Strong Opponent of Walnut Anthracnose—Bacillus velezensis and Its Transcriptome Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Tested Fungal, Bacteria and PLANT Materials
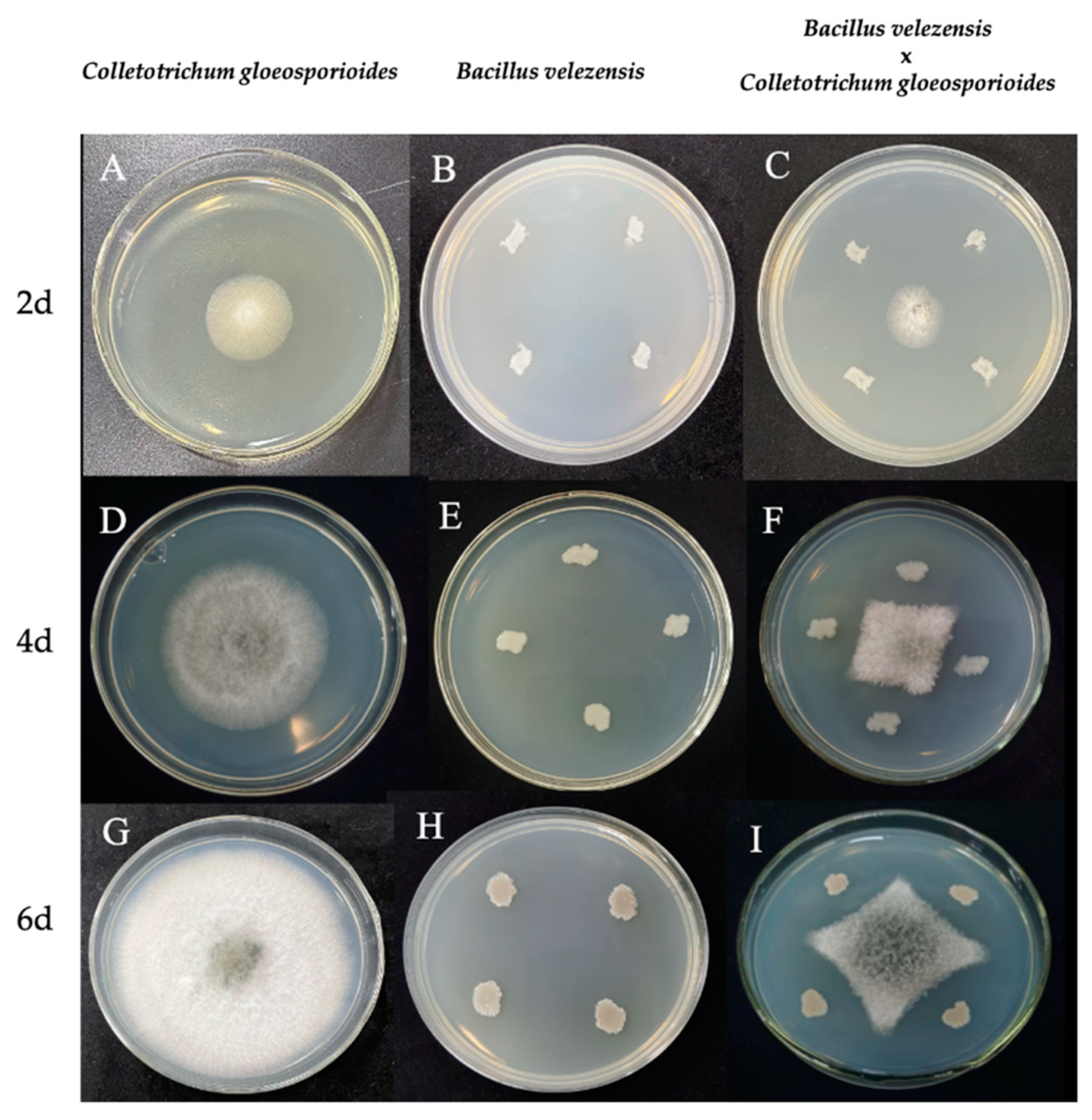
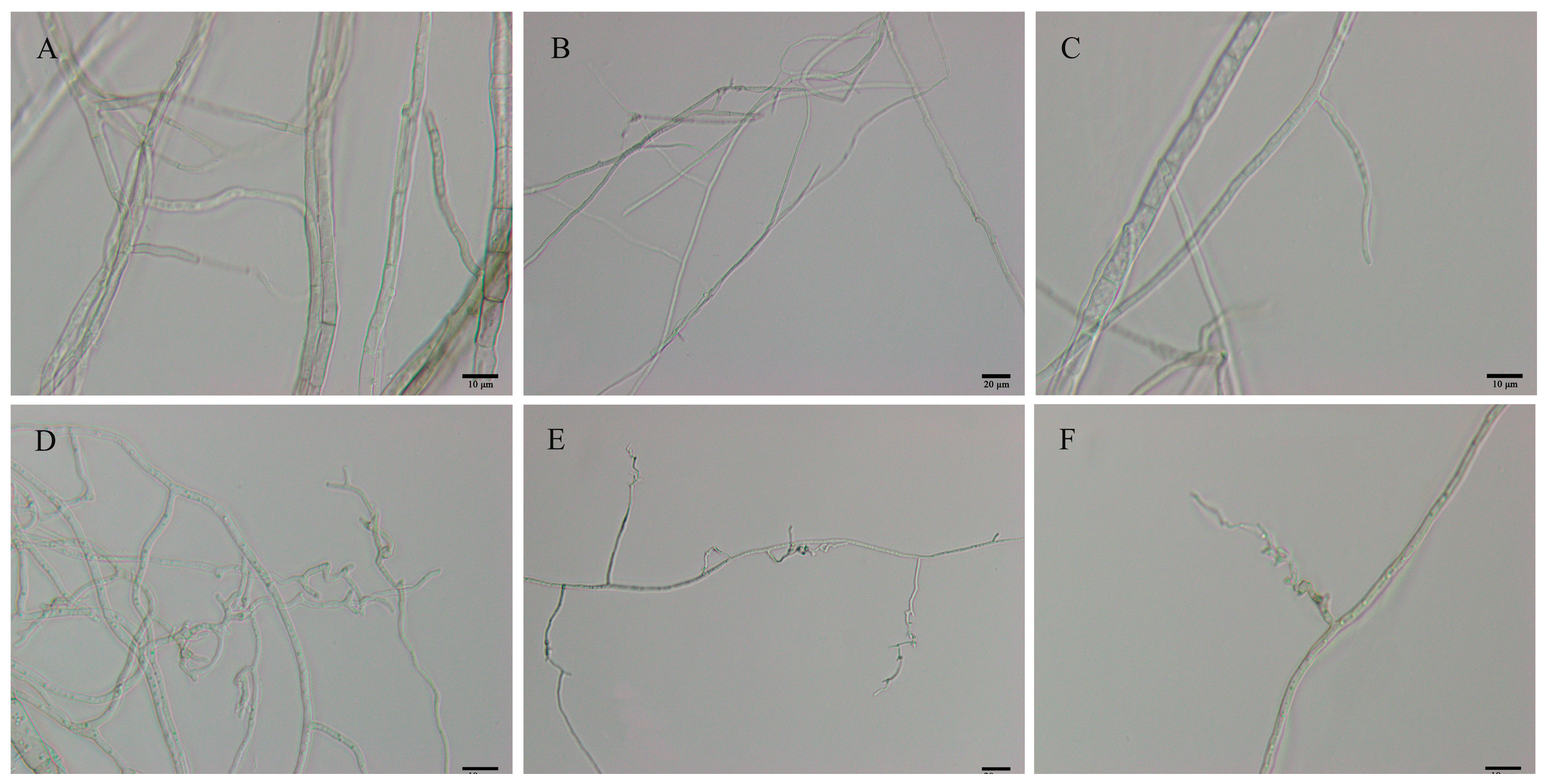
2.2. Effect of B. velezensis on Pathogenicity of C. gloeosporioides
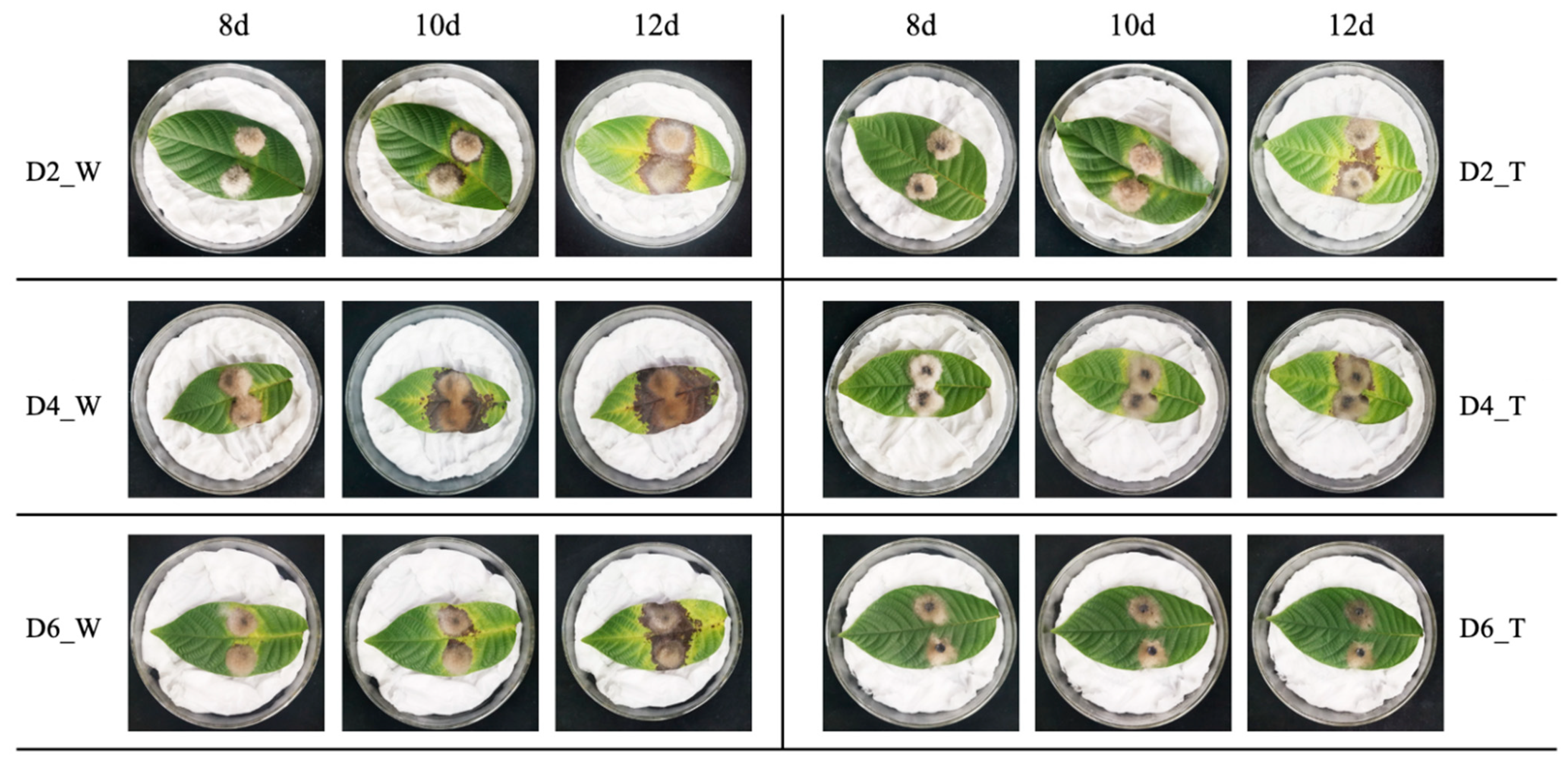
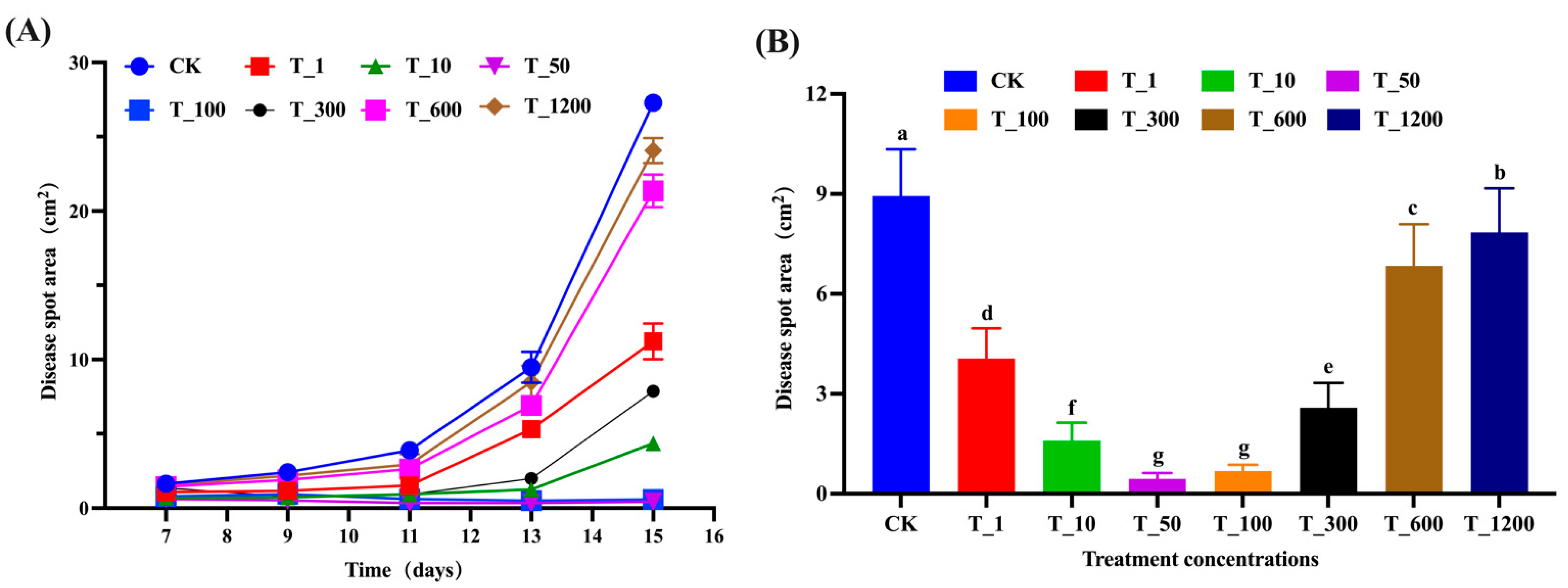
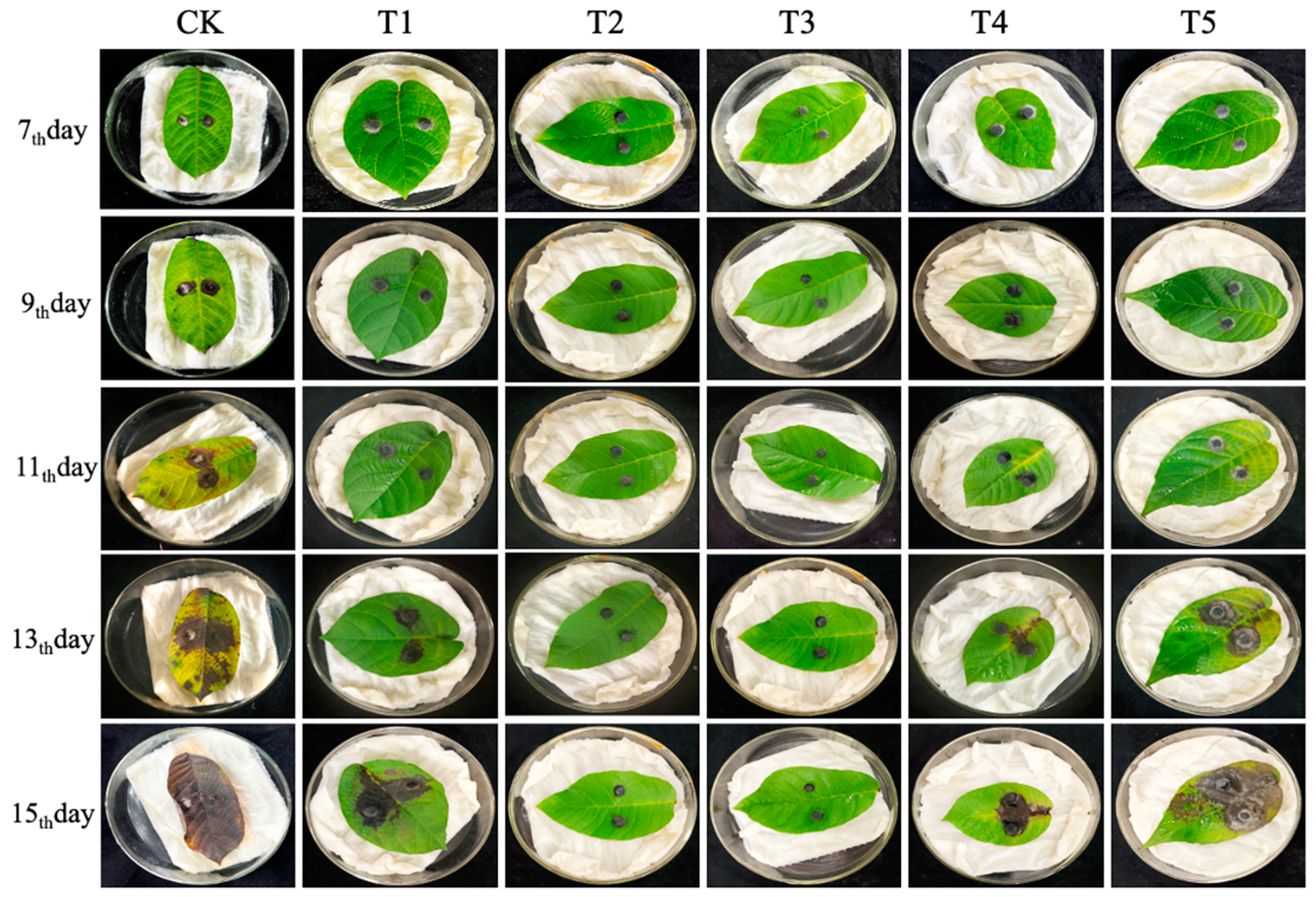
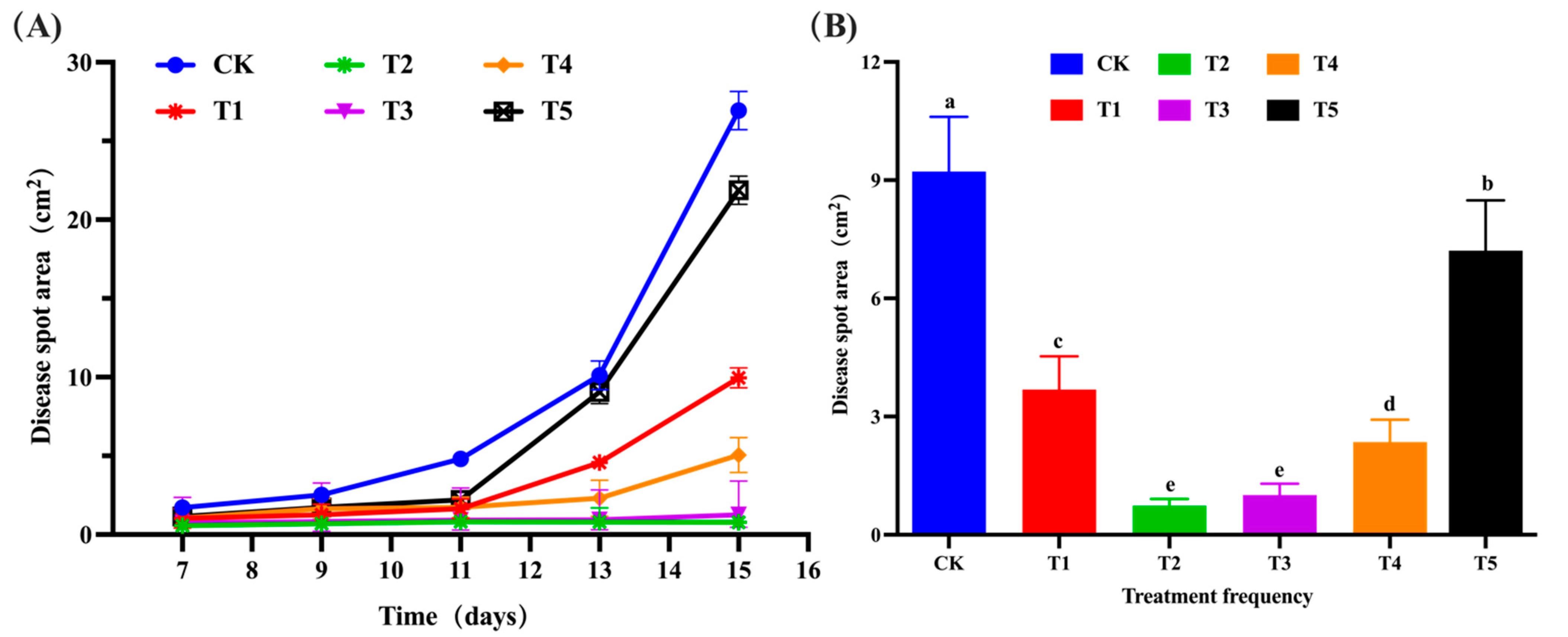
2.3. Control Effect of B. velezensis against C. gloeosporioides on Detached Leaves and Fruits
2.4. Transcriptional Analysis of B. velezensis
2.4.1. RNA Extraction, Library Preparation and Sequencing
2.4.2. Quality Control and Data Analysis
2.4.3. Quantitative and Differential Expression Analysis of Gene Expression
2.4.4. Quantitative Real-Time PCR
3. Results
3.1. Effect of B. velezensis on Mycelial Growth and Pathogenicity of C. gloeosporioides
3.2. Control Effect of B. velezensis on Detached Leaves and Fruits of Walnut
3.3. Transcriptome Results
3.3.1. Sequencing Data Statistics
3.3.2. Analysis of Gene Expression
3.3.3. Gene Annotation Results
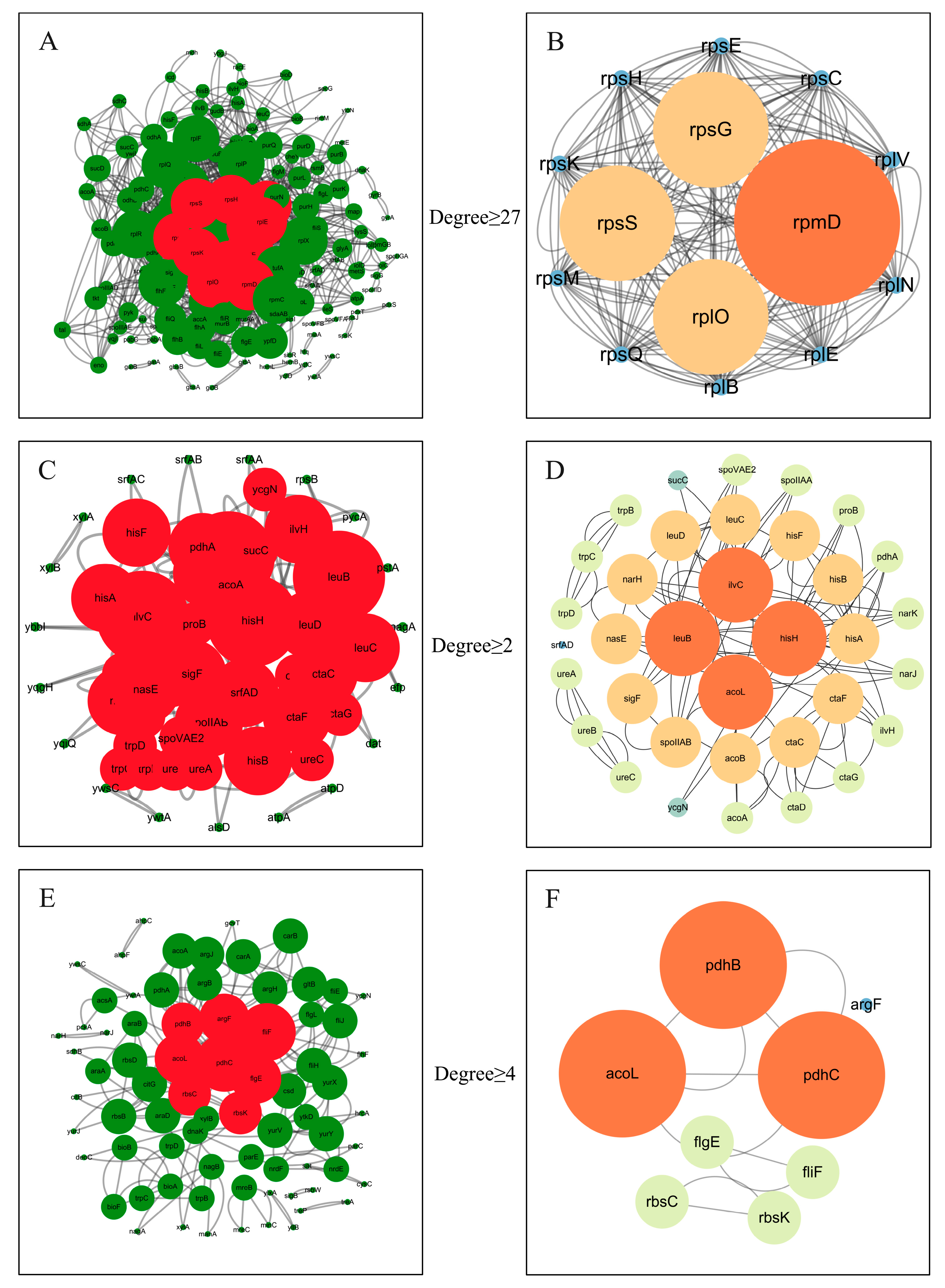
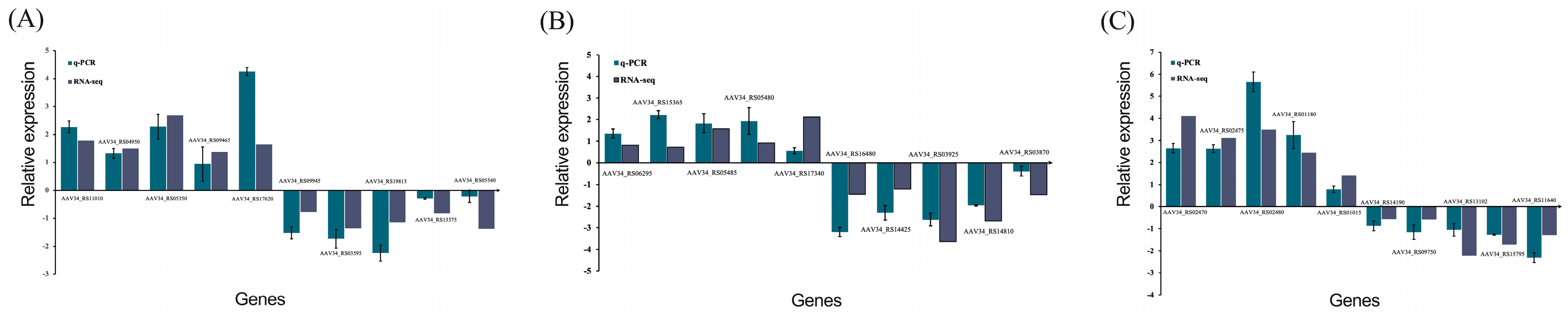
3.3.4. PPI Network Analysis and qRT-PCR Verification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guney, M.; Kafkas, S.; Keles, S.; Zarifikhosroshahi, M.; Gundesli, M.A.; Ercisli, S.; Necas, T.; Bujdoso, G. Genetic diversity among some walnut (Juglans regia L.) genotypes by SSR markers. Sustainability 2021, 13, 6830. [Google Scholar] [CrossRef]
- Claudia, S.G.; Ciudad, C.J.; Noe, V.; Maria, I.P. Health benefits of walnut polyphenols: An exploration beyond their lipid profile. Crit. Rev. Food Sci. 2017, 57, 3373–3383. [Google Scholar] [CrossRef]
- Holasou, H.A.; Jalaly, H.M.; Mohammadi, R.; Panahi, B. Genetic diversity and structure of superior spring frost tolerant genotypes of Persian walnut (Juglans regia L.) in East Azerbaijan province of Iran, characterized using inter simple sequence repeat (ISSR) markers. Genet. Resour. Crop Evol. 2023, 70, 539–548. [Google Scholar] [CrossRef]
- Pardaev, G.Y.; Normamatov, R. Economic and nutritional values of walnut: The main reason for development of walnut in Uzbekistan. J. Nuts 2021, 12, 103–112. [Google Scholar] [CrossRef]
- Daniele, D.L.; José, F.C.D.; Cyrielle, M.; Morgane, C.; Djiby, K.; Michel, G.; Agnes, V.; Patrice, N.; Sabrina, S.; Gaetan, L.F.; et al. Combined metabarcoding and multi-locus approach for genetic characterization of Colletotrichum species associated with common walnut (Juglans regia) anthracnose in France. Sci. Rep. 2018, 8, 10765. [Google Scholar] [CrossRef]
- Han, H.W.; Woeste, K.E.; Hu, Y.F.; Liang, D.; Liu, Q.; Zhao, P.; Liu, J.C. Genetic diversity and population structure of common walnut (Juglans regia) in China based on EST-SSRs and the nuclear gene phenylalanine ammonialyase (PAL). Tree Genet. Genomes 2016, 12, 111. [Google Scholar] [CrossRef]
- Xu, X.W.; Xu, X.H.; Liu, X.; Chen, J.Y.; Wang, Y.X. Reserach progress of walnut anthracnose. Hubei Agric. Sci. 2020, 1, 16–19. (In Chinese) [Google Scholar] [CrossRef]
- Fang, H.C.; Liu, X.; Dong, Y.H.; Feng, S.; Zhou, R.; Wang, C.X.; Ma, X.M.; Liu, J.N.; Yang, K.Q. Transcriptome and proteome analysis of walnut (Juglans regia L.) fruit in response to infection by Colletotrichum gloeosporioides. BMC Plant Biol. 2021, 21, 249. [Google Scholar] [CrossRef]
- Zhu, Y.; Yin, Y.; Yang, K.; Li, J.; Sang, Y.; Long, H.; Shu, F. Construction of a highdensity genetic map using specifc length amplifed fragment markers and identifcation of a quantitative trait locus for anthracnose resistance in walnut (Juglans regia L.). BMC Genom. 2015, 16, 614. [Google Scholar] [CrossRef]
- Jiao, C.; Chen, L.; Sun, C.; Jiang, Y.; Zhai, L.M.; Liu, H.B.; Shen, Z.Y. Evaluating national ecological risk of agricultural pesticides from 2004 to 2017 in China. Environ. Pollut. 2020, 259, 112778. [Google Scholar] [CrossRef]
- Wei, L.L.; Zheng, H.H.; Zhang, P.C.; Chen, W.C.; Zheng, J.Q.; Chen, C.J.; Cao, A.B. Molecular and biochemical characterization of Colletotrichum gloeosporioides isolates resistant to azoxystrobin from grape in China. Plant Pathol. 2021, 70, 1300–1309. [Google Scholar] [CrossRef]
- Chechi, A.; Stahlecker, J.; Dowling, M.; Schnabel, G. Diversity in species composition and fungicide resistance profiles in Colletotrichum isolates from apples. Pestic. Biochem. Phys. 2019, 158, 18–24. [Google Scholar] [CrossRef]
- Tai, H.K.; Zhang, F.; Xiao, C.; Tang, R.; Liu, Z.; Bai, S.X.; Wang, Z.Y. Toxicity of chemical pesticides commonly used in maize to Trichogramma ostriniae (Hymenoptera: Trichogrammatidae), an egg parasitoid of Asian corn borer. Ecotoxicol. Environ. Saf. 2022, 241, 113802. [Google Scholar] [CrossRef]
- Lata, R.; Komal, T.; Neha, K.; Neelam, S.; Sukhbir, S.; Ajmer, S.G.; Arun, L.S.; Jyotsna, K. An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 2021, 283, 124657. [Google Scholar] [CrossRef]
- Pacifico, M.G.; Eckstein, B.; Bettiol, W. Screening of Bacillus for the development of bioprotectants for the control of Fusarium oxysporum f. sp. vasinfectum and meloidogye incognita. Biol. Control 2021, 164, 104764. [Google Scholar] [CrossRef]
- Liu, Y.; Teng, K.; Wang, T.; Dong, E.; Zhang, M.; Tao, Y.; Zhong, J. Antimicrobial Bacillus velezensis HC6: Production of three kinds of lipopeptides and biocontrol potential in maize. J. Appl. Microbiol. 2020, 128, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.S.; Wang, F.G.; Qi, Z.Q.; Qiao, J.Q.; Du, Y.; Yu, J.J.; Yu, M.N.; Liang, D.; Song, T.Q.; Yan, P.X.; et al. Iturins produced by Bacillus velezensis Jt84 play a key role in the biocontrol of rice blast disease. Biol. Control 2022, 174, 105001. [Google Scholar] [CrossRef]
- Jin, P.F.; Wang, H.N.; Tan, Z.; Xuan, Z.; Dahar, G.Y.; Li, Q.X.; Miao, W.G.; Liu, W.B. Antifungal mechanism of bacillomycin D from Bacillus velezensis HN-2 against Colletotrichum gloeosporioides Penz. Pestic. Biochem. Phys. 2020, 163, 102–107. [Google Scholar] [CrossRef]
- Laird, M.; Piccoli, D.; Weselowski, B.; McDowell, T.; Renaud, J.; MacDonald, J.; Yuan, Z.C. Surfactin-producing Bacillus velezensis 1B-23 and Bacillus sp. 1D-12 protect tomato against bacterial canker caused by Clavibacter michiganensis subsp. Michiganensis. J. Plant Pathol. 2020, 2, 451–458. [Google Scholar] [CrossRef]
- Rabbee, M.F.; Islam, N.; Baek, K.H. Biocontrol of citrus bacterial canker caused by Xanthomonas citri subsp. citri by Bacillus velezensis. Saudi J. Biol. Sci. 2022, 29, 2363–2371. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Zhang, W.; Cai, X.; Zhu, T.; Xue, Y.; Liu, C. Bacillus velezensis CC09: A potential ‘Vaccine’ for controlling wheat diseases. Mol. Plant-Microbe Interact. 2018, 31, 623–632. [Google Scholar] [CrossRef]
- Sandhya, N.; Riteshri, S.; Dhiraj, P.; Hareshkumar, K. Genome analysis uncovers the prolific antagonistic and plant growth-prmoting potential of endophyte Bacillus velezensis K1. Gene 2022, 836, 146671. [Google Scholar] [CrossRef]
- Wei, J.B.; Zhao, J.; Suo, M.; Wu, H.; Zhao, M.; Yang, H.Y. Biocontrol mechanisms of Bacillus velezensis against Fusarium oxysporum from Panax ginseng. Biol. Control 2023, 182, 105222. [Google Scholar] [CrossRef]
- Jia, H.L.; Huang, S.W.; Cheng, S.; Zhang, X.W.; Chen, X.; Zhang, Y.S.; Wang, J.; Wu, L.F. Novel mechanisms of selenite reduction in Bacillus subtilis 168: Confirmation of multiple-pathway mediated remediation based on transcriptome analysis. J. Hazard. Mater. 2022, 433, 128834. [Google Scholar] [CrossRef]
- Meng, F.Q.; Zhu, X.Y.; Nie, T.; Lu, F.X.; Bie, X.M.; Lu, Y.J.; Trouth, F.; Lu, Z.X. Enhanced expression of pullulanase in Bacillus subtilis by new strong promoters mined from transcriptome data, both alone and in combination. Front. Microbiol. 2018, 9, 2635. [Google Scholar] [CrossRef]
- Jiang, C.H.; Yao, X.F.; Mi, D.D.; Li, Z.J.; Yang, B.Y.; Zheng, Y.; Qi, Y.J.; Guo, J.H. Comparative transcriptome analysis reveals the biocontrol mechanism of Bacillus velezensis F21 Against Fusarium wilt on watermelon. Front. Microbiol. 2019, 10, 652. [Google Scholar] [CrossRef]
- Ryu, C.M.; Farag, M.A.; Hu, C.H.; Reddy, M.S.; Kloepper, J.W.; Paré, P.W. Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol. 2004, 134, 1017–1026. [Google Scholar] [CrossRef]
- Yan, Z.N.; Reddy, M.S.; Ryu, C.M.; McInroy, J.A.; Wilson, M.; Kloepper, J.W. Induced systemic protection against tomato late blight elicited by plant growth-promoting rhizobacteria. Phytopathology 2002, 92, 1329–1333. [Google Scholar] [CrossRef]
- Liu, R.H.; Li, J.Y.; Zhang, F.R.; Zheng, D.; Chang, Y.L.; Xu, L.S.; Huang, L.L. Biocontrol activity of Bacillus velezensis D4 against apple Valsa canker. Biol. Control 2021, 163, 104760. [Google Scholar] [CrossRef]
- Liang, N.; Charron, J.B.; Jabaji, S. Comparative transcriptome analysis reveals the biocontrol mechanism of Bacillus velezensis E68 against Fusarium graminearum DAOMC 180378, the causal agent of Fusarium head blight. PLoS ONE 2023, 18, e0277983. [Google Scholar] [CrossRef]
- Caroline, P.; Maud, D.; Camille, M.; Dominique, L.C.; Stéphane, A.; Romain, B. Biofilm formation and synthesis of antimicrobial compounds by the biocontrol agent Bacillus velezensis QST713 in an Agaricus bisporus compost micromodel. Appl. Environ. Microbiol. 2019, 85, e00327-19. [Google Scholar] [CrossRef]
- Harun-Or-Rashid, M.; Kim, H.J.; Yeom, S.I.; Yu, H.A.; Manir, M.M.; Moon, S.S.; Kang, Y.J.; Chung, Y.R. Bacillus velezensis YC7010 enhances plant defenses against brown planthopper through transcriptomic and metabolic changes in rice. Front. Plant Sci. 2018, 9, 1904. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xiao, Q.L.; Yang, H.J.; Huang, J.G.; Li, Y. Characterization of a new Bacillus velezensis as a powerful biocontrol agent against tomato gray mold. Pest. Biochem. Physiol. 2022, 187, 105199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Zhao, M.X.; Chen, W.; Yu, H.L.; Jia, W.T.; Pan, H.Y.; Zhang, X.H. Multi-Omics Techniques for Analysis Antifungal Mechanisms of Lipopeptides Produced by Bacillus velezensis GS-1 against Magnaporthe oryzae In Vitro. Int. J. Mol. Sci. 2022, 23, 3762. [Google Scholar] [CrossRef]
- Wang, Q.H.; Ji, Y.P.; Qu, Y.Y.; Qi, Y.K.; Li, D.W.; Liu, Z.Y.; Wu, X.Q. The response strategies of Colletotrichum gloeosporioides s.s. due to the stress caused by biological control agent amyloliquefaciens deciphered by transcriptome analyses. Biol. Control 2020, 150, 104372. [Google Scholar] [CrossRef]
- Sun, L.L. Study on the Effect of Temperature Stress on the Flavor Metabolism of Bacillus Amyloliquefaciens JP-1 Based on Transcriptomics; Guizhou University: Guiyang, China, 2020; (In Chinese). [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yeimmy, P.R.; Chiara, R.; Carlos, D.G.T.; Clemencia, C.L. Green management of postharvest anthracnose caused by Colletotrichum gloeosporioides. J. Fungi 2023, 9, 623. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, W.T.; Lu, R.S.; Chen, M.; Liu, J.; Sun, X.Q.; Zhang, Y.M. Genome sequence resource for Colletotrichum gloeosporioides, an important pathogenic fungus causing anthracnose of Dioscorea alata. Plant Dis. 2023, 107, 893–895. [Google Scholar] [CrossRef]
- Zakaria, L. Diversity of Colletotrichum species associated with anthracnose disease in tropical fruit crops—A review. Agriculture 2021, 11, 297. [Google Scholar] [CrossRef]
- Huang, H.Y.; Wu, Z.Q.; Tian, C.M.; Liang, Y.M.; You, C.J.; Chen, L. Identification and characterization of the endophytic bacterium Bacillus atrophaeus XW2, antagonistic towards Colletotrichum gloeosporioides. Ann. Microbiol. 2015, 65, 1361–1371. [Google Scholar] [CrossRef]
- Zhang, T.F.; Wen, G.Q.; Song, B.; Chen, Z.Y.; Jiang, S.J. Transcriptome profiling reveals the underlying mechanism of grape post-harvest pathogen Penicillium olsonii against the metabolites of Bacillus velezensis. Front. Microbiol. 2023, 13, 1019800. [Google Scholar] [CrossRef]
- Huang, L.; Li, Q.C.; Hou, Y.; Li, G.Q.; Yang, J.Y.; Li, D.W.; Ye, J.R. Bacillus velezensis strain HYEB5-6 as a potential biocontrol agent against anthracnose on Euonymus japonicus. Biocontrol. Sci. Technol. 2017, 27, 636–653. [Google Scholar] [CrossRef]
- Ge, H.L.; Zhou, M.; Lv, D.Z.; Wang, M.Y.; Xie, D.F.; Yang, X.F.; Dong, C.Z.; Li, S.H.; Lin, P. Novel segmented concentration addition method to predict mixture hormesis of chlortetracycline hydrochloride and oxytetracycline hydrochloride to aliivibrio fischeri. Int. J. Mol. Sci. 2020, 21, 481. [Google Scholar] [CrossRef] [PubMed]
- Ayyanath, M.M.; Cutler, G.C.; Scott-Dupree, C.D.; Sibley, P.K. Transgenerational shifts in reproduction hormesis in green peach aphid exposed to low concentrations of imidacloprid. PLoS ONE 2013, 8, e74532. [Google Scholar] [CrossRef]
- Ashwini, N.; Srividya, S. Potentiality of Bacillus subtilis as biocontrol agent for management of anthracnose disease of chilli caused by Colletotrichum gloeosporioides OGC1. 3 Biotech 2014, 4, 127–136. [Google Scholar] [CrossRef]
- Chet, I.; Ordentlich, A.; Shapira, R.; Oppenheim, A. Mechanism of biocontrol of soil borne plant pathogens by rhizobacteria. Plant Soil 1990, 129, 85–92. [Google Scholar] [CrossRef]
- Majed, R.; Faille, C.; Kallassy, M.; Gohar, M. Bacillus cereus biofilms-same, only different. Front. Microbiol. 2016, 7, 1054. [Google Scholar] [CrossRef]
- Chaban, B.; Hughes, H.V.; Beeby, M. The flagellum in bacterial pathogens: For motility and a whole lot more. Semin. Cell Dev. Biol. 2015, 46, 91–103. [Google Scholar] [CrossRef]
- Bacon, C.W.; Hinton, D.M. Endophytic and biological control potential of Bacillus mojavensis and related species. Biol. Control 2002, 23, 274–284. [Google Scholar] [CrossRef]
- Liu, X.Y.; Ren, B.; Chen, M.; Wang, H.B.; Kokare, C.R.; Zhou, X.L.; Wang, J.D.; Dai, H.Q.; Song, F.H.; Liu, M.; et al. Production and characterization of a group of bioemulsifiers from the marine Bacillus velezensis strain H3. Appl. Microbiol. Biotechnol. 2010, 87, 1881–1893. [Google Scholar] [CrossRef]
- Gao, X.Y.; Liu, Y.; Miao, L.L.; Li, E.W.; Sun, G.X.; Liu, Y.; Liu, Z.P. Characterization and mechanism of anti-Aeromonas salmonicida activity of a marine probiotic strain, Bacillus velezensis V4. Appl. Microbiol. Biotechnol. 2017, 101, 3759–3768. [Google Scholar] [CrossRef]
- Li, B.; Li, Q.; Xu, Z.H.; Zhang, N.; Shen, Q.R.; Zhang, R.F. Responses of beneficial Bacillus amyloliquefaciens SQR9 to different soilborne fungal pathogens through the alteration of antifungal compounds production. Front. Microbiol. 2014, 5, 636. [Google Scholar] [CrossRef] [PubMed]
- Raza, W.; Ling, N.; Zhang, R.F.; Huang, Q.W.; Xu, Y.C.; Shen, Q.R. Success evaluation of the biological control of Fusarium wilts of cucumber, banana, and tomato since 2000 and future research strategies. Crit. Rev. Biotechnol. 2017, 37, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Raza, W.; Shen, Q.R.; Huang, Q.W. Antifungal activity of Bacillus amyloliquefaciens NJN-6 volatile compounds against Fusarium oxysporum f. sp. cubense. Appl. Environ. Microbiol. 2012, 78, 5942–5944. [Google Scholar] [CrossRef]
- Deng, Q.; Wang, R.D.; Sun, D.F.; Sun, L.J.; Wang, Y.L.; Pu, Y.H.; Fang, Z.J.; Xu, D.F.; Liu, Y.; Ye, R.Y.; et al. Complete genome of Bacillus velezensis CMT-6 and comparative genome analysis reveals lipopeptide diversity. Biochem. Genet. 2020, 58, 1–15. [Google Scholar] [CrossRef]
- Théatre, A.; Hoste, A.C.R.; Rigolet, A.; Benneceur, I.; Bechet, M.; Ongena, M.; Deleu, M.; Jacques, P. Bacillus sp.: A Remarkable Source of Bioactive Lipopeptides. Adv. Biochem. Eng. Biot. 2022, 181, 123–179. [Google Scholar] [CrossRef]
- Xu, W.F. Study on the Biological Control Effects and Mechanisms of Mulberry Endophytic Bacillus Subtilis 7PJ-16 against Mulberry Fruit Sclerotiniosis; Southwest University Chongqing: Chongqing, China, 2020. (In Chinese) [Google Scholar]
- Yuan, P.H.; Lyu, X.Q.; Liu, Y.F.; Li, J.H.; Liu, L.; Du, G.C. Regulation of plasma membrane homeostasis to increase the accumulation of menaquinone-4 in Bacillus subtilis. Food Ferment. Ind. 2021, 47, 1–7. (In Chinese) [Google Scholar] [CrossRef]
- Douwe, V.S.; Giuliano, G.; Paola, C.; Francesca, D.F.; Sebo, W.; Gérard, V.; Guido, G. Characterization of the srfA locus of Bacillus subtilis: Only the valine-activating domain of srfA is involved in the establishment of genetic competence. Mol. Microbiol. 1993, 8, 833–841. [Google Scholar] [CrossRef]
- Mujtaba, A.S.A.; Sayyed, R.Z.; Mir, M.I.; Khan, M.Y.; Bee, H.; Alkhanani, M.F.; Shafiul, H.; Rahman, M.A.T.A.; Péter, P. Induction of systemic resistance in Maize and antibiofilm activity of surfactin from Bacillus velezensis MS20. Front. Microbiol. 2022, 13, 879739. [Google Scholar] [CrossRef]
- Duarte, I.O.; Hissa, D.C.; Quintela, B.C.S.F.; Rabelo, M.C.; Oliveira, F.A.S.; Lima, N.C.B.; Melo, V.M.M. Genomic analysis of surfactant-producing Bacillus vallismortis TIM68: First glimpse at species pangenome and prediction of new plipastatin-like lipopeptide. Appl. Biochem. Biotechnol. 2023, 195, 753–771. [Google Scholar] [CrossRef]
- Nakano, M.M.; Marahiel, M.A.; Zuber, P. Identification of a genetic locus required for biosynthesis of the lipopeptide antibiotic surfactin in Bacillus subtilis. J. Bacteriol. 1988, 170, 5662–5668. [Google Scholar] [CrossRef]
- Joanna, C.; Iain, D.C.; Michael, D.Y. NMR studies of the interactions of SpoIIAA with its partner proteins that regulate sporulation in Bacillus subtilis. J. Mol. Biol. 2001, 314, 359–364. [Google Scholar] [CrossRef]
- Zhao, Z.M.; Xi, J.T.; Xu, J.F.; Ma, L.T.; Zhao, J. Enhancement of Bacillus subtilis growth and sporulation by two-stage solid-state fermentation strategy. Processes 2019, 7, 644. [Google Scholar] [CrossRef]
- Nicholson, W.L.; Munakata, N.; Horneck, G.; Melosh, H.J.; Setlow, P. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. 2000, 64, 548–572. [Google Scholar] [CrossRef]
- Setlow, P. Spores of Bacillus subtilis: Their resistance to and killing by radiation, heat and chemicals. J. Appl. Microbiol. 2006, 101, 514–525. [Google Scholar] [CrossRef]
- Mojardín, L.; Salas, M. Global transcriptional analysis of virus-host interactions between phage ϕ29 and Bacillus subtilis. J. Virol. 2016, 90, 9293–9304. [Google Scholar] [CrossRef]
- Ueno, T.; Oosawa, K.; Aizawa, S.I. M ring, S ring and proximal rod of the flagellar basal body of Salmonella typhimurium are composed of subunits of a single protein, FliF. J. Mol. Biol. 1992, 227, 672–677. [Google Scholar] [CrossRef]
- Morimoto, Y.V.; Ito, M.; Hiraoka, K.D.; Che, Y.S.; Bai, F.; Kami-ike, N.; Namba, K.; Minamino, T. Assembly and stoichiometry of FliF and FlhA in Salmonella flagellar basal body. Mol. Microbiol. 2014, 91, 1214–1226. [Google Scholar] [CrossRef]
- You, Y.; Ye, F.; Mao, W.; Yang, H.; Lai, J.J.; Deng, S. An overview of the structure and function of the flagellar hook FlgE protein. World J. Microbiol. Biotechnol. 2023, 39, 126. [Google Scholar] [CrossRef]
- Tohru, M.; Miki, K.; Keiichi, N. Directional switching mechanism of the bacterial flagellar motor. Comput. Struct. Biotechnol. J. 2019, 17, 1075–1081. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Zhu, T. Strong Opponent of Walnut Anthracnose—Bacillus velezensis and Its Transcriptome Analysis. Microorganisms 2023, 11, 1885. https://doi.org/10.3390/microorganisms11081885
Wang L, Zhu T. Strong Opponent of Walnut Anthracnose—Bacillus velezensis and Its Transcriptome Analysis. Microorganisms. 2023; 11(8):1885. https://doi.org/10.3390/microorganisms11081885
Chicago/Turabian StyleWang, Linmin, and Tianhui Zhu. 2023. "Strong Opponent of Walnut Anthracnose—Bacillus velezensis and Its Transcriptome Analysis" Microorganisms 11, no. 8: 1885. https://doi.org/10.3390/microorganisms11081885
APA StyleWang, L., & Zhu, T. (2023). Strong Opponent of Walnut Anthracnose—Bacillus velezensis and Its Transcriptome Analysis. Microorganisms, 11(8), 1885. https://doi.org/10.3390/microorganisms11081885




