Biochemical, Antioxidant Properties and Antimicrobial Activity of Steno-Endemic Origanum onites
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Samples
2.2. Extraction
2.3. Antibacterial and Antifungal Activity Test
2.4. Antioxidant Activity Test
2.5. Gas Chromatography-Mass Spectroscopy Method (GC-MS)
2.6. Statistics
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 3670–3695. [Google Scholar] [CrossRef]
- Rates, S.M.K. Plants as source of drugs. Toxicon 2001, 39, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [PubMed]
- Fabricant, D.S.; Farnsworth, N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001, 109, 69–75. [Google Scholar] [PubMed]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277. [Google Scholar]
- Aminov, R.I. A brief history of the antibiotic era: Lessons learned and challenges for the future. Front. Microbiol. 2010, 1, 134. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Baser, K.H.C. Aromatic biodiversity among the flowering plant taxa of Turkey. Pure Appl. Chem. 2002, 74, 527–545. [Google Scholar] [CrossRef]
- Skoula, M.; Harborne, J.B. The taxonomy and chemistry of Origanum. In Medicinal and Aromatic Plants–Industrial Profiles–Oregan, The Genera Origanum and Lipia; Kintzios, S.E., Ed.; Taylor & Francis/CRC Press: Boca Raton, FL, USA, 2002; pp. 67–108. [Google Scholar]
- Bagci, Y.; Saadia, Z.; Özcan, M.M. Aroma profile of Origanum vulgare L. subsp. viride (Boiss.) Hayek, Satureja hortensis L. and Thymbra sintenisii Bornm. & Aznav. subsp. isaurica PH Davis used as condiment and herbal tea in Turkey. J. Essent. Oil-Bear. Plants 2005, 8, 304–311. [Google Scholar]
- Baser, K.H.C.; Özek, T.; Tümen, G.; Sezik, E. Composition of the essential oils of Turkish Origanum species with commercial importance. J. Essent. Oil Res. 1993, 5, 619–623. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Lim, Y.Y.; Chong, K.L.; Tan, J.B.L.; Wong, S.K. Antioxidant properties of tropical and temperate herbal teas. J. Food Comp. Anal. 2010, 23, 185–189. [Google Scholar] [CrossRef]
- Bulut, G.; Tuzlaci, E. An ethnobotanical study of medicinal plants in Turgutlu (Manisa-Turkey). J. Ethnopharmacol. 2013, 149, 633–647. [Google Scholar] [CrossRef] [PubMed]
- Sargın, S.A.; Akçiçek, E.; Selvi, S. An ethnobotanical study of medicinal plants used by the local people of Alaşehir (Manisa) in Turkey. J. Ethnopharmacol. 2013, 150, 860–874. [Google Scholar] [CrossRef]
- Ugulu, I.; Baslar, S.; Yorek, N.; Dogan, Y. The investigation and quantitative ethnobotanical evaluation of medicinal plants used around Izmir province, Turkey. J. Med. Plant Res. 2009, 3, 345–367. [Google Scholar]
- Vokou, D.; Kokkini, S.; Bessiere, J.M. Origanum onites (Lamiaceae) in Greece: Distribution, volatile oil yield, and composition. Econ. Bot. 1988, 42, 407–412. [Google Scholar] [CrossRef]
- Tepe, B.; Daferera, D.; Sokmen, A.; Sokmen, M.; Polissiou, M. Antimicrobial and antioxidant activities of the essential oil and various extracts of Salvia tomentosa Miller (Lamiaceae). Food Chem. 2005, 90, 333–340. [Google Scholar] [CrossRef]
- Kordali, S.; Cakir, A.; Ozer, H.; Cakmakci, R.; Kesdek, M.; Mete, E. Antifungal, phytotoxic and insecticidal properties of essential oil isolated from Turkish Origanum acutidens and its three components, carvacrol, thymol and p-cymene. Bioresour. Technol. 2005, 96, 2038–2046. [Google Scholar] [CrossRef]
- Sokmen, M.; Serkedjieva, J.; Daferera, D.; Gulluce, M.; Polissiou, M.; Tepe, B.; Akpulat, H.A.; Sahin, F.; Sokmen, A. In vitro antioxidant, antimicrobial, and antiviral activities of the essential oil and various extracts from herbal parts and callus cultures of Origanum acutidens. J. Agric. Food Chem. 2004, 52, 3309–3312. [Google Scholar] [CrossRef]
- Özcan, M.M.; Chalchat, J.C. Chemical composition and antifungal activity of rosemary (Rosmarinus officinalis L.) oil from Turkey. Int. J. Food Sci. Nutr. 2008, 59, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Albayrak, S.; Aksoy, A.; Sagdic, O.; Budak, U. Antioxidant and antimicrobial activities of different extracts of some medicinal herbs consumed as tea and spices in Turkey. J. Food Biochem. 2010, 34, 835–868. [Google Scholar] [CrossRef]
- Erenler, R.; Demirtas, I.; Karan, T.; Gul, F.; Kayir, O.; Karakoc, O.C. Chemical constituents, quantitative analysis and insecticidal activities of plant extract and essential oil from Origanum onites L. Trends Phytochem. Res. 2018, 2, 91–96. [Google Scholar]
- Anraku, M.; Gebicki, J.M.; Iohara, D.; Tomida, H.; Uekama, K.; Maruyama, T.; Hirayama, F.; Otagiri, M. Antioxidant activities of chitosans and its derivatives in in vitro and in vivo studies. Carbohydr. Polym. 2018, 199, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Kosakowska, O.; Węglarz, Z.; Pióro-Jabrucka, E.; Przybył, J.L.; Krasniewska, K.; Gniewosz, M.; Błażek, K. Antioxidant and Antibacterial Activity of Essential Oils and Hydroethanolic Extracts of Greek Oregano (O. vulgare L. subsp. hirtum (Link) Ietswaart) and Common Oregano (O. vulgare L. subsp. vulgare). Molecules 2021, 26, 988. [Google Scholar] [CrossRef]
- Kaurinovic, B.; Popovic, M.; Vlaisavljevic, S.; Trivic, S. Antioxidant Capacity of Ocimum basilicum L. and Origanum vulgare L. Extracts. Molecules 2011, 16, 7401–7414. [Google Scholar] [CrossRef]
- Quiroga, P.R.; Asensio, C.M.; Nepote, V. Antioxidant effects of the monoterpenes carvacrol, thymol and sabinene hydrate on chemical and sensory stability of roasted sunflower seeds. J. Sci. Food Agric. 2015, 95, 471–479. [Google Scholar] [CrossRef]
- Tabanca, N.; Kırımer, N.; Demirci, B.; Demirci, F.; Başer, K.H.C. Composition and antimicrobial activity of the essential oils of Micromeria cristata subsp. phrygia and the enantiomeric distribution of borneol. J. Agric. Food Chem. 2001, 49, 4300–4303. [Google Scholar] [CrossRef]
- Bernardo, L.R.; Ferreira, L.K.D.P.; Ferreira, L.A.M.P.; Vieira, C.I.D.; Alves, A.F.; Figueiredo, P.T.R.; Piuvezam, M.R. 4-Carvomenthenol, a monoterpene of essential oils, and its underlying effects on anti-inflammatory activity and immediate hypersensitivity reaction. Braz. J. Pharm. Sci. 2023, 58, e20780. [Google Scholar] [CrossRef]
- El Gazzar, M.; El Mezayen, R.; Marecki, J.C.; Nicolls, M.R.; Canastar, A.; Dreskin, S.C. Anti-inflammatory effect of thymoquinone in a mouse model of allergic lung inflammation. Int. Immunopharmacol. 2006, 6, 1135–1142. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Samarghandian, S.; Shahri, A.M.P.; Samini, F. The neuroprotective effects of thymoquinone: A review. Dose-Response 2018, 16, 1559325818761455. [Google Scholar] [CrossRef] [PubMed]
- Guarda, A.; Rubilar, J.F.; Miltz, J.; Galotto, M.J. The antimicrobial activity of microencapsulated thymol and carvacrol. Int. J. Food Microbiol. 2011, 146, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Yanishlieva, N.V.; Marinova, E.M.; Gordon, M.H.; Raneva, V.G. Antioxidant activity and mechanism of action of thymol and carvacrol in two lipid systems. Food Chem. 1999, 64, 59–66. [Google Scholar] [CrossRef]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Damasceno, S.R.; Oliveira, F.R.A.; Carvalho, N.S.; Brito, C.F.; Silva, I.S.; Sousa, F.B.M.; Silva, R.O.; Sousa, D.P.; Barbosa, A.L.R.; Freitas, R.M.; et al. Carvacryl acetate, a derivative of carvacrol, reduces nociceptive and inflammatory response in mice. Life Sci. 2014, 94, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.J.; Jwa, S.K. Inhibitory effects of β-caryophyllene on Streptococcus mutans biofilm. Arch. Oral Biol. 2018, 88, 42–46. [Google Scholar] [CrossRef]
- Legault, J.; Pichette, A. Potentiating effect of β-caryophyllene on anticancer activity of α-humulene, isocaryophyllene and paclitaxel. J. Pharm. Pharmacol. 2007, 59, 1643–1647. [Google Scholar] [CrossRef]
- Yeo, S.K.; Ali, A.Y.; Hayward, O.A.; Turnham, D.; Jackson, T.; Bowen, I.D.; Clarkson, R. β-Bisabolene, a sesquiterpene from the essential oil extract of opoponax (Commiphora guidottii), exhibits cytotoxicity in breast cancer cell lines. Phytother. Res. 2016, 30, 418–425. [Google Scholar] [CrossRef]
- Nascimento, A.M.; Brandao, M.G.; Oliveira, G.B.; Fortes, I.C.; Chartone-Souza, E. Synergistic bactericidal activity of Eremanthus erythropappus oil or β-bisabolene with ampicillin against Staphylococcus aureus. Antonie Leeuwenhoek 2007, 92, 95–100. [Google Scholar] [CrossRef]
- Ahsan, T.; Chen, J.; Zhao, X.; Irfan, M.; Wu, Y. Extraction and identification of bioactive compounds (eicosane and dibutyl phthalate) produced by Streptomyces strain KX852460 for the biological control of Rhizoctonia solani AG-3 strain KX852461 to control target spot disease in tobacco leaf. AMB Express 2017, 7, 1–9. [Google Scholar] [CrossRef]
- He, J.; Huang, S.; Sun, X.; Han, L.; Chang, C.; Zhang, W.; Zhong, Q. Carvacrol loaded solid lipid nanoparticles of propylene glycol monopalmitate and glyceryl monostearate: Preparation, characterization, and synergistic antimicrobial activity. Nanomaterials 2019, 9, 1162. [Google Scholar] [CrossRef]
- Winn, W., Jr.; Allen, S.; Janda, W.; Koneman, E.; Procop, G.; Schreckenberger, P.; Woods, G. Koneman’s Color Atlas and Textbook of Diagnostic Microbiology, 6th ed.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2006; pp. 353–355. [Google Scholar]
- Seifert, H.; Strate, A.; Pulverer, G. Nosocomial bacteremia due to Acinetobacter baumannii: Clinical features, epidemiology, and predictors of mortality. Medicine 1995, 74, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Bergogne-Berezin, E. Importance of Acinetobacter spp. In Acinetobacter Biology and Pathogenesis. Infectious Agents and Pathogenesis; Bergogne-Berezin, E., Friedman, H., Bendinelli, M., Eds.; Springer: New York, NY, USA, 2008; pp. 1–18. [Google Scholar]
- Gaynes, R.; Edwards, J.R.; National Nosocomial Infections Surveillance System. Overview of nosocomial infections caused by gram-negative bacilli. Clin. Infect. Dis. 2005, 41, 848–854. [Google Scholar]
- Metan, G.; Alp, E.; Aygen, B.; Sumerkan, B. Acinetobacter baumannii meningitis in post-neurosurgical patients: Clinical outcome and impact of carbapenem resistance. J. Antimicrob. Chemother. 2007, 60, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Peleg, A.Y.; Seifert, H.; Paterson, D.L. Acinetobacter baumannii: Emergence of a successful pathogen. Clin. Microbiol. Rev. 2008, 21, 538–582. [Google Scholar] [CrossRef] [PubMed]
- Ozgen, A.; Tan Erkoc, T.; Tastan, O.F.; Pehlevan, F. Phylogenetic Analysis of Origanum vulgare and Its Antioxidant and Antimicrobial Activity. Gazi Univ. J. Sci. 2021, 34, 311–325. [Google Scholar] [CrossRef]
- Canlı, K.; Yetgin, A.; Benek, A.; Bozyel, M.E.; Altuner, E.M. In Vitro Antimicrobial Activity Screening of Ethanol Extract of Lavandula stoechas and Investigation of Its Biochemical Composition. Adv. Pharmacol. Pharm. Sci. 2019, 2019, 3201458. [Google Scholar] [CrossRef]
- Klare, I.; Konstabel, C.; Badstübner, D.; Werner, G.; Witte, W. Occurrence and Spread of Antibiotic Resistances in Enterococcus faecium. Int. J. Food Microbiol. 2003, 88, 269–290. [Google Scholar] [CrossRef]
- Zhou, X.; Willems, R.J.; Friedrich, A.W.; Rossen, J.W.; Bathoorn, E. Enterococcus faecium: From Microbiological Insights to Practical Recommendations for Infection Control and Diagnostics. Antimicrob. Resist. Infect. Control 2020, 9, 1–13. [Google Scholar] [CrossRef]
- Shiadeh, S.M.J.; Pormohammad, A.; Hashemi, A.; Lak, P. Global Prevalence of Antibiotic Resistance in Blood-Isolated Enterococcus faecalis and Enterococcus faecium: A Systematic Review and Meta-Analysis. Infect. Drug Resist. 2019, 12, 2713. [Google Scholar] [CrossRef]
- Sener, I.; Gur, M.; Verep, D.; Guney, K.; Altuner, E.M. Antimicrobial Activities and Some Flavonoids in Extracts of Some Medicinal Plants. Indian J. Pharm. Educ. Res. 2017, 51, 20. [Google Scholar] [CrossRef]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.J.; Cheng, Z. Antibiotic Resistance in Pseudomonas aeruginosa: Mechanisms and Alternative Therapeutic Strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Vena, A.; Croxatto, A.; Righi, E.; Guery, B. How to Manage Pseudomonas aeruginosa Infections. Drugs Context 2018, 7, 212527. [Google Scholar] [CrossRef] [PubMed]
- Thi, M.T.T.; Wibowo, D.; Rehm, B.H. Pseudomonas aeruginosa Biofilms. Int. J. Mol. Sci. 2020, 21, 8671. [Google Scholar] [CrossRef]
- Ahmad, I.H.; Mohammed, S.A.S.; Rana, M.J.; Salam, Y.A.Z.; Waheed, J.J.; Nidal, A.A.Z. Antimicrobial Activities of Six Plants Used in Traditional Arabic Palestinian Herbal Medicine. Afr. J. Microbiol. Res. 2014, 8, 3501–3507. [Google Scholar] [CrossRef]
- Lee, Y.; Puumala, E.; Robbins, N.; Cowen, L.E. Antifungal Drug Resistance: Molecular Mechanisms in Candida albicans and Beyond. Chem. Rev. 2020, 121, 3390–3411. [Google Scholar] [CrossRef]
- d’Enfert, C.; Kaune, A.K.; Alaban, L.R.; Chakraborty, S.; Cole, N.; Delavy, M.; Kosmala, D.; Marsaux, B.; Frois-Martins, R.; Morelli, M.; et al. The Impact of the Fungus-Host-Microbiota Interplay upon Candida albicans Infections: Current Knowledge and New Perspectives. FEMS Microbiol. Rev. 2021, 45, fuaa060. [Google Scholar] [CrossRef]
- Kerbouche, L.; Hazzit, M.; Ferhat, M.A.; Baaliouamer, A.; Miguel, M.G. Biological Activities of Essential Oils and Ethanol Extracts of Teucrium polium subsp. capitatum (L.) Briq. and Origanum floribundum Munby. J. Essent. Oil Bear. Plants 2015, 18, 1197–1208. [Google Scholar] [CrossRef]
- Tunca-Pinarli, Y.; Benek, A.; Turu, D.; Bozyel, M.E.; Canli, K.; Altuner, E.M. Biological Activities and Biochemical Composition of Endemic Achillea fraasii. Microorganisms 2023, 11, 978. [Google Scholar] [CrossRef]
- Andrews, J.M. BSAC Standardized Disc Susceptibility Testing Method (Version 6). J. Antimicrob. Chemother. 2003, 60, 20–41. [Google Scholar] [CrossRef] [PubMed]
- Mensor, L.L.; Menezes, F.S.; Leitão, G.G.; Reis, A.S.; Santos, T.C.D.; Coube, C.S.; Leitão, S.G. Screening of Brazilian Plant Extracts for Antioxidant Activity by the Use of DPPH Free Radical Method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Bharat, C.R.; Krishna, G.D. GC-MS Analysis of Young Leaves of Allophylus cobbe (L.) Raeusch. and Allophylus serratus (Roxb.) Kurz. Indian J. Pharm. Educ. Res. 2017, 51, 472–479. [Google Scholar] [CrossRef]

| No | Microorganisms | 50 µL 1 | 100 µL 1 | 200 µL 1 | Gen | Amp | Tob |
|---|---|---|---|---|---|---|---|
| 1 | Bacillus subtilis DSMZ 1971 | 17.00 ± 0.00 | 19.00 ± 0.00 | 27.00 ± 0.00 | 30 | 41 | 26 |
| 2 | Candida albicans DSMZ 1386 | 12.00 ± 0.00 | 15.00 ± 0.58 | 17.00 ± 0.00 | 12 | 0 | 13 |
| 3 | Enterobacter aerogenes ATCC 13048 | 10.00 ± 0.00 | 14.00 ± 0.00 | 17.00 ± 0.00 | 24 | 0 | 18 |
| 4 | Enterococcus faecalis ATCC 29212 | 11.00 ± 0.00 | 14.00 ± 0.00 | 18.00 ± 0.58 | 12 | 14 | 8 |
| 5 | Escherichia coli ATCC 25922 | 10.00 ± 0.00 | 14.00 ± 0.00 | 18.00 ± 0.00 | 22 | 6 | 20 |
| 6 | Listeria monocytogenes ATCC 7644 | 10.00 ± 0.00 | 13.00 ± 0.00 | 28.00 ± 0.00 | 28 | 23 | 24 |
| 7 | Pseudomonas aeruginosa DSMZ 50071 | 18.00 ± 0.00 | 24.00 ± 0.00 | 15.00 ± 0.00 | 15 | 0 | 22 |
| 8 | Pseudomonas fluorescens P1 | 19.00 ± 0.58 | 24.00 ± 0.00 | 18.00 ± 0.00 | 13 | 14 | 12 |
| 9 | Salmonella enteritidis ATCC 13076 | 11.00 ± 0.00 | 16.00 ± 0.00 | 19.00 ± 0.00 | 21 | 16 | 15 |
| 10 | Salmonella typhimurium SL 1344 | 11.00 ± 0.00 | 12.00 ± 0.00 | 21.00 ± 0.00 | 24 | 13 | 15 |
| 11 | Staphylococcus aureus ATCC 25923 | 20.00 ± 0.00 | 23.00 ± 0.00 | 36.00 ± 0.58 | 21 | 25 | 14 |
| 12 | Staphylococcus epidermidis DSMZ 20044 | 18.00 ± 0.00 | 19.00 ± 0.00 | 29.00 ± 0.00 | 22 | 24 | 20 |
| 13 | Enterococcus durans (FI) | 12.00 ± 0.00 | 16.00 ± 0.00 | 20.00 ± 0.00 | 11 | 28 | 13 |
| 14 | Enterococcus faecium (FI) | 50.00 ± 0.00 | 52.00 ± 0.00 | 42.00 ± 0.00 | 28 | 32 | 15 |
| 15 | Klebsiella pneumoniae (FI) | 12.00 ± 0.00 | 15.00 ± 1.15 | 16.00 ± 0.00 | 19 | 6 | 23 |
| 16 | Listeria innocua (FI) | 11.00 ± 0.00 | 13.00 ± 0.00 | 19.00 ± 0.58 | 13 | 13 | 15 |
| 17 | Salmonella infantis (FI) | 11.00 ± 0.00 | 13.00 ± 0.00 | 19.00 ± 0.00 | 17 | 14 | 14 |
| 18 | Salmonella kentucky (FI) | 11.00 ± 0.00 | 15.00 ± 0.00 | 20.00 ± 0.00 | 12 | 15 | 16 |
| 19 | Escherichia coli (FI) | 11.00 ± 0.00 | 16.00 ± 0.00 | 16.00 ± 0.00 | 20 | 0 | 0 |
| 20 | Staphylococcus aureus (CI) | 25.00 ± 0.00 | 26.00 ± 0.00 | 36.00 ± 0.00 | 22 | 0 | 18 |
| 21 | Shigella boydii (CI) | 11.00 ± 0.00 | 17.00 ± 0.00 | 17.00 ± 0.00 | 20 | 0 | 18 |
| 22 | Candida tropicalis (CI) | 28.00 ± 0.00 | 31.00 ± 0.00 | 31.00 ± 0.00 | 0 | 0 | 0 |
| 23 | Escherichia coli (MDR) | 10.00 ± 0.00 | 16.00 ± 0.00 | 19.00 ± 0.00 | 8 | 0 | 9 |
| 24 | Klebsiella pneumoniae (MDR) | 11.00 ± 0.00 | 16.00 ± 0.00 | 14.00 ± 0.00 | 15 | 8 | 20 |
| 25 | Acinetobacter baumannii (MDR) | 12.00 ± 0.00 | 17.00 ± 0.00 | 19.00 ± 0.00 | 0 | 0 | 0 |
| 26 | Enterobacter aerogenes (MDR) | 11.00 ± 0.00 | 11.00 ± 0.00 | 15.00 ± 0.00 | 16 | 0 | 18 |
| 27 | Serratia odorifera (MDR) | 10.00 ± 0.00 | 15.00 ± 0.00 | 19.00 ± 0.00 | 7 | 0 | 9 |
| 28 | Proteus vulgaris (MDR) | 11.00 ± 0.00 | 14.00 ± 0.00 | 18.00 ± 0.00 | 11 | 9 | 11 |
| 29 | Streptococcus pneumoniae (MDR) | 11.00 ± 0.00 | 13.00 ± 0.00 | 18.00 ± 0.00 | 10 | 9 | 8 |
| 30 | Staphylococcus aureus (MRSA + MDR) | 29.00 ± 0.00 | 29.00 ± 1.15 | 29.00 ± 0.00 | 22 | 22 | 21 |
| Pearson Correlation Test | Confidence Interval for the Differences in Means | ||||
|---|---|---|---|---|---|
| Microorganisms | Correlation | p-Value | EMM 1 | Lower CL 2 | Upper CL 3 |
| Bacillus subtilis DSMZ 1971 | 0.9897 | 0.0913 | 21.0 | 15.79 | 26.2 |
| Candida albicans DSMZ 1386 | 0.9538 | 0.1942 | 14.7 | 9.46 | 19.9 |
| Enterobacter aerogenes ATCC 13048 | 0.9631 | 0.1734 | 13.7 | 8.46 | 18.9 |
| Enterococcus faecalis ATCC 29212 | 0.9942 | 0.0686 | 14.3 | 9.12 | 19.5 |
| Escherichia coli ATCC 25922 | 0.9820 | 0.1210 | 14.0 | 8.79 | 19.2 |
| Listeria monocytogenes ATCC 7644 | 0.9843 | 0.1129 | 17.0 | 11.79 | 22.2 |
| Pseudomonas aeruginosa DSMZ 50071 | −0.5000 | 0.6667 | 19.0 | 13.79 | 24.2 |
| Pseudomonas fluorescens P1 | −0.3394 | 0.7795 | 20.3 | 15.12 | 25.5 |
| Salmonella enteritidis ATCC 13076 | 0.9449 | 0.2123 | 15.3 | 10.12 | 20.5 |
| Salmonella typhimurium SL 1344 | 0.9707 | 0.1544 | 14.7 | 9.46 | 19.9 |
| Staphylococcus aureus ATCC 25923 | 0.9878 | 0.0994 | 26.3 | 21.12 | 31.5 |
| Staphylococcus epidermidis DSMZ 20044 | 0.9686 | 0.1599 | 22.0 | 16.79 | 27.2 |
| Enterococcus durans (FI) | 0.9820 | 0.1210 | 16.0 | 10.79 | 21.2 |
| Enterococcus faecium (FI) | −0.8660 | 0.3333 | 48.0 | 42.79 | 53.2 |
| Klebsiella pneumoniae (FI) | 0.8910 | 0.3000 | 14.3 | 9.12 | 19.5 |
| Listeria innocua (FI) | 0.9960 | 0.0579 | 14.3 | 9.12 | 19.5 |
| Salmonella infantis (FI) | 0.9960 | 0.0579 | 14.3 | 9.12 | 19.5 |
| Salmonella kentucky (FI) | 0.9921 | 0.0803 | 15.3 | 10.12 | 20.5 |
| Escherichia coli (FI) | 0.7559 | 0.4544 | 14.3 | 9.12 | 19.5 |
| Staphylococcus aureus (CI) | 0.9686 | 0.1599 | 29.0 | 23.79 | 34.2 |
| Shigella boydii (CI) | 0.7559 | 0.4544 | 15.0 | 9.79 | 20.2 |
| Candida tropicalis (CI) | 0.7559 | 0.4544 | 30.0 | 24.79 | 35.2 |
| Escherichia coli (MDR) | 0.9286 | 0.2421 | 15.0 | 9.79 | 20.2 |
| Klebsiella pneumoniae (MDR) | 0.4336 | 0.7145 | 13.7 | 8.46 | 18.9 |
| Acinetobacter baumannii (MDR) | 0.9078 | 0.2755 | 16.0 | 10.79 | 21.2 |
| Enterobacter aerogenes (MDR) | 0.9449 | 0.2123 | 12.3 | 7.12 | 17.5 |
| Serratia odorifera (MDR) | 0.9679 | 0.1618 | 14.7 | 9.46 | 19.9 |
| Proteus vulgaris (MDR) | 0.9942 | 0.0687 | 14.3 | 9.12 | 19.5 |
| Streptococcus pneumoniae (MDR) | 0.9986 | 0.0334 | 14.0 | 8.79 | 19.2 |
| Staphylococcus aureus (MRSA + MDR) | NA | NA | 29.0 | 23.79 | 34.2 |
| Antibiotic | Ec | Kp | Ab | Ee | So | Pv | Sp | Sa |
|---|---|---|---|---|---|---|---|---|
| Gentamicin | 8 | 15 | - | 16 | 7 | 11 | 10 | 22 |
| Tobramycin | 9 | 20 | - | 18 | 9 | 11 | 8 | 21 |
| Ciprofloxacin | 7 | 21 | - | 32 | 23 | 42 | 42 | 27 |
| Cefazolin | - | - | - | 11 | - | - | - | 26 |
| Clindamycin | - | - | - | - | - | 9 | 9 | 38 |
| Chloramphenicol | 26 | 25 | 9 | 31 | 28 | 22 | 22 | 30 |
| Ceftriaxone | - | 22 | - | 32 | 8 | 23 | 26 | 19 |
| Ampicillin | 8 | 8 | 8 | - | - | 9 | 9 | 22 |
| Cephalothin | - | - | - | - | - | - | - | 28 |
| Cefuroxime | - | 9 | - | 18 | - | 20 | 19 | 31 |
| Vancomycin | 8 | - | 8 | - | 8 | - | 9 | 19 |
| Amoxicillin/Clavulanic acid | 12 | - | - | - | 13 | 9 | 10 | 25 |
| Trimethoprim/Sulfamethoxazole | - | - | - | 30 | - | 30 | 8 | 30 |
| Clarithromycin | - | 8 | - | 15 | - | 10 | 10 | 15 |
| Aztreonam | 9 | 29 | - | 33 | 16 | 37 | 40 | - |
| Piperacillin/Tazobactam | 20 | 27 | - | 15 | 22 | 32 | 31 | 23 |
| Ampicillin/Sulbactam | 8 | - | - | - | 10 | 12 | 15 | 23 |
| Ceftazidime | 12 | 15 | - | 31 | 21 | 25 | 27 | 19 |
| Rifampicin | - | - | 10 | 8 | 9 | 13 | 11 | 36 |
| Oxacillin | - | - | - | 8 | - | - | - | 17 |
| Piperacillin | - | 14 | - | - | 8 | 24 | 24 | 21 |
| Linezolid | - | - | - | - | - | 11 | 13 | 33 |
| Teicoplanin | 8 | 8 | - | 8 | 8 | 8 | 9 | 18 |
| Amikacin | 20 | 25 | 8 | 29 | 18 | 26 | 29 | 25 |
| Polymyxin B | 16 | 15 | 16 | 14 | 14 | 12 | 10 | 9 |
| Cefoxitin | 8 | 8 | 8 | - | 19 | 12 | 10 | 20 |
| Imipenem | 34 | 27 | 9 | 28 | 29 | 26 | 30 | 56 |
| Sulbactam/Cefoperazone | 10 | 13 | - | 9 | 10 | 16 | 13 | 20 |
| Colistin sulfate | 14 | 20 | 13 | 13 | 10 | - | 9 | 8 |
| Furazolidone | 29 | 28 | 10 | 25 | 23 | 12 | 13 | 17 |
| Optochin | 8 | 8 | - | 8 | 8 | 8 | 8 | - |
| Bacitracin | - | 8 | - | 8 | - | - | - | 8 |
| Cefotaxime | - | 19 | - | 30 | - | 22 | 14 | 22 |
| No | Microorganisms | MIC (mg/mL) |
|---|---|---|
| 1 | Bacillus subtilis DSMZ 1971 | 4.28 |
| 2 | Candida albicans DSMZ 1386 | 4.28 |
| 3 | Enterobacter aerogenes ATCC 13048 | 17.15 |
| 4 | Enterococcus faecalis ATCC 29212 | 17.15 |
| 5 | Escherichia coli ATCC 25922 | 17.15 |
| 6 | Listeria monocytogenes ATCC 7644 | 8.57 |
| 7 | Pseudomonas aeruginosa DSMZ 50071 | 17.15 |
| 8 | Pseudomonas fluorescens P1 | 17.15 |
| 9 | Salmonella enteritidis ATCC 13076 | 34.3 |
| 10 | Salmonella typhimurium SL 1344 | 34.3 |
| 11 | Staphylococcus aureus ATCC 25923 | 17.15 |
| 12 | Staphylococcus epidermidis DSMZ 20044 | 17.15 |
| 13 | Enterococcus durans (FI) | 8.57 |
| 14 | Enterococcus faecium (FI) | 17.15 |
| 15 | Klebsiella pneumoniae (FI) | 34.3 |
| 16 | Listeria innocua (FI) | 4.28 |
| 17 | Salmonella infantis (FI) | 17.15 |
| 18 | Salmonella kentucky (FI) | 17.15 |
| 19 | Escherichia coli (FI) | 8.57 |
| 20 | Staphylococcus aureus (CI) | 17.15 |
| 21 | Shigella boydii (CI) | 34.3 |
| 22 | Candida tropicalis (CI) | 34.3 |
| 23 | Escherichia coli (MDR) | 34.3 |
| 24 | Klebsiella pneumoniae (MDR) | 34.3 |
| 25 | Acinetobacter baumannii (MDR) | 17.15 |
| 26 | Enterobacter aerogenes (MDR) | 8.57 |
| 27 | Serratia odorifera (MDR) | 8.57 |
| 28 | Proteus vulgaris (MDR) | 17.15 |
| 29 | Streptococcus pneumoniae (MDR) | 17.15 |
| 30 | Staphylococcus aureus (MRSA + MDR) | 17.15 |
| Concentrations (µg/mL) | OOEt (%) | Ascorbic Acid (%) |
|---|---|---|
| 200.000 | 99.34 | 94.67 |
| 100.000 | 98.66 | 93.39 |
| 50.000 | 93.11 | 92.08 |
| 25.000 | 90.08 | 90.09 |
| 12.500 | 90.62 | 69.94 |
| 6.250 | 88.75 | 35.79 |
| 3.125 | 88.65 | 17.70 |
| 1.075 | 59.83 | 8.74 |
| No | RT | Chemical Structures | Compound Name | Formula | MW (g/mol) | Area (%) | Known Activity |
|---|---|---|---|---|---|---|---|
| 1 | 14.269 | 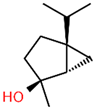 | Sabinene hydrate | C10H18O | 154.249 | 0.75 | Antioxidant activity [29] |
| 2 | 15.487 | 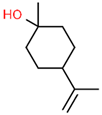 | β -Terpineol | C10H18O | 154.249 | 0.26 | - |
| 3 | 18.004 |  | Borneol | C10H18O | 154.249 | 1.00 | Antibacterial activity [30] |
| 4 | 18.451 | 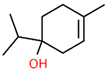 | 4-Carvomenthenol | C10H18O | 154.249 | 0.79 | Anti-inflammatory activity [31] |
| 5 | 21.089 | 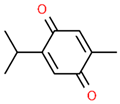 | Thymoquinone | C10H12O2 | 164.201 | 1.09 | Neuroprotective and Anti-inflammatory effects [32,33] |
| 6 | 22.597 |  | Thymol | C10H14O | 150.218 | 0.38 | Antioxidant and antimicrobial activity [34,35,36] |
| 7 | 23.003 |  | Carvacrol | C10H14O | 150.218 | 82.34 | Antioxidant and antimicrobial activity [34,35,36] |
| 8 | 23.192 | 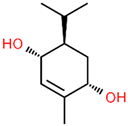 | 2-Methyl-5-(propan-2-ylidene)cyclohexane-1,4-diol | C10H18O2 | 170.249 | 2.49 | - |
| 9 | 25.261 | 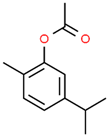 | Carvacrol acetate | C12H16O2 | 192.254 | 0.15 | Anti-inflammatory and anti-nociceptive activity [37] |
| 10 | 26.738 | 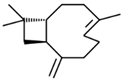 | Caryophyllene | C15H24 | 204.351 | 0.63 | Antibiofilm and anticancer activity [38,39] |
| 11 | 29.504 |  | β-Bisabolene | C15H24 | 204.351 | 0.39 | Anticancer and bactericidal activity [40,41] |
| 12 | 38.574 | 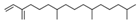 | Neophytadiene | C20H38 | 278.516 | 0.38 | - |
| 13 | 45.801 | - | Unknown | - | - | 1.79 | - |
| 14 | 53.518 | - | Unknown | - | - | 1.00 | - |
| 15 | 59.292 |  | Eicosane | C20H42 | 282.547 | 0.66 | Antifungal activity [42] |
| 16 | 59.698 |  | Glyceryl Monostearate | C21H42O4 | 358.556 | 5.64 | Antibacterial activity [43] |
| 17 | 64.101 |  | Docosane | C22H46 | 310.601 | 0.34 | - |
| 18 | 65.957 |  | Octadecane | C18H38 | 254.494 | 0.25 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canli, K.; Bozyel, M.E.; Turu, D.; Benek, A.; Simsek, O.; Altuner, E.M. Biochemical, Antioxidant Properties and Antimicrobial Activity of Steno-Endemic Origanum onites. Microorganisms 2023, 11, 1987. https://doi.org/10.3390/microorganisms11081987
Canli K, Bozyel ME, Turu D, Benek A, Simsek O, Altuner EM. Biochemical, Antioxidant Properties and Antimicrobial Activity of Steno-Endemic Origanum onites. Microorganisms. 2023; 11(8):1987. https://doi.org/10.3390/microorganisms11081987
Chicago/Turabian StyleCanli, Kerem, Mustafa Eray Bozyel, Dilay Turu, Atakan Benek, Ozcan Simsek, and Ergin Murat Altuner. 2023. "Biochemical, Antioxidant Properties and Antimicrobial Activity of Steno-Endemic Origanum onites" Microorganisms 11, no. 8: 1987. https://doi.org/10.3390/microorganisms11081987
APA StyleCanli, K., Bozyel, M. E., Turu, D., Benek, A., Simsek, O., & Altuner, E. M. (2023). Biochemical, Antioxidant Properties and Antimicrobial Activity of Steno-Endemic Origanum onites. Microorganisms, 11(8), 1987. https://doi.org/10.3390/microorganisms11081987









