Antifungal Activity of Hop Leaf Extracts and Xanthohumol on Two Strains of Venturia inaequalis with Different Sensitivities to Triazoles
Abstract
:1. Introduction
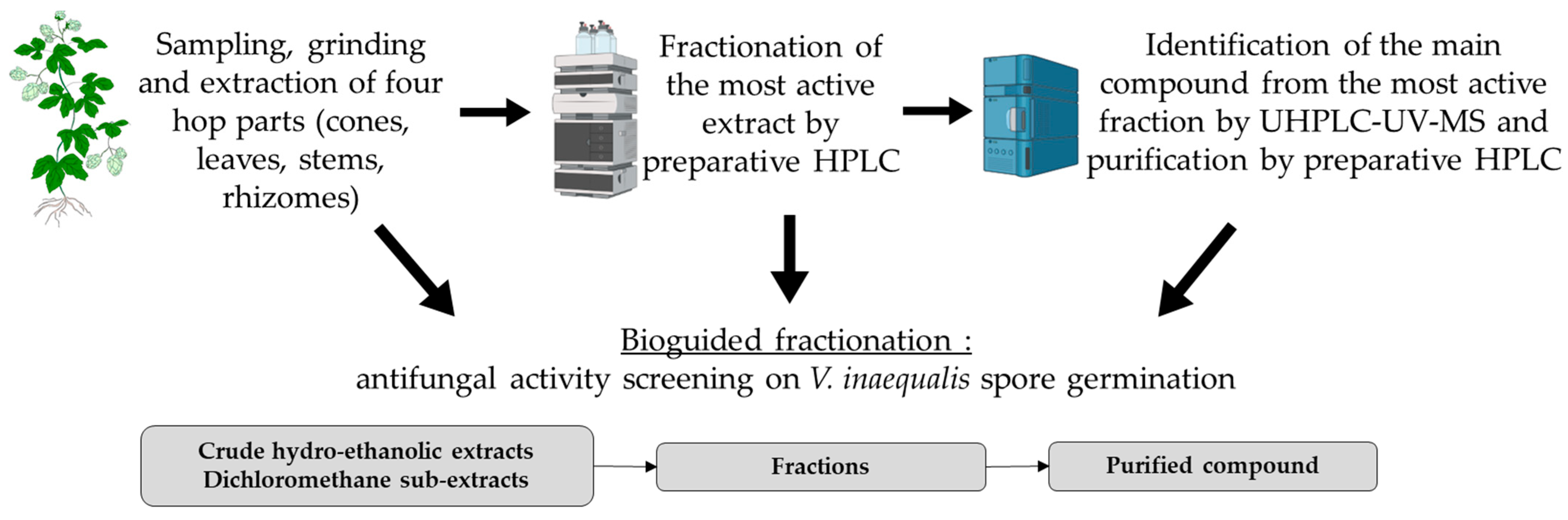
2. Materials and Methods
2.1. Hop Phytochemistry
2.1.1. Preparation of Extracts from 4 Hop Parts
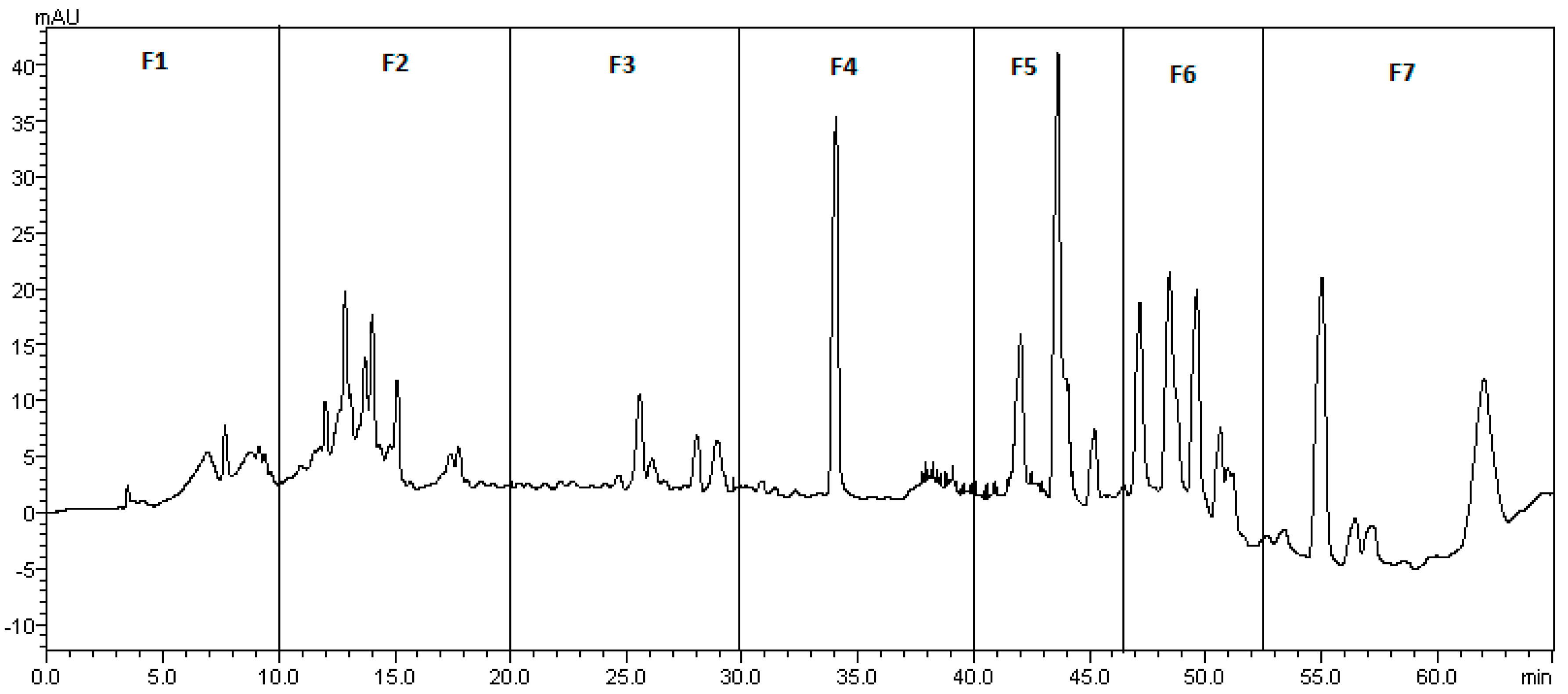
2.1.2. Fractionation of Hop Leaf DSE by Preparative HPLC
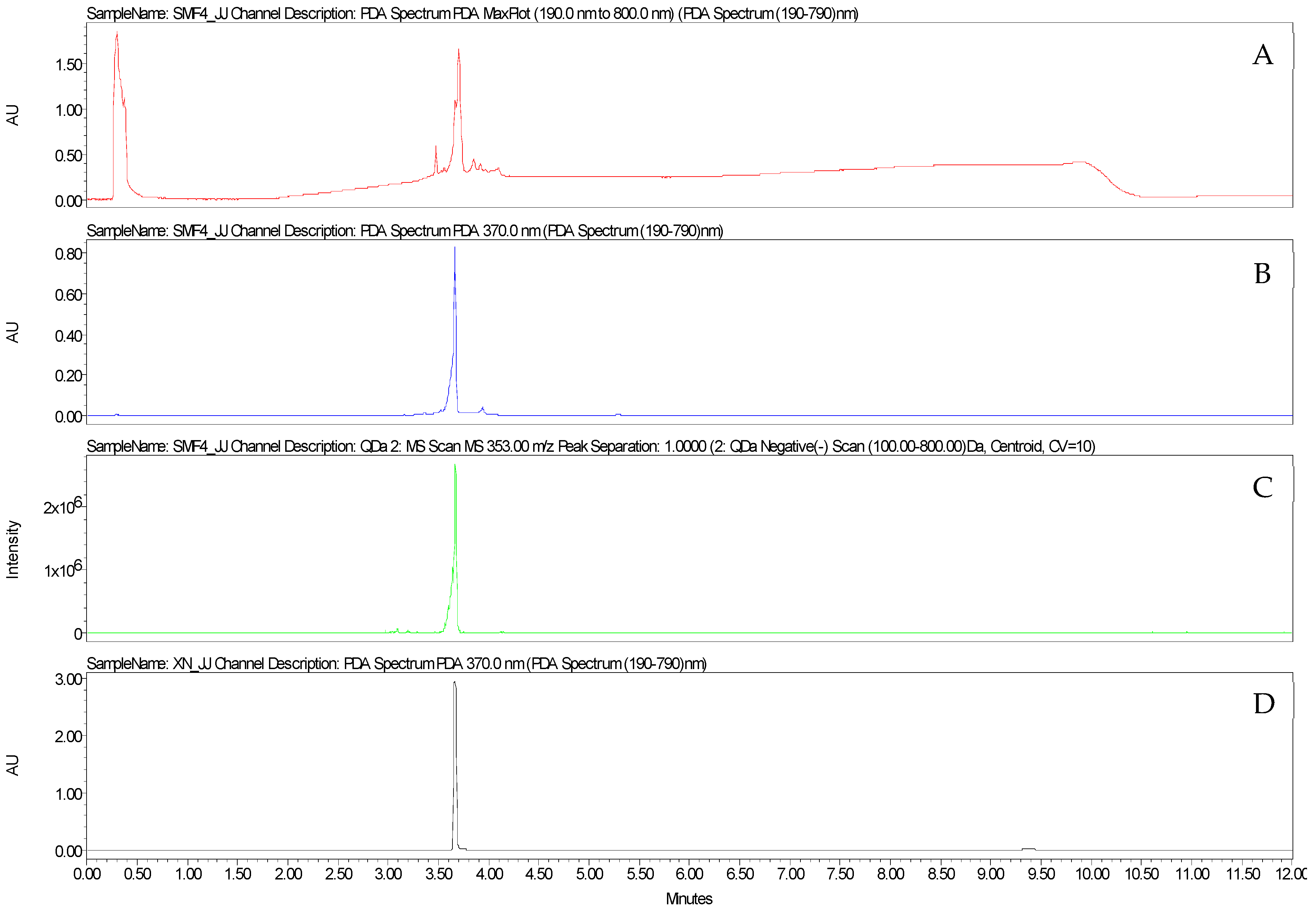
2.1.3. Purification of Xanthohumol from Hop Leaf DSE
2.1.4. Purification of Xanthohumol from Hop Cone DSE
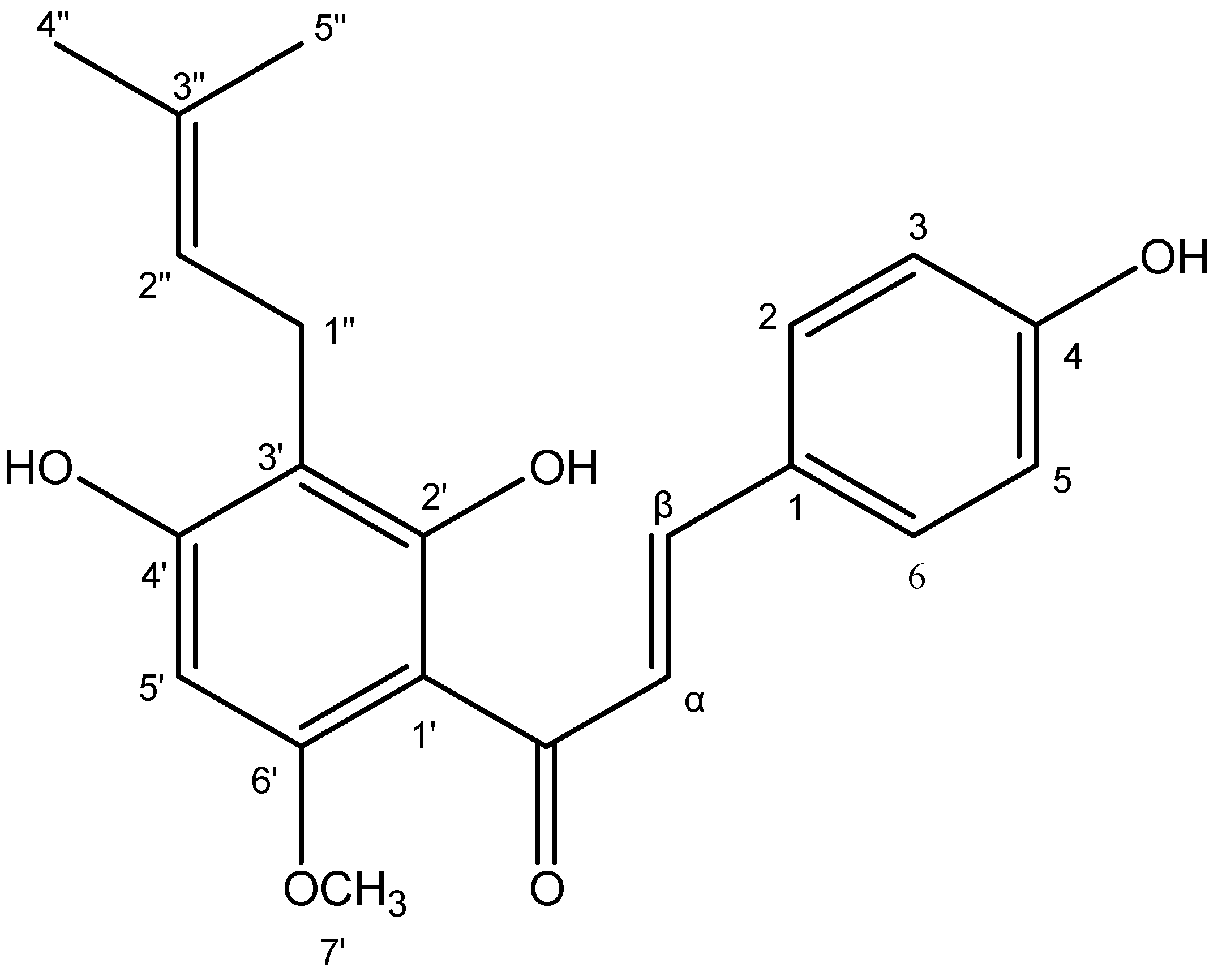
2.1.5. Structural Identification of Xanthohumol from Hop Cones by NMR
2.1.6. UHPLC-UV-MS Analysis
2.2. Antifungal Activity against Two Strains of Venturia inaequalis
2.2.1. Culture Conditions and Inoculum Preparation
2.2.2. In Vitro Assays
2.2.3. Data Analyses
3. Results
3.1. Characterization of Strain Sensitivity to Triazoles and Copper Sulphate
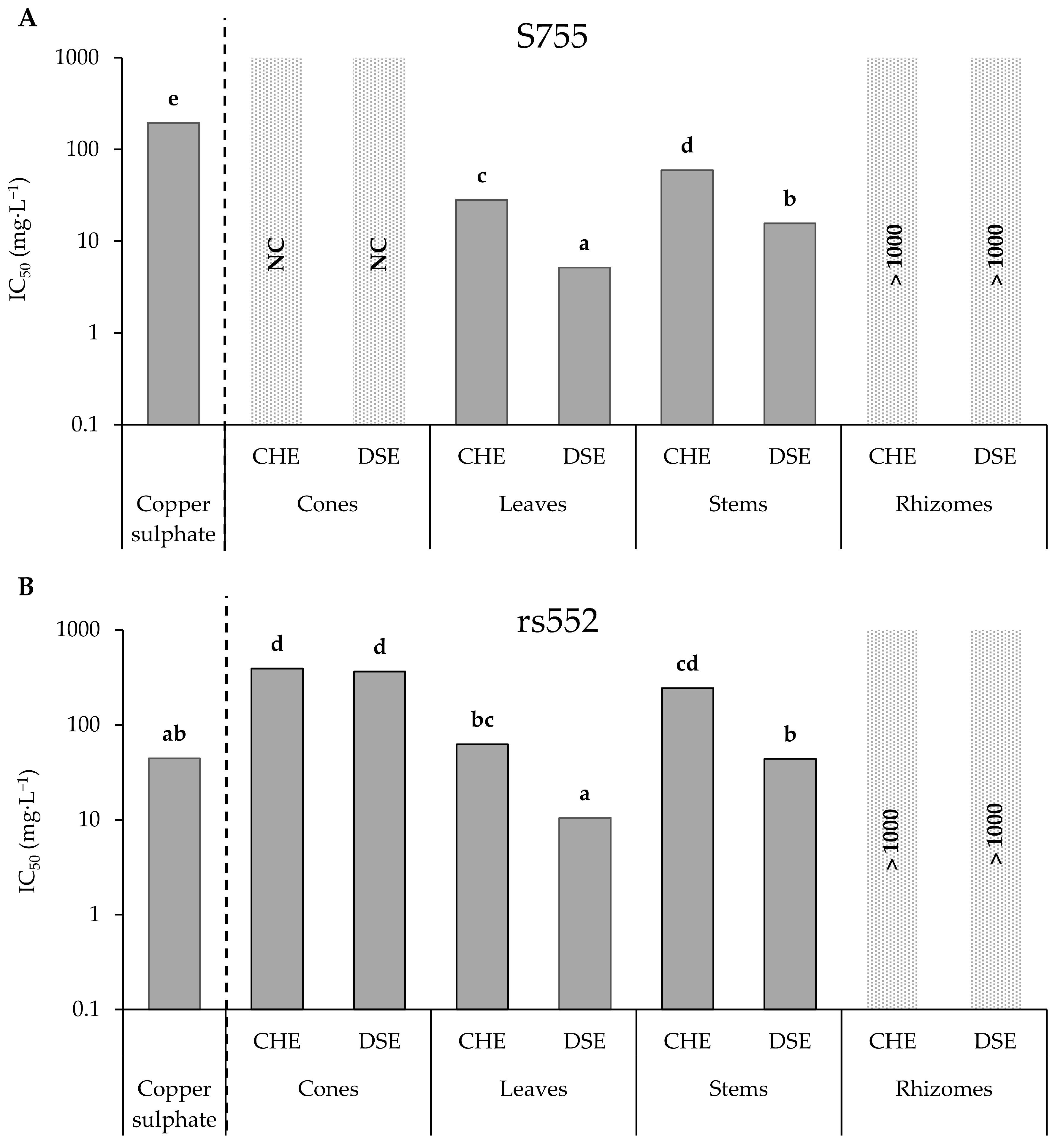
3.2. Screening of Hop Crude Extracts and Apolar Sub-Extracts
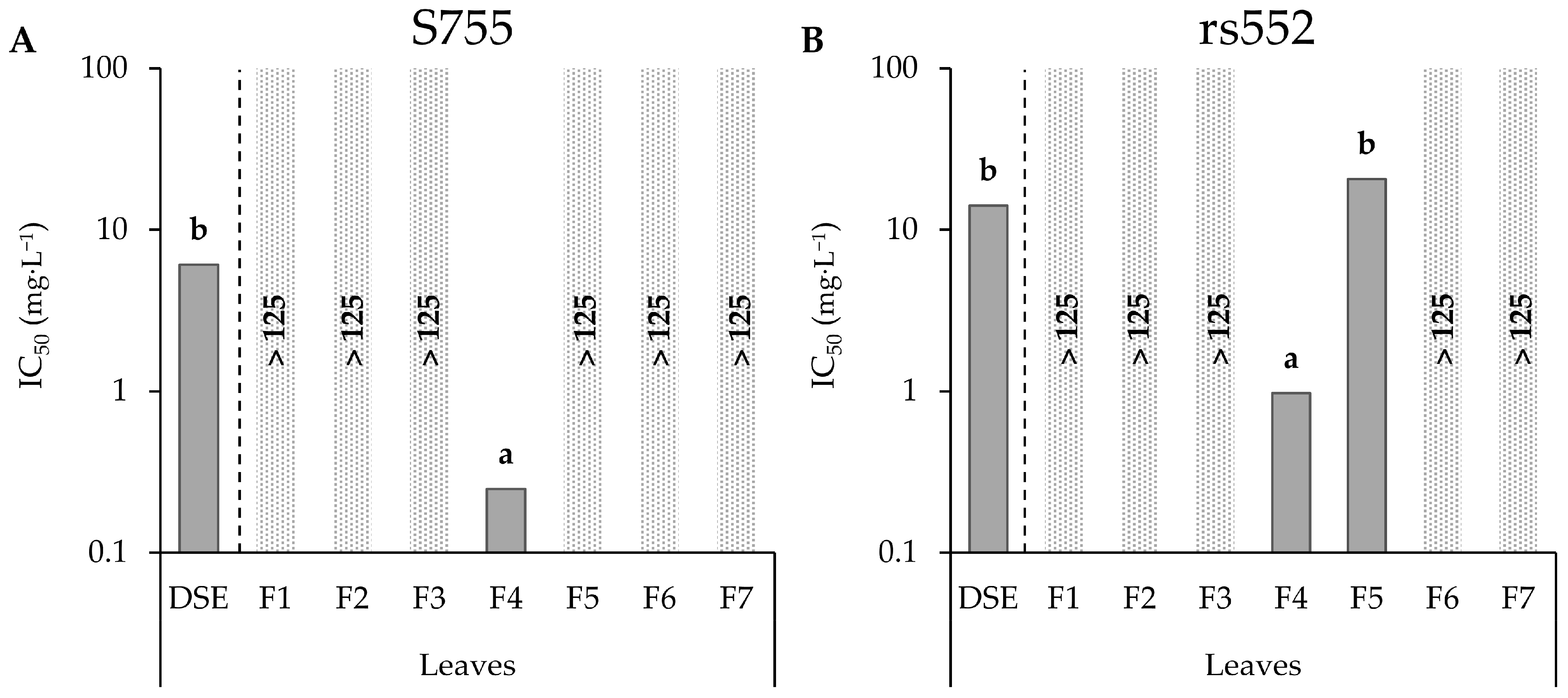
3.3. Bioguided Fractionation of Leaf DSE
3.4. Comparison of the Strain Sensitivities to Hop Extracts, Fraction and Xanthohumol
4. Discussion
4.1. Different Sensitivities in V. inaequalis Strains Are Noticed with Compounds of Plant Origin
4.2. Antifungal Properties of Hop on V. inaequalis
4.3. Hop Leaves, a Promising Source of Antifungal Agents?
4.4. The Case of Xanthohumol: A Metabolite with Interesting Antimicrobial Properties
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schubert, K.; Ritschel, A.; Braun, U. A Monograph of Fusicladium s.Lat. (Hyphomycetes). Schlechtendalia 2003, 9, 67. [Google Scholar]
- Bowen, J.K.; Mesarich, C.H.; Bus, V.G.M.; Beresford, R.M.; Plummer, K.M.; Templeton, M.D. Venturia inaequalis: The Causal Agent of Apple Scab. Mol. Plant Pathol. 2011, 12, 105–122. [Google Scholar] [CrossRef]
- Jha, G.; Thakur, K.; Thakur, P. The Venturia Apple Pathosystem: Pathogenicity Mechanisms and Plant Defense Responses. J. Biomed. Biotechnol. 2009, 2009, 680160. [Google Scholar] [CrossRef] [Green Version]
- MacHardy, W.E. Apple Scab: Biology, Epidemiology, and Management; American Phytopathological Society (APS Press): Saint Paul, MN, USA, 1996. [Google Scholar]
- Bus, V.G.M.; Rikkerink, E.H.A.; Caffier, V.; Durel, C.-E.; Plummer, K.M. Revision of the Nomenclature of the Differential Host-Pathogen Interactions of Venturia Inaequalis and Malus. Annu. Rev. Phytopathol. 2011, 49, 391–413. [Google Scholar] [CrossRef] [Green Version]
- Holb, I.J.; Abonyi, F.; Buurma, J.; Heijne, B. On-Farm and on-Station Evaluations of Three Orchard Management Approaches against Apple Scab and Apple Powdery Mildew. Crop. Prot. 2017, 97, 109–118. [Google Scholar] [CrossRef]
- Russell, P.E. Sensitivity Baselines in Fungicide Resistance Research and Management: FRAC Monograph No. 3; Crop Life International: Brussels, Belgium, 2003. [Google Scholar]
- Chapman, K.S.; Sundin, G.W.; Beckerman, J.L. Identification of Resistance to Multiple Fungicides in Field Populations of Venturia inaequalis. Plant Dis. 2011, 95, 921–926. [Google Scholar] [CrossRef] [Green Version]
- Köller, W.; Wilcox, W.F.; Barnard, J.; Jones, A.L.; Braun, P.G. Detection and Quantification of Resistance of Venturia naequalis Populations to Sterol Demethylation Inhibitors. Phytopathology 1997, 87, 184–190. [Google Scholar] [CrossRef] [Green Version]
- Holb, I.J.; De Jong, P.F.; Heijne, B. Efficacy and Phytotoxicity of Lime Sulphur in Organic Apple Production. Ann. Appl. Biol. 2003, 142, 225–233. [Google Scholar] [CrossRef]
- Damalas, C.A.; Koutroubas, S.D. Current Status and Recent Developments in Biopesticide Use. Agriculture 2018, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Karabín, M.; Hudcová, T.; Jelínek, L.; Dostálek, P. Biologically Active Compounds from Hops and Prospects for Their Use. Compr. Rev. Food Sci. Food Saf. 2016, 15, 542–567. [Google Scholar] [CrossRef] [Green Version]
- Korpelainen, H.; Pietiläinen, M. Hop (Humulus lupulus L.): Traditional and Present Use, and Future Potential. Econ. Bot. 2021, 75, 302–322. [Google Scholar] [CrossRef]
- Zanoli, P.; Zavatti, M. Pharmacognostic and Pharmacological Profile of Humulus lupulus L. J. Ethnopharmacol. 2008, 116, 383–396. [Google Scholar] [CrossRef]
- Abram, V.; Čeh, B.; Vidmar, M.; Hercezi, M.; Lazić, N.; Bucik, V.; Možina, S.S.; Košir, I.J.; Kač, M.; Demšar, L.; et al. A Comparison of Antioxidant and Antimicrobial Activity between Hop Leaves and Hop Cones. Ind. Crop. Prod. 2015, 64, 124–134. [Google Scholar] [CrossRef]
- Campalani, C.; Chioggia, F.; Amadio, E.; Gallo, M.; Rizzolio, F.; Selva, M.; Perosa, A. Supercritical CO2 Extraction of Natural Antibacterials from Low Value Weeds and Agro-Waste. J. CO2 Util. 2020, 40, 101198. [Google Scholar] [CrossRef]
- Gerhäuser, C. Broad Spectrum Anti-Infective Potential of Xanthohumol from Hop (Humulus lupulus L.) in Comparison with Activities of Other Hop Constituents and Xanthohumol Metabolites. Mol. Nutr. Food Res. 2005, 49, 827–831. [Google Scholar] [CrossRef]
- Mizobuchi, S.; Sato, Y. A New Flavanone with Antifungal Activity Isolated from Hops. Agric. Biol. Chem. 1984, 48, 2771–2775. [Google Scholar] [CrossRef] [Green Version]
- Reher, T.; Van Kerckvoorde, V.; Verheyden, L.; Wenseleers, T.; Beliën, T.; Bylemans, D.; Martens, J.A. Evaluation of Hop (Humulus lupulus) as a Repellent for the Management of Drosophila Suzukii. Crop. Prot. 2019, 124, 104839. [Google Scholar] [CrossRef]
- Paventi, G.; de Acutis, L.; De Cristofaro, A.; Pistillo, M.; Germinara, G.S.; Rotundo, G. Biological Activity of Humulus lupulus (L.) Essential Oil and Its Main Components against Sitophilus granarius (L.). Biomolecules 2020, 10, 1108. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, A.; Mitton, G.; Szawarski, N.; Cooley, H.; Ramos, F.; Meroi Arcerito, F.; Brasesco, C.; Ramirez, C.; Gende, L.; Eguaras, M.; et al. Essential Oils from Humulus lupulus as Novel Control Agents against Varroa Destructor. Ind. Crop. Prod. 2020, 158, 113043. [Google Scholar] [CrossRef]
- Bocquet, L.; Rivière, C.; Dermont, C.; Samaillie, J.; Hilbert, J.-L.; Halama, P.; Siah, A.; Sahpaz, S. Antifungal Activity of Hop Extracts and Compounds against the Wheat Pathogen Zymoseptoria tritici. Ind. Crop. Prod. 2018, 122, 290–297. [Google Scholar] [CrossRef]
- Alonso-Esteban, J.I.; Pinela, J.; Barros, L.; Ćirić, A.; Soković, M.; Calhelha, R.C.; Torija-Isasa, E.; de Cortes Sánchez-Mata, M.; Ferreira, I.C.F.R. Phenolic Composition and Antioxidant, Antimicrobial and Cytotoxic Properties of Hop (Humulus lupulus L.) Seeds. Ind. Crop. Prod. 2019, 134, 154–159. [Google Scholar] [CrossRef]
- Bartmańska, A.; Wałecka-Zacharska, E.; Tronina, T.; Popłoński, J.; Sordon, S.; Brzezowska, E.; Bania, J.; Huszcza, E. Antimicrobial Properties of Spent Hops Extracts, Flavonoids Isolated Therefrom, and Their Derivatives. Molecules 2018, 23, 2059. [Google Scholar] [CrossRef] [Green Version]
- Berne, S.; Kovačević, N.; Kastelec, D.; Javornik, B.; Radišek, S. Hop Polyphenols in Relation to Verticillium Wilt Resistance and Their Antifungal Activity. Plants 2020, 9, 1318. [Google Scholar] [CrossRef]
- Jiang, H.; Zhong, S.; Schwarz, P.; Chen, B.; Rao, J. Antifungal Activity, Mycotoxin Inhibitory Efficacy, and Mode of Action of Hop Essential Oil Nanoemulsion against Fusarium graminearum. Food Chem. 2023, 400, 134016. [Google Scholar] [CrossRef]
- Yan, Y.-F.; Wu, T.-L.; Du, S.-S.; Wu, Z.-R.; Hu, Y.-M.; Zhang, Z.-J.; Zhao, W.-B.; Yang, C.-J.; Liu, Y.-Q. The Antifungal Mechanism of Isoxanthohumol from Humulus lupulus Linn. Int. J. Mol. Sci. 2021, 22, 10853. [Google Scholar] [CrossRef] [PubMed]
- Jacquin, J.; Moureu, S.; Deweer, C.; Hakem, A.; Paguet, A.-S.; Bonneau, N.; Bordage, S.; Dermont, C.; Sahpaz, S.; Muchembled, J.; et al. Hop (Humulus lupulus L.) Specialized Metabolites: Extraction, Purification, Characterization in Different Plant Parts and In Vitro Evaluation of Anti-Oomycete Activities against Phytophthora Infestans. Agronomy 2022, 12, 2826. [Google Scholar] [CrossRef]
- Afonso, S.; Arrobas, M.; Pereira, E.L.; Rodrigues, M.Â. Recycling Nutrient-Rich Hop Leaves by Composting with Wheat Straw and Farmyard Manure in Suitable Mixtures. J. Environ. Manag. 2021, 284, 112105. [Google Scholar] [CrossRef]
- Gardea-Torresdey, J.; Hejazi, M.; Tiemann, K.; Parsons, J.G.; Duarte-Gardea, M.; Henning, J. Use of Hop (Humulus lupulus) Agricultural by-Products for the Reduction of Aqueous Lead(II) Environmental Health Hazards. J. Hazard. Mater. 2002, 91, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Bocquet, L.; Sahpaz, S.; Bonneau, N.; Beaufay, C.; Mahieux, S.; Samaillie, J.; Roumy, V.; Jacquin, J.; Bordage, S.; Hennebelle, T.; et al. Phenolic Compounds from Humulus lupulus as Natural Antimicrobial Products: New Weapons in the Fight against Methicillin Resistant Staphylococcus Aureus, Leishmania Mexicana and Trypanosoma Brucei Strains. Molecules 2019, 24, 1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muchembled, J.; Deweer, C.; Sahmer, K.; Halama, P. Antifungal Activity of Essential Oils on Two Venturia inaequalis Strains with Different Sensitivities to Tebuconazole. Environ. Sci. Pollut. Res. Int. 2018, 25, 29921–29928. [Google Scholar] [CrossRef]
- Price, C.L.; Parker, J.E.; Warrilow, A.G.; Kelly, D.E.; Kelly, S.L. Azole Fungicides—Understanding Resistance Mechanisms in Agricultural Fungal Pathogens: Mode of Action and Resistance Mechanisms to Azole Fungicides. Pest Manag. Sci. 2015, 71, 1054–1058. [Google Scholar] [CrossRef]
- Hulvey, J.; Popko, J.T.; Sang, H.; Berg, A.; Jung, G. Overexpression of ShCYP51B and ShatrD in Sclerotinia Homoeocarpa Isolates Exhibiting Practical Field Resistance to a Demethylation Inhibitor Fungicide. Appl. Environ. Microbiol. 2012, 78, 6674–6682. [Google Scholar] [CrossRef] [Green Version]
- Villani, S.M.; Biggs, A.R.; Cooley, D.R.; Raes, J.J.; Cox, K.D. Prevalence of Myclobutanil Resistance and Difenoconazole Insensitivity in Populations of Venturia inaequalis. Plant Dis. 2015, 99, 1526–1536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaegashi, H.; Hirayama, K.; Akahira, T.; Ito, T. Point Mutation in CYP51A1 of Venturia inaequalis Is Associated with Low Sensitivity to Sterol Demethylation Inhibitors. J. Gen. Plant Pathol. 2020, 86, 245–249. [Google Scholar] [CrossRef]
- Parker, J.E.; Warrilow, A.G.S.; Price, C.L.; Mullins, J.G.L.; Kelly, D.E.; Kelly, S.L. Resistance to Antifungals That Target CYP51. J. Chem. Biol. 2014, 7, 143–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cervantes, C.; Gutierrez-Corona, F. Copper Resistance Mechanisms in Bacteria and Fungi. FEMS Microbiol. Rev. 1994, 14, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Gajewska, J.; Floryszak-Wieczorek, J.; Sobieszczuk-Nowicka, E.; Mattoo, A.; Arasimowicz-Jelonek, M. Fungal and Oomycete Pathogens and Heavy Metals: An Inglorious Couple in the Environment. IMA Fungus 2022, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Antsotegi-Uskola, M.; Markina-Iñarrairaegui, A.; Ugalde, U. New Insights into Copper Homeostasis in Filamentous Fungi. Int. Microbiol. 2020, 23, 65–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceh, B.; Kac, M.; Košir, I.; Abram, V.; Ceh, B.; Kac, M.; Košir, I.J.; Abram, V. Relationships between Xanthohumol and Polyphenol Content in Hop Leaves and Hop Cones with Regard to Water Supply and Cultivar. Int. J. Mol. Sci. 2007, 8, 989–1000. [Google Scholar] [CrossRef] [Green Version]
- Arruda, T.R.; Pinheiro, P.F.; Silva, P.I.; Bernardes, P.C. A New Perspective of a Well-Recognized Raw Material: Phenolic Content, Antioxidant and Antimicrobial Activities and α- and β-Acids Profile of Brazilian Hop (Humulus lupulus L.) Extracts. LWT 2021, 141, 110905. [Google Scholar] [CrossRef]
- Schoss, K.; Kočevar Glavač, N.; Dolenc Koce, J.; Anžlovar, S. Supercritical CO2 Plant Extracts Show Antifungal Activities against Crop-Borne Fungi. Molecules 2022, 27, 1132. [Google Scholar] [CrossRef] [PubMed]
- Macchioni, V.; Picchi, V.; Carbone, K. Hop Leaves as an Alternative Source of Health-Active Compounds: Effect of Genotype and Drying Conditions. Plants 2022, 11, 99. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.; Yang, Y. Properties of Natural Cellulose Fibers from Hop Stems. Carbohydr. Polym. 2009, 77, 898–902. [Google Scholar] [CrossRef]
- Mishra, A.K.; Kocábek, T.; Sukumari Nath, V.; Awasthi, P.; Shrestha, A.; Kumar Killi, U.; Jakse, J.; Patzak, J.; Krofta, K.; Matoušek, J. Dissection of Dynamic Transcriptome Landscape of Leaf, Bract, and Lupulin Gland in Hop (Humulus lupulus L.). Int. J. Mol. Sci. 2019, 21, 233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morcol, T.B.; Matthews, P.D.; Kennelly, E.J. Differences in Leaf Chemistry and Glandular Trichome Density between Wild Southwestern American Hop (Humulus neomexicanus) and Commercial Hop Cultivars. J. Agric. Food Chem. 2021, 69, 7798–7814. [Google Scholar] [CrossRef]
- Stanius, Ž.; Dūdėnas, M.; Kaškonienė, V.; Stankevičius, M.; Skrzydlewska, E.; Drevinskas, T.; Ragažinskienė, O.; Obelevičius, K.; Maruška, A. Analysis of the Leaves and Cones of Lithuanian Hops (Humulus lupulus L.) Varieties by Chromatographic and Spectrophotometric Methods. Molecules 2022, 27, 2705. [Google Scholar] [CrossRef]
- Mizobuchi, S.; Sato, Y. Antifungal Activities of Hop Bitter Resins and Related Compounds. Agric. Biol. Chem. 1985, 49, 399–403. [Google Scholar] [CrossRef]


| Tested Substance | Strain | IC50 (mg·L−1) | Statistical Analysis | Difference between Strains |
|---|---|---|---|---|
| Tebuconazole | S755 | 0.009 | a | Yes |
| rs552 | 1.45 | b | ||
| Difenoconazole | S755 | <0.0001 | a | Yes |
| rs552 | 0.06 | b | ||
| Copper sulphate | S755 | 194.6 | b | Yes |
| rs552 | 44.5 | a |
| Hop Parts | Extracts, Fractions or Purified Metabolite | Strains | IC50 (mg·L−1) | Statistical Analysis | Difference between Strains |
|---|---|---|---|---|---|
| Cones | CHE | S755 | NC | - | Yes |
| rs552 | 389.7 | - | |||
| DSE | S755 | NC | - | Yes | |
| rs552 | 361.6 | - | |||
| Leaves | CHE | S755 | 28.2 | a | Yes |
| rs552 | 62.5 | b | |||
| DSE | S755 | 5.2 | a | Yes | |
| rs552 | 10.5 | b | |||
| Stems | CHE | S755 | 59.3 | a | Yes |
| rs552 | 242.1 | b | |||
| DSE | S755 | 15.6 | a | Yes | |
| rs552 | 43.7 | b | |||
| Rhizomes | CHE | S755 | NA | - | No |
| rs552 | NA | - | |||
| DSE | S755 | NA | - | No | |
| rs552 | NA | - | |||
| Leaves | F1 | S755 | NA | - | No |
| rs552 | NA | - | |||
| F2 | S755 | NA | - | No | |
| rs552 | NA | - | |||
| F3 | S755 | NA | - | No | |
| rs552 | NA | - | |||
| F4 | S755 | 0.25 | a | Yes | |
| rs552 | 0.97 | b | |||
| F5 | S755 | NC | - | Yes | |
| rs552 | 20.6 | - | |||
| F6 | S755 | NA | - | No | |
| rs552 | NA | - | |||
| F7 | S755 | NA | - | No | |
| rs552 | NA | - | |||
| Leaves | Xanthohumol 99% | S755 | 1.6 | a | Yes |
| rs552 | 5.1 | b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moureu, S.; Jacquin, J.; Samaillie, J.; Deweer, C.; Rivière, C.; Muchembled, J. Antifungal Activity of Hop Leaf Extracts and Xanthohumol on Two Strains of Venturia inaequalis with Different Sensitivities to Triazoles. Microorganisms 2023, 11, 1605. https://doi.org/10.3390/microorganisms11061605
Moureu S, Jacquin J, Samaillie J, Deweer C, Rivière C, Muchembled J. Antifungal Activity of Hop Leaf Extracts and Xanthohumol on Two Strains of Venturia inaequalis with Different Sensitivities to Triazoles. Microorganisms. 2023; 11(6):1605. https://doi.org/10.3390/microorganisms11061605
Chicago/Turabian StyleMoureu, Sophie, Justine Jacquin, Jennifer Samaillie, Caroline Deweer, Céline Rivière, and Jérôme Muchembled. 2023. "Antifungal Activity of Hop Leaf Extracts and Xanthohumol on Two Strains of Venturia inaequalis with Different Sensitivities to Triazoles" Microorganisms 11, no. 6: 1605. https://doi.org/10.3390/microorganisms11061605







