Nitric Oxide Detection Using a Chemical Trap Method for Applications in Bacterial Systems †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Growth Conditions
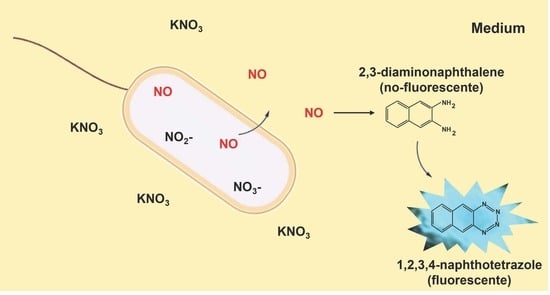
2.2. Determining NO Production in Bacteria via the Greiss Reaction and Fluorescence
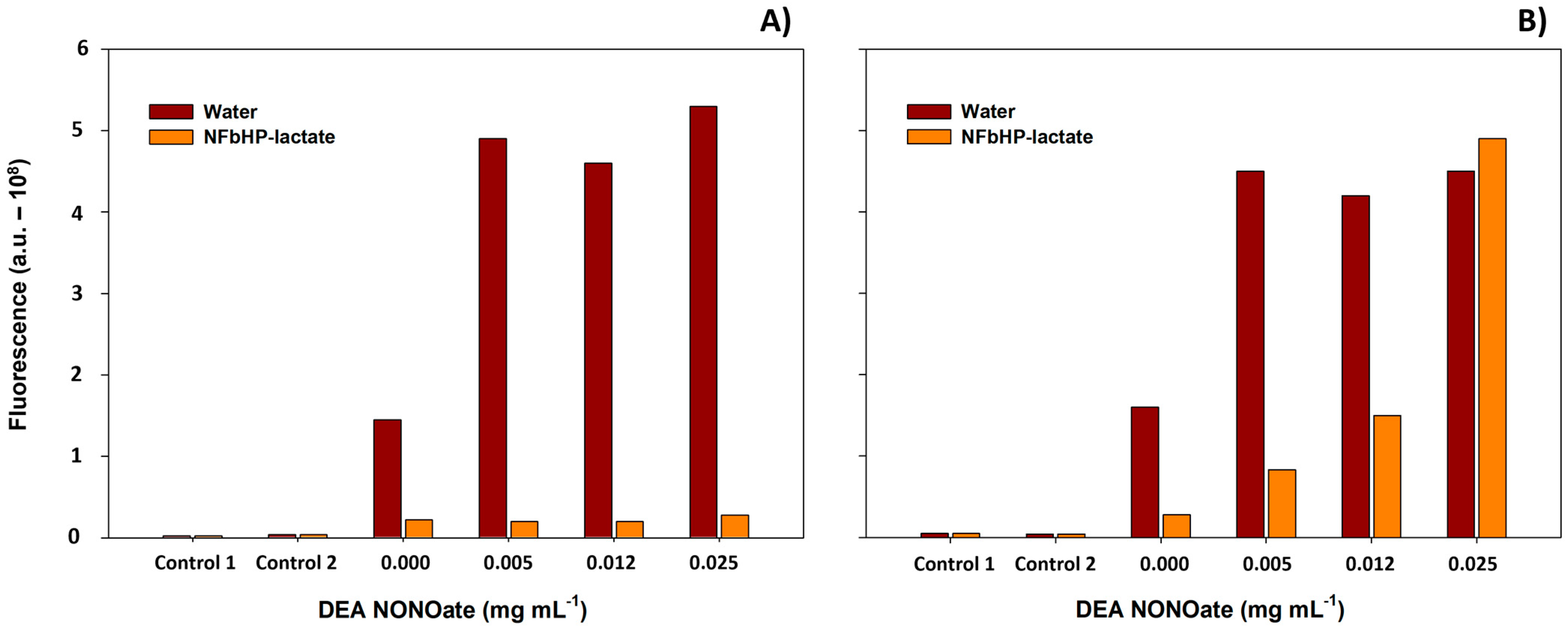
2.3. Monitoring NO Production in the NFbHP-NH4Cl-Lactate Medium
2.4. Monitoring NO Production in Bacteria by Chromatography
2.5. Statistical Analysis
3. Results
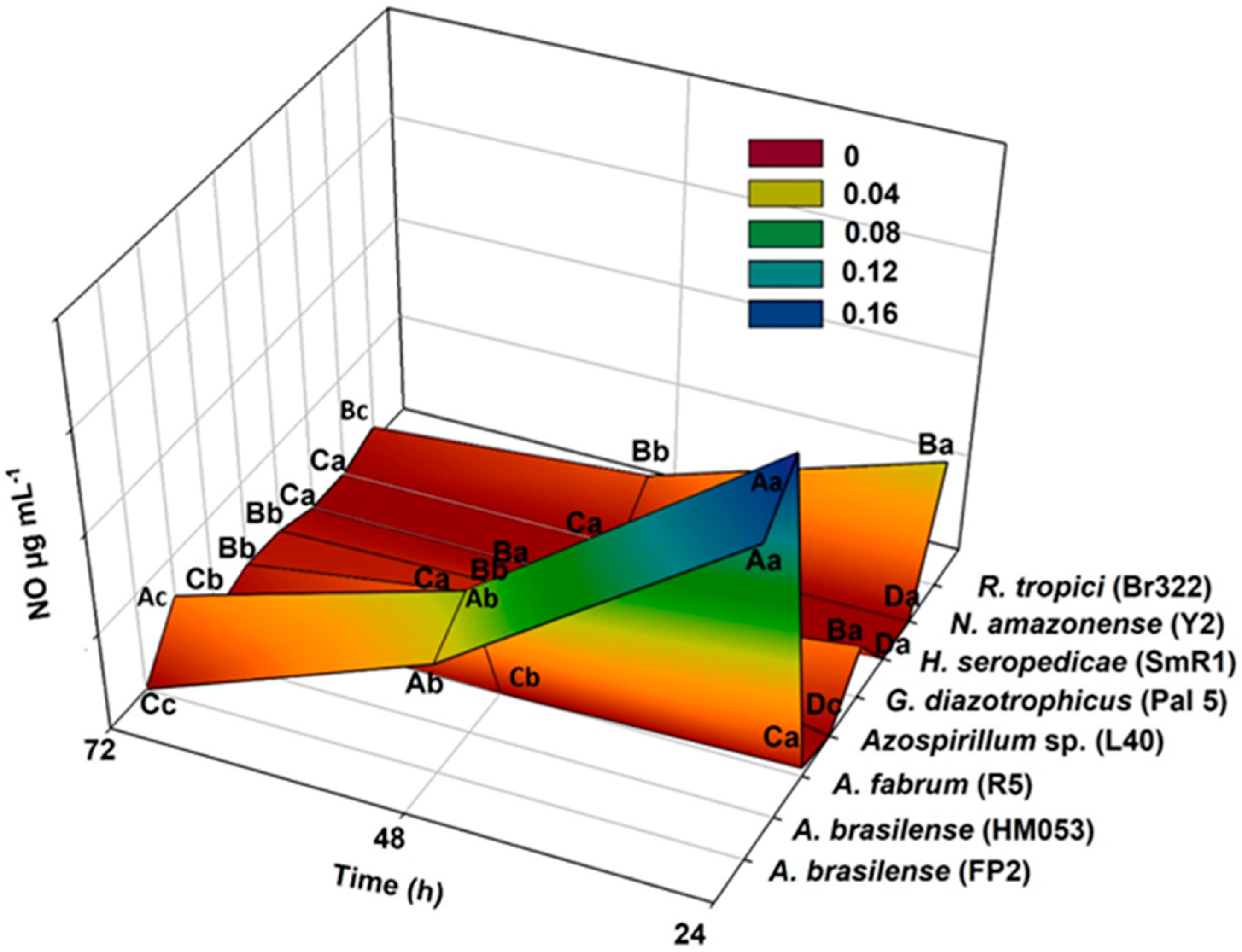
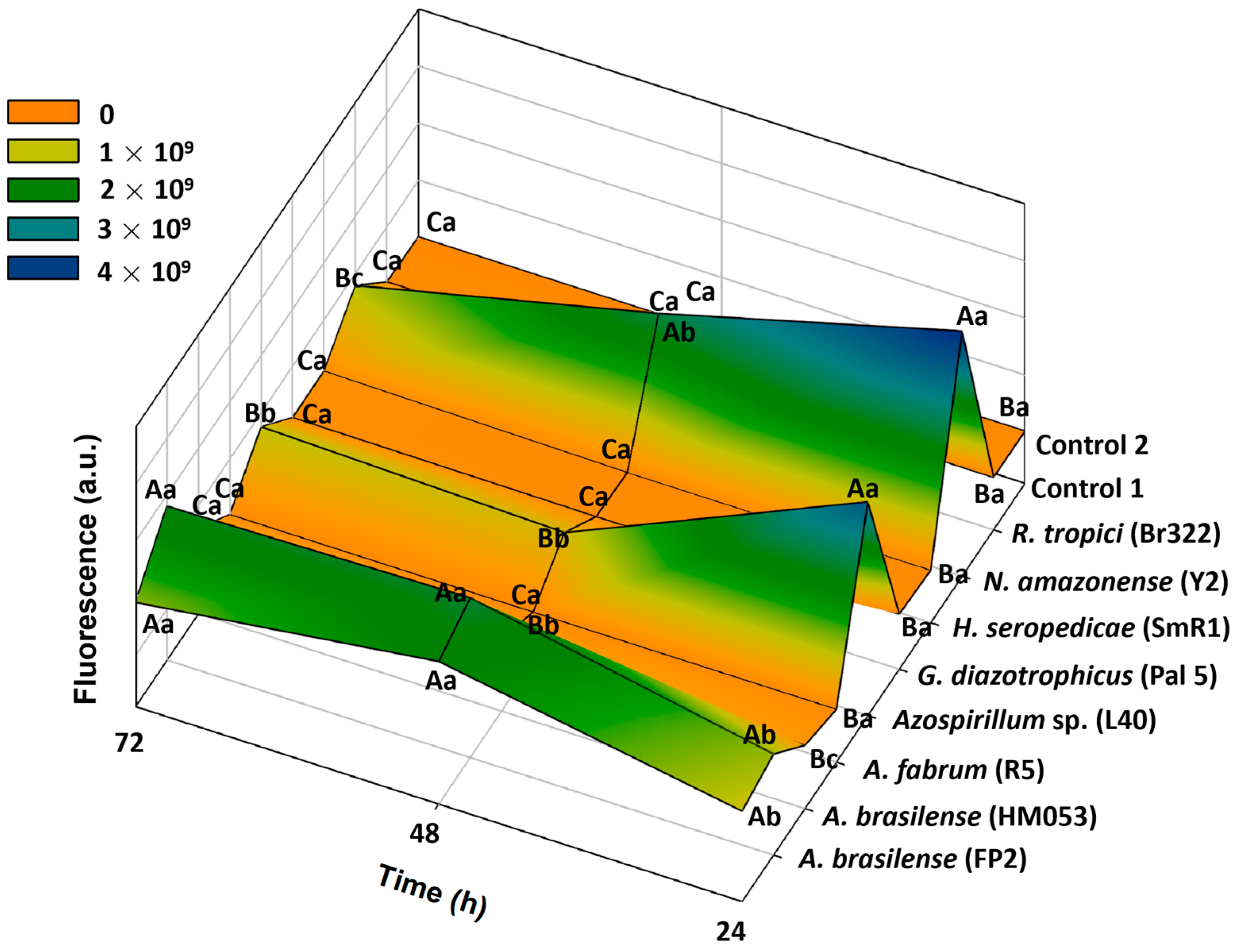
3.1. Greiss and 2,3-Diaminonaphthalene (DAN) Assays
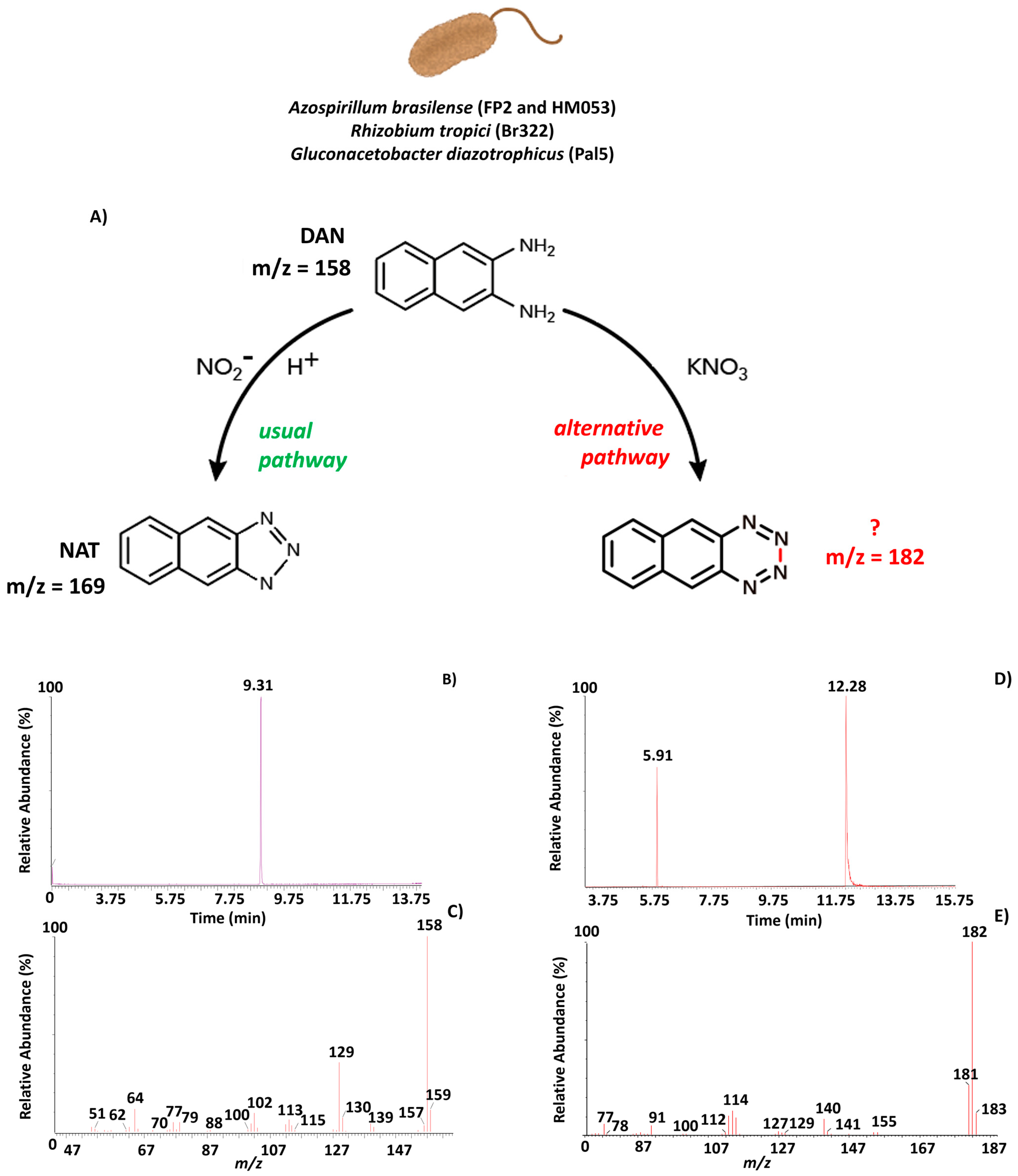
3.2. GC-MS Analyses
3.3. Validation Method
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vessey, J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil. 2003, 255, 571–586. [Google Scholar] [CrossRef]
- Dobbelaere, S.; Vanderleyden, J.; Okon, Y. Plant Growth-Promoting Effects of Diazotrophs in th1-e Rhizosphere. Crit. Rev. Plant Sci. 2003, 22, 107–149. [Google Scholar] [CrossRef]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef] [PubMed]
- Hungria, M.; Campo, R.J.; Mendes, I.C. Fixação Biológica do N2 na Cultura da Soja; Embrapa Soja: Londrina, Brazil, 2001; p. 35. [Google Scholar]
- Balandreau, J.; Villard, V.; Cournoyer, B.; Coenye, T.; Laevens, S.; Vandamme, P. Burkholderia cepacia Genomovar III Is a Common Plant-Associated Bacterium. Appl. Environ. Microbiol. 2011, 67, 982–985. [Google Scholar] [CrossRef]
- Baldani, J.I.; Baldani, V.L.D.; Seldin, L.; Döbereiner, J. Characterization of Herbaspirillum seropedicae gen. nov., sp. nov., a Root-Associated Nitrogen-Fixing Bacterium. Int. J. Syst. Bacteriol. 1986, 36, 86–93. [Google Scholar] [CrossRef]
- Bilal, R.; Rasul, G.; Qureshi, J.A.; Malik, K.A. Characterization of Azospirillum and related diazotrophs associated with roots of plants growing in saline soils. Microbiol. Biotechnol. 1990, 6, 46–52. [Google Scholar] [CrossRef]
- Gillis, M.; Kersters, K.; Hoste, B.; Janssens, D.; Kroppenstedt, R.M.; Stephan, M.P.; Teixeira, K.R.S.; Döbereiner, J.; De Ley, J. Acetobacter diazotrophicus sp. nov., a Nitrogen-Fixing Acetic Acid Bacterium Associated with Sugarcane. Int. J. Syst. Bacteriol. 1989, 39, 361–364. [Google Scholar] [CrossRef]
- Reinhold-Hurek, B.; Hurek, T.; Gillis, M.; Hoste, B.; Vancanney, M.; Kersters, K.; De Ley, J. Azoarcus gen. nov., Nitrogen-Fixing Proteobacteria Associated with Roots of Kallar Grass (Leptochloa fusca (L.) Kunth), and Description of 2 Species, Azoarcus indigens sp. nov. and Azoarcus communis sp. nov. Int. J. Syst. Bacteriol. 1993, 43, 574–584. [Google Scholar] [CrossRef]
- Yanni, Y.G.; Dazzo, F.B. Enhancement of rice production using endophytic strains of Rhizobium leguminosarum bv. trifolii in extensive field inoculation trials within the Egypt Nile delta. Plant Soil. 2010, 336, 129–142. [Google Scholar] [CrossRef]
- Spanning, R.; Richardson, D.J.; Ferguson, S.J. Chapter 1—Introduction to the Biochemistry and Molecular Biology of Denitrification. In Biology of the Nitrogen Cycle; Elsevier: Amsterdam, The Netherlands, 2007; pp. 3–20. [Google Scholar]
- Klotz, M.G.; Stein, L.Y. Nitrifier genomics and evolution of the nitrogen cycle. FEMS Microbiol. Lett. 2008, 278, 146–156. [Google Scholar] [CrossRef]
- Braker, G.; Tiedje, J.M. Nitric oxide reductase (norB) genes from pure cultures and environmental samples. Appl. Environ. Microbiol. 2003, 69, 3476–3483. [Google Scholar] [CrossRef] [PubMed]
- Lamattina, L.; García-Mata, C.; Graziano, M.; Pagnussat, G. Nitric Oxide: The Versatility of an Extensive Signal Molecule. Annu. Rev. Plant Biol. 2003, 54, 109–136. [Google Scholar] [CrossRef] [PubMed]
- Gusarov, I.; Shatalin, K.; Starodubtseva, M.; Nudler, E. Endogenous nitric oxide protects bacteria against a wide spectrum of antibiotics. Science 2009, 325, 1380–1384. [Google Scholar] [CrossRef]
- Salas, A.; Cabrera, J.J.; Jiménez-Leiva, A.; Mesa, S.; Bedmar, E.J.; Richardson, D.J.; Gates, A.J.; Delgado, M. Bacterial nitric oxide metabolism: Recent insights in rhizobia. Adv. Microb. Physiol. 2021, 78, 259–315. [Google Scholar]
- Signorelli, S.; Sainz, M.; Rosa, S.T.; Monza, J. The role of nitric oxide in nitrogen fixation by legumes. Front. Plant Sci. 2020, 11, 521. [Google Scholar] [CrossRef] [PubMed]
- Cassán, F.D.; Okon, Y.; Creus, C.M. Handbook for Azospirillum: Technical Issues and Protocols; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- Molina-Favero, C.; Creus, C.M.; Simontacchi, M.; Puntarulo, S.; Lamattina, L. Aerobic nitric oxide production by Azospirillum brasilense Sp245 and its influence on root architecture in tomato. Mol. Plant-Microbe Interact. 2008, 21, 1001–1009. [Google Scholar] [CrossRef]
- Creus, C.M.; Graziano, M.; Casanovas, E.M.; Pereyra, M.A.; Simontacchi, M.; Puntarulo, S.; Barassi, C.A.; Lamattina, L. Nitric oxide is involved in the Azospirillum brasilense-induced lateral root formation in tomato. Planta 2005, 221, 297–303. [Google Scholar] [CrossRef]
- Nagano, T. Practical methods for detection of nitric oxide. Luminescence 1999, 14, 283–290. [Google Scholar] [CrossRef]
- Bryan, N.S.; Grisham, M.B. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic. Biol. Med. 2007, 43, 645–657. [Google Scholar] [CrossRef]
- Miles, A.M.; Wink, D.A.; Cook, J.C.; Grisham, M.B. Determination of nitric oxide using fluorescence spectroscopy. Methods Enzymol. 1996, 268, 105–120. [Google Scholar]
- Wada, M.; Morinaka, C.; Ikenaga, T.; Kuroda, N.; Nakashima, K. A Simple HPLC-Fluorescence Detection of Nitric Oxide in Cultivated Plant Cells by in situ Derivatization with 2,3-Diaminonaphthalene. Anal. Sci. 2002, 18, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Kováčik, J.; Babula, P.; Klejdus, B.; Hedbavny, J.; Jarosová, M. Unexpected behavior of some nitric oxide modulators under cadmium excess in plant tissue. PLoS ONE 2014, 9, e91685. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Meininger, C.J.; Wu, G. Rapid determination of nitrite by reversed-phase high-performance liquid chromatography with fluorescence detection. J. Chrom. B Biom. Sci. Appl. 2000, 746, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Filippovich, S.Y.; Onufriev, M.V.; Bachurina, G.P.; Kristisky, M.S. The Role of Nitrogen Oxide in Photomorphogenesis in Neurospora crassa. Appl. Biochem. Microbiol. 2019, 55, 427–433. [Google Scholar] [CrossRef]
- Yu, H.; Bonetti, J.; Gaucher, C.; Fries, I.; Vernex-Loset, L.; Leroy, P.; Chaimbault, P. Higher-energy collision-induced dissociation for the quantification by liquid chromatography/tandem ion trap mass spectrometry of nitric oxide metabolites coming from S-nitroso-glutathione in an in vitro model of the intestinal barrier. Rapid Commun. Mass Spectrom. 2019, 33, 1–11. [Google Scholar] [CrossRef]
- Orlandi, V.T.; Bolognese, F.; Rolando, B.; Guglielmo, S.; Lazzarato, L.; Fruttero, R. Anti-Pseudomonas activity of 3-nitro-4-phenylfuroxan. Microbiology 2018, 164, 1557–1566. [Google Scholar] [CrossRef]
- Mallick, N.; Rai, L.C.; Mohn, F.H.; Soeder, C.J. Studies on nitric oxide (NO) formation by the green alga Scenedesmus obliquus and the diazotrophic cyanobacterium Anabaena doliolum. Chemosphere 1999, 39, 1601–1610. [Google Scholar] [CrossRef]
- Berger, A.; Boscari, A.; Frendo, P.; Brouquisse, R. Nitric oxide signaling, metabolism and toxicity in nitrogen-fixing symbiosis. J. Exp. Bot. 2019, 70, 4505–4520. [Google Scholar] [CrossRef]
- Hérouart, D.; Baudouin, E.; Frendo, P.; Harrison, J.; Santos, R.; Jamet, A.; Van de Sype, G.; Touati, D.; Puppo, A. Reactive oxygen species, nitric oxide and glutathione: A key role in the establishment of the legume–Rhizobium symbiosis? Plant Physiol. Biochem. 2002, 40, 619–624. [Google Scholar] [CrossRef]
- Griess, P.J. Bemerkungen zu der abhandlung der H.H. Weselsky und Benedikt “Ueber einige azoverbindungen”. Berichte Dtsch. Chem. Ges. 1879, 12, 426–428. [Google Scholar] [CrossRef]
- Amenta, M.; Molina-Favero, C.; Creus, C.M.; Lamattina, L. Chapter 9: Nitric oxide in Azospirillum and Related Bacteria: Production and Effects. In Handbook for Azospirillum; Cassán, F.D., Okon, Y., Creus, C.M., Eds.; Springer: Cham, Switzerland, 2015; pp. 155–180. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1996, 72, 248–254. [Google Scholar] [CrossRef]
- Shimoda, Y.; Nagata, M.; Suzuki, A.; Abe, M.; Sato, S.; Kato, T.; Tabata, S.; Higashi, S.; Uchiumi, T. Symbiotic rhizobium and nitric oxide induce gene expression of non-symbiotic hemoglobin in Lotus japonicus. Plant Cell Physiol. 2005, 46, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Nagata, M.; Murakami, E.; Shimoda, Y.; Shimoda-Sasakura, F.; Kucho, K.; Suzuki, A.; Abe, M.; Higashi, S.; Uchiumi, T. Expression of a class 1 hemoglobin gene and production of nitric oxide in response to symbiotic and pathogenic bacteria in Lotus japonicas. Mol. Plant Microbe Interact. 2008, 21, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, E.; Pieuchot, L.; Engler, G.; Pauly, N.; Puppo, A. Nitric oxide is formed in Medicago truncatula-Sinorhizobium meliloti functional nodules. Mol. Plant-Microbe Interact. 2006, 19, 970–975. [Google Scholar] [CrossRef]
- Del Giudice, J.; Cam, Y.; Damiani, I.; Fung-Chat, F.; Meilhoc, E.; Bruand, C.; Brouquisse, R.; Puppo, A.; Boscari, A. Nitric oxide is required for an optimal establishment of the Medicago truncatula–Sinorhizobium meliloti symbiosis. New Phytol. 2001, 191, 405–417. [Google Scholar] [CrossRef]
- Richardson, D.J.; Berks, B.C.; Russell, D.A.; Spiro, S.; Taylor, C.J. Functional, biochemical and genetic diversity of prokaryotic nitrate reductases. Cell. Mol. Life Sci. 2001, 58, 165–178. [Google Scholar] [CrossRef]
- Ji, X.B.; Hollocher, T.C. Mechanism for nitrosation of 2, 3-diaminonaphthalene by Escherichia coli: Enzymatic production of NO followed by O2-dependent chemical nitrosation. Appl. Environ. Microbiol. 1988, 54, 1791–1794. [Google Scholar] [CrossRef]
- Ji, X.B.; Hollocher, T.C. Reduction of nitrite to nitric oxide by enteric bacteria. Biochem. Biophys. Res. Commun. 1988, 157, 106–108. [Google Scholar] [CrossRef]
- Jetten, M.S.; Logemann, S.; Muyzer, G.; Robertson, L.A.; Vries, S.; van Loosdrecht, M.C.; Kuenen, J.G. Novel principles in the microbial conversion of nitrogen compounds. Antonie Leeuwenhoek 1997, 71, 75–93. [Google Scholar] [CrossRef]
- Jang, J.; Sakai, Y.; Senoo, K.; Ishii, S. Potentially mobile denitrification genes identified in Azospirillum sp. strain TSH58. Appl. Environ. Microbiol. 2019, 85, e02474-18. [Google Scholar] [CrossRef]
- Petrova, L.P.; Varshalomidze, O.E.; Shelud’ko, A.V.; Katsy, E.I. Localization of denitrification genes in plasmid DNA of bacteria Azospirillum brasilense. Russ. J. Genet. 2010, 46, 801–807. [Google Scholar] [CrossRef]
- Crane, B.R.; Sudhamsu, J.; Patel, B.A. Bacterial nitric oxide synthases. Annu. Rev. Biochem. 2010, 79, 445–470. [Google Scholar] [CrossRef] [PubMed]
- Arruebarrena Di Palma, A.; Pereyra, C.M.; Ramirez, L.M.; Xiqui Vazquez, M.L.; Baca, B.E.; Pereyra, M.A.; Lamattina, L.; Creus, M.C. Denitrification-derived nitric oxide modulates biofilm formation in Azospirillum brasilense. FEMS Microbiol. Lett. 2013, 338, 77–85. [Google Scholar] [CrossRef]
- Rondina, A.B.L.; Sanzovo, A.W.S.; Guimarães, G.S.; Wendling, J.R.; Nogueira, M.A.; Hungria, M. Changes in root morphological traits in soybean co-inoculated with Bradyrhizobium spp. and Azospirillum brasilense or treated with A. brasilense exudates. Biol. Fertil. Soils 2020, 56, 537–549. [Google Scholar] [CrossRef]
- Vacheron, J.; Desbrosses, G.; Bouffaud, M.L.; Touraine, B.; Moënne-Loccoz, Y.; Muller, D.; Legendre, L.; Wisniewski-Dyé, F.; Prigent-Combaret, C. Plant growth-promoting rhizobacteria and root system functioning. Front. Plant Sci. 2013, 4, 356. [Google Scholar] [CrossRef]
- Pagnussat, G.C.; Lanteri, M.L.; Lamattina, L. Nitric oxide and cyclic GMP are messengers in the indole acetic acid-induced adventitious rooting process. Plant Physiol. 2003, 132, 1241–1248. [Google Scholar] [CrossRef]
- Pham, T.M.; Bui, X.D.; Trang, L.V.K.; Le, T.; Nguyen, M.L.; Trinh, D.M.; Phuong, T.D.; Khoo, K.S.; Chew, K.W.; Show, P.L. Isolation of indole-3-acetic acid-producing Azospirillum brasilense from Vietnamese wet rice: Co-immobilization of isolate and microalgae as a sustainable biorefinery. J. Biotechnol. 2022, 349, 12–20. [Google Scholar] [CrossRef]
- Molina, R.; Rivera, D.; Mora, V.; López, G.; Rosas, S.; Spaepen, S.; Vanderleyden, J.; Cassán, F. Regulation of IAA biosynthesis in Azospirillum brasilense under environmental stress conditions. Curr. Microbiol. 2018, 75, 1408–1418. [Google Scholar] [CrossRef]
- Rao, M.; Smith, B.C.; Marletta, M.A. Nitric oxide mediates biofilm formation and symbiosis in Silicibacter sp. strain TrichCH4B. mBio 2015, 6, e00206-15. [Google Scholar] [CrossRef]
- Koul, V.; Adholeya, A.; Kochar, M. Sphere of influence of indole acetic acid and nitric oxide in bacteria. J. Basic Microbiol. 2015, 55, 543–553. [Google Scholar] [CrossRef]
- Puppo, A.; Pauly, N.; Boscari, A.; Mandon, K.; Brouquisse, R. Hydrogen peroxide and nitric oxide: Key regulators of the Legume-Rhizobium and mycorrhizal symbioses. Antioxid. Redox Signal. 2013, 18, 2202–2219. [Google Scholar] [CrossRef]
- Pedrosa, F.O.; Yates, M.G. Regulation of nitrogen fixation (nif) genes of Azospirillum brasilense by nifA and ntrC (glnG) type genes. FEMS Microbiol. Lett. 1984, 55, 95–101. [Google Scholar] [CrossRef]
- Kloos, K.; Mergel, A.; Rösch, C.; Bothe, H. Denitrification within the genus Azospirillum and other associative bacteria. Aust. J. Plant Physiol. 2001, 28, 991–998. [Google Scholar] [CrossRef]
- Möller, M.N.; Rios, N.; Trujillo, M.; Radi, R.; Denicola, A.; Alvarez, B. Detection and quantification of nitric oxide–derived oxidants in biological systems. J. Biol. Chem. 2019, 294, 14776–14802. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.L.; Adolfsen, K.J.; Brynildsen, M.P. Deciphering nitric oxide stress in bacteria with quantitative modeling. Curr. Opin. Microbiol. 2014, 19, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Yarullina, D.R.; Il’inskaia, O.N.; Aganov, A.V.; Silkin, N.I.; Zverev, D.G. Alternative pathways of nitric oxide formation in lactobacilli: Evidence for nitric oxide synthase activity by EPR. Microbiology 2006, 75, 634–638. [Google Scholar] [CrossRef]
- Miles, A.M.; Chen, Y.; Owens, M.W.; Grisham, M.B. Fluorometric determination of nitric oxide. Methods 1995, 7, 40–47. [Google Scholar] [CrossRef]
- Brew, F.; Forsythe, S. The rapid nitrosation of 2, 3-diaminonaphthalene by gastric isolates of Neisseria subflava. Lett. Appl. Microbiol. 1990, 10, 39–42. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, M.S.; Santos, K.F.D.N.; de Paula, R.M.; Vitorino, L.C.; Bessa, L.A.; Greer, A.; Di Mascio, P.; de Souza, J.C.P.; Martin-Didonet, C.C.G. Nitric Oxide Detection Using a Chemical Trap Method for Applications in Bacterial Systems. Microorganisms 2023, 11, 2210. https://doi.org/10.3390/microorganisms11092210
Oliveira MS, Santos KFDN, de Paula RM, Vitorino LC, Bessa LA, Greer A, Di Mascio P, de Souza JCP, Martin-Didonet CCG. Nitric Oxide Detection Using a Chemical Trap Method for Applications in Bacterial Systems. Microorganisms. 2023; 11(9):2210. https://doi.org/10.3390/microorganisms11092210
Chicago/Turabian StyleOliveira, Marilene Silva, Karina F. D. N. Santos, Railane Monteiro de Paula, Luciana C. Vitorino, Layara A. Bessa, Alexander Greer, Paolo Di Mascio, João C. P. de Souza, and Claudia C. G. Martin-Didonet. 2023. "Nitric Oxide Detection Using a Chemical Trap Method for Applications in Bacterial Systems" Microorganisms 11, no. 9: 2210. https://doi.org/10.3390/microorganisms11092210