Biofilm-Forming Capacity and Drug Resistance of Different Gardnerella Subgroups Associated with Bacterial Vaginosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Vaginal Specimens and Ethical Approval
2.2. Culture Conditions and Strain Isolation
2.3. Strain Identification
2.4. In Vitro Biofilm Formation and Quantification at Different Stages
2.5. cpn60 Sequencing of Gardnerella Strains and Detection of Virulence Factors
2.6. Minimal Inhibitory Concentration Assays
2.7. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Gardnerella Strains
3.2. cpn60 Typing of Gardnerella Strains and Biofilm Formation
3.3. Growth Curves of the Gardnerella Strains
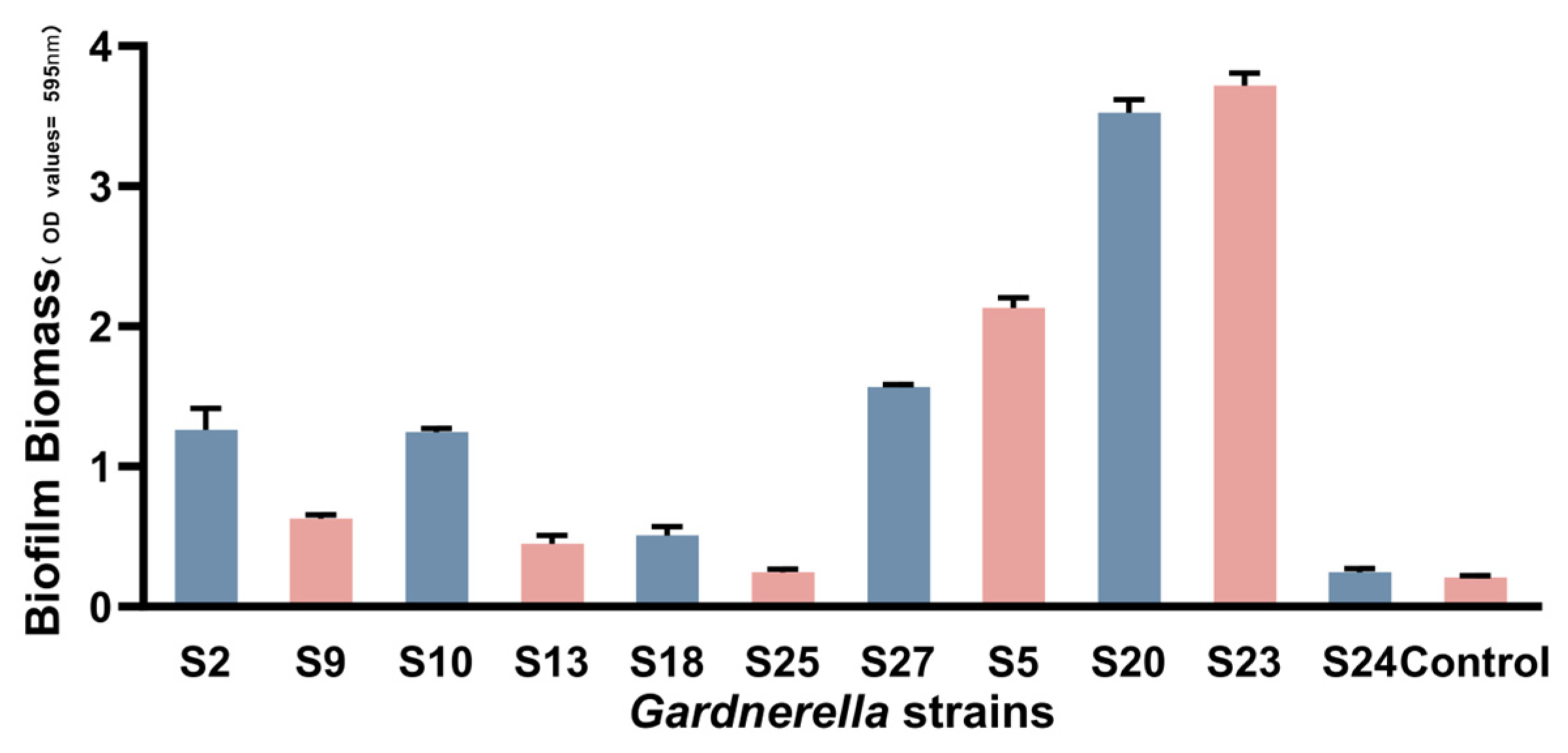
3.4. Quantification of Gardnerella Biofilm Formation at Different Times
3.5. The MIC of Gardnerella spp. Planktonic Cells and Biofilms in Response to Metronidazole and Clindamycin
3.6. Genomic Extraction of Gardnerella Isolates and Detection of Virulence Factor-Related Genes
4. Discussion
5. Limitations
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kenyon, C.; Colebunders, R.; Crucitti, T. The global epidemiology of bacterial vaginosis: A systematic review. Am. J. Obstet. Gynecol. 2013, 209, 505–523. [Google Scholar] [CrossRef] [PubMed]
- Ravel, J.; Moreno, I.; Simón, C. Bacterial vaginosis and its association with infertility, endometritis, and pelvic inflammatory disease. Am. J. Obstet. Gynecol. 2021, 224, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Guo, R.; Wang, J.; Zhou, W.; Ling, Z. Contribution of Lactobacillus iners to Vaginal Health and Diseases: A Systematic Review. Front. Cell. Infect. Microbiol. 2021, 11, 792787. [Google Scholar] [CrossRef]
- Pacha-Herrera, D.; Erazo-Garcia, M.P.; Cueva, D.F.; Orellana, M.; Borja-Serrano, P.; Arboleda, C.; Tejera, E.; Machado, A. Clustering Analysis of the Multi-Microbial Consortium by Lactobacillus Species Against Vaginal Dysbiosis among Ecuadorian Women. Front. Cell. Infect. Microbiol. 2022, 12, 863208. [Google Scholar] [CrossRef] [PubMed]
- Workowski, K.A.; Bachmann, L.H.; Chan, P.A.; Johnston, C.M.; Muzny, C.A.; Park, I.; Reno, H.; Zenilman, J.M.; Bolan, G.A. Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR Recomm. Rep. 2021, 70, 1–187. [Google Scholar] [CrossRef]
- Muñoz-Barreno, A.; Cabezas-Mera, F.; Tejera, E.; Machado, A. Comparative Effectiveness of Treatments for Bacterial Vaginosis: A Network Meta-Analysis. Antibiotics 2021, 10, 978. [Google Scholar] [CrossRef]
- Bostwick, D.G.; Woody, J.; Hunt, C.; Budd, W. Antimicrobial resistance genes and modelling of treatment failure in bacterial vaginosis: Clinical study of 289 symptomatic women. J. Med. Microbiol. 2016, 65, 377–386. [Google Scholar] [CrossRef]
- Rosca, A.S.; Castro, J.; França, Â.; Vaneechoutte, M.; Cerca, N. Gardnerella Vaginalis Dominates Multi-Species Biofilms in both Pre-Conditioned and Competitive In Vitro Biofilm Formation Models. Microb. Ecol. 2021, 84, 1278–1287. [Google Scholar] [CrossRef]
- Machado, A.; Cerca, N. Influence of Biofilm Formation by Gardnerella vaginalis and Other Anaerobes on Bacterial Vaginosis. J. Infect. Dis. 2015, 212, 1856–1861. [Google Scholar] [CrossRef]
- Muzny, C.A.; Taylor, C.M.; Swords, W.E.; Tamhane, A.; Chattopadhyay, D.; Cerca, N.; Schwebke, J.R. An Updated Conceptual Model on the Pathogenesis of Bacterial Vaginosis. J. Infect. Dis. 2019, 220, 1399–1405. [Google Scholar] [CrossRef]
- Harwich, M.D., Jr.; Alves, J.M.; Buck, G.A.; Strauss, J.F., 3rd; Patterson, J.L.; Oki, A.T.; Girerd, P.H.; Jefferson, K.K. Drawing the line between commensal and pathogenic Gardnerella vaginalis through genome analysis and virulence studies. BMC Genom. 2010, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.; Alves, P.; Sousa, C.; Cereija, T.; França, Â.; Jefferson, K.K.; Cerca, N. Using an in-vitro biofilm model to assess the virulence potential of bacterial vaginosis or non-bacterial vaginosis Gardnerella vaginalis isolates. Sci. Rep. 2015, 5, 11640. [Google Scholar] [CrossRef] [PubMed]
- Shvartsman, E.; Hill, J.E.; Sandstrom, P.; MacDonald, K.S. Gardnerella Revisited: Species Heterogeneity, Virulence Factors, Mucosal Immune Responses, and Contributions to Bacterial Vaginosis. Infect. Immun. 2023, 91, e0039022. [Google Scholar] [CrossRef] [PubMed]
- Vaneechoutte, M.; Guschin, A.; Van Simaey, L.; Gansemans, Y.; Van Nieuwerburgh, F.; Cools, P. Emended description of Gardnerella vaginalis and description of Gardnerella leopoldii sp. nov., Gardnerella piotii sp. nov. and Gardnerella swidsinskii sp. nov., with delineation of 13 genomic species within the genus Gardnerella. Int. J. Syst. Evol. Microbiol. 2019, 69, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.E.; Goh, S.H.; Money, D.M.; Doyle, M.; Li, A.; Crosby, W.L.; Links, M.; Leung, A.; Chan, D.; Hemmingsen, S.M. Characterization of vaginal microflora of healthy, nonpregnant women by chaperonin-60 sequence-based methods. Am. J. Obstet. Gynecol. 2005, 193, 682–692. [Google Scholar] [CrossRef]
- Schellenberg, J.J.; Links, M.G.; Hill, J.E.; Dumonceaux, T.J.; Kimani, J.; Jaoko, W.; Wachihi, C.; Mungai, J.N.; Peters, G.A.; Tyler, S.; et al. Molecular definition of vaginal microbiota in East African commercial sex workers. Appl. Environ. Microbiol. 2011, 77, 4066–4074. [Google Scholar] [CrossRef]
- Shvartsman, E.; Perciani, C.T.; Richmond, M.E.I.; Russell, J.N.H.; Tough, R.H.; Vancuren, S.J.; Hill, J.E.; Kavi, I.; Jaoko, W.; McKinnon, L.R.; et al. Gardnerella subgroup dominant microbiomes are associated with divergent cervicovaginal immune responses in a longitudinal cohort of Kenyan women. Front. Immunol. 2022, 13, 974195. [Google Scholar] [CrossRef]
- Mehta, S.D.; Nannini, D.R.; Otieno, F.; Green, S.J.; Agingu, W.; Landay, A.; Zheng, Y.; Hou, L. Host Genetic Factors Associated with Vaginal Microbiome Composition in Kenyan Women. mSystems 2020, 5, e00502-20. [Google Scholar] [CrossRef]
- Qin, H.; Xiao, B. Research Progress on the Correlation Between Gardnerella Typing and Bacterial Vaginosis. Front. Cell. Infect. Microbiol. 2022, 12, 858155. [Google Scholar] [CrossRef]
- Aroutcheva, A.A.; Simoes, J.A.; Behbakht, K.; Faro, S. Gardnerella vaginalis isolated from patients with bacterial vaginosis and from patients with healthy vaginal ecosystems. Clin. Infect. Dis. 2001, 33, 1022–1027. [Google Scholar] [CrossRef]
- Castro, J.; Martins, A.P.; Rodrigues, M.E.; Cerca, N. Lactobacillus crispatus represses vaginolysin expression by BV associated Gardnerella vaginalis and reduces cell cytotoxicity. Anaerobe 2018, 50, 60–63. [Google Scholar] [CrossRef]
- Castro, J.; Jefferson, K.K.; Cerca, N. Genetic Heterogeneity and Taxonomic Diversity among Gardnerella Species. Trends Microbiol. 2020, 28, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Amsel, R.; Totten, P.A.; Spiegel, C.A.; Chen, K.C.; Eschenbach, D.; Holmes, K.K. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am. J. Med. 1983, 74, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Nugent, R.P.; Krohn, M.A.; Hillier, S.L. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J. Clin. Microbiol. 1991, 29, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Stepanovic, S.; Vukovic, D.; Dakic, I.; Savic, B.; Svabic-Vlahovic, M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef]
- Hardy, L.; Jespers, V.; Van den Bulck, M.; Buyze, J.; Mwambarangwe, L.; Musengamana, V.; Vaneechoutte, M.; Crucitti, T. The presence of the putative Gardnerella vaginalis sialidase A gene in vaginal specimens is associated with bacterial vaginosis biofilm. PLoS ONE 2017, 12, e0172522. [Google Scholar] [CrossRef]
- Gelber, S.E.; Aguilar, J.L.; Lewis, K.L.; Ratner, A.J. Functional and phylogenetic characterization of Vaginolysin, the human-specific cytolysin from Gardnerella vaginalis. J. Bacteriol. 2008, 190, 3896–3903. [Google Scholar] [CrossRef]
- Nowak, R.G.; Randis, T.M.; Desai, P.; He, X.; Robinson, C.K.; Rath, J.M.; Glover, E.D.; Ratner, A.J.; Ravel, J.; Brotman, R.M. Higher Levels of a Cytotoxic Protein, Vaginolysin, in Lactobacillus-Deficient Community State Types at the Vaginal Mucosa. Sex. Transm. Dis. 2018, 45, e14–e17. [Google Scholar] [CrossRef]
- Brook, I.; Wexler, H.M.; Goldstein, E.J. Antianaerobic antimicrobials: Spectrum and susceptibility testing. Clin. Microbiol. Rev. 2013, 26, 526–546. [Google Scholar] [CrossRef]
- Swidsinski, A.; Mendling, W.; Loening-Baucke, V.; Ladhoff, A.; Swidsinski, S.; Hale, L.P.; Lochs, H. Adherent biofilms in bacterial vaginosis. Obstet. Gynecol. 2005, 106, 1013–1023. [Google Scholar] [CrossRef]
- Rosca, A.S.; Castro, J.; Sousa, L.G.V.; Cerca, N. Gardnerella and vaginal health: The truth is out there. FEMS Microbiol. Rev. 2020, 44, 73–105. [Google Scholar] [CrossRef] [PubMed]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Redelinghuys, M.J.; Geldenhuys, J.; Jung, H.; Kock, M.M. Bacterial Vaginosis: Current Diagnostic Avenues and Future Opportunities. Front. Cell. Infect. Microbiol. 2020, 10, 354. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Figueroa, C.; Estrada-Moreno, A.K.; Vences-Velázquez, A.; Cortés-Sarabia, K. One-Step Staining Method for the Identification of Clue Cells and Bacterial Morphotypes Associated with Bacterial Vaginosis. Microbiol. Spectr. 2022, 10, e0192721. [Google Scholar] [CrossRef]
- Swidsinski, A.; Loening-Baucke, V.; Swidsinski, S.; Sobel, J.D.; Dörffel, Y.; Guschin, A. Clue Cells and Pseudo Clue Cells in Different Morphotypes of Bacterial Vaginosis. Front. Cell. Infect. Microbiol. 2022, 12, 905739. [Google Scholar] [CrossRef]
- Khan, S.; Voordouw, M.J.; Hill, J.E. Competition Among Gardnerella Subgroups from the Human Vaginal Microbiome. Front. Cell. Infect. Microbiol. 2019, 9, 374. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.; Rosca, A.S.; Muzny, C.A.; Cerca, N. Atopobium vaginae and Prevotella bivia Are Able to Incorporate and Influence Gene Expression in a Pre-Formed Gardnerella vaginalis Biofilm. Pathogens 2021, 10, 247. [Google Scholar] [CrossRef]
- Jung, H.S.; Ehlers, M.M.; Lombaard, H.; Redelinghuys, M.J.; Kock, M.M. Etiology of bacterial vaginosis and polymicrobial biofilm formation. Crit. Rev. Microbiol. 2017, 43, 651–667. [Google Scholar] [CrossRef]
- Castro, J.; Machado, D.; Cerca, N. Unveiling the role of Gardnerella vaginalis in polymicrobial Bacterial Vaginosis biofilms: The impact of other vaginal pathogens living as neighbors. ISME J. 2019, 13, 1306–1317. [Google Scholar] [CrossRef]
- Patterson, J.L.; Girerd, P.H.; Karjane, N.W.; Jefferson, K.K. Effect of biofilm phenotype on resistance of Gardnerella vaginalis to hydrogen peroxide and lactic acid. Am. J. Obstet. Gynecol. 2007, 197, e171–e177. [Google Scholar] [CrossRef]
- Swidsinski, S.; Moll, W.M.; Swidsinski, A. Bacterial Vaginosis—Vaginal Polymicrobial Biofilms and Dysbiosis. Dtsch. Arztebl. Int. 2023, 20, 347. [Google Scholar] [CrossRef]
- Jayaprakash, P.T.; Schellenberg, J.J.; Hill, J.E. Resolution and characterization of distinct cpn60-based subgroups of Gardnerella vaginalis in the vaginal microbiota. PLoS ONE 2012, 7, e43009. [Google Scholar] [CrossRef] [PubMed]
- Schellenberg, J.J.; Paramel Jayaprakash, T.; Withana Gamage, N.; Patterson, M.H.; Vaneechoutte, M.; Hill, J.E. Gardnerella vaginalis Subgroups Defined by cpn60 Sequencing and Sialidase Activity in Isolates from Canada, Belgium and Kenya. PLoS ONE 2016, 11, e0146510. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Earl, J.; Retchless, A.; Hillier, S.L.; Rabe, L.K.; Cherpes, T.L.; Powell, E.; Janto, B.; Eutsey, R.; Hiller, N.L.; et al. Comparative genomic analyses of 17 clinical isolates of Gardnerella vaginalis provide evidence of multiple genetically isolated clades consistent with subspeciation into genovars. J. Bacteriol. 2012, 194, 3922–3937. [Google Scholar] [CrossRef] [PubMed]
- Balashov, S.V.; Mordechai, E.; Adelson, M.E.; Gygax, S.E. Identification, quantification and subtyping of Gardnerella vaginalis in noncultured clinical vaginal samples by quantitative PCR. J. Med. Microbiol. 2014, 63, 162–175. [Google Scholar] [CrossRef]
- Albert, A.Y.; Chaban, B.; Wagner, E.C.; Schellenberg, J.J.; Links, M.G.; van Schalkwyk, J.; Reid, G.; Hemmingsen, S.M.; Hill, J.E.; Money, D. A Study of the Vaginal Microbiome in Healthy Canadian Women Utilizing cpn60-Based Molecular Profiling Reveals Distinct Gardnerella Subgroup Community State Types. PLoS ONE 2015, 10, e0135620. [Google Scholar] [CrossRef]
- Hilbert, D.W.; Schuyler, J.A.; Adelson, M.E.; Mordechai, E.; Sobel, J.D.; Gygax, S.E. Gardnerella vaginalis population dynamics in bacterial vaginosis. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1269–1278. [Google Scholar] [CrossRef]
- Swidsinski, A.; Mendling, W.; Loening-Baucke, V.; Swidsinski, S.; Dörffel, Y.; Scholze, J.; Lochs, H.; Verstraelen, H. An adherent Gardnerella vaginalis biofilm persists on the vaginal epithelium after standard therapy with oral metronidazole. Am. J. Obstet. Gynecol. 2008, 198, e91–e96. [Google Scholar] [CrossRef]
- Rosca, A.S.; Castro, J.; Sousa, L.G.V.; França, A.; Vaneechoutte, M.; Cerca, N. In vitro interactions within a biofilm containing three species found in bacterial vaginosis (BV) support the higher antimicrobial tolerance associated with BV recurrence. J. Antimicrob. Chemother. 2022, 77, 2183–2190. [Google Scholar] [CrossRef]
- Janulaitiene, M.; Paliulyte, V.; Grinceviciene, S.; Zakareviciene, J.; Vladisauskiene, A.; Marcinkute, A.; Pleckaityte, M. Prevalence and distribution of Gardnerella vaginalis subgroups in women with and without bacterial vaginosis. BMC Infect. Dis. 2017, 17, 394. [Google Scholar] [CrossRef]
- Vodstrcil, L.A.; Twin, J.; Garland, S.M.; Fairley, C.K.; Hocking, J.S.; Law, M.G.; Plummer, E.L.; Fethers, K.A.; Chow, E.P.; Tabrizi, S.N.; et al. The influence of sexual activity on the vaginal microbiota and Gardnerella vaginalis clade diversity in young women. PLoS ONE 2017, 12, e0171856. [Google Scholar] [CrossRef]
- Hill, J.E.; Albert, A.Y.K. Resolution and Cooccurrence Patterns of Gardnerella leopoldii, G. swidsinskii, G. piotii, and G. vaginalis within the Vaginal Microbiome. Infect. Immun. 2019, 87, 10–128. [Google Scholar] [CrossRef] [PubMed]
- Verstraelen, H.; Swidsinski, A. The biofilm in bacterial vaginosis: Implications for epidemiology, diagnosis and treatment. Curr. Opin. Infect. Dis. 2013, 26, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Machado, D.; Castro, J.; Palmeira-de-Oliveira, A.; Martinez-de-Oliveira, J.; Cerca, N. Bacterial Vaginosis Biofilms: Challenges to Current Therapies and Emerging Solutions. Front. Microbiol. 2015, 6, 1528. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.L.; Gottschick, C.; Bhuju, S.; Masur, C.; Abels, C.; Wagner-Döbler, I. Metatranscriptome Analysis of the Vaginal Microbiota Reveals Potential Mechanisms for Protection against Metronidazole in Bacterial Vaginosis. mSphere 2018, 3, 10–128. [Google Scholar] [CrossRef]
- Belizário, J.E.; Napolitano, M. Human microbiomes and their roles in dysbiosis, common diseases, and novel therapeutic approaches. Front. Microbiol. 2015, 6, 1050. [Google Scholar] [CrossRef]
- Verstraelen, H.; Swidsinski, A. The biofilm in bacterial vaginosis: Implications for epidemiology, diagnosis and treatment: 2018 update. Curr. Opin. Infect. Dis. 2019, 32, 38–42. [Google Scholar] [CrossRef]
- Castro, J.; Cerca, N. BV and non-BV associated Gardnerella vaginalis establish similar synergistic interactions with other BV-associated microorganisms in dual-species biofilms. Anaerobe 2015, 36, 56–59. [Google Scholar] [CrossRef]
- Azeredo, J.; Azevedo, N.F.; Briandet, R.; Cerca, N.; Coenye, T.; Costa, A.R.; Desvaux, M.; Di Bonaventura, G.; Hébraud, M.; Jaglic, Z.; et al. Critical review on biofilm methods. Crit. Rev. Microbiol. 2017, 43, 313–351. [Google Scholar] [CrossRef]


| Clinical Isolate | Age | Symptoms | Treatment BT/AT | Clue Cells BT/AT | Nugent * Scores | Diagnosis | Treatment Outcomes |
|---|---|---|---|---|---|---|---|
| S2 | 44 | Abnormal discharge | BT | +/+ | 8 | BV | Uncured |
| S5 | 39 | Abnormal discharge, odor | BT | −/− | 10 | BV | Cure |
| S9 | 26 | Abnormal discharge | BT | −/− | 8 | BV | Cure |
| S10 | 42 | Abnormal discharge | BT | −/− | 8 | BV | Cure |
| S13 | 38 | Abnormal discharge, itching | BT | +/− | 7 | BV | Cure |
| S18 | 31 | Abnormal discharge | BT | +/+ | 7 | BV | Uncured |
| S25 | 37 | Abnormal discharge | BT | +/+ | 8 | BV | Uncured |
| S27 | 49 | Abnormal discharge | BT | +/− | 8 | BV | Uncured |
| S20 | 41 | None | AT | +/− | 4 | Intermediate | − |
| S23 | 36 | Abnormal discharge | AT | −/− | 1 | Nomal | − |
| S24 | 24 | None | AT | −/− | 0 | Nomal | − |
| cpn60 Typing | Clincal Isolate | Biofilm (Mean ± SD) (OD595) | Mean in Subgroup (Mean ± SD) (OD595) |
|---|---|---|---|
| Subgroup A | S2 | 1.262 ± 0.152 (S) | 0.7223 ± 0.4293 |
| S9 | 0.627 ± 0.029 (M) | ||
| S10 | 1.244 ± 0.026 (S) | ||
| S13 | 0.449 ± 0.058 (W) | ||
| S18 | 0.507 ± 0.065 (M) | ||
| S25 | 0.245 ± 0.023 (W) | ||
| Subgroup B | S27 | 1.567 ± 0.019 (S) | − |
| Subgroup D | S5 | 2.132 ± 0.072 (S) | − |
| Subgroup | Strains | Biofilm | Metronidazole (μg/mL) MIC | Clindamycin (μg/mL) MIC | ||||
|---|---|---|---|---|---|---|---|---|
| Planktonic Cells | 24 h Biofilm | 48 h Biofilm | Planktonic Cells | 24 h Biofilm | 48 h Biofilm | |||
| Subgroup A | S2 | S | <0.125 | 1 | 1 | <0.0625 | <0.0625 | 0.125 |
| S9 | M | 128 | >256 | >256 | <0.0625 | >128 | >128 | |
| S10 | S | 64 | 128 | 256 | 0.25 | >128 | >128 | |
| S13 | W | 128 | >256 | >256 | <0.0625 | >128 | >128 | |
| S18 | M | 128 | 32 | >256 | 64 | >128 | >128 | |
| S25 | W | <0.125 | 1 | 1 | <0.0625 | <0.0625 | 0.125 | |
| Subgroup B | S27 | S | 128 | 256 | 256 | <0.0625 | 0.125 | 0.125 |
| Subgroup D | S5 | S | 64 | 64 | 128 | <0.0625 | <0.0625 | 0.125 |
| Strains | S20 | S23 | S24 | S25 |
|---|---|---|---|---|
| Size(bp) | 1,686,096 | 1,685,505 | 1,549,419 | 1,582,566 |
| CDS | 1316 | 1359 | 1290 | 1286 |
| Plasmid | NO | NO | NO | NO |
| Island | 6 | 6 | 6 | 6 |
| GC % | 41.31 | 41.81 | 42.55 | 42.74 |
| tRNA | 45 | 45 | 45 | 45 |
| rRNA | 6 | 6 | 6 | 6 |
| CRISPER | 1 | 1 | 0 | 0 |
| Metabolism | 376 | 414 | 281 | 259 |
| Genetic Information Processing | 276 | 214 | 270 | 208 |
| Environmental Information Processing | 82 | 80 | 114 | 106 |
| Cellular Processes | 58 | 58 | 102 | 110 |
| Organismal Systems | 28 | 19 | 36 | 25 |
| Human Diseases | 31 | 30 | 62 | 44 |
| HMPREF0424_0103 | + | + | − | − |
| HMPREF0424_1109 | + | + | − | − |
| HMPREF0424_0125 | + | + | − | − |
| HMPREF0424_0821 | + | + | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, H.; Liu, Y.; Zhai, Z.; Xiao, B. Biofilm-Forming Capacity and Drug Resistance of Different Gardnerella Subgroups Associated with Bacterial Vaginosis. Microorganisms 2023, 11, 2186. https://doi.org/10.3390/microorganisms11092186
Qin H, Liu Y, Zhai Z, Xiao B. Biofilm-Forming Capacity and Drug Resistance of Different Gardnerella Subgroups Associated with Bacterial Vaginosis. Microorganisms. 2023; 11(9):2186. https://doi.org/10.3390/microorganisms11092186
Chicago/Turabian StyleQin, Hanyu, Yun Liu, Zhengyuan Zhai, and Bingbing Xiao. 2023. "Biofilm-Forming Capacity and Drug Resistance of Different Gardnerella Subgroups Associated with Bacterial Vaginosis" Microorganisms 11, no. 9: 2186. https://doi.org/10.3390/microorganisms11092186
APA StyleQin, H., Liu, Y., Zhai, Z., & Xiao, B. (2023). Biofilm-Forming Capacity and Drug Resistance of Different Gardnerella Subgroups Associated with Bacterial Vaginosis. Microorganisms, 11(9), 2186. https://doi.org/10.3390/microorganisms11092186








