Stimulation of the Defense Mechanisms of Potatoes to a Late Blight Causative Agent When Treated with Bacillus subtilis Bacteria and Chitosan Composites with Hydroxycinnamic Acids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant, Microbe, and Insect Material
2.2. Obtaining Conjugates of Chitosan with Hydroxycinnamic Acids
2.3. Hydrogen Peroxide Content Measurement
2.4. SOD Activity
2.5. Peroxidase (PO) and Catalase (CAT) Activity
2.6. Native Electrophoresis of PO
2.7. Proline Content
2.8. RNA Extraction and Real-Time PCR (qPCR)
2.9. Statistical Analysis
3. Results
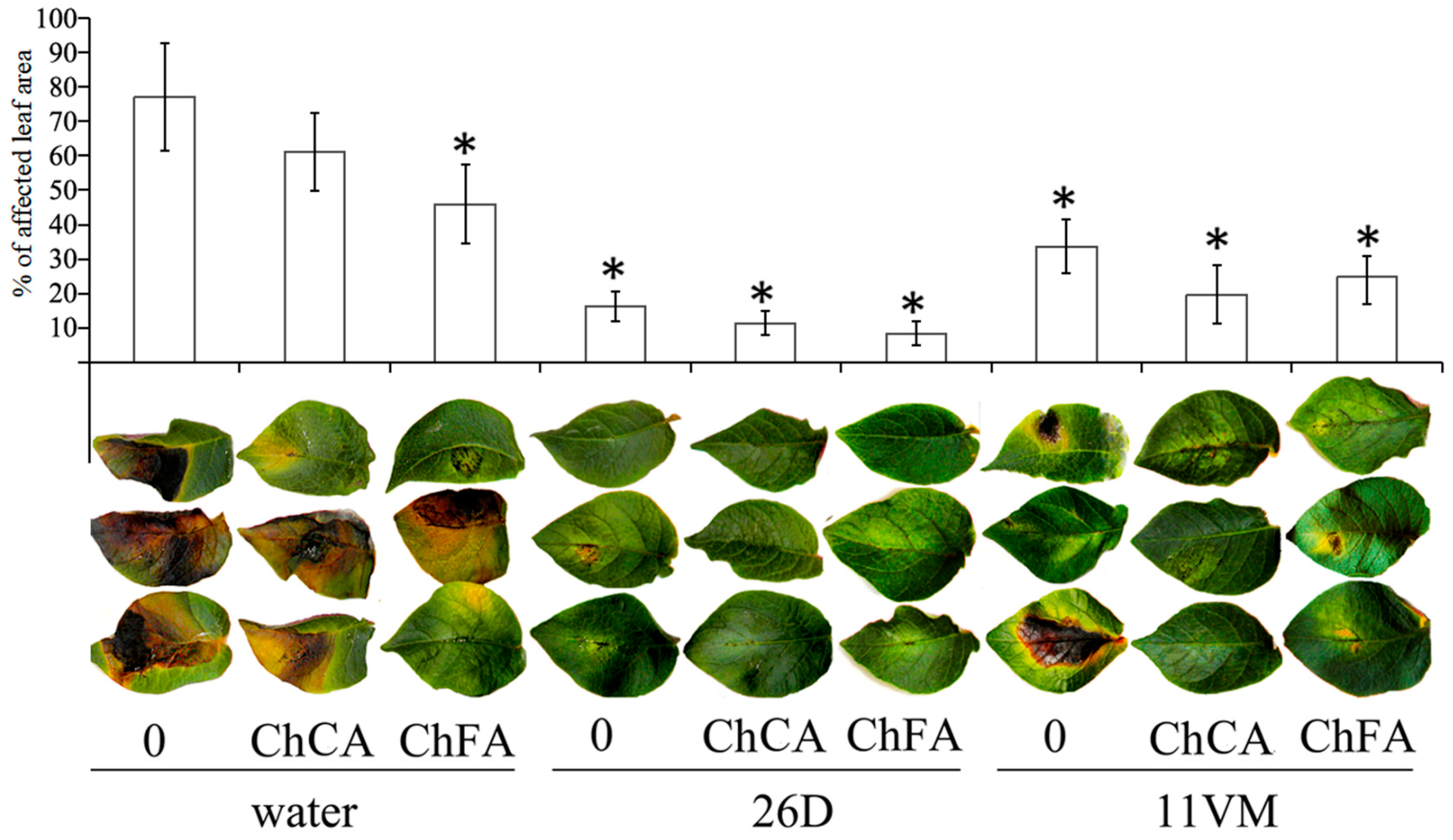
3.1. Susceptibility of Leaves to P. infestans
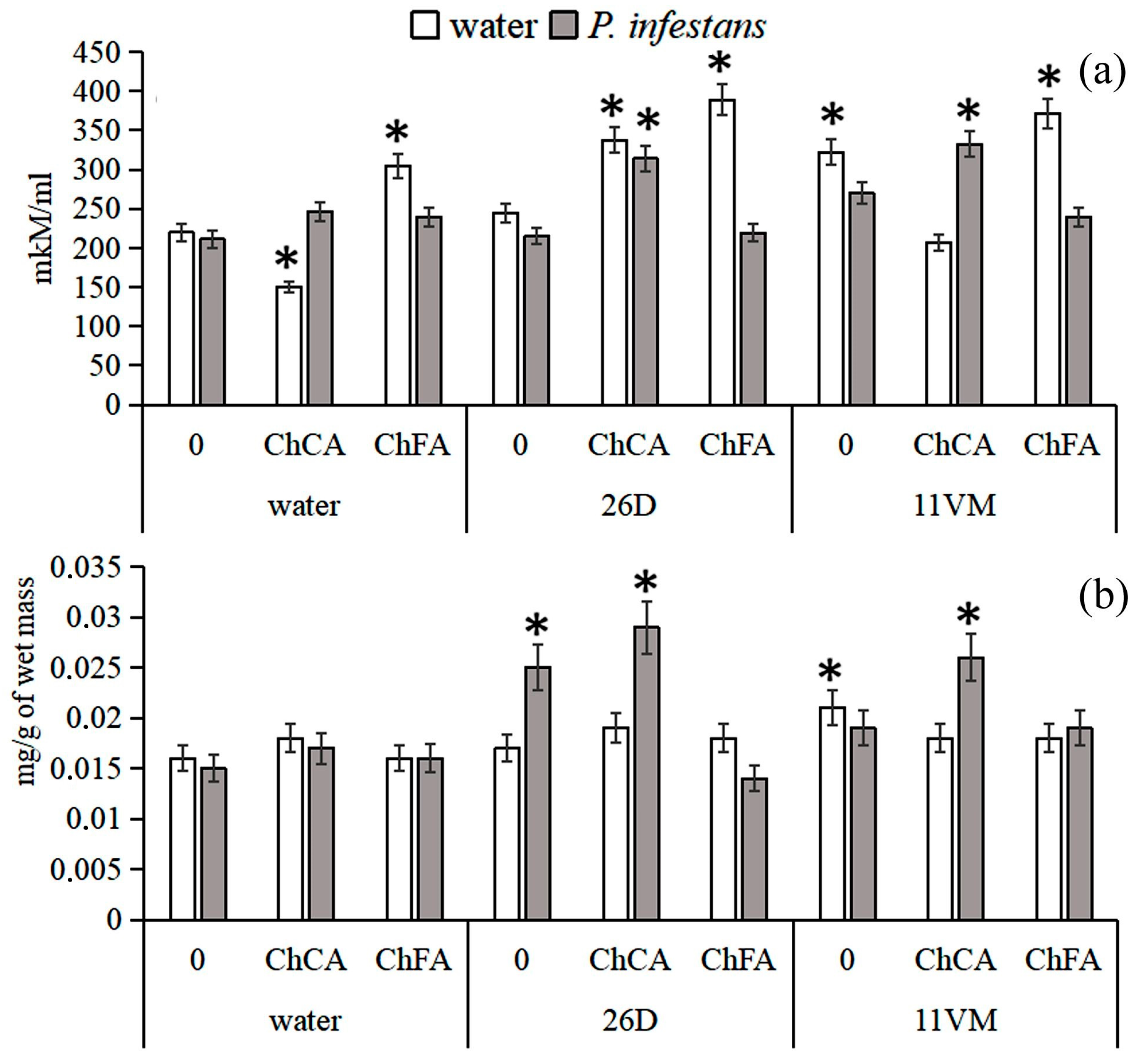
3.2. Content of Proline and H2O2
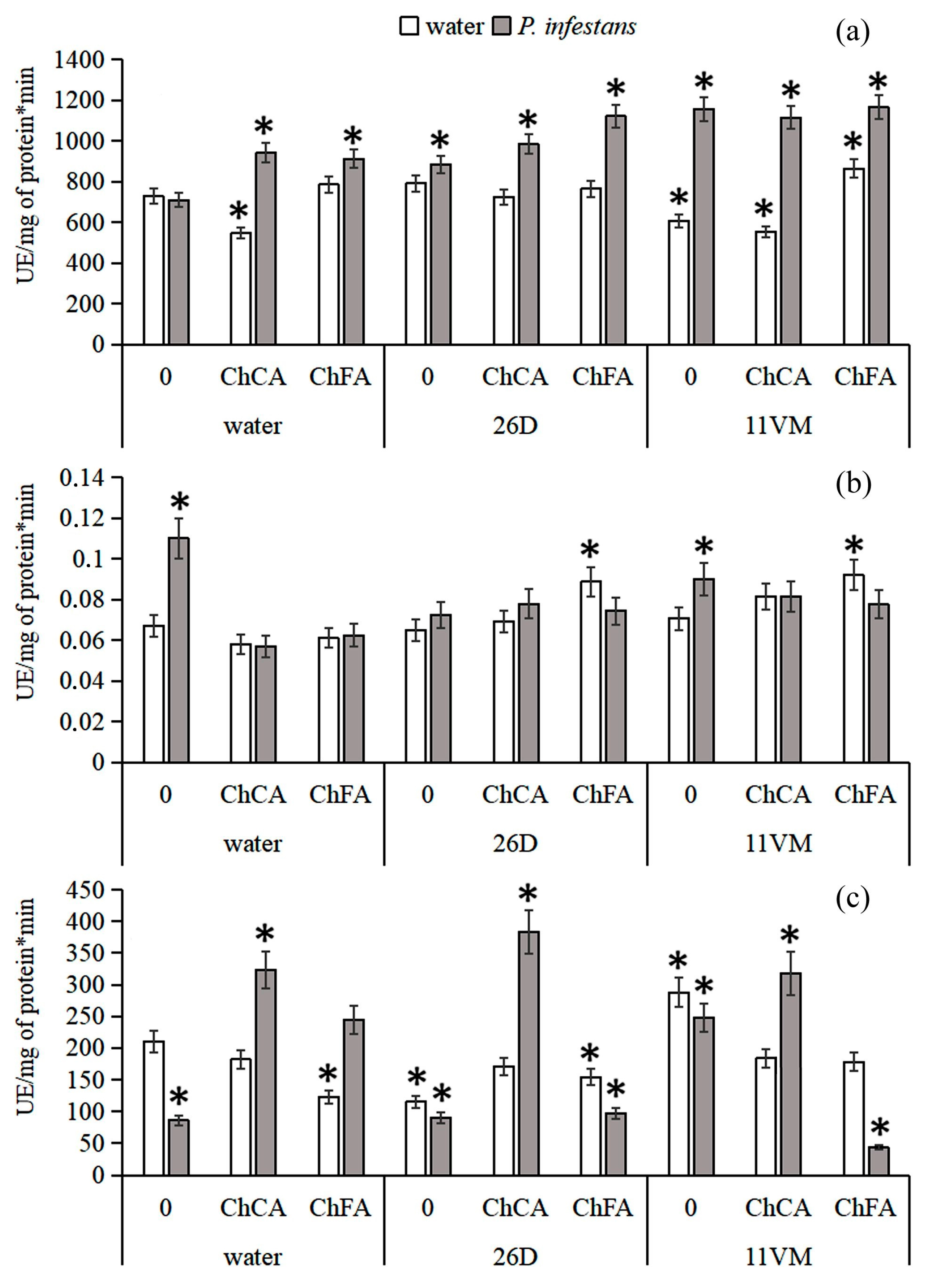
3.3. Activity of Antioxidant Enzymes
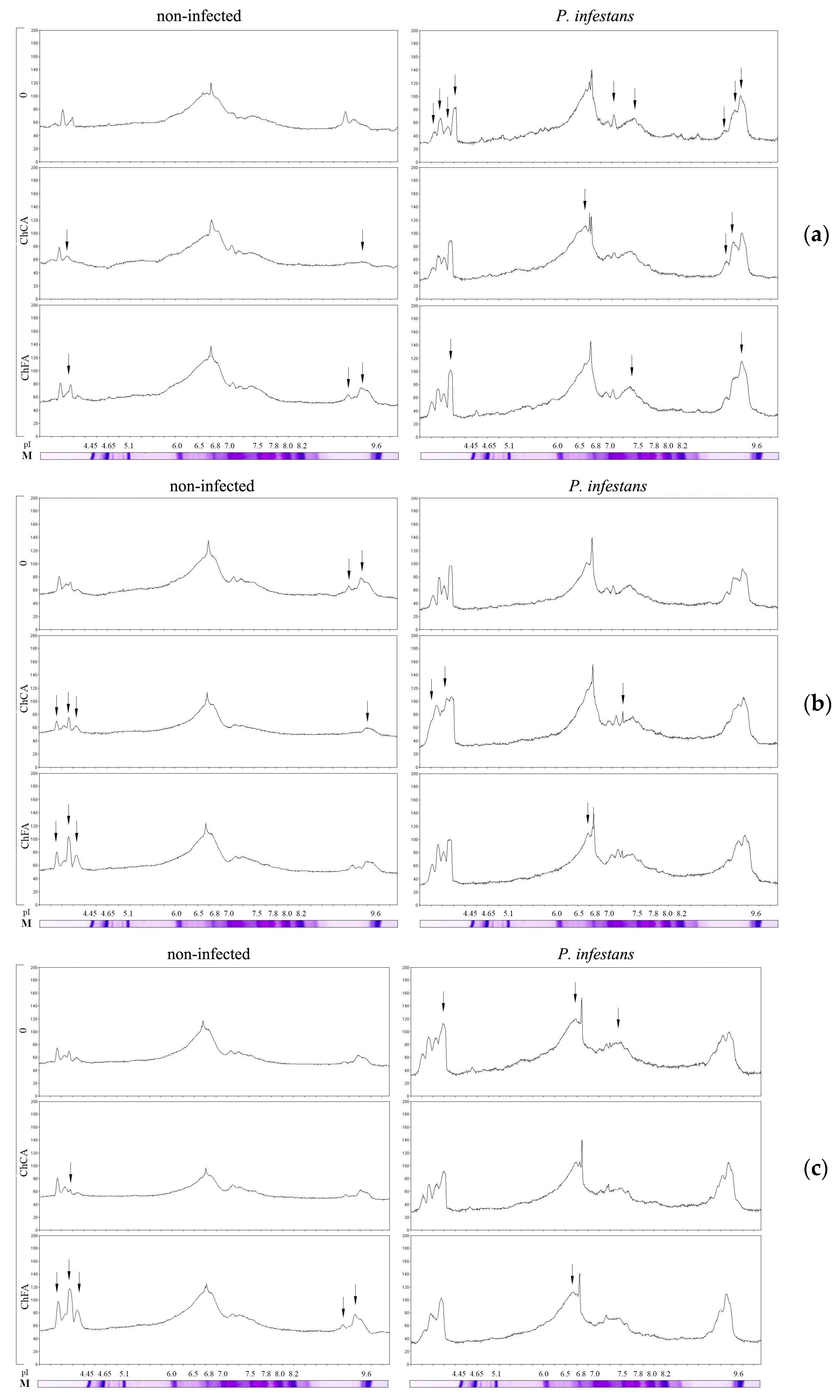
3.4. Activity of PO Isoenzymes
3.5. Transcriptional Activity of PR Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rkhaila, A.; Chtouki, T.; Erguig, H.; El Haloui, N.; Ounine, K. Chemical Proprieties of Biopolymers (Chitin/Chitosan) and Their Synergic Effects with Endophytic Bacillus Species: Unlimited Applications in Agriculture. Molecules 2021, 26, 1117. [Google Scholar] [CrossRef]
- Kumawat, K.C.; Razdan, N.; Saharan, K. Rhizospheric microbiome: Bio-based emerging strategies for sustainable agriculture development and future perspectives. Microbiol. Res. 2022, 254, 126901. [Google Scholar] [CrossRef]
- Pinski, A.; Betekhtin, A.; Hupert-Kocurek, K.; Mur, L.A.J.; Hasterok, R. Defining the Genetic Basis of Plant-Endophytic Bacteria Interactions. Int. J. Mol. Sci. 2019, 20, 1947. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.; Mallubhotla, S. Diversity, Antimicrobial Activity, and Antibiotic Susceptibility Pattern of Endophytic Bacteria Sourced from Cordia dichotoma L. Front. Microbiol. 2022, 13, 879386. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, N.; Mitra, R.; Pal, S.; Ganguly, R.; Acharya, K.; Minkina, T.; Sarkar, A.; Keswani, C. Biopesticide Consumption in India: Insights into the Current Trends. Agriculture 2023, 13, 557. [Google Scholar] [CrossRef]
- Chandler, D.; Bailey, A.S.; Tatchell, G.M.; Davidson, G.; Greaves, J.; Grant, W.P. The development, regulation and use of biopesticides for integrated pest management. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 1987–1998. [Google Scholar] [CrossRef]
- Kocięcka, J.; Liberacki, D. The Potential of Using Chitosan on Cereal Crops in the Face of Climate Change. Plants 2021, 10, 1160. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.; Prasad, J.; Das, S.; Dwivedy, A.K. Essential Oils and Their Application in Food Safety. Front. Sustain. Food Syst. 2021, 5, 653420. [Google Scholar] [CrossRef]
- Fitza, K.N.E.; Payn, K.G.; Steenkamp, E.T.; Myburg, A.A.; Naidoo, S. Chitosan Application Improves Resistance to Fusarium circinatum in Pinus patula. S. Afr. J. Bot. 2013, 85, 70–78. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, C.; Ferreira, N.; Lourenço, L. Production of Low Molecular Weight Chitosan and Chitooligosaccharides (COS): A Review. Polymers 2021, 13, 2466. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Benchamas, G.; Huang, G.L.; Huang, S.Y.; Huang, H.L. Preparation and biological activities of chitosan oligosaccharides. Trends Food Sci. Tech. 2021, 107, 38–44. [Google Scholar] [CrossRef]
- Yang, G.S.; Sun, H.H.; Cao, R.; Liu, Q.; Mao, X.Z. Characterization of a novel glycoside hydrolase family 46 chitosanase, Csn-BAC, from Bacillus sp. MD-5. Int. J. Biol. Macromol. 2020, 146, 518–523. [Google Scholar] [CrossRef]
- Palazzini, J.; Reynoso, A.; Yerkovich, N.; Zachetti, V.; Ramírez, M.; Chulze, S. Combination of Bacillus velezensis RC218 and Chitosan to Control Fusarium Head Blight on Bread and Durum Wheat under Greenhouse and Field Conditions. Toxins 2022, 14, 499. [Google Scholar] [CrossRef]
- Ahmed, A.S.; Ezziyyani, M.; Sánchez, C.P.; Candela, M.E. Effect of chitin on biological control activity of Bacillus spp. and Trichoderma harzianum against root rot disease in pepper (Capsicum annuum) plants. Eur. J. Plant Pathol. 2003, 109, 633–637. [Google Scholar] [CrossRef]
- Brzezinska, M.S.; Kalwasińska, A.; Świątczak, J.; Żero, K.; Jankiewicz, U. Exploring the properties of chitinolytic Bacillus isolates for the pathogens biological control. Microb. Pathog. 2020, 148, 104462. [Google Scholar] [CrossRef]
- Agbodjato, N.A.; Noumavo, P.A.; Adjanohoun, A.; Agbessi, L.; Baba-Moussa, L. Synergistic effects of plant growth promoting rhizobacteria and chitosan on in vitro seeds germination, greenhouse growth, and nutrient uptake of maize (Zea mays L.). Biotechnol. Res. Int. 2016, 2016, 7830182. [Google Scholar] [CrossRef]
- Chen, Q.; Qi, Y.; Jiang, Y.; Quan, W.; Luo, H.; Wu, K.; Li, S.; Ouyang, Q. Progress in Research of Chitosan Chemical Modification Technologies and Their Applications. Mar. Drugs 2022, 20, 536. [Google Scholar] [CrossRef]
- Di Santo, M.C.; D’Antoni, C.L.; Domínguez Rubio, A.P.; Alaimo, A.; Pérez, O.E. Chitosan-tripolyphosphate nanoparticles designed to encapsulate polyphenolic compounds for biomedical and pharmaceutical applications–A review. Biomed. Pharmacother. 2021, 142, 111970. [Google Scholar] [CrossRef]
- Qi, J.; Song, C.P.; Wang, B.; Zhou, J.; Kangasjärvi, J.; Zhu, J.K.; Gong, Z. Reactive oxygen species signaling and stomatal movement in plant responses to drought stress and pathogen attack. J. Integr. Plant Biol. 2018, 60, 805–826. [Google Scholar] [CrossRef] [Green Version]
- Fortunato, S.; Lasorella, C.; Dipierro, N.; Vita, F.; de Pinto, M.C. Redox Signaling in Plant Heat Stress Response. Antioxidants 2023, 12, 605. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.; Iqbal, J.; Naseer, S.; Yaseen, T.; Shaukat, M.; Bibi, H.; Ahmad, Y.; Daud, H.; Abbasi, N.L.; Mahmood, T. Molecular Mechanisms of Plant Tolerance to Heat Stress: Current Landscape and Future Perspectives. Plant Cell Rep. 2021, 40, 2247–2271. [Google Scholar] [CrossRef] [PubMed]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; et al. Recent Developments in Enzymatic Antioxidant Defence Mechanism in Plants with Special Reference to Abiotic Stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Mahomoodally, M.F.; Daphne Désiré, A.-L.; Elodie Rosette, M.A.-L. Chapter 2.2–Catalase. In Antioxidants Effects in Health; Seyed, M.N., Silva, A.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 81–90. [Google Scholar] [CrossRef]
- Bakalova, S.; Nikolova, A.; Wedera, D. Isoenzyme profiles of peroxidase, catalase and superoxide dismutase as affected by dehydration stress and ABA during germination of wheat seeds. J. Plant Physiol. 2004, 30, 64–77. [Google Scholar] [CrossRef]
- Luna, C.M.; Pastori, G.M.; Driscoll, S.; Groten, K.; Bernard, S.; Foyer, C.H. Drought controls on H2O2 accumulation, catalase (CAT) activity and CAT gene expression in wheat. J. Exp. Bot. 2005, 56, 417–423. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Huang, B. Drought and Heat Stress Injury to Two Cool-Season Turfgrasses in Relation to Antioxidant Metabolism and Lipid Peroxidation. Crop. Sci. 2001, 41, 436–442. [Google Scholar] [CrossRef]
- Fornera, S.; Walde, P. Spectrophotometric quantification of horseradish peroxidase with o-phenylenediamine. Anal. Biochem. 2010, 407, 293–295. [Google Scholar] [CrossRef]
- Karthika, S.; Remya, M.; Varghese, S.; Dhanraj, N.D.; Sali, S.; Rebello, S.; Jose, S.M.; Jisha, M.S. Bacillus tequilensis PKDN31 and Bacillus licheniformis PKDL10-As double headed swords to combat Fusarium oxysporum f. sp. lycopersici induced tomato wilt. Microb. Pathog. 2022, 172, 105784. [Google Scholar] [CrossRef]
- Samaras, A.; Kamou, N.; Tzelepis, G.; Karamanoli, K.; Menkissoglu-Spiroudi, U.; Karaoglanidis, G.S. Root Transcriptional and Metabolic Dynamics Induced by the Plant Growth Promoting Rhizobacterium (PGPR) Bacillus subtilis Mbi600 on Cucumber Plants. Plants 2022, 11, 1218. [Google Scholar] [CrossRef]
- Fabro, G.; Kovács, I.; Pavel, V.; Szabados, L.; Alvarez, M.E. Proline accumulation and AtP5CS2 gene activation are induced by plant-pathogen incompatible interactions in Arabidopsis. Mol. Plant Microb. Intrract. 2004, 17, 343–350. [Google Scholar] [CrossRef] [Green Version]
- Lastochkina, O.; Seifikalhor, M.; Aliniaeifard, S.; Baymiev, A.; Pusenkova, L.; Garipova, S.; Kulabuhova, D.; Maksimov, I. Bacillus Spp.: Efficient Biotic Strategy to Control Postharvest Diseases of Fruits and Vegetables. Plants 2019, 8, 97. [Google Scholar] [CrossRef] [Green Version]
- Sorokan, A.; Cherepanova, E.; Burkhanova, G.; Veselova, S.; Rumyantsev, S.; Alekseev, V.; Mardanshin, I.; Sarvarova, E.; Khairullin, R.; Benkovskaya, G.; et al. Endophytic Bacillus spp. as a Prospective Biological Tool for Control of Viral Diseases and Non-vector Leptinotarsa decemlineata Say. in Solanum tuberosum L. Front. Microbiol. 2020, 11, 569457. [Google Scholar] [CrossRef]
- Catalog of Strains and Isolates of the Collection of Endophytic Microorganisms IBG UFRC RAS. Available online: http://ibg.anrb.ru/wp-content/uploads/2019/04/Katalog-endofit.doc (accessed on 25 June 2023). (In Russian).
- Yarullina, L.G.; Burkhanova, G.F.; Cherepanova, E.A.; Sorokan, A.V.; Zaikina, E.A.; Tsvetkov, V.O.; Mardanshin, I.S.; Kalatskaya, J.N.; Balyuk, N.V. Effect of Bacillus subtilis and signaling molecules on the state of the pro/antioxidant system and the expression of protective protein genes in potato plants upon phytophthorosis and a moisture deficit. Appl. Biochem. Microbiol. 2021, 57, 760–769. [Google Scholar] [CrossRef]
- Kraskouski, A.; Nikalaichuk, V.; Kulikouskaya, V.; Hileuskaya, K.; Kalatskaja, J.; Nedved, H.; Laman, N.; Agabekov, V. Synthesis and properties of hydrogel particles based on chitosan-ferulic acid conjugates. Soft Mater. 2021, 19, 495–502. [Google Scholar] [CrossRef]
- Bindschedler, L.V.; Minibayeva, F.; Gardner, S.L.; Gerrish, C.; Davies, D.R.; Bolwell, G.P. Early signalling events in the apoplastic oxidative burst in suspension cultured French bean cells involve cAMP and Ca2+. New Phytol. 2001, 151, 185–194. [Google Scholar] [CrossRef]
- Bonnet, M.; Camares, O.; Veisseire, P. Effect of Zinc and influence of Acromonium lolli on growth parameters, chlorophyll fluorescence and antioxidant enzyme activities of rye grass (Lolium perenne L. cv. Apollo). J. Exp. Bot. 2000, 51, 945–953. [Google Scholar]
- Minibayeva, F.; Kolesnikov, O.; Chasov, A.; Beckett, R.P.; Lüthje, S.; Vylegzhanina, N.; Buck, F.; Böttger, M. Wound-induced apoplastic peroxidase activities: Their roles in the production and detoxification of reactive oxygen species. Plant Cell Environ. 2009, 32, 497–508. [Google Scholar] [CrossRef]
- Maksimov, I.V.; Sorokan, A.V.; Cherepanova, E.A.; Surina, O.B.; Troshina, N.B.; Yarullina, L.G. Effects of salicylic and jasmonic acids on the components of pro/antioxidant system in potato plants infected with late blight. Russ. J. Plant Physiol. 2011, 58, 299–306. [Google Scholar] [CrossRef]
- Kruger, N.J. The Bradford Method for Protein Quantitation. In The Protein Protocols Handbook; Springer Protocols Handbooks; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2009; pp. 17–24. [Google Scholar]
- Lastochkina, O.V.; Aliniaeifard, S.; Seifikalhor, M.; Yuldashev, R.; Pusenkova, L.; Garipova, S. Plant growth promoting bacteria–Biotic strategy to cope with abiotic stresses in wheat. In Wheat Production in Changing Environments. Responses, Adaptation and Tolerance; Hasanuzzaman, M., Nahar, K., Hossain, A., Eds.; Springer: Singapore, 2019; pp. 579–614. [Google Scholar]
- Bolwell, G.P.; Bindschedler, L.V.; Blee, K.A.; Butt, V.S.; Davies, D.R.; Gardner, S.L.; Gerrish, C.; Minibayeva, F. The apoplastic oxidative burst in response to biotic stress in plants: A tree component system. J. Exp. Bot. 2002, 53, 1367–1376. [Google Scholar]
- Galvez-Valdivieso, G.; Fryer, M.J.; Lawson, T.; Slattery, K.; Truman, W.; Smirnoff, N.; Asami, T.; Davies, W.J.; Jones, A.M.; Baker, N.R. The High Light Response in Arabidopsis Involves ABA Signaling between Vascular and Bundle Sheath Cells. Plant Cell 2009, 21, 2143–2162. [Google Scholar] [CrossRef] [Green Version]
- Jia, X.; Rajib, M.; Yin, H. Recognition Pattern, Functional Mechanism and Application of Chitin and Chitosan Oligosaccharides in Sustainable Agriculture. Curr. Pharm. Des. 2020, 26, 3508–3521. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, M.; Hasanuzzaman, M.; Rahman, M.; Khan, M.D.; Bhowmik, P.; Mahmud, N.U.; Tanveer, M.; Islam, T. Mechanism of Plant Growth Promotion and Disease Suppression by Chitosan Biopolymer. Agriculture 2020, 10, 624. [Google Scholar] [CrossRef]
- Siquet, C.; Paiva-Martins, F.; Lima, J.L.; Reis, S.; Borges, F. Antioxidant profile of dihydroxy- and trihydroxyphenolic acids–A structure–activity relationship study. Free Radic. Res. 2006, 40, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Rivero, R.M.; Ruiz, J.M.; Garcia, P.C.; Lopez-Lefebre, L.R.; Sanchez, E.; Romero, L. Resistance to Cold and Heat Stress: Accumulation of Phenolic Compounds in Tomato and Watermelon Plants. Plant Sci. 2001, 160, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Krol, A.; Amarowicz, R.; Weidner, S. Changes in the composition of phenolic compounds and antioxidant properties of grapevine roots and leaves (Vitis vinifera L.) under continuous of long-term drought stress. Acta Physiol. Plant. 2014, 36, 1491–1499. [Google Scholar] [CrossRef] [Green Version]
- Budna, G.A.; Lima, R.B.; Zanardo, D.Y.; dos Santos, W.D.; Ferrarese, M.; Ferrarese-Filho, O. Exogenous caffeic acid inhibits the growth and enhances the lignification of the roots of soybean (Glycine max). J. Plant Physiol. 2011, 168, 1627–1633. [Google Scholar] [CrossRef]
- Pfannschmidt, T.; Bräutigam, K.; Wagner, R.; Dietzel, L.; Schröter, Y. Potential regulation of gene expression in photosynthetic cells by redox and energy state: Approaches towards better understanding. Ann. Bot. 2009, 103, 599–607. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S.; Dubey, R.S. Inhibition of ribonuclease and protease activities in arsenic exposed rice seedlings: Role of proline as enzyme protectant. J. Plant Physiol. 2006, 163, 927–936. [Google Scholar] [CrossRef]
- Muley, A.B.; Shingote, P.R.; Patil, A.P.; Dalvi, S.G.; Suprasanna, P. Gamma radiation degradation of chitosan for application in growth promotion and induction of stress tolerance in potato (Solanum tuberosum L.). Carbohydr. Polym. 2019, 210, 289–301. [Google Scholar] [CrossRef]
- Rahman, M.; Mukta, J.A.; Sabir, A.A.; Gupta, D.R.; Mohi-Ud-Din, M.; Hasanuzzaman, M.; Miah, M.G.; Rahman, M.; Islam, M.T. Chitosan biopolymer promotes yield and stimulates accumulation of antioxidants in strawberry fruit. PLoS ONE 2018, 13, e0203769. [Google Scholar] [CrossRef]
- Puzina, T.I.; Makeeva, I.Y. Participation of caffeic acid in regulation of the production process in Solanum tuberosum L. Agrochemistry 2015, 6, 53–58. (In Russian) [Google Scholar]
- Kolupaev, Y.E.; Karpets, Y.V.; Kabashnikova, L.F. Antioxidative system of plants: Cellular compartmentalization, protective and signaling functions, mechanisms of regulation (review). Appl. Biochem. Microbiol. 2019, 55, 441–459. [Google Scholar] [CrossRef]
- Smirnoff, N.; Arnaud, D. Hydrogen peroxide metabolism and functions in plants. New Phytol. 2019, 221, 1197–1214. [Google Scholar] [CrossRef] [Green Version]
- Rizhsky, L.; Liang, H.; Mittler, R. The water-water cycle is essential for chloroplast protection in the absence of stress. J. Biol. Chem. 2003, 278, 38921–38925. [Google Scholar] [CrossRef] [Green Version]
- Pradedova, E.V.; Isheeva, O.D.; Salyaev, R.K. Antioxidant defense enzymes in cell vacuoles of red beet roots. Russ. J. Plant Physiol. 2011, 58, 36–44. [Google Scholar] [CrossRef]
- Gazaryan, I.G.; Khushpulyan, D.M.; Tishkov, V.I. Structure and mechanism of action of plant peroxidases. Adv. Biol. Chem. 2006, 46, 303–322. (In Russian) [Google Scholar]
- Sathyamurthy, B.; Dama, G.; Balasubramanian, A.; Vvs, S. Evaluation on antioxidant activity of curry leaf extracts in cisplatin induced wistar rats. World J. Pharm. Res. 2015, 4, 927–939. [Google Scholar]
- Cosio, C.; Dunand, C. Specific functions of individual class III peroxidase genes. J. Exp. Bot. 2009, 60, 391–408. [Google Scholar] [CrossRef]
- Spoel, S.H.; Johnson, J.S.; Dong, X. Regulation of tradeoffs between plant defenses against pathogens with different lifestyles. Proc. Nat. Acad. Sci. USA 2007, 104, 18842–18847. [Google Scholar] [CrossRef]
- Marjamaa, K.; Kukkola, E.M.; Fagerstedt, K.V. The role of xylem class III peroxidases in lignification. J. Exp. Bot. 2009, 60, 367–376. [Google Scholar] [CrossRef]
- Conrath, U.; Beckers, G.J.M.; Flors, V.; García-Agustín, P.; Jakab, G.; Mauch, F.; Newman, M.-A.; Pieterse, C.M.J.; Poinssot, B.; Pozo, M.J. Priming: Getting ready for battle. Mol. Plant Microbe Interact. 2006, 19, 1062–1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veselova, S.V.; Nuzhnaya, T.V.; Maksimov, I.V. Role of Jasmonic Acid in Interaction of Plants with Plant Growth Promoting Rhizobacteria during Fungal Pathogenesis. In Jasmonic Acid: Biosynthesis, Functions and Role in Plant Development, Series Plant Science Research and Practices; Morrison, L., Ed.; Nova Sci. Publishers: Hauppauge, NY, USA, 2015; pp. 33–66. [Google Scholar]
- Glazebrook, J. Contrasting Mechanisms of Defense Against Biotrophic and Necrotrophic Pathogens. Annu. Rev. Phytopathol. 2005, 43, 205. [Google Scholar] [CrossRef]
- Gimenez-Ibanez, S.; Solano, R. Nuclear jasmonate and salicylate signaling and crosstalk in defense against pathogens. Front. Plant Sci. 2013, 4, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasyukova, N.I.; Ozeretskovskaya, O.L. Jasmonate-dependent defense signaling in plant tissues. Russ. J. Plant Physiol. 2009, 56, 581–590. [Google Scholar] [CrossRef]
- Martinez-Medina, A.; Flors, V.; Heil, M.; Mauch-Mani, B.; Corné, M.J.; Pieterse, C.M.J. Recognizing Plant Defense Priming. Trends Plant Sci. 2016, 21, 818–822. [Google Scholar] [CrossRef] [Green Version]

| Gene Products | Genes | GenBank Accession Number | Sequence (5′-3′) | |
|---|---|---|---|---|
| Forward Primers | Reverse Primer | |||
| Actin | StAct | X55749 | gat-ggt-gtc-agc-cac-ac | att-cca-gca-gct-tcc-att-cc |
| PR1 | StPR1 | AY050221 | tgg-gtg-gtg-gtt-cat-ttc-ttg-t | cat-tta-att-cct-tac-aca-tca-taa-g |
| Thaumatin-like, PR5 | StPR5 | AY737317 | ccc-gtt-tga-cat-tga-cct-ttg | cga-ata-cgg-tgg-aac-atg-ga |
| Proteinase inhibitor, PR6 | StPR6 | JX683427 | ggg-aaa-gaa-tat-gct-caa-gtt-at | aat-tct-cca-tca-tct-tcc-act-g |
| Peroxidase, PR9 | StPR9 | M21334 | gta-atc-ctg-ccg-cac-aac-t | gca-gca-aaa-tct-cca-agg-aa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yarullina, L.; Cherepanova, E.A.; Burkhanova, G.F.; Sorokan, A.V.; Zaikina, E.A.; Tsvetkov, V.O.; Mardanshin, I.S.; Fatkullin, I.Y.; Kalatskaja, J.N.; Yalouskaya, N.A.; et al. Stimulation of the Defense Mechanisms of Potatoes to a Late Blight Causative Agent When Treated with Bacillus subtilis Bacteria and Chitosan Composites with Hydroxycinnamic Acids. Microorganisms 2023, 11, 1993. https://doi.org/10.3390/microorganisms11081993
Yarullina L, Cherepanova EA, Burkhanova GF, Sorokan AV, Zaikina EA, Tsvetkov VO, Mardanshin IS, Fatkullin IY, Kalatskaja JN, Yalouskaya NA, et al. Stimulation of the Defense Mechanisms of Potatoes to a Late Blight Causative Agent When Treated with Bacillus subtilis Bacteria and Chitosan Composites with Hydroxycinnamic Acids. Microorganisms. 2023; 11(8):1993. https://doi.org/10.3390/microorganisms11081993
Chicago/Turabian StyleYarullina, Liubov, Ekaterina A. Cherepanova, Guzel F. Burkhanova, Antonina V. Sorokan, Evgenia A. Zaikina, Vyacheslav O. Tsvetkov, Ildar S. Mardanshin, Ildus Y. Fatkullin, Joanna N. Kalatskaja, Ninel A. Yalouskaya, and et al. 2023. "Stimulation of the Defense Mechanisms of Potatoes to a Late Blight Causative Agent When Treated with Bacillus subtilis Bacteria and Chitosan Composites with Hydroxycinnamic Acids" Microorganisms 11, no. 8: 1993. https://doi.org/10.3390/microorganisms11081993
APA StyleYarullina, L., Cherepanova, E. A., Burkhanova, G. F., Sorokan, A. V., Zaikina, E. A., Tsvetkov, V. O., Mardanshin, I. S., Fatkullin, I. Y., Kalatskaja, J. N., Yalouskaya, N. A., & Nikalaichuk, V. V. (2023). Stimulation of the Defense Mechanisms of Potatoes to a Late Blight Causative Agent When Treated with Bacillus subtilis Bacteria and Chitosan Composites with Hydroxycinnamic Acids. Microorganisms, 11(8), 1993. https://doi.org/10.3390/microorganisms11081993






