Enhancing the Mn-Removal Efficiency of Acid-Mine Bacterial Consortium: Performance Optimization and Mechanism Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Acclimation of Mn(II)-Oxidizing Consortium
2.2. Optimization of the Culture Conditions
2.3. Batch Experiments
2.4. Microbial Community Analysis
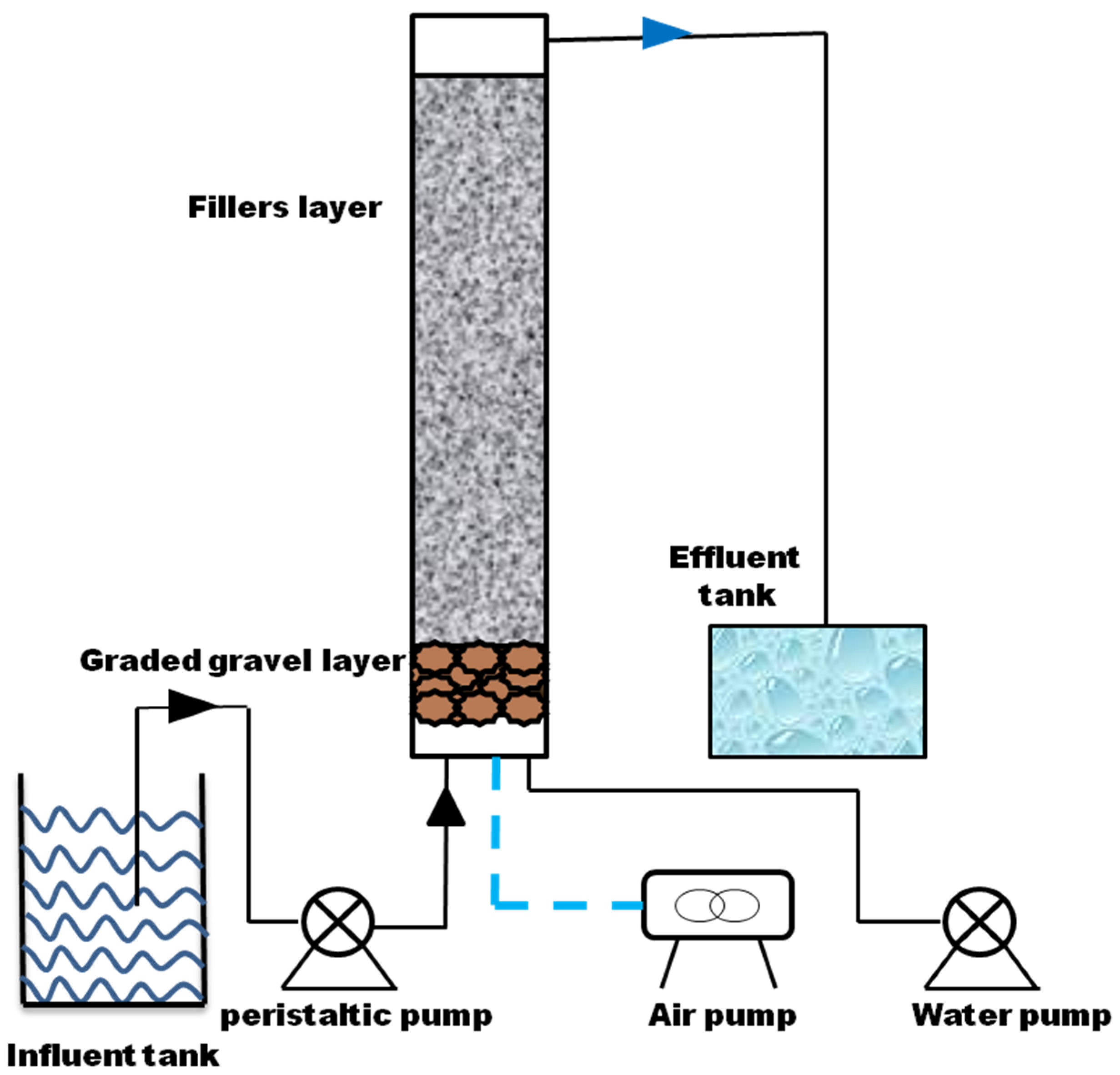
2.5. Column Experiment
2.6. Analytical Methods
3. Results and Discussion
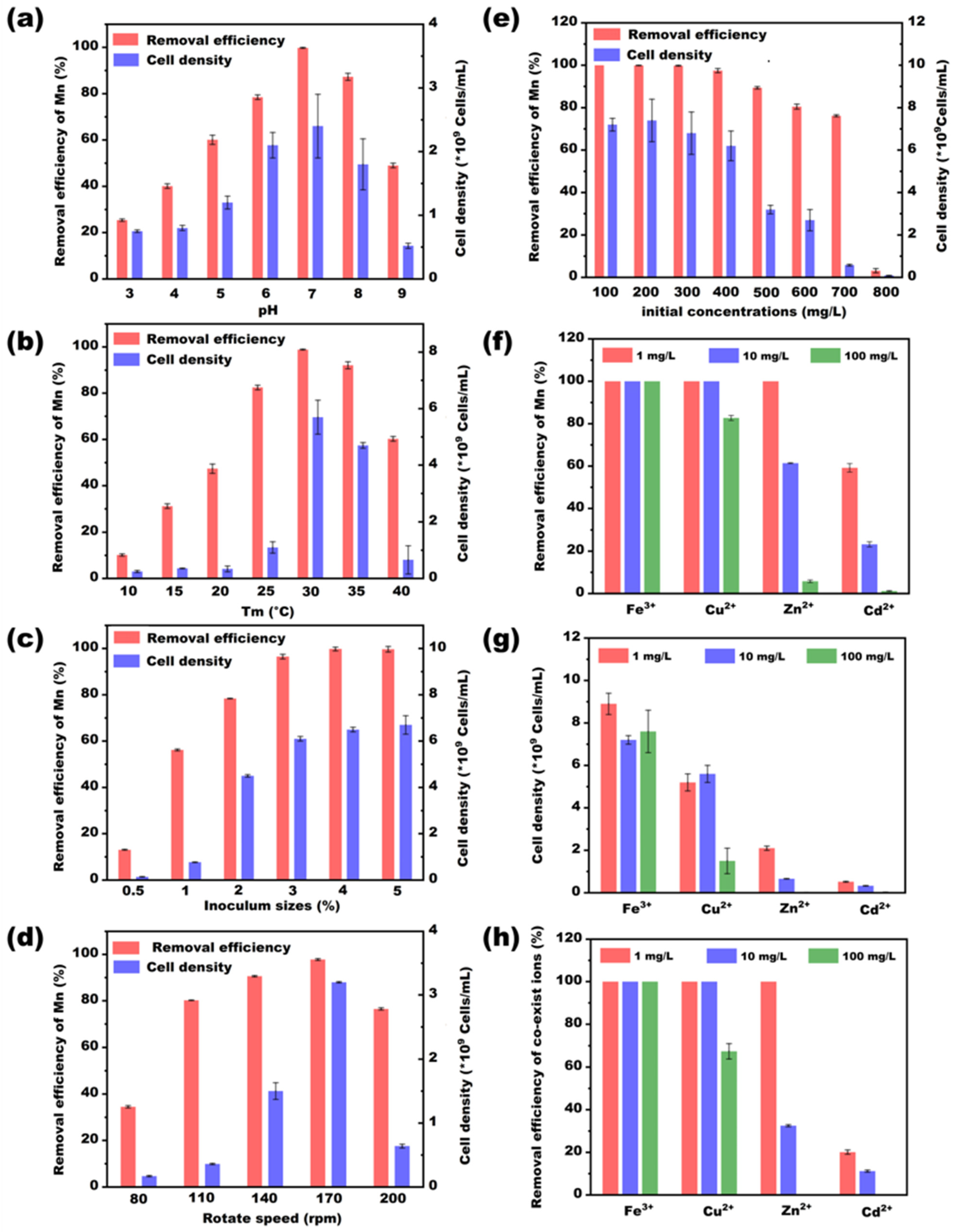
3.1. Effects of Environmental Factors on Bacterial Growth and Mn Removal
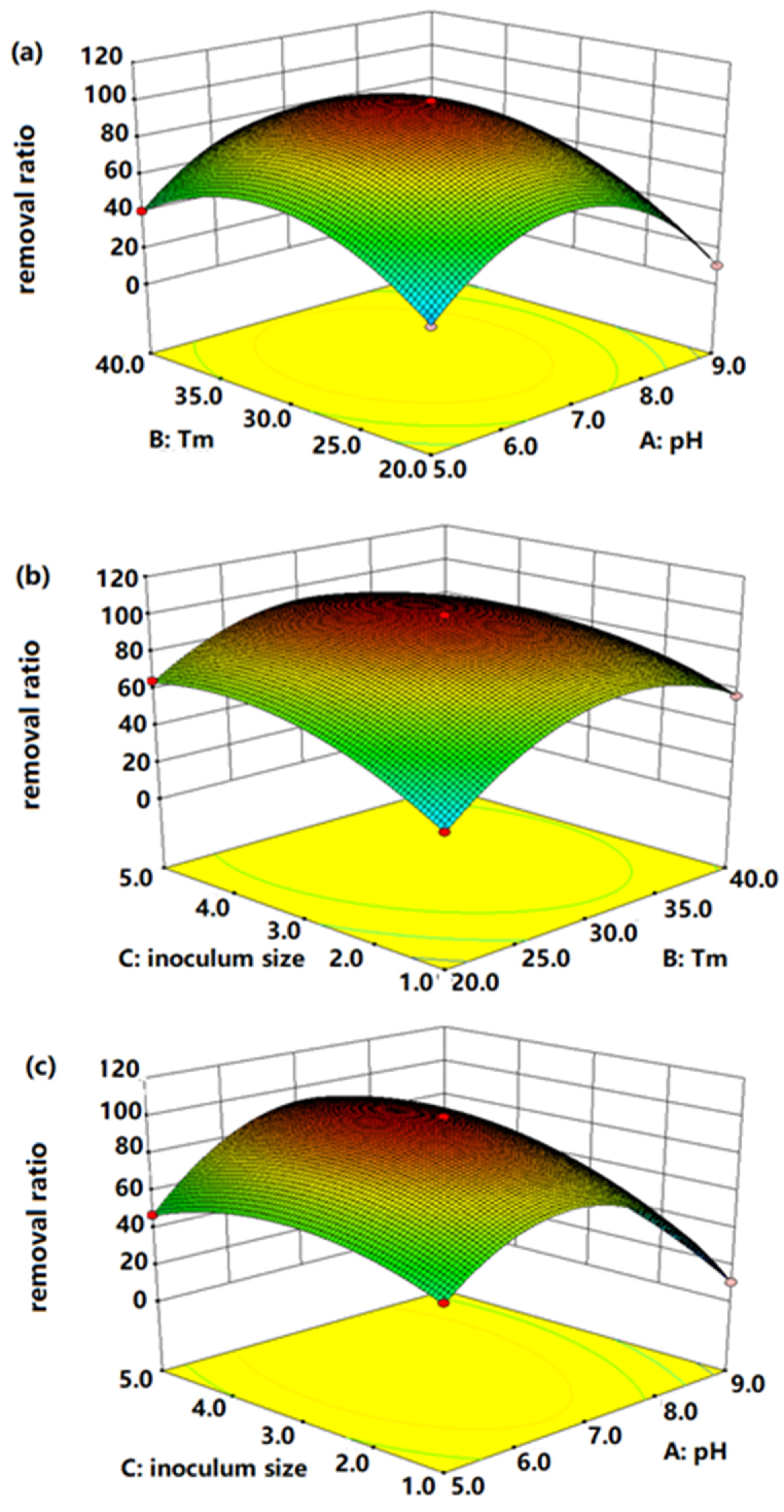
3.2. Optimization Conditions for Mn-Removal Efficiency
− 6.75 × BC − 44.82 × A2 − 29 × B2 − 16.17 × C2
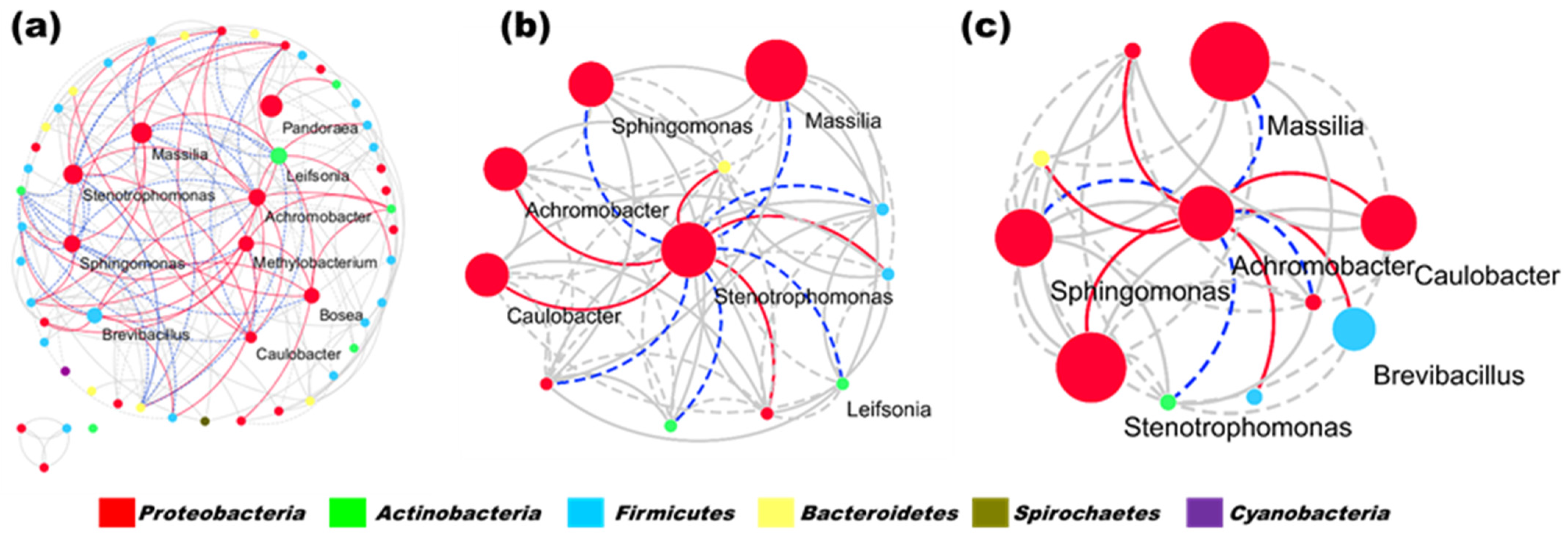
3.3. Dynamic Changes in Microbial Community
3.4. Possible Removal Mechanisms
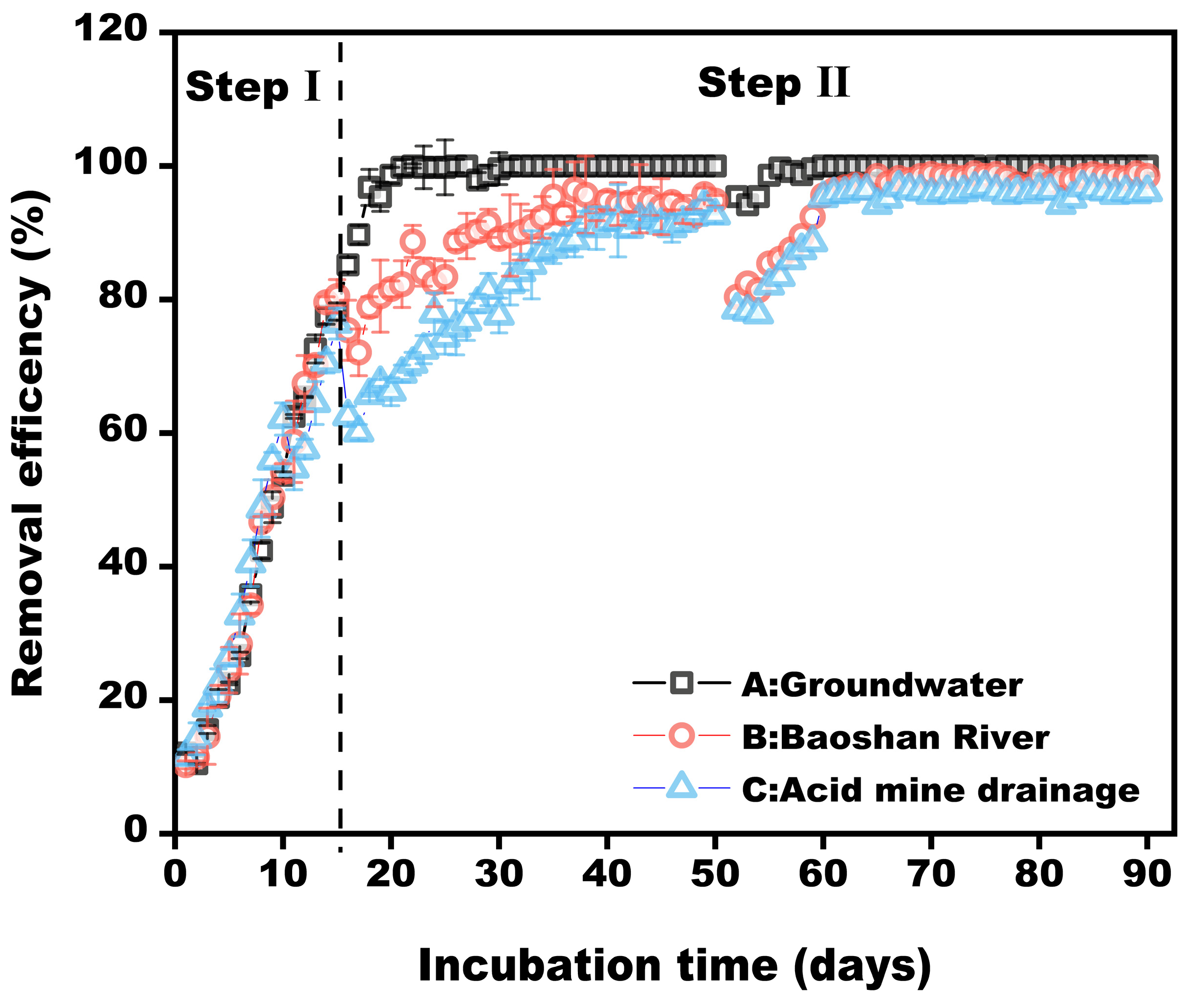
3.5. Performance of Bacterial Consortium in the Real Wastewater
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rudi, N.N.; Muhamad, M.S.; Chuan, L.T.; Alipal, J.; Omar, S.; Hamidon, N.; Abdul Hamid, N.H.; Sunar, N.M.; Ali, R.; Harun, H. Evolution of adsorption process for manganese removal in water via agricultural waste adsorbents. Heliyon 2020, 6, 05049. [Google Scholar] [CrossRef]
- An, Q.; Zhang, C.; Zhao, B.; Li, Z.; Deng, S.; Wang, T.; Jin, L. Insight into synergies between Acinetobacter sp. AL-6 and pomelo peel biochar in a hybrid process for highly efficient manganese removal. Sci. Total Environ. 2021, 793, 148609. [Google Scholar] [CrossRef]
- Pachana, P.K.; Rattanasak, U.; Nuithitikul, K.; Jitsangiam, P.; Chindaprasirt, P. Sustainable utilization of water treatment residue as a porous geopolymer for iron and manganese removals from groundwater. J. Environ. Manag. 2022, 302, 114036. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; Yan, Z.S.; Du, X.; Bai, L.M.; Yu, H.R.; Ding, A.; Li, G.B.; Liang, H.; Aminabhavi, T.M. Removal of manganese from groundwater in the ripened sand filtration: Biological oxidation versus chemical auto-catalytic oxidation. Chem. Eng. J. 2020, 382, 123033. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, Y.; Feng, K.; Wang, X.H.; Liu, B.F.; Xie, G.J.; Xing, D.F. Self-regulating microbiome networks ensure functional resilience of biofilms in sand biofilters during manganese load fluctuations. Water Res. 2021, 188, 116473. [Google Scholar] [CrossRef]
- Bai, Y.H.; Su, J.F.; Ali, A.; Wen, Q.; Chang, Q.; Gao, Z.H.; Wang, Y. Efficient removal of nitrate, manganese, and tetracycline in a novel loofah immobilized bioreactor: Performance, microbial diversity, and functional genes. Bioresour. Technol. 2022, 344, 126228. [Google Scholar] [CrossRef]
- He, Y.H.; Xu Dietrich, A.M.; Jin, Q.; Lin, T.T.; Yu, D.J.; Huang, H.B. Cellulose adsorbent produced from the processing waste of brewer’s spent grain for efficient removal of Mn and Pb from contaminated water. Food Bioprod. Process. 2022, 135, 227–237. [Google Scholar] [CrossRef]
- Rudi, N.N.; Apandi, M.N.; Muhamad, M.S.; Mohamed, S.N.; Apandi, A.M.; Chuan, T.L.; Nagarajah, R.; Omar, S. Chemical Treatment of Banana Blossom Peels Adsorbent as New Approach for Manganese Removal: Isotherm and Kinetic Studies. Sustainability 2023, 15, 10223. [Google Scholar] [CrossRef]
- Goodwill, J.E.; Mai, X.; Jiang, Y.; Reckhow, D.A.; Tobiason, J.E. Oxidation of manganese(II) with ferrate: Stoichiometry, kinetics, products and impact of organic carbon. Chemosphere 2016, 159, 457–464. [Google Scholar] [CrossRef]
- Lin, S.; Mackey, H.R.; Hao, T.; Guo, G.; van Loosdrecht, M.C.M.; Chen, G. Biological sulfur oxidation in wastewater treatment: A review of emerging opportunities. Water Res. 2018, 143, 399–415. [Google Scholar] [CrossRef]
- Wang, G.C.; Liu, Y.; Wu, M.H.; Zong, W.J.; Yi, X.L.; Zhan, J.J.; Liu, L.F.; Zhou, H. Coupling the phenolic oxidation capacities of a bacterial consortium and in situ-generated manganese oxides in a moving bed biofilm reactor (MBBR). Water Res. 2019, 166, 115047. [Google Scholar] [CrossRef]
- Shoiful, A.; Ohta, T.; Kambara, H.; Matsushita, S.; Kindaichi, T.; Ozaki, N.; Aoi, Y.; Imachi, H.; Ohashi, A. Multiple organic substrates support Mn(II) removal with enrichment of Mn(II)-oxidizing bacteria. J. Environ. Manag. 2020, 259, 109771. [Google Scholar] [CrossRef]
- Zhou, H.; Fu, C. Manganese-oxidizing microbes and biogenic manganese oxides: Characterization, Mn(II) oxidation mechanism and environmental relevance. Rev. Environ. Sci. Bio Technol. 2020, 19, 489–507. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Tani, S.; Fujiwara, M.; Nakamura, M.; Sumitani, J.I.; Kawaguchi, T. Biogenic manganese oxides combined with 1-hydroxybenzotriazol and an Mn(II)-oxidizing enzyme from Pleosporales sp. Mn1 oxidize 3,4-dimethoxytoluene to yield 3,4-dimethoxybenzaldehyde. J. Biosci. Bioeng. 2021, 131, 475–482. [Google Scholar] [CrossRef]
- Liang, D.H.; Hu, Y.Y.; Cheng, J.H.; Chen, Y.C. Enhanced performance of sulfamethoxazole degradation using Achromobacter sp. JL9 with in-situ generated biogenic manganese oxides. Bioresour. Technol. 2021, 333, 125089. [Google Scholar] [CrossRef]
- Su, J.M.; Deng, L.; Huang, L.B.; Guo, S.J.; Liu, F.; He, J. Catalytic oxidation of manganese(II) by multicopper oxidase CueO and characterization of the biogenic Mn oxide. Water Res. 2014, 56, 304–313. [Google Scholar] [CrossRef]
- Abu Hasan, H.; Sheikh Abdullah, S.R.; Kamarudin, S.K.; Tan Kofli, N. Effective curves of completing simultaneous ammonium and manganese removal in polluted water using a biological aerated filter. Ind. Eng. Chem. 2015, 30, 153–159. [Google Scholar] [CrossRef]
- Tang, W.W.; Gong, J.M.; Wu, L.J.; Li, Y.F.; Zhang, M.T.; Zeng, X.P. DGGE diversity of manganese mine samples and isolation of a Lysinibacillus sp. efficient in removal of high Mn (II) concentrations. Chemosphere 2016, 165, 277–283. [Google Scholar] [CrossRef]
- Chang, Q.; Ali, A.; Su, J.F.; Wen, Q.; Bai, Y.H.; Gao, Z.H. Simultaneous removal of nitrate, manganese, and tetracycline by Zoogloea sp. MFQ7: Adsorption mechanism of tetracycline by biological precipitation. Bioresour. Technol. 2021, 340, 125690. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Kurniawan, S.B.; Iwuozor, K.O.; Aniagor, C.O.; Ajala, O.J.; Oba, S.N.; Iwuchukwu, F.U.; Ahmadi, S.; Igwegbe, C.A. A review of treatment technologies for the mitigation of the toxic environmental effects of acid mine drainage (AMD). Process Saf. Environ. Prot. 2022, 157, 37–58. [Google Scholar] [CrossRef]
- Bressanin, L.A.; Diniz, A.A.M.; de Souza, K.R.D.; Florentino, L.A.; da Silva, A.B.; Magalhaes, P.C.; Pasqual, M.; de Souza, T.C. Diazotrophic bacteria improve Hymenaea courbaril seedlings growth and survival in iron mine tailings. J. Environ. Manag. 2022, 321, 115985. [Google Scholar] [CrossRef] [PubMed]
- Mayanna, S.; Peacock, C.L.; Schäffner, F.; Grawunder, A.; Merten, D.; Kothe, E.; Büchel, G. Biogenic precipitation of manganese oxides and enrichment of heavy metals at acidic soil pH. Chem. Geol. 2015, 402, 6–17. [Google Scholar] [CrossRef]
- Barboza, N.R.; Amorim, S.S.; Santos, P.A.; Reis, F.D.; Cordeiro, M.M.; Guerra-Sa, R.; Leao, V.A. Indirect Manganese Removal by Stenotrophomonas sp. and Lysinibacillus sp. Isolated from Brazilian Mine Water. BioMed Res. Int. 2015, 2015, 925972. [Google Scholar] [CrossRef] [PubMed]
- Akob, D.M.; Bohu, T.; Beyer, A.; Schäffner, F.; Händel, M.; Johnson, C.A.; Merten, D.; Büchel, G.; Totsche, K.U.; Küsel, K.; et al. Identification of Mn(II)-Oxidizing Bacteria from a Low-pH Contaminated Former Uranium Mine. Appl. Environ. Microbiol. 2014, 80, 5086–5097. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Zhang, P.; Zhang, J.; Zhou, Y.; Yang, Y.; Mao, Q.; Tsang, D.C.W.; Nunez-Delgado, A.; Luo, L. Spatial variation of sediment bacterial community in an acid mine drainage contaminated area and surrounding river basin. J. Environ. Manag. 2019, 251, 109542. [Google Scholar] [CrossRef]
- Hou, D.M.; Zhang, P.; Wei, D.N.; Zhang, J.C.; Yan, B.H.; Cao, L.Y.; Zhou, Y.Y.; Luo, L. Simultaneous removal of iron and manganese from acid mine drainage by acclimated bacteria. J. Hazard. Mater. 2020, 396, 122631. [Google Scholar] [CrossRef]
- Tran, T.N.; Kim, D.G.; Ko, S. Efficient removal of 17α-ethinylestradiol from secondary wastewater treatment effluent by a biofilm process incorporating biogenic manganese oxide and Pseudomonas putida strain MnB1. J. Hazard. Mater. 2020, 398, 122810. [Google Scholar] [CrossRef]
- Hu, J.; Yan, J.B.; Wu, L.; Bao, Y.Z.; Yu, D.Q.; Li, J. Insight into halotolerance of a robust heterotrophic nitrifying and aerobic denitrifying bacterium Halomonas salifodinae. Bioresour. Technol. 2022, 351, 126925. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Zhan, W.H.; Ren, Q.; Cheng, S.S.; Wang, J.N.; Ma, X.Y.; Zhang, C.S.; Wang, Y.S. Biodegradation of di-(2-ethylhexyl) phthalate by a newly isolated Gordonia sp. and its application in the remediation of contaminated soils. Sci. Total Environ. 2019, 689, 645–651. [Google Scholar] [CrossRef]
- Wang, Y.; Su, J.F.; Ali, A.; Chang, Q.; Bai, Y.H.; Gao, Z.H. Enhanced nitrate, manganese, and phenol removal by polyvinyl alcohol/sodium alginate with biochar gel beads immobilized bioreactor: Performance, mechanism, and bacterial diversity. Bioresour. Technol. 2022, 348, 126818. [Google Scholar] [CrossRef]
- Ruan, X.L.; Ge, S.J.; Jiao, Z.Q.; Zhan, W.H.; Wang, Y.Y. Bioaccumulation and risk assessment of potential toxic elements in the soil-vegetable system as influenced by historical wastewater irrigation. Agr. Water Manag. 2023, 279, 108197. [Google Scholar] [CrossRef]
- He, Z.F.; Zhang, Q.Y.; Wei, Z.; Zhu, Y.H.; Pan, X.L. Simultaneous removal of As(III) and Cu(II) from real bottom ash leachates by manganese-oxidizing aerobic granular sludge: Performance and mechanisms. Sci. Total Environ. 2020, 700, 134510. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Liu, G.Y.; Qu, F.S.; Li, K.; Shao, S.L.; Li, G.B.; Liang, H. Removal of iron, manganese and ammonia from groundwater using a PAC-MBR system: The anti-pollution ability, microbial population and membrane fouling. Desalination 2017, 403, 97–106. [Google Scholar] [CrossRef]
- Zhou, H.; Pan, H.X.; Xu, J.Q.; Xu, W.P.; Liu, L.F. Acclimation of a marine microbial consortium for efficient Mn(II) oxidation and manganese containing particle production. J. Hazard. Mater. 2016, 304, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.J.; Xing, Y.H.; Qin, X.X.; Li, X.; Liu, S.; Luo, X.S.; Huang, Q.Y.; Chen, W.L. A manganese-oxidizing bacterial consortium and its biogenic Mn oxides for dye decolorization and heavy metal adsorption. Chemosphere 2020, 253, 126627. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.M.; Shao, Z.Z.; Liu, Y.J.; Wang, G.J. Removal of multi-heavy metals using biogenic manganese oxides generated by a deep-sea sedimentary bacterium Brachybacterium sp. strain Mn32. Microbiology 2009, 155, 1989–1996. [Google Scholar] [CrossRef] [PubMed]
- Hullo, M.F.; Moszer, I.; Danchin, A.; Martin-Verstraete, I. CotA of Bacillus subtilis is a copper-dependent laccase. J. Bacteriol. 2001, 183, 5426–5430. [Google Scholar] [CrossRef]
- Qi, J.; Song, Y.J.; Liang, J.S.; Bai, Y.H.; Hu, C.Z.; Liu, H.J.; Qu, J.H. Growth inhibition of Microcystis aeruginosa by sand-filter prevalent manganese-oxidizing bacterium. Sep. Purif. Technol. 2021, 256, 117808. [Google Scholar] [CrossRef]
- Filippidou, S.; Sathiyanarayanan, G.; Junier, T.; Muñoz Rufatt, P.; Jeanneret, N.; Wunderlin, T.; Sieber, N.; Dorador, C.; Junier, P. Manganese-II oxidation and Copper-II resistance in endospore forming Firmicutes isolated from uncontaminated environmental sites. AIMS Environ. Sci. 2016, 3, 220–238. [Google Scholar]
- Zhao, S.; Chang, Y.; Liu, J.; Sangeetha, T.; Feng, Y.; Liu, D.; Xu, C. Removal of antibiotic resistance genes and mobile genetic elements in a three-stage pig manure management system: The implications of microbial community structure. J. Environ. Manag. 2022, 323, 116185. [Google Scholar] [CrossRef]
- Yang, H.; Li, D.; Zeng, H.P.; Zhang, J. Autotrophic nitrogen conversion process and microbial population distribution in biofilter that simultaneously removes Fe, Mn and ammonia from groundwater. Int. Biodeterior. Biodegrad. 2018, 135, 53–61. [Google Scholar] [CrossRef]
- Sarkar, A.; Paul, D.; Kazy, S.K.; Sar, P. Molecular analysis of microbial community in arsenic-rich groundwater of Kolsor, West Bengal. J. Environ. Sci. Health. Part A Toxic Hazard. Subst. Environ. Eng. 2016, 51, 229–239. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Giaramida, L.; Reich, P.B.; Khachane, A.N.; Hamonts, K.; Edwards, C.; Lawton, L.A.; Singh, B.K. Lack of functional redundancy in the relationship between microbial diversity and ecosystem functioning. J. Ecol. 2016, 104, 936–946. [Google Scholar] [CrossRef]
- Su, J.F.; Liang, D.H.; Lian, T.T. Comparison of denitrification performance by bacterium Achromobacter sp. A14 under different electron donor conditions. Chem. Eng. J. 2018, 333, 320–326. [Google Scholar] [CrossRef]
- Yin, Y.J.; Li, D.; Wang, Y.Q.; Xu, Z.H.; Xu, G.J.; Zhao, Z.Q.; Li, S.; Song, L.Y. Concurrent Removal of Mn (II) and Cr (VI) by Achromobacter sp. TY3-4. Geomicrobiol. J. 2019, 36, 317–325. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, X.H.; Liu, B.F.; Xie, G.J.; Xing, D.F. Characterization of manganese oxidation by Brevibacillus at different ecological conditions. Chemosphere 2018, 205, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.P.; Zhang, M.T.; Liu, Y.Y.; Tang, W.W. Manganese(II) oxidation by the multi-copper oxidase CopA from Brevibacillus panacihumi MK-8. Enzyme Microb. Technol. 2018, 117, 79–83. [Google Scholar] [CrossRef]
- Furuta, S.; Ikegaya, H.; Hashimoto, H.; Ichise, S.; Kohno, T.; Miyata, N.; Takada, J. Formation of Filamentous Mn Oxide Particles by the Alphaproteobacterium Bosea sp. Strain BIWAKO-01. Geomicrobiol. J. 2014, 32, 666–676. [Google Scholar] [CrossRef]
- Lu, K.; Wang, T.T.; Zhai, L.; Wu, W.; Dong, S.P.; Gao, S.X.; Mao, L. Adsorption behavior and mechanism of Fe-Mn binary oxide nanoparticles: Adsorption of methylene blue. J. Colloid. Interf. Sci. 2019, 539, 553–562. [Google Scholar] [CrossRef]
- Peng, L.; Deng, X.Z.; Song, H.J.; Tan, X.K.; Gu, J.D.; Luo, S.; Lei, M. Manganese enhances the immobilization of trace cadmium from irrigation water in biological soil crust. Ecotoxicol. Environ. Saf. 2019, 168, 369–377. [Google Scholar] [CrossRef]
- Xiao, Z.Y.; Bao, Y.X.; Li, Z.X.; Huai, X.D.; Wang, M.H.; Liu, P.; Wang, L. The Construction of Hollow Cobalt-Nickel Phosphate Nanocages through a Controllable Etching Strategy for High Supercapacitor Performances. ACS Appl. Energy Mater. 2019, 2, 1089–1092. [Google Scholar] [CrossRef]
- Villalobos, M.; Toner, B.; Bargar, J.; Sposito, G. Characterization of the manganese oxide produced by Pseudomonas putida strain MnB1. Geochim. Cosmochim. Acta 2003, 67, 2649–2662. [Google Scholar] [CrossRef]
- Pei, Y.J.; Chen, X.; Xiong, D.D.; Liao, S.J.; Wang, G.J. Removal and Recovery of Toxic Silver Ion Using Deep-Sea Bacterial Generated Biogenic Manganese Oxides. PLoS ONE 2013, 8, e81627. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.S.; Liu, J.; Deng, Y.H.; Tian, Y.Y.; Zhang, G.J.; Liao, J.L.; Yang, J.J.; Yang, Y.Y.; Liu, N.; Sun, Q. Uranium(VI) adsorption from aqueous solutions by microorganism-graphene oxide composites via an immobilization approach. J. Cleaner Prod. 2019, 236, 117624. [Google Scholar] [CrossRef]
- Wei, D.N.; Li, B.Y.; Luo, L.; Zheng, Y.X.; Huang, L.H.; Zhang, J.C.; Yang, Y.; Huang, H.L. Simultaneous adsorption and oxidation of antimonite onto nano zero-valent iron sludge-based biochar: Indispensable role of reactive oxygen species and redox-active moieties. J. Hazard. Mater. 2020, 391, 122057. [Google Scholar] [CrossRef]
- Guo, M.; Balamurugan, J.; Li, X.Y.; Kim, N.H.; Lee, J.H. Hierarchical 3D Cobalt-Doped Fe3O4 Nanospheres@NG Hybrid as an Advanced Anode Material for High-Performance Asymmetric Supercapacitors. Small 2017, 13, 1701275. [Google Scholar] [CrossRef]
- Godlewska, P.; Bogusz, A.; Dobrzyńska, J.; Dobrowolski, R.; Oleszczuk, P. Engineered biochar modified with iron as a new adsorbent for treatment of water contaminated by selenium. J. Saudi Chem. Soc. 2020, 24, 824–834. [Google Scholar] [CrossRef]
- Xia, F.T.; Song, Z.X.; Liu, X.; Liu, X.; Yang, Y.H.; Zhang, Q.L.; Peng, J.H. Improved catalytic activity and N2 selectivity of Fe–Mn–Ox catalyst for selective catalytic reduction of NO by NH3 at low temperature. Res. Chem. Intermed. 2018, 44, 2703–2717. [Google Scholar] [CrossRef]
- Chang, Q.; Ali, A.; Su, J.F.; Wen, Q.; Bai, Y.H.; Gao, Z.H.; Xiong, R.B. Efficient removal of nitrate, manganese, and tetracycline by a polyvinyl alcohol/sodium alginate with sponge cube immobilized bioreactor. Bioresour. Technol. 2021, 331, 125065. [Google Scholar] [CrossRef]
- Zhang, L.J.; Yang, Y.; Wu, S.X.; Xia, F.; Han, X.; Xu, X.J.; Deng, S.; Jiang, Y.H. Insights into the synergistic removal mechanisms of thallium(I) by biogenic manganese oxides in a wide pH range. Sci. Total Environ. 2022, 831, 154865. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; Chen, Z.; Wu, Z.; Chu, X.; Qing, S.; Xu, L.; Yang, K.; Meng, Q.; Cheng, H.; et al. Effects of salinity on anoxic–oxic system performance, microbial community dynamics and co-occurrence network during treating wastewater. Chem. Eng. J. 2023, 461, 141969. [Google Scholar] [CrossRef]
- Sun, W.; Xiao, E.; Kalin, M.; Krumins, V.; Dong, Y.; Ning, Z.; Liu, T.; Sun, M.; Zhao, Y.; Wu, S.; et al. Remediation of antimony-rich mine waters: Assessment of antimony removal and shifts in the microbial community of an onsite field-scale bioreactor. Environ. Pollut. 2016, 215, 213–222. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, D.; Zhang, L.; Li, C.; Chen, L.; Zou, J. Enhancing the Mn-Removal Efficiency of Acid-Mine Bacterial Consortium: Performance Optimization and Mechanism Study. Microorganisms 2023, 11, 2185. https://doi.org/10.3390/microorganisms11092185
Hou D, Zhang L, Li C, Chen L, Zou J. Enhancing the Mn-Removal Efficiency of Acid-Mine Bacterial Consortium: Performance Optimization and Mechanism Study. Microorganisms. 2023; 11(9):2185. https://doi.org/10.3390/microorganisms11092185
Chicago/Turabian StyleHou, Dongmei, Lan Zhang, Chuncheng Li, Lutong Chen, and Jianping Zou. 2023. "Enhancing the Mn-Removal Efficiency of Acid-Mine Bacterial Consortium: Performance Optimization and Mechanism Study" Microorganisms 11, no. 9: 2185. https://doi.org/10.3390/microorganisms11092185
APA StyleHou, D., Zhang, L., Li, C., Chen, L., & Zou, J. (2023). Enhancing the Mn-Removal Efficiency of Acid-Mine Bacterial Consortium: Performance Optimization and Mechanism Study. Microorganisms, 11(9), 2185. https://doi.org/10.3390/microorganisms11092185






