First Report of Campylobacter jejuni Strains Belonging to ST-21 Clonal Complex Isolated from Human, Poultry and Wild Birds in Croatia: Antimicrobial Resistance and Genetic Distance
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparing the Bacterial Isolates
2.1.1. Campylobacter Isolates
2.1.2. Species Confirmation
2.1.3. Antimicrobial Susceptibility Testing (AST)
2.2. Whole Genome Sequencing
2.2.1. Genomics
2.2.2. Genetic Distance Analysis
2.2.3. Identification of Antibiotic Resistance Genes
3. Results
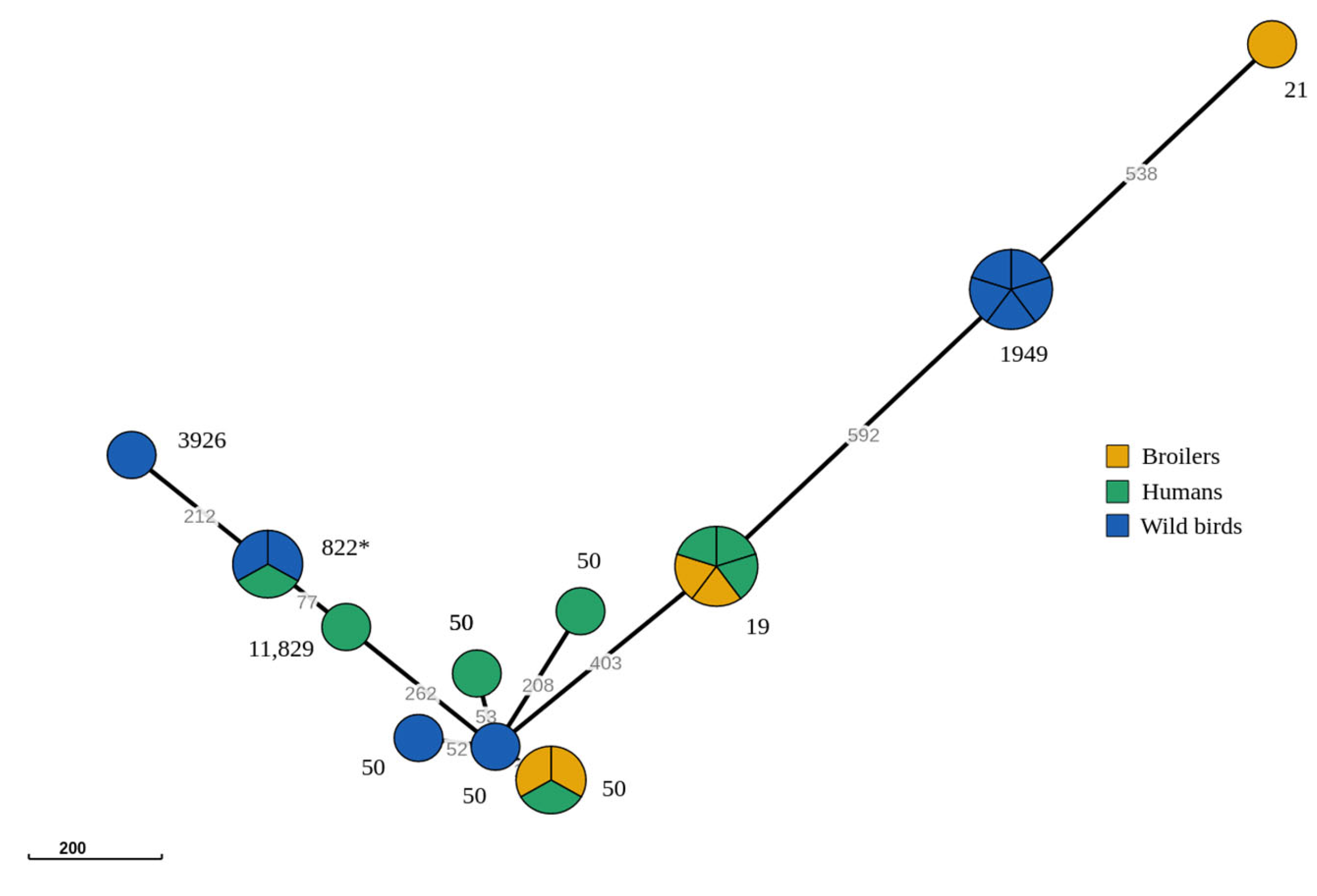
3.1. PCR Identification and Genetic Distance Analysis
3.2. Genomic Relationship amongst C. jejuni ST-21 CC Strains
3.3. Antibiotic Resistance Phenotypes
3.4. Identification of Antibiotic Resistance Genes and Mutations
3.5. Correlations between Phenotypic and Genotypic Resistance
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- ECDC: European Centre for Disease Prevention and Control. Assessing the Health Burden of Infections with Antibiotic-Resistant Bacteria in the EU/EEA, 2016–2020; ECDC: Stockholm, Sweden, 2022. [Google Scholar]
- Jonas, O.B.; Irwin, A.; Berthe, F.C.J.; Le Gall, F.G.; Marquez, P.V. Drug-Resistant Infections: A Threat to Our Economic Future (Vol. 2): Final Report (English); HNP/Agriculture Global Antimicrobial Resistance Initiative; World Bank: Washington, DC, USA, 2017; Available online: http://documents.worldbank.org/curated/en/323311493396993758/final-report (accessed on 29 December 2022).
- EFSA (European Food Safety Authority); ECDC (European Centre for Disease Prevention and Control). The European Union One Health 2021 Zoonoses Report. EFSA J. 2022, 20, e07666. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Campylobacteriosis. In Annual Epidemiological Report for 2021; ECDC: Stockholm, Sweden, 2022. [Google Scholar]
- Pergola, S.; Franciosini, M.P.; Comitini, F.; Ciani, M.; De Luca, S.; Bellucci, S.; Menchetti, L.; Casagrande Proietti, P. Genetic diversity and antimicrobial resistance profiles of Campylobacter coli and Campylobacter jejuni isolated from broiler chicken in farms and at time of slaughter in central Italy. J. Appl. Microbiol. 2017, 122, 1348–1356. [Google Scholar] [CrossRef]
- Iannetti, S.; Calistri, P.; Di Serafino, G.; Marotta, F.; Alessiani, A.; Antoci, S.; Neri, D.; Perilli, M.; Iannitto, G.; Iannetti, L.; et al. Campylobacter jejuni and Campylobacter coli: Prevalence, contamination levels, genetic diversity and antibiotic resistance in Italy. Vet. Ital. 2020, 56, 23–34. [Google Scholar]
- Popa, S.A.; Morar, A.; Ban-Cucerzan, A.; Tîrziu, E.; Herman, V.; Sallam, K.I.; Morar, D.; Acaroz, U.; Imre, M.; Florea, T.; et al. Occurrence of Campylobacter spp. and Phenotypic Antimicrobial Resistance Profiles of Campylobacter jejuni in Slaughtered Broiler Chickens in North-Western Romania. Antibiotics 2022, 11, 1713. [Google Scholar] [CrossRef] [PubMed]
- Jolley, K.A.; Bray, J.E.; Maiden, M.C.J. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res. 2018, 3, 124. [Google Scholar] [CrossRef] [PubMed]
- Šoprek, S.; Duvnjak, S.; Kompes, G.; Jurinović, L.; Tambić Andrašević, A. Resistome Analysis of Campylobacter jejuni Strains Isolated from Human Stool and Primary Sterile Samples in Croatia. Microorganisms 2022, 10, 1410. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs, Consolidated Version. Available online: http://data.europa.eu/eli/reg/2005/2073/oj (accessed on 2 February 2023).
- Commission Regulation (EC) No 2160/2003 of the European Parliament and of the Council of 17 November 2003 on the Control of Salmonella and Other Specified Food-Borne Zoonotic Agents. Available online: http://data.europa.eu/eli/reg/2003/2160/oj (accessed on 2 February 2023).
- Wang, G.; Clark, C.G.; Taylor, T.M.; Pucknell, C.; Barton, C.; Price, L.; Woodward, D.L.; Rodgers, F.G. Colony multiplex PCR assay for identification and differentiation of Campylobacter jejuni, C. coli, C. lari, C. upsaliensis, and C. fetus subsp. fetus. J. Clin. Microbiol. 2002, 40, 4744–4747. [Google Scholar] [CrossRef] [PubMed]
- EUCAST. Cut off MIC and Zone Distributions and ECOFFs. Available online: https://www.eucast.org/mic_distributions_and_ecoffs/ (accessed on 29 December 2022).
- EFSA. Technical report on the methodological approach used for the assessment of the control measures for Category A diseases in the context of the new Animal Health Law. EFSA J. 2020, 17, EN-1988. [Google Scholar] [CrossRef]
- Cody, A.J.; Bray, J.E.; Jolley, K.A.; McCarthy, N.D.; Maiden, M.C.J. Core Genome Multilocus Sequence Typing Scheme for Stable, Comparative Analyses of Campylobacter jejuni and C. coli Human Disease Isolates. J. Clin. Microbiol. 2017, 55, 2086–2097. [Google Scholar] [CrossRef] [PubMed]
- Alfredson, D.A.; Korolik, V. Isolation and expression of a novel molecular class D beta-lactamase, OXA-61, from Campylobacter jejuni. Antimicrob. Agents Chemother. 2005, 49, 2515–2518. [Google Scholar] [CrossRef] [PubMed]
- Lucain, C.; Goossens, H.; Pechere, J.C. Beta-lactamases in Campylobacter jejuni. In Campylobacter III; Pearson, A.D., Skirrow, M.B., Lior, H., Rowe, B., Eds.; Public Health Laboratory Service: London, UK, 1985; pp. 36–37. [Google Scholar]
- Puljko, A.; Milaković, M.; Križanović, S.; Kosić-Vukšić, J.; Babić, I.; Petrić, I.; Maravić, A.; Jelić, M.; Udiković-Kolić, N. Prevalence of enteric opportunistic pathogens and extended-spectrum cephalosporin- and carbapenem-resistant coliforms and genes in wastewater from municipal wastewater treatment plants in Croatia. J. Hazard. Mater. 2022, 427, 128155. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.R. Emerging carbapenemases: A global perspective. Int. J. Antimicrob. Agents 2010, 36, S8–S14. [Google Scholar] [CrossRef] [PubMed]
- Jianlong, W.; Libing, C.; László, W.; Erzsébet, T. Occurrence and fate of antibiotics, antibiotic resistant genes (ARGs) and antibiotic resistant bacteria (ARB) in municipal wastewater treatment plant: An overview. Sci. Total Environ. 2020, 744, 140997. [Google Scholar] [CrossRef]
- Martín-Maldonado, B.; Rodríguez-Alcázar, P.; Fernández-Novo, A.; González, F.; Pastor, N.; López, I.; Suárez, L.; Moraleda, V.; Aranaz, A. Urban Birds as Antimicrobial Resistance Sentinels: White Storks Showed Higher Multidrug-Resistant Escherichia coli Levels Than Seagulls in Central Spain. Animals 2022, 12, 2714. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, K.; Osek, J. Antimicrobial resistance mechanisms among Campylobacter. Biomed Res. Int. 2013, 2013, 340605. [Google Scholar] [CrossRef]
- Marotta, F.; Garofolo, G.; di Marcantonio, L.; Di Serafino, G.; Neri, D.; Romantini, R.; Sacchini, L.; Alessiani, A.; Di Donato, G.; Nuvoloni, R.; et al. Antimicrobial resistance genotypes and phenotypes of Campylobacter jejuni isolated in Italy from humans, birds from wild and urban habitats, and poultry. PLoS ONE 2019, 14, e0223804. [Google Scholar] [CrossRef]
- Habib, I.; Miller, W.G.; Uyttendaele, M.; Houf, K.; De Zutter, L. Clonal population structure and antimicrobial resistance of Campylobacter jejuni in chicken meat from Belgium. Appl. Environ. Microbiol. 2009, 75, 4264–4272. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-H.; Harrison, L.; Mukherjee, S.; Strain, E.; McDermott, P.; Zhang, Q.; Zhao, S. Core Genome Multilocus Sequence Typing for Food Animal Source Attribution of Human Campylobacter jejuni Infections. Pathogens 2020, 9, 532. [Google Scholar] [CrossRef] [PubMed]
| Isolate ID | Source | Year of Isolation | Specimen | MLST | cgMLST |
|---|---|---|---|---|---|
| ZG01 | human | 2021 | stool | 19 | cgST-2499 |
| ZG02 | human | 2021 | stool | 19 | cgST-2499 |
| ZG03 | human | 2021 | stool | 50 | cgST-10207 |
| ZG04 | human | 2021 | stool | 19 | cgST-2499 |
| ZG06 | human | 2021 | stool | 822 | cgST-115 |
| ZG07 | human | 2021 | stool | 2787 | cgST-35061 |
| ZGI07 | human | 2021 | blood | 11,829 | cgST-115 |
| ZG12 | human | 2021 | stool | 50 | cgST-294 |
| 1c258 | Larus michahellis, Yellow-legged Gull | 2021 | cloacal swab | 50 | cgST-35358 |
| 1c381 | Larus michahellis, Yellow-legged Gull | 2022 | cloacal swab | 3926 | cgST-27074 |
| 1c402 | Ciconia ciconia, White Stork | 2022 | cloacal swab | 822 | cgST-115 |
| 1c404 | Ciconia ciconia, White Stork | 2022 | cloacal swab | 1949 | cgST-7821 |
| 1c405 | Ciconia ciconia, White Stork | 2022 | cloacal swab | ST-50 | cgST-35358 |
| 1c406 | Ciconia ciconia, White Stork | 2022 | cloacal swab | 1949 | cgST-7821 |
| 1c410 | Ciconia ciconia, White Stork | 2022 | cloacal swab | 1949 | cgST-7821 |
| 1c411 | Ciconia ciconia, White Stork | 2022 | cloacal swab | 1949 | cgST-7821 |
| 1c412 | Ciconia ciconia, White Stork | 2022 | cloacal swab | 1949 | cgST-7821 |
| 1c423 | Ciconia ciconia, White Stork | 2022 | cloacal swab | 822 | cgST-115 |
| CP01 | broiler | 2021 | cloacal swab | 19 | cgST-2499 |
| CP03 | broiler | 2021 | cloacal swab | 19 | cgST-2499 |
| CP10 | broiler | 2021 | cloacal swab | 21 | cgST-36048 |
| CP11 | broiler | 2021 | cloacal swab | 50 | cgST-294 |
| CP14 | broiler | 2021 | cloacal swab | 50 | cgST-294 |
| Antimicrobial Agent (Class)—MIC | ||||||
|---|---|---|---|---|---|---|
| Isolate ID | Erythromycin (Macrolide) | Ciprofloxacin (Fluoroquinolones) | Tetracycline (Tetracycline) | Gentamicin (Aminoglycoside) | Chloramphenicol (Amphenicol) | Ertapenem (Carbapenem) |
| ZG01 | ≤1 | 16 | ≤0.5 | ≤0.25 | 4 | ≤0.12 |
| ZG02 | ≤1 | 8 | ≤0.5 | 0.5 | ≤2 | 0.25 |
| ZG03 | ≤1 | 8 | ≤0.5 | 0.5 | ≤2 | 0.5 |
| ZG04 | ≤1 | 16 | ≤0.5 | 0.5 | ≤2 | ≤0.12 |
| ZG06 | ≤1 | ≤0.12 | ≤0.5 | 0.5 | ≤2 | ≤0.12 |
| ZG07 | ≤1 | 8 | ≤0.5 | ≤0.25 | ≤2 | ≤0.12 |
| ZGI07 | ≤1 | ≤0.12 | ≤0.5 | 0.5 | ≤2 | ≤0.12 |
| ZG12 | ≤1 | 8 | ≤0.5 | 0.5 | ≤2 | 0.5 |
| 1c258 | ≤1 | 0.12 | ≤0.5 | 0.5 | ≤2 | 0.25 |
| 1c381 | ≤1 | 8 | ≤0.5 | 0.5 | ≤2 | ≤0.12 |
| 1c402 | 4 | ≤0.12 | ≤0.5 | 0.5 | ≤2 | ≤0.12 |
| 1c404 | ≤1 | 8 | ≤0.5 | ≤0.25 | ≤2 | ≤0.12 |
| 1c405 | ≤1 | 8 | ≤0.5 | 0.5 | ≤2 | ≤0.12 |
| 1c406 | ≤1 | 8 | ≤0.5 | ≤0.25 | ≤2 | ≤0.12 |
| 1c410 | ≤1 | 8 | ≤0.5 | 0.5 | ≤2 | ≤0.12 |
| 1c411 | ≤1 | 8 | ≤0.5 | ≤0.25 | ≤2 | 0.25 |
| 1c412 | ≤1 | 8 | ≤0.5 | ≤0.25 | ≤2 | 0.25 |
| 1c423 | ≤1 | ≤0.12 | ≤0.5 | 0.5 | ≤2 | ≤0.12 |
| CP01 | ≤1 | 16 | ≤0.5 | 0.5 | 4 | 0.25 |
| CP03 | 4 | 8 | ≤0.5 | ≤0.25 | ≤2 | ≤0.12 |
| CP10 | ≤1 | 16 | ≤0.5 | 0.5 | 4 | 0.25 |
| CP11 | ≤1 | 8 | ≤0.5 | ≤0.25 | ≤2 | 0.25 |
| CP14 | ≤1 | 8 | ≤0.5 | ≤0.25 | ≤2 | 0.25 |
| Isolate ID | Antimicrobial Agents (Class) and Resistance Determinants | |||
|---|---|---|---|---|
| Ciprofloxacin (Fluoroquinolones) | RD | Ertapenem (Carbapenem) | RD | |
| ZG01 | NW | gyrA T86I | W | OXA-580 |
| ZG02 | NW | gyrA T86I | W | OXA-193 |
| ZG03 | NW | gyrA T86I | W | OXA-193 |
| ZG04 | NW | gyrA T86I | W | OXA-193 |
| ZG06 | W | - | W | OXA-193 |
| ZG07 | NW | gyrA T86I | W | OXA-580 |
| ZGI07 | W | - | W | OXA-193 |
| ZG12 | NW | gyrA T86I | W | - |
| 1c258 | W | - | W | OXA-193 |
| 1c381 | NW | gyrA T86I | W | OXA-580 |
| 1c402 | W | - | W | OXA-580 |
| 1c404 | NW | gyrA T86I | W | OXA-193 |
| 1c405 | NW | gyrA T86I | W | OXA-193 |
| 1c406 | NW | gyrA T86I | W | OXA-193 |
| 1c410 | NW | gyrA T86I | W | OXA-193 |
| 1c411 | NW | gyrA T86I | W | OXA-193 |
| 1c412 | NW | gyrA T86I | W | OXA-193 |
| 1c423 | W | - | W | OXA-580 |
| CP01 | NW | gyrA T86I | W | OXA-193 |
| CP03 | NW | gyrA T86I | W | OXA-193 |
| CP10 | NW | gyrA T86I | W | OXA-193 |
| CP11 | NW | gyrA T86I | W | OXA-193 |
| CP14 | NW | gyrA T86I | W | OXA-193 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šoprek, S.; Ujević, J.; Kompes, G.; Jurinović, L.; Tambić Andrašević, A. First Report of Campylobacter jejuni Strains Belonging to ST-21 Clonal Complex Isolated from Human, Poultry and Wild Birds in Croatia: Antimicrobial Resistance and Genetic Distance. Microorganisms 2023, 11, 1884. https://doi.org/10.3390/microorganisms11081884
Šoprek S, Ujević J, Kompes G, Jurinović L, Tambić Andrašević A. First Report of Campylobacter jejuni Strains Belonging to ST-21 Clonal Complex Isolated from Human, Poultry and Wild Birds in Croatia: Antimicrobial Resistance and Genetic Distance. Microorganisms. 2023; 11(8):1884. https://doi.org/10.3390/microorganisms11081884
Chicago/Turabian StyleŠoprek, Silvija, Josip Ujević, Gordan Kompes, Luka Jurinović, and Arjana Tambić Andrašević. 2023. "First Report of Campylobacter jejuni Strains Belonging to ST-21 Clonal Complex Isolated from Human, Poultry and Wild Birds in Croatia: Antimicrobial Resistance and Genetic Distance" Microorganisms 11, no. 8: 1884. https://doi.org/10.3390/microorganisms11081884
APA StyleŠoprek, S., Ujević, J., Kompes, G., Jurinović, L., & Tambić Andrašević, A. (2023). First Report of Campylobacter jejuni Strains Belonging to ST-21 Clonal Complex Isolated from Human, Poultry and Wild Birds in Croatia: Antimicrobial Resistance and Genetic Distance. Microorganisms, 11(8), 1884. https://doi.org/10.3390/microorganisms11081884





