‘Follow the Water’: Microbial Water Acquisition in Desert Soils
Abstract
1. Introduction
2. Desert Soils and Their Water-Holding Capacity
3. Sources of Bioavailable Water in Desert Ecosystems
3.1. Rainfall
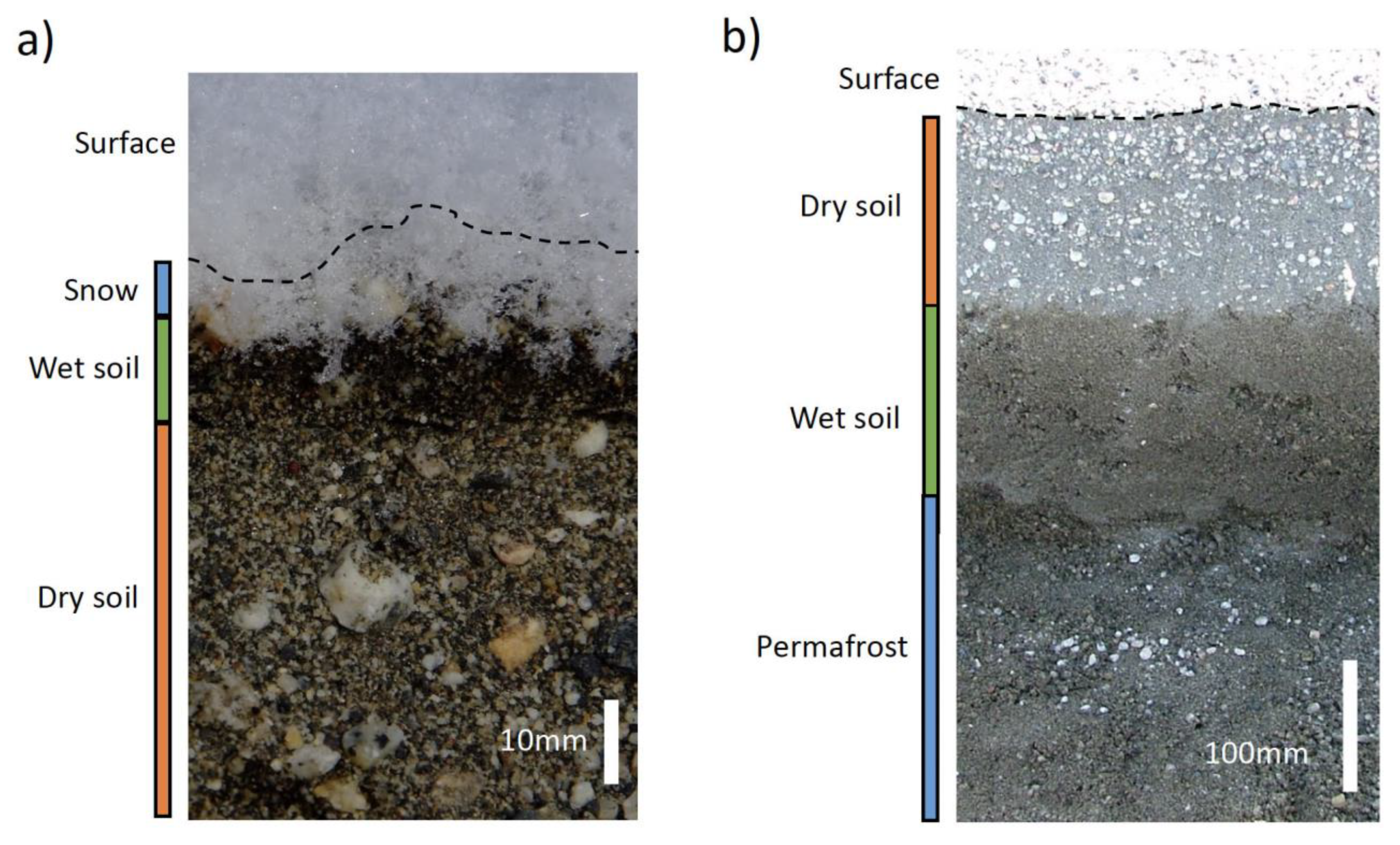
3.2. Glacial Ice
3.3. Dew and Fog
3.4. Groundwater
3.5. Adsorption of Water from the Atmosphere
3.6. Hygroscopic Minerals and Surfaces
3.7. In Situ Hydro-Genesis
4. Implications of Climate Change
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daniel, R.M.; Finney, J.L.; Stoneham, M. The molecular basis of life: Is life possible without water? Philosoph. Trans. R. Soc. Lond. B Biol. Sci. 2004, 359, 1141–1328. [Google Scholar]
- Williford, K.H.; Farley, K.A.; Stack, K.M.; Allwood, A.C.; Beaty, D.; Beegle, L.W.; Bhartia, R.; Brown, A.J.; de la Torre Juarez, M.; Hamran, S.E.; et al. The NASA Mars 2020 Rover Mission and the Search for Extraterrestrial Life. Chapter 11; In From Habitability to Life on Mars; Elsevier Publisher: Amsterdam, The Netherlands, 2018; pp. 275–308. [Google Scholar]
- Rummel, J.D.; Beaty, D.W.; Jones, M.A.; Bakermans, C.; Barlow, N.G.; Boston, P.J.; Chevrier, V.F.; Clark, B.C.; De Vera, J.-P.P.; Gough, R.; et al. A new analysis of Mars “Special Regions”: Findings of the Second MEPAG Special Regions Science Analysis Group (SR-SAG2). Astrobiology 2014, 14, 887–968. [Google Scholar] [CrossRef] [PubMed]
- Pointing, S.B.; Belnap, J. Microbial colonization and controls in dryland systems. Nat. Rev. Microbiol. 2012, 10, 551–562. [Google Scholar] [CrossRef]
- Cary, S.C.; McDonald, I.R.; Barrett, J.E.; Cowan, D.A. On the rocks: The microbiology of Antarctic Dry Valley soils. Nat. Rev. Microbiol. 2010, 8, 129–138. [Google Scholar] [CrossRef]
- Tiao, G.; Lee, C.K.; McDonald, I.R.; Cowan, D.A.; Cary, S.C. Rapid microbial response to the presence of an ancient relic in the Antarctic Dry Valleys. Nat. Commun. 2012, 3, 660. [Google Scholar] [CrossRef]
- Lebre, P.; De Maayer, P.; Cowan, D.A. Xerotolerant prokaryotes: Surviving through a dry spell. Nat. Rev. Microbiol. 2017, 15, 285–296. [Google Scholar] [CrossRef]
- Brown, A.D. Microbial water stress. Bacteriol. Rev. 1976, 40, 803–846. [Google Scholar] [CrossRef]
- Stevenson, A.; Hamill, P.G.; O’Kane, C.J.; Kminek, G.; Rummel, J.D.; Voytek, M.A.; Dijksterhuis, J.; Hallsworth, J.E. Aspergillus penicillioides differentiation and cell division at 0.585 water activity. Environ. Microbiol. 2017, 19, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, A.; Burkhardt, J.; Cockell, C.S.; Cray, J.A.; Dijksterhuis, J.; Fox-Powell, M.; Kee, T.P.; Kminek, G.; McGenity, T.J.; Timmis, K.N.; et al. Multiplication of microbes below 0.690 water activity: Implications for terrestrial and extra-terrestrial life. Environ. Microbiol. 2015, 17, 257–277. [Google Scholar] [CrossRef]
- Gómez-Silva, B. Lithobiontic life: Atacama rocks are well and alive. Anton. Leeuwenhoek 2018, 111, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Cowan, D.A.; Hopkins, D.W.; Jones, B.E.; Maggs-Kölling, G.; Majewska, R.; Ramond, J.-B. Microbiomics of Namib Desert habitats. Extremophiles 2020, 24, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Mehda, S.; Muñoz-Martín, M..; Oustani, M.; Hamdi-Aïssa, B.; Perona, E.; Mateo, P. Lithic cyanobacterial communities in the polyextreme Sahara Desert: Implications for the search for the limits of life. Environ. Microbiol. 2022, 24, 451–474. [Google Scholar] [CrossRef] [PubMed]
- Meslier, V.; Casero, M.C.; Dailey, M.; Wierzchos, J.; Ascaso, C.; Artieda, O.; McCullough, P.R.; DiRuggiero, J. Fundamental drivers for endolithic microbial community assemblies in the hyperarid Atacama Desert. Environ. Microbiol. 2018, 20, 1765–1781. [Google Scholar] [CrossRef]
- Archer, S.D.J.; Lee, K.C.; Caruso, T.; Alcami, A.; Araya, J.G.; Cary, S.C.; Cowan, D.A.; Etchebehere, C.; Gantsetseg, B.; Gomez-Silva, B.; et al. Contribution of soil bacteria to the atmosphere across biomes. STOTEN 2023, 871, 162137. [Google Scholar] [CrossRef]
- Santl-Temkiv, T.; Amato, P.; Casamayor, E.O.; Lee, P.K.H.; Pointing, S.B. Microbial ecology of the atmosphere. FEMS Microbiol. Rev. 2022, 46, fuac009. [Google Scholar] [CrossRef] [PubMed]
- Manabe, S.; Wetherald, R.T. Thermal equilibrium of the atmosphere with a given distribution of relative humidity. In The Warming Papers: A Scientific Foundation for the Climate Change Forcast; Archer, D., Pierrehumbert, R., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2011; pp. 94–115. [Google Scholar]
- Yang, Y.; Zhao, W.; Xiao, X. The upper temperature limit of life under high hydrostatic pressure in the deep biosphere. Deep-Sea Res. Part I Oceanogr. Res. Pap. 2021, 176, 103604. [Google Scholar] [CrossRef]
- McKay, C.P.; Davila, A.F.; Sun, H.J. Cold and Dry Limits of Life. In Astrobiology: An Evolutionary Approach; CRC Press: Boca Raton, FL, USA, 2014; pp. 271–281. [Google Scholar]
- Goordial, J.; Davila, A.; Lacelle, D.; Pollard, W.; Marinova, M.M.; Greer, C.W.; DiRuggiero, J.; McKay, C.P.; Whyte, L.G. Nearing the cold-arid limits of microbial life in permafrost of an upper Dry Valley, Antarctica. ISME J. 2016, 10, 1613–1624. [Google Scholar] [CrossRef]
- Dragone, N.B.; Henley, J.B.; Holland-Moritz, H.; Diaz, M.; Hogg, I.D.; Lyons, W.B.; Wall, D.H.; Adams, B.J.; Fierer, N. Elevational constraints on the composition and genomic attributes of microbial communities in Antarctic soils. mSystems 2022, 7, e01330-21. [Google Scholar] [CrossRef]
- Varsadiya, M.; Urich, T.; Hugelius, G.; Bárta, J. Microbiome structure and functional potential in permafrost soils of the Western Canadian Arctic. FEMS Microb. Ecol. 2021, 97, fiab008. [Google Scholar] [CrossRef]
- Altshuler, I.; Hamel, J.; Turney, S. Species interactions and distinct microbial communities in high Arctic permafrost affected cryosols are associated with the CH4 and CO2 gas fluxes. Environ. Microbiol. 2019, 21, 3711–3727. [Google Scholar] [CrossRef]
- Altshuler, I.; Raymond-Bouchard, I.; Magnuson, E.; Tremblay, J.; Greer, C.W.; Whyte, L.G. Unique high Arctic methane metabolizing community revealed through in situ 13CH4-DNA-SIP enrichment in concert with genome binning. Sci. Rep. 2022, 12, e1160. [Google Scholar] [CrossRef]
- Morozova, D.; Wagner, D. Highly resistant methanogenic archaea from Siberian permafrost as candidates for the possible life on Mars. Int. J. Astrobiol. 2007, 6, 59–87. [Google Scholar]
- Grinberg, M.; Orevi, T.; Steinberg, S.; Kashtan, N. Bacterial survival in microscopic surface wetness. eLife 2019, 8, e48508. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, J.; Hunsche, M. Breath figures on leaf surfaces-formation and effects of microscopic leaf wetness. Front. Plant Sci. 2013, 4, 422. [Google Scholar] [CrossRef]
- Qu, E.; Omelon, C.; Oren, O.; Meslier, V.; Cowan, D.A.; Maggs-Kölling, G.; DiRuggiero, J. Trophic selective pressures organize the composition of endolithic microbial communities from global deserts. Front. Microbiol. 2020, 10, 2952. [Google Scholar] [CrossRef]
- Davila, A.F.; Hawes, I.; Ascaso, C.; Wierzchos, J. Salt deliquescence drives photosynthesis in the hyperarid Atacama Desert. Environ. Microbiol. Rep. 2013, 5, 583–587. [Google Scholar] [CrossRef] [PubMed]
- DiRuggiero, J.J.; Wierzchos, C.K.; Robinson, T.; Souterre, T.; Ravel, J.; Artieda, O.; Souza-Egipsy, V.; Ascaso, C. Microbial colonization of chasmoendolithic habitats in the hyper-arid zone of the Atacama Desert. Biogeosciences 2013, 10, 2439–2450. [Google Scholar] [CrossRef]
- Greve, P.; Roderick, M.L.; Ukkola, A.M.; Wada, Y. The Aridity Index under global warming. Environ. Res. Lett. 2019, 14, 124006. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Wolf, A.B.; Vos, M.; de Boer, W.; Kowalchuk, G.A. Impact of matric potential and pore size distribution on growth dynamics of filamentous and non-filamentous soil bacteria. PLoS ONE 2013, 8, e83661. [Google Scholar] [CrossRef]
- Eckardt, F.D.; Soderberg, K.; Coop, L.J. The nature of moisture at Gobabeb, in the central Namib Desert. J. Arid Environ. 2013, 93, 7–19. [Google Scholar] [CrossRef]
- Obryk, M.K.; Doran, P.T.; Fountain, A.G.; Myers, M.; McKay, C.P. Climate from the McMurdo Dry Valleys, Antarctica, 1986–2017: Surface air temperature trends and redefined summer season. J. Geophys. Res. Atmos. 2020, 125, e2019JD032180. [Google Scholar] [CrossRef]
- Fountain, A.G.; Nylen, T.H.; Monaghan, A.; Basagic, H.J.; Bromwich, D. Snow in the McMurdo Dry Valleys, Antarctica. Int. J. Climatol. 2010, 30, 633–642. [Google Scholar] [CrossRef]
- Hopkins, D.W.; Sparrow, A.D.; Novis, P.M.; Gregorich, E.; Elberling, B.; Greenfield, L. Controls on the distribution of productivity and organic resources in Antarctic Dry Valley soils. Proc. R. Soc. B 2006, 273, 2687–2695. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.J. Endolithic microbial life in extreme cold climate; Snow is required, but perhaps less is more. Biology 2013, 2, 693–701. [Google Scholar] [CrossRef]
- Weber, B.; Belnap, J.; Budel, B.; Antoninka, A.J.; Barger, N.N.; Chaudhary, V.B.; Darrouzet-Nardi, A.; Eldridge, D.J.; Faist, A.M.; Ferrenberg, S.; et al. What is a biocrust? A refined, contemporary definition for a broadening research community. Biol. Rev. Camb. Philos. Soc. 2022, 97, 1768–1785. [Google Scholar] [CrossRef]
- Francis, M.L.; Fey, M.V.; Prinsloo, H.P.; Ellis, F.; Mills, A.; Medinski, T. Soils of Namaqualand: Compensations for aridity. J. Arid Environ. 2007, 70, 588–603. [Google Scholar] [CrossRef]
- Alvarez, A. Sepiolite: Properties and uses. In Palygorskite–Sepiolite: Occurrences, Genesis and Uses. Developments in Sedimentology; Singer, A., Galan, E., Eds.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1984; Volume 37, pp. 253–287. [Google Scholar]
- Francis, M.L. Effect of sepiolite and palygorskite on plant available water in Arenosols of Namaqualand, South Africa. Geoderma Reg. 2019, 17, e00022. [Google Scholar] [CrossRef]
- Eckardt, F. Saline Soils. In Encyclopaedia of Engineering Geology; Encyclopaedia of Earth Sciences, Series; Bobrowsky, P.T., Marker, B., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Cosby, B.J.; Hornberger, G.M.; Clapp, R.B.; Ginn, T. A statistical exploration of the relationships of soil moisture characteristics to the physical properties of soils. Water Resour. Res. 1984, 20, 682–690. [Google Scholar] [CrossRef]
- Pfeiffer, M.; Morgan, A.; Heimsath, A.; Jordan, T.; Howard, A.; Amundson, R. Century scale rainfall in the absolute Atacama Desert: Landscape response and implications for past and future rainfall. Quart. Sci. Rev. 2021, 254, 106797. [Google Scholar] [CrossRef]
- Schulze-Makuch, D.; Wagner, D.; Kounaves, S.P.; Mangelsdorf, K.; Devine, K.G.; de Vera, J.-P.; Schmitt-Kopplin, P.; Grossart, H.-P.; Parro, V.; Kaupenjohann, M.; et al. Transitory microbial habitat in the hyperarid Atacama Desert. Proc. Natl. Acad. Sci. USA 2018, 115, 2670–2675. [Google Scholar] [CrossRef]
- Warren-Rhodes, K.A.; Lee, K.C.; Archer, S.D.J.; Cabrol, N.; Ng-Boyle, L.; Wettergreen, D.; Zacny, K.; Pointing, S.B.; NASA Life in the Atacama Project Team. Subsurface microbial habitats in an extreme desert Mars-analog environment. Front. Microbiol. 2019, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.; Valverde, A.; Ramond, J.B.; Makhalanyane, T.P.; Jansson, J.K.; Hopkins, D.W.; Aspray, T.J.; Seely, M.; Trindade, M.I.; Cowan, D.A. Temporal dynamics of hot desert microbial communities reveal structural and functional responses to water input. Sci. Rep. 2016, 6, 34434. [Google Scholar] [CrossRef] [PubMed]
- Hotaling, S.; Lutz, S.; Dial, R.J.; Anesio, A.M.; Benning, L.G.; Fountain, A.G.; Kelley, J.L.; McCutcheon, J.; Skiles, S.M.; Takeuchi, N.; et al. Biological albedo reduction on ice sheets, glaciers, and snowfields. Earth Sci. Rev. 2021, 220, 103728. [Google Scholar] [CrossRef]
- Anesio, A.M.; Lutz, S.; Chrismas, N.A.M.; Benning, L.G. The microbiome of glaciers and ice sheets. Biofilms Microbiomes 2017, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Skidmore, M.L.; Foght, J.M.; Sharp, M.J. Microbial life beneath a high Arctic glacier. Appl. Environ. Microbiol. 2000, 66, 3214–3220. [Google Scholar] [CrossRef]
- Robinson, P.J. Temporal trends in United States dew point temperature. Int. J. Climatol. 2000, 20, 985–1002. [Google Scholar] [CrossRef]
- Zangvil, A. Six years of dew observations in the Negev Desert, Israel. J. Arid Environ. 1996, 32, 361–371. [Google Scholar] [CrossRef]
- Linacre, E.T. Climate Data and Resources; Routledge: London, UK, 1992; 366p. [Google Scholar]
- Zhuang, Y.; Zhao, W.; Luo, L.; Wang, L. Dew formation characteristics in the gravel desert ecosystem and its ecological roles on Reaumuria soongorica. J. Hydrol. 2021, 603, 126932. [Google Scholar] [CrossRef]
- Lange, O.L.; Geiger, I.L.; Schulze, E.D. Ecophysiological investigations on lichens of the Negev desert. Oecologia 1977, 28, 247–259. [Google Scholar] [CrossRef]
- Pintado, A.; Sancho, L.G.; Green, T.G.A.; Blanquer, J.M.; Lázaro, R. Functional ecology of the biological soil crust in semiarid SE Spain: Sun and shade populations of Diploschistes diacapsis (Ach.). Lumbsch. Lichenologist 2005, 37, 425–432. [Google Scholar] [CrossRef]
- Del Prado, R.; Sancho, L.G. Dew as a key factor for the distribution pattern of the lichen species Teloschistes lacunosus in the Tabernas Desert (Spain). Flora 2007, 202, 417–428. [Google Scholar] [CrossRef]
- Cereceda, P.; Larrain, H.; Osses, P.; Farías, M.; Egaña, I. The spatial and temporal variability of fog and its relation to fog oases in the Atacama Desert, Chile. Atmos. Res. 2008, 87, 312–323. [Google Scholar] [CrossRef]
- Cermak, J. Low clouds and fog along the South-Western African coast—Satellite-based retrieval and spatial patterns. Atmos. Res. 2012, 116, 15–21. [Google Scholar] [CrossRef]
- Schieferstein, B.; Loris, K. Ecological investigations on lichen fields of the Central Namib. Vegetatio 1992, 98, 113–128. [Google Scholar] [CrossRef]
- Hinchliffe, G.; Bollard-Breen, B.; Cowan, D.A.; Doshi, A.; Gillman, L.N.; Maggs-Kolling, G.; Rios, A.D.L.; Pointing, S.B. Advanced photogrammetry to assess lichen colonization in the hyper-arid Namib Desert. Front. Microbiol. 2017, 8, 2083. [Google Scholar] [CrossRef] [PubMed]
- Roth-Nebelsick, A.; Ebner, M.; Miranda, T.; Gottschalk, V.; Voigt, D.; Gorb, S.; Stegmaier, T.; Sarsour, J.; Linke, M.; Konrad, W. Leaf surface structures enable the endemic Namib desert grass Stipagrostis sabulicola to irrigate itself with fog water. J. R. Soc. Interface 2012, 9, 1965–1974. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, W.J.; Seely, M.K. Fog basking by the Namib Desert beetle, Onymacris unguicularis. Nature 1976, 262, 284–285. [Google Scholar] [CrossRef]
- Wierzchos, J.; Cámara, B.; De Los Ríos, A.; Davila, A.F.; Almazo, I.M.S.; Artieda, O.; Wierzchos, K.; Gómez-Silva, B.; Mckay, C.; Ascaso, C. Microbial colonization of Ca-sulfate crusts in the hyperarid core of the Atacama Desert: Implications for the search for life on Mars. Geobiology 2010, 9, 44–60. [Google Scholar] [CrossRef] [PubMed]
- Azúa-Bustos, A.; González-Silva, C.; Mancilla, R.A.; Salas, L.; Gómez-Silva, B.; McKay, C.P.; Vicuña, R. Hypolithic cyanobacteria supported mainly by fog in the coastal range of the Atacama Desert. Microb. Ecol. 2011, 61, 568–581. [Google Scholar] [CrossRef]
- Warren-Rhodes, K.A.; McKay, C.P.; Boyle, L.N.; Wing, M.R.; Kiekebusch, E.M.; Cowan, D.A.; Stomeo, F.; Pointing, S.B.; Kaseke, K.F.; Eckardt, F.; et al. Physical ecology of hypolithic communities in the central Namib desert: The role of fog, rain, rock habitat and light. J. Geophys. Res. 2013, 118, 1451–1460. [Google Scholar] [CrossRef]
- Uritskiy, G.; Tisza, M.J.; Gelsinger, D.R.; Munn, A.; Taylor, J.; DiRuggiero, J. Cellular life from the three domains and viruses are transcriptionally active in a hypersaline desert community. Environ. Microbiol. 2021, 23, 3401–3417. [Google Scholar] [CrossRef]
- Marasco, R.; Mosqueira, M.J.; Fusi, M.; Ramond, J.-B.; Merlino, G.; Booth, J.M.; Maggs-Kölling, G.; Cowan, D.; Daffonchio, D. Rhizosheath microbial community assembly of sympatric desert speargrasses is independent of the plant host. Microbiome 2018, 6, 215. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.K.; Wierzchos, J.; Black, C.; Crits-Christoph, A.; Ma, B.; Ravel, J.; Ascaso, C.; Artieda, O.; Valea, S.; Roldán, M.; et al. Microbial diversity and the presence of algae in halite endolithic communities are correlated to atmospheric moisture in the hyper-arid zone of the Atacama Desert. Environ. Microbiol. 2015, 17, 299. [Google Scholar] [CrossRef]
- Uritskiy, G.; Munn, A.; Dailey, M.; Gelsinger, D.R.; Getsin, S.; Davila, A.; McCullough, P.R.; Taylor, J.; DiRuggiero, J. Environmental factors driving spatial heterogeneity in desert halophile microbial communities. Front. Microbiol. 2020, 11, 578660. [Google Scholar] [CrossRef]
- Mikucki, J.; Auken, E.; Tulaczyk, S.; Virginia, R.A.; Schamper, C.; Sørensen, K.I.; Doran, P.T.; Dugan, H.; Foley, N. Deep groundwater and potential subsurface habitats beneath an Antarctic Dry Valley. Nat. Commun. 2015, 6, 6831. [Google Scholar] [CrossRef]
- Batista, R.F.; Reichert, J.; Holthusen, D.; Batistão, A.C.; Daher, M.; Schünemann, A.L.; Filho, E.I.F.; Schaefer, C.E.G.R.; Francelino, M.R. Freeze–thaw cycles affecting rheological properties of Antarctic soils. Geoderma 2022, 428, 116220. [Google Scholar] [CrossRef]
- Evans, D.D.; Thames, J.L. Water in Desert Ecosystems; US/IBP Synthesis Series; Academic Press: Cambridge, MA, USA, 1981; Volume 11, 280p. [Google Scholar]
- Du, C.; Yu, J.; Wang, P.; Zhang, Y. Analysing the mechanisms of soil water and vapour transport in the desert vadose zone of the extremely arid region of northern China. J. Hydrol. 2018, 558, 592–606. [Google Scholar] [CrossRef]
- Lange, O.L.; Green, T.C.A.; Melzer, B.; Meyer, A.; Zellner, H. Water relations and CO2 exchange of the terrestrial lichen Teloschistes capensis in the Namib fog desert: Measurements during two seasons in the field and under controlled conditions. Flora 2006, 201, 268–280. [Google Scholar] [CrossRef]
- Glaser, D.M.; Hartnett, H.E.; Finn, D.R.; Perez-Montaño, S.; Cadillo-Quiroz, H.; Desch, S. Water vapor adsorption provides daily, sustainable water to soils of the hyperarid Atacama Desert. Astrobiology 2022, 22, 1222. [Google Scholar] [CrossRef]
- Kaur, N.; Dey, P. Bacterial exopolysaccharides as as emerging bioactive macromolecules: From fundamentals to applications. Res. Microbiol. 2023, 174, 104024. [Google Scholar] [CrossRef]
- Fleming, H.-C.; Wingender, J. Relevance of microbial extracellular polymeric substances (EPSs)—Part I: Structural and ecological aspects. Water Sci. Technol. 2001, 43, 1–8. [Google Scholar] [CrossRef]
- Wu, B.; Wang, H.; Li, W.; Dai, X.; Chai, X. Influential mechanism of water occurrence states of waste-activated sludge: Potential linkage between water-holding capacity and molecular compositions of EPS. Water Res. 2022, 213, 118169. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-L.; Wang, L.-F.; Ren, X.-M.; Ye, X.-D.; Li, W.-W.; Yuan, S.-J.; Sun, M.; Sheng, G.P.; Yu, H.-Q.; Wang, X.K. pH dependence of structure and surface properties of microbial EPS. Environ. Sci. Technol. 2012, 46, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Razafindralambo, H.; Blecker, C.; N’yapo, C.; Thonart, P.; Delvigne, F. Stochastic exposure to sub-lethal high temperature enhances exopolysaccharides (EPS) excretion and improves Bifidobacterium bifidum cell survival to freeze–drying. Biochem. Eng. J. 2014, 188, 85–94. [Google Scholar] [CrossRef]
- Baubin, C.; Ran, N.; Siebner, H.; Gillor, O. Divergence of biocrust active bacterial communities in the Negev Desert during a hydration-desiccation cycle. Microb. Ecol. 2023, 86, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Costa, O.Y.A.; Raaijmakers, J.M.; Kuramae, E.E. Microbial extracellular polymeric substances: Ecological function and impact on soil aggregation. Front. Microbiol. 2018, 9, 1636. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Da Costa, M.S.; Santos, H.; Galinski, E.A. An overview of the role and diversity of compatible solutes in Bacteria and Archaea. Adv. Biochem. Eng. Biotechnol. 1998, 61, 118–153. [Google Scholar]
- Wyatt, T.T.; Golovina, E.A.; van Leeuwen, M.R.; Hallsworth, J.E.; Wösten, H.A.; Dijksterhuis, J. Decreases in bulk water and mannitol and accumulation of trehelose and trehelose-based oligosaccharides define a two-stage maturation process towards extreme stress resistance in ascospores of Neosartorya fischerii (Aspergillus fischerii). Environ. Microbiol. 2014, 17, 383–394. [Google Scholar] [CrossRef]
- Fakes, M.G.; Dali, M.V.; Haby, T.A.; Morris, K.R.; Varia, S.A.; Serajuddin, A.T. Moisture sorption behaviour of selected bulking agents used in lyophilized products. PDA J. Pharm. Sci. Technol. 2000, 54, 144–149. [Google Scholar]
- Bosch, J.; Marais, E.; Maggs-Kölling, G.; Ramond, J.B.; Lebre, P.H.; Eckardt, F.; Cowan, D.A. Water inputs across the Namib Desert: Implications for dryland edaphic microbiology. Front. Biogeogr. 2022, 14, e55302. [Google Scholar] [CrossRef]
- Ewing, G.E. Thin film water. J. Phys. Chem. B 2004, 108, 15953–15961. [Google Scholar] [CrossRef]
- Wang, J.; Kalinichev, A.G.; Kirkpatrick, R.J. Effects of substrate structure and composition on the structure, dynamics, and energetics of water at mineral surfaces: A molecular dynamics modeling study. Geochim. Cosmochim. Acta 2006, 70, 562–582. [Google Scholar] [CrossRef]
- Mohlmann, D. Widen the belt of habitability. Orig. Life Evol. Biosph. 2012, 42, 93–100. [Google Scholar] [CrossRef]
- Mohlmann, D. Are nanometric films of liquid undercooled interfacial water bio-relevant? Cryobiology 2009, 58, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Qvit-Raz, N.; Jurkevitch, E.; Belkin, S. Drop-size soda lakes: Transient microbial habitats on a salt-secreting desert tree. Genetics 2008, 178, 1615–1622. [Google Scholar] [CrossRef]
- Burch, A.Y.; Zeisler, V.; Yokota, K.; Schreiber, L.; Lindow, S.E. The hygroscopic biosurfactant syringafactin produced by Pseudomonas syringae enhances fitness on leaf surfaces during fluctuating humidity. Environ. Microbiol. 2014, 16, 2086–2098. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, A.; Hallsworth, J.E. Water and temperature relations of soil Actinobacteria. Environ. Microbiol. Rep. 2014, 6, 744–755. [Google Scholar] [CrossRef]
- Azua-Bustos, A.; Fairén, A.G.; Silva, C.G.; Carrizo, D.; Fernández-Martínez, M..; Arenas-Fajardo, C.; Fernández-Sampedro, M.; Gil-Lozano, C.; Sánchez-García, L.; Ascaso, C.; et al. Inhabited subsurface wet smectites in the hyperarid core of the Atacama Desert as an analog for the search for life on Mars. Sci. Rep. 2020, 10, 19183. [Google Scholar] [CrossRef]
- Huang, W.; Erteki, E.; Wang, T.; Cruz, L.; Dailey, M.; DiRuggiero, J.; Kisailus, D. Mechanism of water extraction from gypsum rock by desert colonizing microorganisms. Proc. Natl. Acad. Sci. USA 2020, 117, 10681–10687. [Google Scholar] [CrossRef]
- Kreuzer-Martin, H.W.; Ehrlinger, J.R.; Hegg, E.L. Oxygen isotopes indicate most intracellular water in log-phase Escherichia coli is derived from metabolism. Proc. Natl. Acad. Sci. USA 2005, 102, 17337–17341. [Google Scholar] [CrossRef]
- Leung, P.M.; Bay, S.; Meier, D.; Chiri, E.; Cowan, D.A.; Gillor, O.; Woebken, D.; Greening, C. Energetic and trophic basis of microbial persistence in desert ecosystems. mSystems 2020, 5, e00495-19. [Google Scholar] [CrossRef]
- Bosch, J.; Varliero, G.; Hallsworth, J.; Dallas, T.D.; Hopkins, D.; Frey, B.; Kong, W.; Lebre, P.; Makhalanyane, T.P.; Cowan, D.A. Microbial anhydrobiosis. Environ. Microbiol. 2021, 23, 6377–6390. [Google Scholar] [CrossRef] [PubMed]
- Katoh, H.; Asthana, R.K.; Ohmori, M. Gene expression in the cyanobacterium Anabaena sp. PCC7120 under desiccation. Microb. Ecol. 2004, 47, 164–174. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, J.C.; Gonçalves, E.R.; Mohn, W.W. Global response to desiccation stress in the soil actinomycete Rhodococcus jostii RHA1. Appl. Environ. Microbiol. 2008, 74, 2627–2636. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, A.; Golovina, E.A.; Gervais, P.; Dupont, S.; Beney, L. Anhydrobiosis: Inside yeast cells. Biotechnol. Adv. 2019, 37, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Greening, C.; Van Wonterghem, I.; Carere, C.R.; Bay, S.K.; Steen, J.A.; Montgomery, K.; Lines, T.; Beardall, J.; van Dorst, J.; et al. Atmospheric trace gases support primary production in Antarctic desert surface soil. Nature 2017, 552, 400–403. [Google Scholar] [CrossRef]
- Jordaan, K.; Lappan, R.; Dong, X.; Aitkenhead, I.J.; Bay, S.K.; Chiri, E.; Wieler, N.; Meredith, L.K.; Cowan, D.A.; Chown, S.L.; et al. Hydrogen-oxidising bacteria are abundant in hot desert soils and strongly stimulated by hydration. mSystems 2020, 5, e01131. [Google Scholar] [CrossRef]
- Ortiz, M.; Leung, P.M.; Shelley, G.; Jirapanjawat, T.; Nauer, P.A.; Van Goethem, M.W.; Bay, S.K.; Islam, Z.F.; Jordaan, K.; Vikram, S.; et al. Multiple energy sources and metabolic strategies sustain microbial diversity in Antarctic desert soils. Proc. Natl. Acad. Sci. USA 2021, 118, e2025322118. [Google Scholar] [CrossRef]
- Ray, A.E.; Zaugg, J.; Benaud, N.; Chelliah, D.S.; Bay, S.; Wong, H.L.; Leung, P.M.; Ji, M.; Terauds, A.; Montgomery, K.; et al. Atmospheric chemosynthesis is phylogenetically and geographically widespread and contributes significantly to carbon fixation throughout cold deserts. ISME J. 2022, 16, 2547–2560. [Google Scholar] [CrossRef]
- Greening, C.; Grinter, R. Microbial oxidation of atmospheric trace gases. Nat. Rev. Microbiol. 2022, 20, 513–528. [Google Scholar] [CrossRef] [PubMed]
- León-Sobrino, C.; Ramond, J.-B.; Maggs-Kölling, G.; Cowan, D.A. Nutrient acquisition, rather than stress response over diel cycles, drives microbial transcription in a hyper-arid Namib Desert soil. Front. Microbiol. 2019, 10, 1045. [Google Scholar] [CrossRef]
- Gunnigle, E.; Frossard, A.; Ramond, J.B.; Guerrero, L.; Seely, M.; Cowan, D.A. Diel-scale temporal dynamics recorded for bacterial groups in Namib Desert soil. Sci. Rep. 2017, 7, 40189. [Google Scholar] [CrossRef]
- Midgley, G.F.; Bond, W.J. Future of African terrestrial biodiversity and ecosystems under anthropogenic climate change. Nat. Clim. Change 2015, 5, 823–829. [Google Scholar] [CrossRef]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future köppen-geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef] [PubMed]
- Corrado, R.; Cherubini, A.M.; Pennetta, C. Early warning signals of desertification transitions in semiarid ecosystems. Phys. Rev. E 2014, 90, 062705. [Google Scholar] [CrossRef] [PubMed]
- Román-Palacios, C.; Wiens, J.J. Recent responses to climate change reveal the drivers of species extinction and survival. Proc. Natl. Acad. Sci. USA 2019, 117, 4211–4217. [Google Scholar] [CrossRef]
- Change, C. The IPCC Scientific Assessment; Houghton, J.T., Jenkins, G., Ephraums, J.J., Eds.; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Olivares, J.; Bedmar, E.J.; Sanjuán, J. Biological nitrogen fixation in the context of global change. Molec. Plant-Microb. Interact. 2013, 26, 486–494. [Google Scholar] [CrossRef]
- Hassol, S.J. Impacts of a Warming Arctic-Arctic Climate Impact Assessment; Cambridge University Press: Cambridge, UK, 2004; 139p. [Google Scholar]
- Barrett, J.E.; Virginia, R.A.; Wall, D.H.; Doran, P.T.; Fountain, A.G.; Welch, K.A.; Lyons, W.B. Persistent effects of a discrete warming event on a polar desert ecosystem. Glob. Change Biol. 2008, 14, 2249–2261. [Google Scholar] [CrossRef]
- Cowan, D.A.; Ah Tow, L. Endangered Antarctic environments. Ann. Rev. Microbiol. 2004, 58, 649–690. [Google Scholar] [CrossRef] [PubMed]
- Gutt, J.; Isla, E.; Xavier, J.C.; Adams, B.J.; Ahn, I.; Cheng, C.C.; Colesie, C.; Cummings, V.J.; di Prisco, G.; Griffiths, H.; et al. Antarctic ecosystems in transition—Life between stresses and opportunities. Biol. Rev. 2021, 96, 798–821. [Google Scholar] [CrossRef]
- Lee, J.R.; Raymond, B.; Bracegirdle, T.J.; Chadès, I.; Fuller, R.A.; Shaw, J.D.; Terauds, A. Climate change drives expansion of Antarctic ice-free habitat. Nature 2017, 547, 49–54. [Google Scholar] [CrossRef]
- Cavicchioli, R.; Ripple, W.J.; Timmis, K.N.; Azam, F.; Bakken, L.R.; Baylis, M.; Behrenfeld, M.J.; Boetius, A.; Boyd, P.W.; Classen, A.T.; et al. Scientists’ warning to humanity: Microorganisms and climate change. Nat. Rev. Microbiol. 2019, 17, 569–586. [Google Scholar] [CrossRef] [PubMed]
- Colby, G.A.; Ruuskanen, M.O.; St. Pierre, K.A.; St. Louis, V.L.; Poulain, A.J.; Aris-Brosou, S. Warming climate is reducing the diversity of dominant microbes in the largest high Arctic lake. Front. Microbiol. 2020, 11, 561194. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.C.; Caruso, T.; Archer, S.D.J.; Gillman, L.N.; Lau, M.C.; Cary, S.C.; Lee, C.K.; Pointing, S.B. Stochastic and deterministic effects of a moisture gradient on soil microbial communities in the McMurdo Dry Valleys of Antarctica. Front. Microbiol. 2018, 9, 2619. [Google Scholar] [CrossRef]
- Niederberger, T.D.; Sohm, J.A.; Tirindelli, J.; Gunderson, T.; Capone, D.G.; Carpenter, E.J.; Cary, S.C. Diverse and highly active diazotrophic assemblages inhabit ephemerally wetted soils of the Antarctic Dry Valleys. FEMS. Microbiol. Ecol. 2012, 82, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Chan, Y.; Lacap, D.C.; Hyde, K.D.; Pointing, S.B.; Farrell, R.L. Low-diversity fungal assemblage in an Antarctic Dry Valleys soil. Polar Biol. 2011, 35, 567–574. [Google Scholar] [CrossRef]
- Philippot, L.; Griffiths, B.S.; Langenheder, S. Microbial community resilience across ecosystems and multiple disturbances. Microbiol. Mol. Biol. Rev. 2021, 85, e00026-20. [Google Scholar] [CrossRef]
- Uritskiy, G.; Getsin, S.; Munn, A.; Gomez-Silva, B.; Davila, A.; Glass, B.; Taylor, J.; DiRuggiero, J. Halophilic microbial community compositional shift after a rare rainfall in the Atacama Desert. ISME J. 2019, 13, 2737–2749. [Google Scholar] [CrossRef]
- Cruz-Martínez, K.; Suttle, K.; Brodie, E.; Power, M.E.; Andersen, G.L.; Banfield, J.F. Despite strong seasonal responses, soil microbial consortia are more resilient to long-term changes in rainfall than overlying grassland. ISME J. 2009, 3, 738–744. [Google Scholar] [CrossRef]
- Hawkes, C.V.; Keitt, T.H. Resilience vs. historical contingency in microbial responses to environmental change. Ecol. Lett. 2015, 18, 612–625. [Google Scholar] [CrossRef]
- Evans, S.E.; Wallenstein, M.D. Climate change alters ecological strategies of soil bacteria. Ecol. Lett. 2014, 17, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Mariotte, P.; Canarini, A.; Dijkstra, F.A. Stoichiometric N:P flexibility and mycorrhizal symbiosis favour plant resistance against drought. J. Ecol. 2017, 105, 958–967. [Google Scholar] [CrossRef]
- Fiodor, A.; Singh, S.; Pranaw, K. The contrivance of plant growth promoting microbes to mitigate climate change impact in agriculture. Microorganisms 2021, 9, 1841. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.; Weinand, T.; Asch, F. Plant-rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ. 2009, 32, 1682–1694. [Google Scholar] [CrossRef] [PubMed]
- De Vries, F.; Liiri, M.; Bjørnlund, L.; Bowker, M.A.; Christensen, S.; Setälä, H.M.; Bardgett, R.D. Land use alters the resistance and resilience of soil food webs to drought. Nat. Clim. Change 2012, 2, 276–280. [Google Scholar] [CrossRef]
- Orwin, K.; Wardle, D.A. Plant species composition effects on belowground properties and the resistance and resilience of the soil microflora to a drying disturbance. Plant Soil 2005, 278, 205–221. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cowan, D.A.; Cary, S.C.; DiRuggiero, J.; Eckardt, F.; Ferrari, B.; Hopkins, D.W.; Lebre, P.H.; Maggs-Kölling, G.; Pointing, S.B.; Ramond, J.-B.; et al. ‘Follow the Water’: Microbial Water Acquisition in Desert Soils. Microorganisms 2023, 11, 1670. https://doi.org/10.3390/microorganisms11071670
Cowan DA, Cary SC, DiRuggiero J, Eckardt F, Ferrari B, Hopkins DW, Lebre PH, Maggs-Kölling G, Pointing SB, Ramond J-B, et al. ‘Follow the Water’: Microbial Water Acquisition in Desert Soils. Microorganisms. 2023; 11(7):1670. https://doi.org/10.3390/microorganisms11071670
Chicago/Turabian StyleCowan, Don A, S. Craig Cary, Jocelyne DiRuggiero, Frank Eckardt, Belinda Ferrari, David W. Hopkins, Pedro H. Lebre, Gillian Maggs-Kölling, Stephen B. Pointing, Jean-Baptiste Ramond, and et al. 2023. "‘Follow the Water’: Microbial Water Acquisition in Desert Soils" Microorganisms 11, no. 7: 1670. https://doi.org/10.3390/microorganisms11071670
APA StyleCowan, D. A., Cary, S. C., DiRuggiero, J., Eckardt, F., Ferrari, B., Hopkins, D. W., Lebre, P. H., Maggs-Kölling, G., Pointing, S. B., Ramond, J.-B., Tribbia, D., & Warren-Rhodes, K. (2023). ‘Follow the Water’: Microbial Water Acquisition in Desert Soils. Microorganisms, 11(7), 1670. https://doi.org/10.3390/microorganisms11071670







