; Höner zu Siederdissen, C.; Tafer, H.; Flamm, C.; Stadler, P.F.; Hofacker, I.L. ViennaRNA Package 2.0. Algorithms Mol. Biol. 2011, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Kotrba, P.; Inui, M.; Yukawa’, A. Bacterial phosphotransferase system (ITS) in carbohydrate uptake and control of carbon metabolism. J. Biosci. Bioeng. 2001, 92, 502–517. [Google Scholar] [CrossRef]
- Moussatova, A.; Kandt, C.; O’Mara, M.L.; Tieleman, D.P. ATP-Binding cassette transporters in Escherichia coli. Biochim. Biophys. Acta-Biomembr. 2008, 1778, 1757–1771. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Blattner, F.R.; Plunkett, G.; Bloch, C.A.; Perna, N.T.; Burland, V.; Riley, M.; Collado-Vides, J.; Glasner, J.D.; Rode, C.K.; Mayhew, G.F.; et al. The complete genome sequence of Escherichia coli K-12. Science 1997, 277, 1453–1462. [Google Scholar] [CrossRef]
- Khare, D.; Oldham, M.L.; Orelle, C.; Davidson, A.L.; Chen, J. Alternating access in maltose transporter mediated by rigid-body rotations. Mol. Cell 2009, 33, 528–536. [Google Scholar] [CrossRef]
- Bao, H.; Duong, F. Discovery of an auto-regulation mechanism for the maltose ABC transporter MalFGK2. PLoS ONE 2012, 7, e34836. [Google Scholar] [CrossRef]
- Hiller, R.M.; von Kügelgen, J.; Bao, H.; Van Hoa, F.D.; Cytrynbaum, E.N. A mathematical model for the kinetics of the MalFGK2 maltose transporter. Bull. Math. Biol. 2020, 82, 62. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.; BÖhm, A.; Lee, S.-J.; Peist, R.; Decker, K.; Boos, W. Network regulation of the Escherichia coli maltose system. J. Mol. Microbiol. Biotechnol. 2002, 4, 301–307. [Google Scholar] [PubMed]
- Richet, E.; Davidson, A.L.; Joly, N. The ABC Transporter MalFGK2 sequesters the MalT transcription factor at the membrane in the absence of cognate substrate. Mol. Microbiol. 2012, 85, 632–647. [Google Scholar] [CrossRef] [PubMed]
- Hogg, R.W.; Voelker, C.; Von Carlowitz, I. Nucleotide sequence and analysis of the Mgl operon of Escherichia coli K12. Mol. Gen. Genet. 1991, 229, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Weickert, M.J.; Adhya, S. Control of transcription of gal repressor and isorepressor genes in Escherichia coli. J. Bacteriol. 1993, 175, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.; Orosz, L.; Sneppen, K.; Adhya, S.; Semsey, S. Relation of Intracellular signal levels and promoter activities in the gal regulon of Escherichia coli. J. Mol. Biol. 2009, 391, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Semsey, S.; Geanacopoulos, M.; Lewis, D.E.A.; Adhya, S. Operator-bound GalR dimers close dna loops by direct interaction: Tetramerization and inducer binding. EMBO J. 2002, 21, 4349–4356. [Google Scholar] [CrossRef] [PubMed]
- Semsey, S.; Krishna, S.; Sneppen, K.; Adhya, S. Signal integration in the galactose network of Escherichia coli. Mol. Microbiol. 2007, 65, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Davejan, P.; Hendargo, K.J.; Javadi-Razaz, I.; Chou, A.; Yee, D.C.; Ghazi, F.; Lam, K.J.K.; Conn, A.M.; Madrigal, A.; et al. Expansion of the Major Facilitator Superfamily (MFS) to include novel transporters as well as transmembrane-acting enzymes. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183277. [Google Scholar] [CrossRef] [PubMed]
- Bidossi, A.; Mulas, L.; Decorosi, F.; Colomba, L.; Ricci, S.; Pozzi, G.; Deutscher, J.; Viti, C.; Oggioni, M.R. A Functional genomics approach to establish the complement of carbohydrate transporters in Streptococcus pneumoniae. PLoS ONE 2012, 7, e33320. [Google Scholar] [CrossRef] [PubMed]
- Henderson, P.J.; Giddens, R.A.; Jones-Mortimer, M.C. Transport of galactose, glucose and their molecular analogues by Escherichia coli K12. Biochem. J. 1977, 162, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Notley-McRobb, L.; Death, A.; Ferenci, T. The Relationship between external glucose concentration and cAMP Levels inside Escherichia coli: Implications for models of phosphotransferase-mediated regulation of adenylate cyclase. Microbiology 1997, 143, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ferenci, T. Regulation of porin-mediated outer membrane permeability by nutrient limitation in Escherichia coli. J. Bacteriol. 1998, 180, 3917–3922. [Google Scholar] [CrossRef]
- Geanacopoulos, M.; Adhya, S. Functional characterization of roles of GalR and GalS as regulators of the gal regulon. J. Bacteriol. 1997, 179, 228–234. [Google Scholar] [CrossRef]
- Death, A.; Ferenci, T. Between feast and famine: Endogenous inducer synthesis in the adaptation of Escherichia coli to growth with limiting carbohydrates. J. Bacteriol. 1994, 176, 5101–5107. [Google Scholar] [CrossRef]
- Notley, L.; Ferenci, T. Differential expression of Mal genes under cAMP and Endogenous inducer control in nutrient-stressed Escherichia coli. Mol. Microbiol. 1995, 16, 121–129. [Google Scholar] [CrossRef]
- Rodriguez, A.; Martínez, J.A.; Báez-Viveros, J.L.; Flores, N.; Hernández-Chávez, G.; Ramírez, O.T.; Gosset, G.; Bolivar, F. Constitutive expression of selected genes from the pentose phosphate and aromatic pathways increases the shikimic acid yield in high-glucose batch cultures of an Escherichia coli strain lacking PTS and pykF. Microb. Cell Factories 2013, 12, 17. [Google Scholar] [CrossRef]
- Chandran, S.S.; Yi, J.; Draths, K.M.; von Daeniken, R.; Weber, W.; Frost, J.W. Phosphoenolpyruvate availability and the biosynthesis of shikimic acid. Biotechnol. Prog. 2003, 19, 808–814. [Google Scholar] [CrossRef]
- Flores, N.; Flores, S.; Escalante, A.; de Anda, R.; Leal, L.; Malpica, R.; Georgellis, D.; Gosset, G.; Bolívar, F. Adaptation for fast growth on glucose by differential expression of central carbon metabolism and gal regulon genes in an Escherichia coli strain lacking the phosphoenolpyruvate:carbohydrate phosphotransferase system. Metab. Eng. 2005, 7, 70–87. [Google Scholar] [CrossRef]
- Aguilar, C.; Escalante, A.; Flores, N.; de Anda, R.; Riveros-McKay, F.; Gosset, G.; Morett, E.; Bolívar, F. Genetic changes during a laboratory adaptive evolution process that allowed fast growth in glucose to an Escherichia coli strain lacking the major glucose transport system. BMC Genom. 2012, 13, 385. [Google Scholar] [CrossRef] [PubMed]
- McCloskey, D.; Xu, S.; Sandberg, T.E.; Brunk, E.; Hefner, Y.; Szubin, R.; Feist, A.M.; Palsson, B.O. Adaptive laboratory evolution resolves energy depletion to maintain high aromatic metabolite phenotypes in Escherichia coli strains lacking the phosphotransferase system. Metab. Eng. 2018, 48, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.A.; Bolívar, F.; Escalante, A. Shikimic acid production in Escherichia coli: From classical metabolic engineering strategies to omics applied to improve its production. Front. Bioeng. Biotechnol. 2015, 3, 145. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, C.; Martínez-Batallar, G.; Flores, N.; Moreno-Avitia, F.; Encarnación, S.; Escalante, A.; Bolívar, F. Analysis of differentially upregulated proteins in ptsHIcrr− and rppH− mutants in Escherichia coli during an adaptive laboratory evolution experiment. Appl. Microbiol. Biotechnol. 2018, 102, 10193–10208. [Google Scholar] [CrossRef]
- Hernández-Montalvo, V.; Martínez, A.; Hernández-Chavez, G.; Bolivar, F.; Valle, F.; Gosset, G. Expression of galP and Glk in a Escherichia coli PTS mutant restores glucose transport and increases glycolytic flux to fermentation products: galP and glk genes restore glucose assimilation capacity in E. coli PTS−. Biotechnol. Bioeng. 2003, 83, 687–694. [Google Scholar] [CrossRef]
- Balderas-Hernandez, V.E.; Sabido-Ramos, A.; Silva, P.; Cabrera-Valladares, N.; Hernandez-Chavez, G.; Baez-Viveros, J.L.; Martinez, A.; Bolivar, F.; Gosset, G. Metabolic engineering for improving anthranilate synthesis from glucose in Escherichia coli. Microb. Cell Factories 2009, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Carmona, S.B.; Flores, N.; Martínez-Romero, E.; Gosset, G.; Bolívar, F.; Escalante, A. Evolution of an Escherichia coli PTS− Strain: A study of reproducibility and dynamics of an adaptive evolutive process. Appl. Microbiol. Biotechnol. 2020, 104, 9309–9325. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Zhang, F.; Li, Y.; Zhang, X.; Li, J.; Yang, P.; Qi, Q. Comparison of individual component deletions in a glucose-specific phosphotransferase system revealed their different applications. Sci. Rep. 2015, 5, 13200. [Google Scholar] [CrossRef]
- Kurgan, G.; Sievert, C.; Flores, A.; Schneider, A.; Billings, T.; Panyon, L.; Morris, C.; Taylor, E.; Kurgan, L.; Cartwright, R.; et al. Parallel experimental evolution reveals a novel repressive control of galP on xylose fermentation in Escherichia coli. Biotechnol. Bioeng. 2019, 116, 2074–2086. [Google Scholar] [CrossRef]
- Guo, Q.; Mei, S.; Xie, C.; Mi, H.; Jiang, Y.; Zhang, S.; Tan, T.; Fan, L. Reprogramming of sugar transport pathways in Escherichia coli Using a permeabilized SecY protein-translocation channel. Biotechnol. Bioeng. 2020, 117, 1738–1746. [Google Scholar] [CrossRef]
- Flores, S.; Gosset, G.; Flores, N.; de Graaf, A.A.; Bolívar, F. Analysis of carbon metabolism in Escherichia coli strains with an inactive phosphotransferase system by 13C labeling and NMR spectroscopy. Metab. Eng. 2002, 4, 124–137. [Google Scholar] [CrossRef]
- Kyselova, L.; Kreitmayer, D.; Kremling, A.; Bettenbrock, K. Type and capacity of glucose transport influences succinate yield in two-stage cultivations. Microb. Cell Factories 2018, 17, 132. [Google Scholar] [CrossRef]
- Jung, H.-M.; Im, D.-K.; Lim, J.H.; Jung, G.Y.; Oh, M.-K. Metabolic perturbations in mutants of glucose transporters and their applications in metabolite production in Escherichia coli. Microb. Cell Factories 2019, 18, 170. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, R.; Noda, S.; Tanaka, T.; Kondo, A. Metabolic engineering of Escherichia coli for shikimate pathway derivative production from glucose–xylose co-substrate. Nat. Commun. 2020, 11, 279. [Google Scholar] [CrossRef] [PubMed]
- Fragoso-Jiménez, J.C.; Gutierrez-Rios, R.M.; Flores, N.; Martinez, A.; Lara, A.R.; Delvigne, F.; Gosset, G. glucose consumption rate-dependent transcriptome profiling of Escherichia coli provides insight on performance as microbial factories. Microb. Cell Factories 2022, 21, 189. [Google Scholar] [CrossRef] [PubMed]

| Gene(s) | Transporter Family | Transported Sugar | PROTEINS | Cellular Location |
|---|---|---|---|---|
| alsBAC | ABC | D-allose | D-allose ABC transporter membrane | P, IM, C |
| araFGH | ABC | L-Arabinose | Arabinose ABC transporter | P, IM, C |
| malEFG-malK | ABC | Maltose/maltodextrine | Maltose ABC transporter | P, IM, C |
| malK | ABC | Maltose/maltotetraose/ maltotriose | Maltose ABC transporter ATP binding subunit | IM |
| mglBAC | ABC | D-galactose/methyl-galactoside | D-galactose/methyl-galactoside ABC transporter | P, IM, C |
| rbsACB | ABC | Ribose/D-xylose | Ribose ABC transporter | P, IM |
| upgBAEC | ABC | sn-Glycerol 3-phosphate | sn-Glycerol 3-phosphate ABC transporter | P, IM, C |
| xylFHG | ABC | D-Xylose | Xylose ABC transporter | P, IM, C |
| yphFED | ABC | Sugar | Putative ABC transporter | P, IM |
| ytfQRT-yjfF | ABC | β-D-Galactofuranose α-D-Galactofuranose | Galactofuranose ABC transporter | P, IM |
| araE | MFS (SP) | Arabinose | Arabinose:H+ symporter | IM |
| dgoT | MFS (ACS) | D-Galactonate | D-Galactonate:H+ symporter | IM |
| fucP | MFS (FHS) | L-Fucose/D-arabinose/ L-galactose | L-fucose:H+ symporter | IM |
| galP | MFS (SP) | D-Galactose | Galactose:H+ symporter | IM |
| garP | MFS (ACS) | Galactarate/D-glucarate | Galactarate/D-glucarate transporter | IM |
| glpT | MFS (OPA) | Glycerol-3-phosphate | sn-glycerol 3-phophate:phosphate antiporter | IM |
| gudP | MFS (ACS) | Galactarate/D-glucarate | Galactarate/D-glucarate transporter | IM |
| lacY | MFS (OHS) | Lactose/melibiose | Lactose/melibiose:H+ symporter | IM |
| lgoT | MFS (ACS) | L-Galactonate | L-Galactonate:H+ symporter | IM |
| setA | MFS (SET) | Lactose | Sugar exporter SetA | IM |
| setB | MFS (SET) | Lactose | Sugar exporter SetB | IM |
| setC | MFS (SET) | Arabinose-like | Putative arabinose exporter | IM |
| uhpC | MFS (OPA) | Sugar phosphate | Inner membrane protein sensing glucose-6-phosphate | IM |
| uhpT | MFS (OPA) | Hexose-6-phosphate | Hexose-6-phosphate:phosphate antiporter | IM |
| xylE | MFS (SP) | Xylose | D-xylose:H+ symporter | IM |
| ydeA | MFS (DHA1) | Arabinose | L-arabinose exporter | |
| agaBCD | PTS | Galactosamine | Galactosamine specific PTS system EIIBCD | IM, C |
| agaV | PTS | n-acetyl-D-galactosamine (galactose) | N-acetyl-D-galactosamine specific PTS system IIB | C |
| ascF | PTS | β-Glucoside (arbutin/cellobiose/salicin) | β-Glucoside specific PTS enzyme IIBC | IM |
| bglF | PTS | β-Glucoside (metil-β-D-glucoside, arbutine, salicin, β-D-glucose) | β-Glucoside specific PTS enzyme II/BglG kinase/BglG phosphatase | IM |
| chbAC | PTS | β-D-Cellobiose/chitobiose (starch, sucrose) | N, N’-diacetyl chitobiose-specific PTS enzyme IIAC | C |
| chbB | PTS | β-D-Cellobiose/chitobiose (starch, sucrose) | N, N’-diacetyl chitobiose-specific PTS enzyme IIB | IM |
| cmtA | PTS | Mannitol (fructose and mannose) | Mannitol-specific PTS enzyme IICB | IM |
| cmtB | PTS | Mannitol (fructose and mannose) | Mannitol-specific PTS enzyme IIA | C |
| fruA | PTS | Fructose and mannose | Fructose-specific PTS multi-phosphoryl transfer protein FruA PTS system EIIBC | IM |
| frvA | PTS | Fructose-like | Putative PTS enzyme IIA | C |
| frvB | PTS | Fructose-like | Putative PTS enzyme IIBC | IM |
| frwB—frwD | PTS | Fructose-like | Fructose-like PTS system EIIB | C |
| frwC | PTS | Fructose-like | Fructose-like PTS system EIIC | IM |
| fryC | PTS | Fructose-like | Fructose-like PTS system EIIC | IM |
| fryB | PTS | Fructose-like | Fructose-like PTS system EIIB | C |
| gatA | PTS | Galactitol | Galactitol-specific PTS system EIIA | C |
| gatB | PTS | Galactitol | Galactitol-specific PTS system EIIB | C |
| glvBC | PTS | α-Glucoside | Alpha-glucoside PTS system EIICB | IM |
| malX | PTS | Maltose/glucose | PTS enzyme IIBC component MalX | IM |
| manYZ | PTS | Mannose | Mannose-specific PTS system EIICD | IM |
| manX | PTS | Mannose | Mannose-specific PTS system EIIAB | IM, C |
| mngA | PTS | 2-O-α-mannosyl-D-glycerate | 2-O-α-mannosyl-D-glycerate specific PTS enzyme IIABC | IM |
| mtlA | PTS | Mannitol (fructose, mannose) | Mannitol-specific PTS enzyme IICBA | IM |
| nagE | PTS | n-Acetylglucosamine | N-acetylglucosamine-specific PTS enzyme II | IM |
| ptsG | PTS | Glucose | Glucose-specific PTS enzyme IIBC component | IM |
| ptsHIcrr | PTS | Glucose | ptsH, phosphor carrier protein HPr ptsI, PTS enzyme I crr, Enzyme IIAGlc | C |
| sgcA | PTS | Galactitol-like | Galactitol-specific PTS system EIIA | C |
| sgcB | PTS | Galactitol-like | Galactitol-specific PTS system EIIB | C |
| sgcC | PTS | Galactitol-like | Galactitol-specific PTS system EIIC | IM |
| srlA | PTS | Glucitol/Sorbitol | Sorbitol specific PTS system IIC2 | IM |
| srlB | PTS | Glucitol/Sorbitol | Sorbitol specific PTS system EIIA | C |
| srlE | PTS | Glucitol/Sorbitol | Sorbitol specific PTS system IIBC1 | IM |
| treB | PTS | Trehalose | Trehalose-specific PTS enzyme IIBC | IM |
| ulaABC | PTS | Ascorbate | L-ascorbate specific PTS system EIICBA | IM, C |
| bglH | OT (C/P) | β-Glycosides | Carbohydrate-specific outer membrane porin, cryptic | OM |
| glpF | OT (MIP) | Glycerol | Glycerol facilitator | IM |
| lamB | OT (C/P) | Maltose | Maltose outer membrane channel/phage lambda receptor protein | OM |
| melB | OT (EDP) | Melibiose | Melibiose:H+/Na+/Li+ symporter | IM |
| ompF | OT (C/P) | Sugar | Outer membrane porin F | OM |
| ompC | OT (C/P) | Sugar | Outer membrane porin C | OM |
| Parental Strain | PTS Mutation | Growth and Relevant Changes in the Expression of Several Genes Involved in Transport Respect the Parental Strain | References | |
|---|---|---|---|---|
| MG1655 | ΔptsG | Aerobic conditions | Anaerobic conditions | [20] |
| Decrement in μ of 73%. Increased expression of galS and down-regulation of galP (0.2 X) and manX (0.5 X). Overexpression of the mgl operon in 10 X. Downregulation of cyaA and increased levels of cAMP: 552.5 X. | Decrement in μ of 70.2%. Increased expression of galS and downregulation of galP. Increased expression of malE (48 X). Overexpression of the mgl operon in 48 X. Down-regulation of cyaA with increased levels of cAMP: 390.9 X. | |||
| JM101 | ΔptsHIcrr | Reduction in μ~85% to 57%. | [73,74] | |
| Overexpression of mglB 13.4 X and lamB 17.6 X. | ||||
| Overexpression of some genes of the gal regulon: galP 12.4 X, galR 3.2X, galS 4.9X. | ||||
| MG1655 | ptsHIcrr KO | Reduction in μ~79%. | [75] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carreón-Rodríguez, O.E.; Gosset, G.; Escalante, A.; Bolívar, F. Glucose Transport in Escherichia coli: From Basics to Transport Engineering. Microorganisms 2023, 11, 1588. https://doi.org/10.3390/microorganisms11061588
Carreón-Rodríguez OE, Gosset G, Escalante A, Bolívar F. Glucose Transport in Escherichia coli: From Basics to Transport Engineering. Microorganisms. 2023; 11(6):1588. https://doi.org/10.3390/microorganisms11061588
Chicago/Turabian StyleCarreón-Rodríguez, Ofelia E., Guillermo Gosset, Adelfo Escalante, and Francisco Bolívar. 2023. "Glucose Transport in Escherichia coli: From Basics to Transport Engineering" Microorganisms 11, no. 6: 1588. https://doi.org/10.3390/microorganisms11061588
APA StyleCarreón-Rodríguez, O. E., Gosset, G., Escalante, A., & Bolívar, F. (2023). Glucose Transport in Escherichia coli: From Basics to Transport Engineering. Microorganisms, 11(6), 1588. https://doi.org/10.3390/microorganisms11061588





