Microbial Recycling of Polylactic Acid Food Packaging Waste into Carboxylates via Hydrolysis and Mixed-Culture Fermentation
Abstract
:1. Introduction
2. Materials and Methods
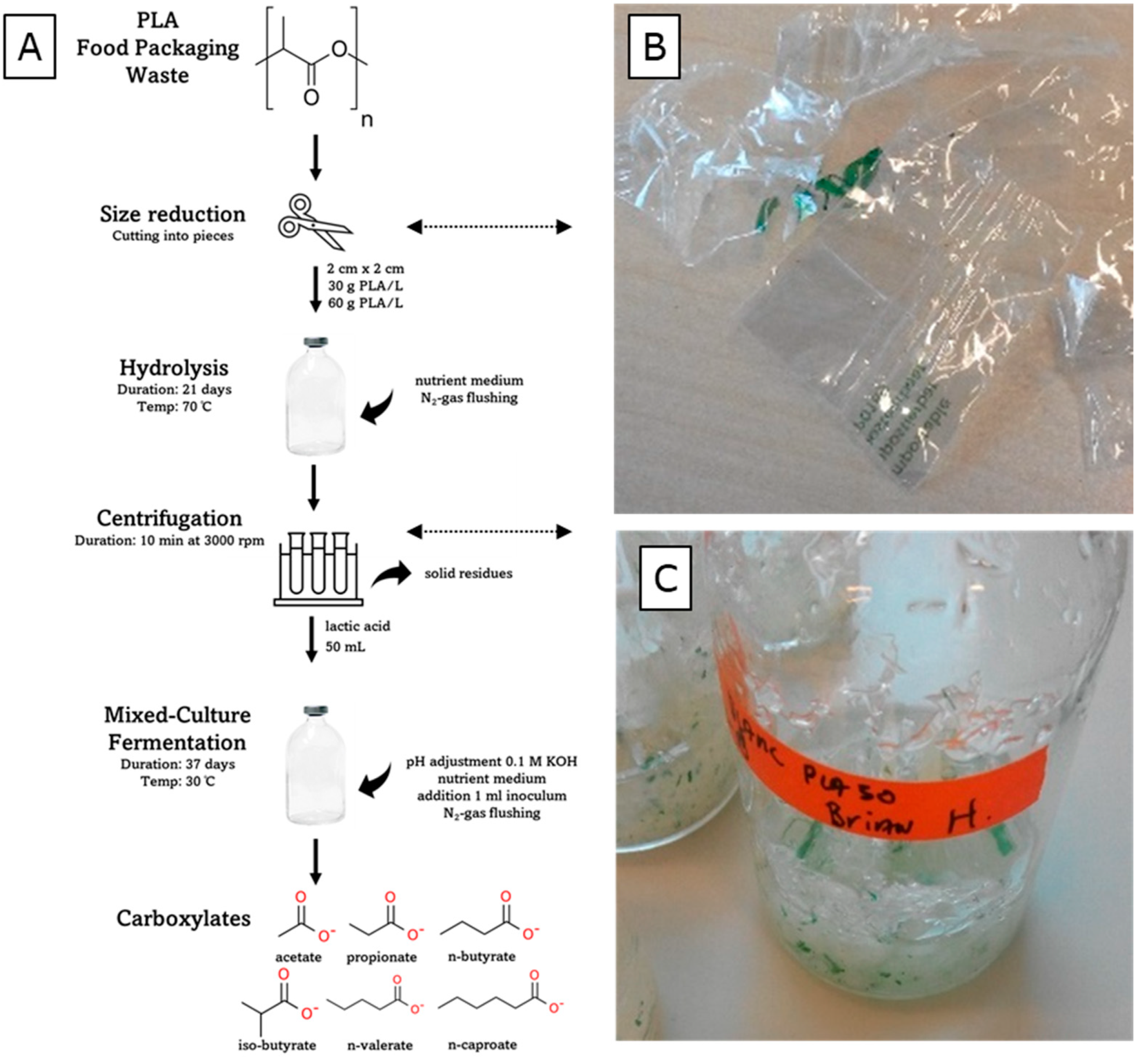
2.1. Materials and Preparation
2.2. Experimental Procedures
2.3. Analytical Methods
3. Results
3.1. PLA Food Packaging Waste Was Hydrolyzed into Lactic Acid within a Microbial Growth Medium
3.2. Lactate Obtained from PLA Food Packaging Waste Was Fermented into a Spectrum of Carboxylates
4. Discussion
4.1. Prospects for Microbial Recycling of End-of-Life PLA-Based Products
4.2. Improving Hydrolysis and Development of PLA and other BDP Converting Bioprocesses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commission. A European Strategy for Plastics in a Circular Economy. 2018. Available online: https://www.europarc.org/wp-content/uploads/2018/01/Eu-plastics-strategy-brochure.pdf (accessed on 16 January 2023).
- European Commission. Closing the Loop—An EU Action Plan for the Circular Economy. 2015. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52015DC0614 (accessed on 6 March 2023).
- Rosenboom, J.-G.; Langer, R.; Traverso, G. Bioplastics for a circular economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef]
- European Bioplastics. Bioplastics Market Data. 2023. Available online: https://www.european-bioplastics.org/market/ (accessed on 16 January 2023).
- Narancic, T.; Verstichel, S.; Chaganti, S.R.; Morales-Gamez, L.; Kenny, S.T.; De Wilde, B.; Padamati, R.B.; O’Connor, K.E. Biodegradable Plastic Blends Create New Possibilities for End-of-Life Management of Plastics but They Are Not a Panacea for Plastic Pollution. Environ. Sci. Technol. 2018, 52, 10441–10452. [Google Scholar] [CrossRef]
- Netherlands Enterprise Agency. Green Deal on Coffee Pads and Tea Bags in Organic Household Waste. 2021. Available online: https://www.greendeals.nl/sites/default/files/2021-05/Green%20Deal%20on%20coffee%20pads%20and%20tea%20bags%20in%20organic%20houshold%20waste.pdf (accessed on 6 March 2023).
- Van den Oever, M.; Molenveld, K.; van der Zee, M.; Bos, H. Bio-Based and Biodegradable Plastics: Facts and Figures: Focus on Food Packaging in the Netherlands; Wageningen Food & Biobased Research: Wageningen, The Netherlands, 2017; Available online: https://edepot.wur.nl/408350 (accessed on 16 January 2023).
- World Biogas Association. Global Potential of Biogas. 2019. Available online: https://www.worldbiogasassociation.org/wp-content/uploads/2019/07/WBA-globalreport-56ppa4_digital.pdf (accessed on 6 March 2023).
- Arslan, D.; Steinbusch, K.J.J.; Diels, L.; Hamelers, H.V.M.; Strik, D.P.B.T.B.; Buisman, C.J.N.; De Wever, H. Selective short-chain carboxylates production: A review of control mechanisms to direct mixed culture fermentations. Crit. Rev. Environ. Sci. Technol. 2016, 46, 592–634. [Google Scholar] [CrossRef]
- Grootscholten, T.I.M.; Strik, D.P.B.T.B.; Steinbusch, K.J.J.; Buisman, C.J.N.; Hamelers, H.V.M. Two-stage medium chain fatty acid (MCFA) production from municipal solid waste and ethanol. Appl. Energy 2014, 116, 223–229. [Google Scholar] [CrossRef]
- Contreras-Dávila, C.A.; Ali, A.; Buisman, C.J.N.; Strik, D.P.B.T.B. Lactate Metabolism and Microbiome Composition Are Affected by Nitrogen Gas Supply in Continuous Lactate-Based Chain Elongation. Fermentation 2021, 7, 41. [Google Scholar] [CrossRef]
- Holtzapple, M.; Lonkar, S.; Granda, C. Producing biofuels via the carboxylate platform. Chem. Eng. Prog. 2015, 111, 52–57. [Google Scholar]
- Holtzapple, M.T.; Wu, H.; Weimer, P.J.; Dalke, R.; Granda, C.B.; Mai, J.; Urgun-Demirtas, M. Microbial communities for valorizing biomass using the carboxylate platform to produce volatile fatty acids: A review. Bioresour. Technol. 2022, 344, 126253. [Google Scholar] [CrossRef]
- Website of ChainCraft. 2022. Available online: https://www.chaincraft.nl/ (accessed on 25 February 2023).
- Website of Afyren. 2022. Available online: https://afyren.com/en/afyren-neoxy/ (accessed on 25 February 2023).
- Strik, D.P.B.T.B.; Ganigué, R.; Angenent, L.T. Editorial: Microbial Chain Elongation-Close the Carbon Loop by Connecting-Communities. Front. Bioeng. Biotechnol. 2022, 10, 894490. [Google Scholar] [CrossRef]
- Jin, Y.; de Leeuw, K.D.; Strik, D.P.B.T.B. Microbial Recycling of Bioplastics via Mixed-Culture Fermentation of Hydrolyzed Polyhydroxyalkanoates into Carboxylates. Materials 2023, 16, 2693. [Google Scholar] [CrossRef]
- Ballerstedt, H.; Tiso, T.; Wierckx, N.; Wei, R.; Averous, L.; Bornscheuer, U.; O’connor, K.; Floehr, T.; Jupke, A.; Klankermayer, J.; et al. MIXed plastics biodegradation and UPcycling using microbial communities: EU Horizon 2020 project MIX-UP started January 2020. Environ. Sci. Eur. 2021, 33, 99. [Google Scholar] [CrossRef]
- Verschoor, J.-A.; Kusumawardhani, H.; Ram, A.F.J.; de Winde, J.H. Toward Microbial Recycling and Upcycling of Plastics: Prospects and Challenges. Front. Microbiol. 2022, 13, 821629. [Google Scholar] [CrossRef] [PubMed]
- García-Depraect, O.; Lebrero, R.; Rodriguez-Vega, S.; Börner, R.A.; Börner, T.; Muñoz, R. Production of volatile fatty acids (VFAs) from five commercial bioplastics via acidogenic fermentation. Bioresour. Technol. 2022, 360, 127655. [Google Scholar] [CrossRef] [PubMed]
- Yagi, H.; Ninomiya, F.; Funabashi, M.; Kunioka, M. Thermophilic anaerobic biodegradation test and analysis of eubacteria involved in anaerobic biodegradation of four specified biodegradable polyesters. Polym. Degrad. Stab. 2013, 98, 1182–1187. [Google Scholar] [CrossRef]
- Mu, L.; Zhang, L.; Ma, J.; Zhu, K.; Chen, C.; Li, A. Enhanced biomethanization of waste polylactic acid plastic by mild hydrothermal pretreatment: Taguchi orthogonal optimization and kinetics modeling. Waste Manag. 2021, 126, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Dávila, C.A.; Esveld, J.; Buisman, C.J.N.; Strik, D.P. nZVI Impacts Substrate Conversion and Microbiome Composition in Chain Elongation From D- and L-Lactate Substrates. Front. Bioeng. Biotechnol. 2021, 9, 666582. [Google Scholar] [CrossRef]
- Contreras-Dávila, C.A.; Carrión, V.J.; Vonk, V.R.; Buisman, C.N.J.; Strik, D.P.B.T.B. Consecutive lactate formation and chain elongation to reduce exogenous chemicals input in repeated-batch food waste fermentation. Water Res. 2020, 169, 115215. [Google Scholar] [CrossRef]
- Lindeboom, R.E.F.; Fermoso, F.G.; Weijma, J.; Zagt, K.; van Lier, J.B. Autogenerative high pressure digestion: Anaerobic digestion and biogas upgrading in a single step reactor system. Water Sci. Technol. 2011, 64, 647–653. [Google Scholar] [CrossRef]
- Roghair, M.; Liu, Y.; Strik, D.P.B.T.B.; Weusthuis, R.A.; Bruins, M.E.; Buisman, C.J.N. Development of an Effective Chain Elongation Process From Acidified Food Waste and Ethanol Into n-Caproate. Front. Bioeng. Biotechnol. 2018, 6, 50. [Google Scholar] [CrossRef]
- Estévez-Alonso, A.; Pei, R.; van Loosdrecht, M.C.; Kleerebezem, R.; Werker, A. Scaling-up microbial community-based polyhydroxyalkanoate production: Status and challenges. Bioresour. Technol. 2021, 327, 124790. [Google Scholar] [CrossRef]
- Afyren. Afyren Annual Financial Report. 2021. Available online: https://afyren.com/wp-content/uploads/2022/04/AFYREN-Annual-financial-report.pdf (accessed on 6 March 2023).
- Nagarajan, V.; Mohanty, A.K.; Misra, M. Perspective on Polylactic Acid (PLA) based Sustainable Materials for Durable Applications: Focus on Toughness and Heat Resistance. ACS Sustain. Chem. Eng. 2016, 4, 2899–2916. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Zhang, Z. Occurrence, effects, and biodegradation of plastic additives in sludge anaerobic digestion: A review. Environ. Pollut. 2021, 287, 117568. [Google Scholar] [CrossRef] [PubMed]
- Maarten van der Zee, K.M. The Fate of (Compostable) Plastic Products in a Full Scale Industrial Organic Waste Treatment Facility. 2020. Available online: https://edepot.wur.nl/514397 (accessed on 6 March 2023).
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, L.; Dombrowski, A.; Völker, C.; Wagner, M. Are bioplastics and plant-based materials safer than conventional plastics? In vitro toxicity and chemical composition. Environ. Int. 2020, 145, 106066. [Google Scholar] [CrossRef] [PubMed]
- Haider, T.P.; Völker, C.; Kramm, J.; Landfester, K.; Wurm, F.R. Plastics of the Future? The Impact of Biodegradable Polymers on the Environment and on Society. Angew. Chem. Int. Ed. 2019, 58, 50–62. [Google Scholar] [CrossRef]
- European Bioplastics. Available online: https://www.european-bioplastics.org/bioplastics/ (accessed on 16 January 2023).
- TotalEnergies Corbion. Bioplastics Product Profile Luminy® PLA for Drinking Bottles, TECP-AS/EXTCP-22Q4-EN-V1. 2022. Available online: https://www.totalenergies-corbion.com/media/enici1qh/luminy-pla-for-drinking-bottles.pdf (accessed on 5 March 2023).
- Total-Corbion. PRESS RELEASE Luminy® PLA Made from Chemically Recycled Feedstock Now Commercially Available. Gorinchem, the Netherlands. 2021. Available online: https://www.totalenergies-corbion.com/media/b24pa55h/211025-total-corbion-pla-chemically-recycling.pdf (accessed on 5 March 2023).
- de Bie, F.; Sart, G.G.D.; Ravard, M.; La Scola, P.; Veras, R. Stay in the Cycle—Rethinking Recycling with PLA Bioplastics. 2022. Available online: https://www.totalenergies-corbion.com/media/xy4a3pcj/stay-in-the-cycle-whitepaper-about-pla-recycling-totalenergies-corbion.pdf (accessed on 5 March 2023).
- McKeown, P.; Jones, M.D. The Chemical Recycling of PLA: A Review. Sustain. Chem. 2020, 1, 1–22. [Google Scholar] [CrossRef]
- Palos, R.; Gutiérrez, A.; Vela, F.J.; Olazar, M.; Arandes, J.M.; Bilbao, J. Waste Refinery: The Valorization of Waste Plastics and End-of-Life Tires in Refinery Units. A Review. Energy Fuels 2021, 35, 3529–3557. [Google Scholar] [CrossRef]
- Van Roijen, E.C.; Miller, S.A. A review of bioplastics at end-of-life: Linking experimental biodegradation studies and life cycle impact assessments. Resour. Conserv. Recycl. 2022, 181, 106236. [Google Scholar] [CrossRef]
- Biofoam-Refill. Available online: https://bigbeanbagcompany.com/shop/biofoam-refill/?v=796834e7a283 (accessed on 5 March 2023).
- Colorfabb. Available online: https://colorfabb.com/natural (accessed on 5 March 2023).
- Bhagia, S.; Bornani, K.; Agrawal, R.; Satlewal, A.; Ďurkovič, J.; Lagaňa, R.; Bhagia, M.; Yoo, C.G.; Zhao, X.; Kunc, V.; et al. Critical review of FDM 3D printing of PLA biocomposites filled with biomass resources, characterization, biodegradability, upcycling and opportunities for biorefineries. Appl. Mater. Today 2021, 24, 101078. [Google Scholar] [CrossRef]
- Gorrasi, G.; Pantani, R. Hydrolysis and Biodegradation of Poly(lactic acid). In Synthesis, Structure and Properties of Poly(lactic acid); Di Lorenzo, M.L., Androsch, R., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 119–151. [Google Scholar] [CrossRef]
- Chauliac, D.; Pullammanappallil, P.C.; Ingram, L.O.; Shanmugam, K.T. A Combined Thermochemical and Microbial Process for Recycling Polylactic Acid Polymer to Optically Pure l-Lactic Acid for Reuse. J. Polym. Environ. 2020, 28, 1503–1512. [Google Scholar] [CrossRef]
- Zhu, X.; Tao, Y.; Liang, C.; Li, X.; Wei, N.; Zhang, W.; Zhou, Y.; Yang, Y.; Bo, T. The synthesis of n-caproate from lactate: A new efficient process for medium-chain carboxylates production. Sci. Rep. 2015, 5, 14360. [Google Scholar] [CrossRef]
- Contreras-Dávila, C.A.; Zuidema, N.; Buisman, C.J.N.; Strik, D.P. Reactor microbiome enriches vegetable oil with n-caproate and n-caprylate for potential functionalized feed additive production via extractive lactate-based chain elongation. Biotechnol. Biofuels 2021, 14, 232. [Google Scholar] [CrossRef] [PubMed]
- Kucek, L.A.; Nguyen, M.; Angenent, L.T. Conversion of l-lactate into n-caproate by a continuously fed reactor microbiome. Water Res. 2016, 93, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Karamanlioglu, M.; Preziosi, R.; Robson, G.D. Abiotic and biotic environmental degradation of the bioplastic polymer poly(lactic acid): A review. Polym. Degrad. Stab. 2017, 137, 122–130. [Google Scholar] [CrossRef]
- Bernat, K.; Kulikowska, D.; Wojnowska-Baryła, I.; Zaborowska, M.; Pasieczna-Patkowska, S. Thermophilic and mesophilic biogas production from PLA-based materials: Possibilities and limitations. Waste Manag. 2021, 119, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-S.; Huang, S.; Strik, D.P.; Buisman, C.J. Isobutyrate biosynthesis via methanol chain elongation: Converting organic wastes to platform chemicals. J. Chem. Technol. Biotechnol. 2017, 92, 1370–1379. [Google Scholar] [CrossRef]
- Liu, B.; Popp, D.; Müller, N.; Sträuber, H.; Harms, H.; Kleinsteuber, S. Three Novel Clostridia Isolates Produce n-Caproate and iso-Butyrate from Lactate: Comparative Genomics of Chain-Elongating Bacteria. Microorganisms 2020, 8, 1970. [Google Scholar] [CrossRef] [PubMed]
- de Leeuw, K.D.; de Smit, S.M.; van Oossanen, S.; Moerland, M.J.; Buisman, C.J.N.; Strik, D.P.B.T.B. Methanol-Based Chain Elongation with Acetate to n-Butyrate and Isobutyrate at Varying Selectivities Dependent on pH. ACS Sustain. Chem. Eng. 2020, 8, 8184–8194. [Google Scholar] [CrossRef]
- Duber, A.; Zagrodnik, R.; Gutowska, N.; Łężyk, M.; Oleskowicz-Popiel, P. Lactate and Ethanol Chain Elongation in the Presence of Lactose: Insight into Product Selectivity and Microbiome Composition. ACS Sustain. Chem. Eng. 2022, 10, 3407–3416. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strik, D.P.B.T.B.; Heusschen, B. Microbial Recycling of Polylactic Acid Food Packaging Waste into Carboxylates via Hydrolysis and Mixed-Culture Fermentation. Microorganisms 2023, 11, 2103. https://doi.org/10.3390/microorganisms11082103
Strik DPBTB, Heusschen B. Microbial Recycling of Polylactic Acid Food Packaging Waste into Carboxylates via Hydrolysis and Mixed-Culture Fermentation. Microorganisms. 2023; 11(8):2103. https://doi.org/10.3390/microorganisms11082103
Chicago/Turabian StyleStrik, David P. B. T. B., and Brian Heusschen. 2023. "Microbial Recycling of Polylactic Acid Food Packaging Waste into Carboxylates via Hydrolysis and Mixed-Culture Fermentation" Microorganisms 11, no. 8: 2103. https://doi.org/10.3390/microorganisms11082103
APA StyleStrik, D. P. B. T. B., & Heusschen, B. (2023). Microbial Recycling of Polylactic Acid Food Packaging Waste into Carboxylates via Hydrolysis and Mixed-Culture Fermentation. Microorganisms, 11(8), 2103. https://doi.org/10.3390/microorganisms11082103






