Invasive Disease Due to Neisseria meningitidis: Surveillance and Trends in Israel Prior to and during the COVID-19 Pandemic
Abstract
:1. Introduction
2. Methods
2.1. Epidemiological Methods
2.2. Laboratory Methods
2.3. Statistical Analysis
3. Results
3.1. Invasive Meningococcal Disease Incidence Trends
3.2. Invasive Meningococcal Disease Case Characteristics
3.3. Distribution of N. meningitidis Serogroups
3.4. N. meningitidis Serogroups Trends According to Age Groups
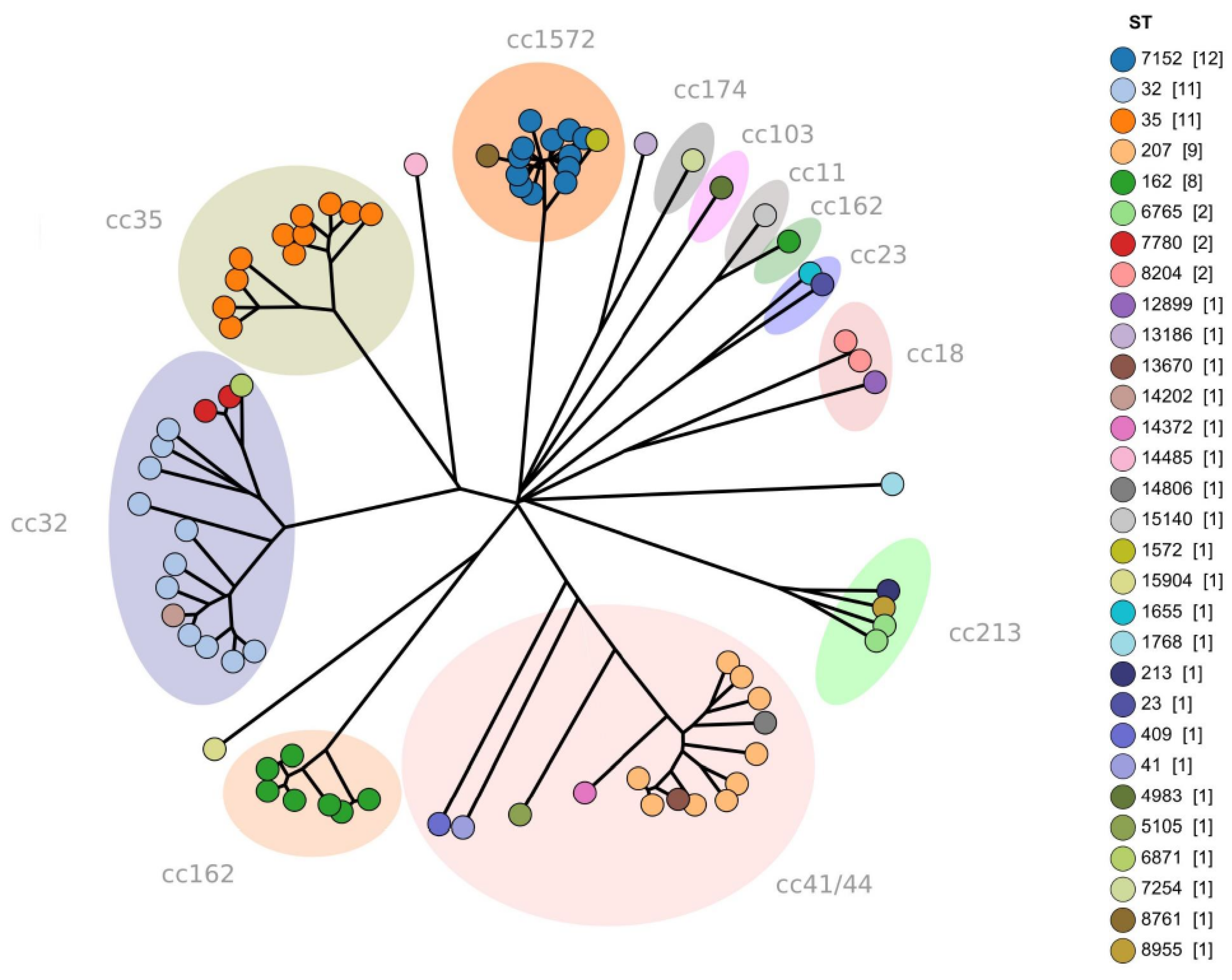
3.5. Core Genome Multi-Locus Sequence Typing (cgMLST) Analysis
3.6. Potential Strain Coverage Analysis
3.7. Trends in Clonal Complexes (CC)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fitzgerald, D.; Waterer, G.W. Invasive Pneumococcal and Meningococcal Disease. Infect. Dis. Clin. N. Am. 2019, 33, 1125–1141. [Google Scholar]
- Parikh, S.R.; Campbell, H.; Bettinger, J.A.; Harrison, L.H.; Marshall, H.S.; Martinon-Torres, F.; Safadi, M.A.; Shao, Z.; Zhu, B.; von Gottberg, A.; et al. The everchanging epidemiology of meningococcal disease worldwide and the potential for prevention through vaccination. J. Infect. 2020, 81, 483–498. [Google Scholar] [PubMed]
- Wang, B.; Santoreneos, R.; Giles, L.; Haji Ali Afzali, H.; Marshall, H. Case fatality rates of invasive meningococcal disease by serogroup and age: A systematic review and meta-analysis. Vaccine 2019, 37, 2768–2782. [Google Scholar] [PubMed]
- World Health Organization. Defeating Meningitis by 2030. Available online: https://www.who.int/initiatives/defeating-meningitis-by-2030 (accessed on 7 July 2023).
- Pinto, T.C.A.; Costa, N.S.; Oliveira, L.M.A. World Meningitis Day and the World Health Organization’s roadmap to defeat bacterial meningitis in the COVID-19 pandemic era. Int. J. Infect. Dis. 2021, 107, 219–220. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shimol, S.; Dagan, R.; Schonmann, Y.; Givon-Lavi, N.; Keller, N.; Block, C.; Kassis, I.; Ephros, M.; Greenberg, D. Dynamics of childhood invasive meningococcal disease in Israel during a 22-year period (1989–2010). Infection 2013, 41, 791–798. [Google Scholar]
- Salama, M.; Kopel, E.; Jaffe, J.; Amitai, Z.; Sheffer, R.; Rahmani, S.; Yuabov, I.; Dardik, L.; Valinsky, L. Surveillance of invasive meningococcal disease in the Tel Aviv District, Israel, 2007–2017. Vaccine 2019, 37, 6186–6191. [Google Scholar] [CrossRef]
- Stein-Zamir, C.; Shoob, H.; Abramson, N.; Block, C.; Keller, N.; Jaffe, J.; Valinsky, L. Invasive meningococcal disease epidemiology and characterization of Neisseria meningitidis serogroups, sequence types, and clones; implication for use of meningococcal vaccines. Hum. Vaccin. Immunother. 2019, 15, 242–248. [Google Scholar]
- Ministry of Health, Israel. Immunization Guidelines. Available online: https://www.health.gov.il/English/Topics/Vaccination/Pages/default.aspx (accessed on 7 July 2023).
- Brueggemann, A.B.; Jansen van Rensburg, M.J.; Shaw, D.; McCarthy, N.D.; Jolley, K.A.; Maiden, M.C.J.; van der Linden, M.P.G.; Amin-Chowdhury, Z.; Bennett, D.E.; Borrow, R.; et al. Changes in the incidence of invasive disease due to Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis during the COVID-19 pandemic in 26 countries and territories in the Invasive Respiratory Infection Surveillance Initiative: A prospective analysis of surveillance data. Lancet Digit. Health 2021, 3, e360–e370. [Google Scholar]
- Steens, A.; Knol, M.J.; Freudenburg-de Graaf, W.; de Melker, H.E.; van der Ende, A.; van Sorge, N.M. Pathogen- and Type-Specific Changes in Invasive Bacterial Disease Epidemiology during the First Year of the COVID-19 Pandemic in The Netherlands. Microorganisms 2022, 10, 972. [Google Scholar]
- Deghmane, A.E.; Taha, M.K. Changes in Invasive Neisseria meningitidis and Haemophilus influenzae Infections in France during the COVID-19 Pandemic. Microorganisms 2022, 10, 907. [Google Scholar]
- Baloche, A.; Dussart, C.; Bedouch, P.; Carrouel, F.; Mick, G. Epidemiology and Clinical Burden of Meningococcal Disease in France: Scoping Review. J. Clin. Med. 2023, 12, 849. [Google Scholar] [PubMed]
- Israel Central Bureau of Statistics. Characterization and Classification of Geographical Units by the Socio-Economic Level of the Population. 2015. Available online: https://www.cbs.gov.il/en/mediarelease/Pages/2018/Characterization-and-Classification-of-Geographical-Units-by-the-Socio-Economic-Level-of-the-Population-2015.aspx (accessed on 7 July 2023).
- Ministry of Health Israel, Weekly Epidemiological Reports. Available online: https://www.gov.il/he/Departments/DynamicCollectors/weekly-epidemiological-report?skip=0 (accessed on 7 July 2023).
- The Central Bureau of Statistics (CBS), Israel. Statistical Abstract of Israel. Available online: https://www.cbs.gov.il/en/Pages/search/yearly.aspx (accessed on 7 July 2023).
- Jolley, K.A.; Bray, J.E.; Maiden, M.C.J. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res. 2018, 3, 124. [Google Scholar] [PubMed]
- Nuttens, C.; Findlow, J.; Balmer, P.; Swerdlow, D.L.; Tin Tin Htar, M. Evolution of invasive meningococcal disease epidemiology in Europe, 2008 to 2017. Euro Surveill. 2022, 27, 2002075. [Google Scholar]
- Centers for Disease Control and Prevention. Meningococcal Disease Surveillance. Available online: https://www.cdc.gov/meningococcal/surveillance/ (accessed on 7 July 2023).
- Guedes, S.; Bricout, H.; Langevin, E.; Tong, S.; Bertrand-Gerentes, I. Epidemiology of invasive meningococcal disease and sequelae in the United Kingdom during the period 2008 to 2017—A secondary database analysis. BMC Public Health 2022, 22, 521. [Google Scholar] [CrossRef] [PubMed]
- Zografaki, I.; Detsis, M.; Del Amo, M.; Iantomasi, R.; Maia, A.; Montuori, E.A.; Mendez, C. Invasive Meningococcal Disease epidemiology and vaccination strategies in four Southern European countries: A review of the available data. Expert. Rev. Vaccines 2023, 22, 545–562. [Google Scholar]
- Whittaker, R.; Dias, J.G.; Ramliden, M.; Ködmön, C.; Economopoulou, A.; Beer, N.; Pastore Celentano, L.; ECDC Network Members for Invasive Meningococcal Disease. The epidemiology of invasive meningococcal disease in EU/EEA countries, 2004–2014. Vaccine 2017, 35, 2034–2041. [Google Scholar]
- Stein-Zamir, C.; Abramson, N.; Zentner, G.; Shoob, H.; Valinsky, L.; Block, C. Invasive meningococcal disease in children in Jerusalem. Epidemiol. Infect. 2008, 136, 782–789. [Google Scholar]
- Stein-Zamir, C.; Shoob, H.; Sokolov, I.; Kunbar, A.; Abramson, N.; Zimmerman, D. The clinical features and long-term sequelae of invasive meningococcal disease in children. Pediatr. Infect. Dis. J. 2014, 33, 777–779. [Google Scholar]
- GBD 2016 Meningitis Collaborators. Global, regional, and national burden of meningitis, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 1061–1082. [Google Scholar]
- GBD 2019 Antimicrobial Resistance Collaborators. Global mortality associated with 33 bacterial pathogens in 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 2221–2248. [Google Scholar]
- Wright, C.; Blake, N.; Glennie, L.; Smith, V.; Bender, R.; Kyu, H.; Wunrow, H.Y.; Liu, L.; Yeung, D.; Knoll, M.D.; et al. The Global Burden of Meningitis in Children: Challenges with Interpreting Global Health Estimates. Microorganisms 2021, 9, 377. [Google Scholar] [CrossRef] [PubMed]
- Green, M.S.; Schwartz, N.; Peer, V. A meta-analytic evaluation of sex differences in meningococcal disease incidence rates in 10 countries. Epidemiol. Infect. 2020, 148, e246. [Google Scholar] [CrossRef] [PubMed]
- Brady, R.C. Meningococcal Infections in Children and Adolescents: Update and Prevention. Adv. Pediatr. 2020, 67, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, Y.L.; Stephens, D.S. A Narrative Review of the W, X, Y, E, and NG of Meningococcal Disease: Emerging Capsular Groups, Pathotypes, and Global Control. Microorganisms 2021, 9, 519. [Google Scholar] [PubMed]
- Sofer-Sali, N.; Roif-Kaminsky, D.; Motro, Y.; Khalfin, B.; Avramovich, E.; Galor, I.; Shlaifer, A.; Sommer, A.; Rutenberg, R.; Sachter, Y.; et al. Prevalence and Characteristics of Carriage of Neisseria meningitidis Among Young Israeli Adults. Open Forum Infect. Dis. 2022, 9, ofac482. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, R.; Bai, X.; Borrow, R.; Caugant, D.A.; Carlos, J.; Ceyhan, M.; Christensen, H.; Climent, Y.; De Wals, P.; Dinleyici, E.C.; et al. The Global Meningococcal Initiative meeting on prevention of meningococcal disease worldwide: Epidemiology, surveillance, hypervirulent strains, antibiotic resistance and high-risk populations. Expert. Rev. Vaccines 2019, 18, 15–30. [Google Scholar]
- Alderson, M.R.; Arkwright, P.D.; Bai, X.; Black, S.; Borrow, R.; Caugant, D.A.; Dinleyici, E.C.; Harrison, L.H.; Lucidarme, J.; McNamara, L.A.; et al. Surveillance and control of meningococcal disease in the COVID-19 era: A Global Meningococcal Initiative review. J. Infect. 2022, 84, 289–296. [Google Scholar]
- Pizza, M.; Bekkat-Berkani, R.; Rappuoli, R. Vaccines against Meningococcal Diseases. Microorganisms 2020, 8, 1521. [Google Scholar]
- Villena, R.; Safadi, M.A.P.; Valenzuela, M.T.; Torres, J.P.; Finn, A.; O’Ryan, M. Global epidemiology of serogroup B meningococcal disease and opportunities for prevention with novel recombinant protein vaccines. Hum. Vaccin. Immunother. 2018, 14, 1042–1057. [Google Scholar] [CrossRef]
- Marten, O.; Koerber, F.; Bloom, D.; Bullinger, M.; Buysse, C.; Christensen, H.; De Wals, P.; Dohna-Schwake, C.; Henneke, P.; Kirchner, M.; et al. DELPHI study on aspects of study design to overcome knowledge gaps on the burden of disease caused by serogroup B invasive meningococcal disease. Health Qual. Life Outcomes 2019, 17, 87. [Google Scholar]
- Gofrit, S.G.; Pikkel, Y.Y.; Levine, H.; Fraifeld, S.; Kahana Merhavi, S.; Friedensohn, L.; Eliahou, R.; Ben-Hur, T.; Honig, A. Characterization of Meningitis and Meningoencephalitis in the Israeli Defense Forces From 2004 to 2015: A Population-Based Study. Front. Neurol. 2022, 13, 887677. [Google Scholar] [CrossRef]
- Mimouni, D.; Bar-Zeev, Y.; Huerta, M.; Balicer, R.D.; Grotto, I.; Ankol, O. Preventive effect of meningococcal vaccination in Israeli military recruits. Am. J. Infect. Control. 2010, 38, 56–58. [Google Scholar] [CrossRef]
- Stein-Zamir, C.; Rishpon, S. The National Immunization Technical Advisory Group in Israel. Isr. J. Health Policy Res. 2021, 10, 7. [Google Scholar] [PubMed]
- Ginsberg, G.M.; Block, C.; Stein-Zamir, C. Cost-utility analysis of a nationwide vaccination programme against serogroup B meningococcal disease in Israel. Int. J. Public. Health 2016, 61, 683–692. [Google Scholar]
- Ministry of Health, Israel. The Expansion of the Health Services Basket for 2023. Available online: https://www.gov.il/he/departments/publications/reports/hbs2023 (accessed on 7 July 2023).
- Ladhani, S.N.; Andrews, N.; Parikh, S.R.; Campbell, H.; White, J.; Edelstein, M.; Bai, X.; Lucidarme, J.; Borrow, R.; Ramsay, M.E. Vaccination of Infants with Meningococcal Group B Vaccine (4CMenB) in England. N. Engl. J. Med. 2020, 382, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Castilla, J.; García Cenoz, M.; Abad, R.; Sánchez-Cambronero, L.; Lorusso, N.; Izquierdo, C.; Cañellas Llabrés, S.; Roig, J.; Malvar, A.; González Carril, F.; et al. Effectiveness of a Meningococcal Group B Vaccine (4CMenB) in Children. N. Engl. J. Med. 2023, 388, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, G.M.; Biffi, G.; Castellazzi, M.L.; Tagliabue, C.; Pinzani, R.; Bosis, S.; Marchisio, P.G. Meningococcal Disease in Pediatric Age: A Focus on Epidemiology and Prevention. Int. J. Environ. Res. Public. Health 2022, 19, 4035. [Google Scholar] [CrossRef] [PubMed]
- Bekkat-Berkani, R.; Fragapane, E.; Preiss, S.; Rappuoli, R.; Sohn, W.Y.; Soumahoro, L.; Vadivelu, K. Public health perspective of a pentavalent meningococcal vaccine combining antigens of MenACWY-CRM and 4CMenB. J. Infect. 2022, 85, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.K.; Bekkat-Berkani, R.; Abitbol, V. Changing patterns of invasive meningococcal disease and future immunization strategies. Hum. Vaccine Immunother. 2023, 19, 2186111. [Google Scholar] [CrossRef]



| Variable | IMD Cases (n = 521) |
|---|---|
| Age (mean ± SD), years | 14 ± 21.8 |
| Age median, years (IQR) | 3 (IQR = 0.6–18.2 years) |
| Age group, years | |
| <1 | 174 (33.4%) |
| 1–4 | 133 (25.5%) |
| 5–9 | 50 (9.6%) |
| 10–17 | 34 (6.5%) |
| 18–44 | 67 (12.9%) |
| ≥45 | 63 (12.1%) |
| Gender | |
| male | 313 (60.1%) |
| female | 208 (39.9%) |
| Socio-economic status | |
| Rank 1–3 | 288 (55.3%) |
| Rank 4–6 | 117 (22.4%) |
| Rank 7–10 | 116 (22.3%) |
| Ethnicity | |
| Jews and others | 406 (77.9%) |
| Arabs | 115 (22.1%) |
| Seasonality | |
| December-March | 238 (45.7%) |
| April–July | 147 (28.2%) |
| August–November | 136 (26.1%) |
| Mortality | 47 (9%) |
| Source of bacterial isolation | |
| Blood culture | 331 (63.5%) |
| CSF | 175 (33.6%) |
| other | 15 (2.9%) |
| Serogroup | |
| B | 326 (62.6%) |
| Y | 80 (15.4%) |
| W135 | 41 (7.9%) |
| C | 29 (5.6%) |
| A | 7 (1.3%) |
| Not allocated | 38 (7.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stein-Zamir, C.; Shoob, H.; Abramson, N.; Valinsky, L.; Jaffe, J.; Maimoun, D.; Amit, S.; Davidovich-Cohen, M. Invasive Disease Due to Neisseria meningitidis: Surveillance and Trends in Israel Prior to and during the COVID-19 Pandemic. Microorganisms 2023, 11, 2212. https://doi.org/10.3390/microorganisms11092212
Stein-Zamir C, Shoob H, Abramson N, Valinsky L, Jaffe J, Maimoun D, Amit S, Davidovich-Cohen M. Invasive Disease Due to Neisseria meningitidis: Surveillance and Trends in Israel Prior to and during the COVID-19 Pandemic. Microorganisms. 2023; 11(9):2212. https://doi.org/10.3390/microorganisms11092212
Chicago/Turabian StyleStein-Zamir, Chen, Hanna Shoob, Nitza Abramson, Lea Valinsky, Joseph Jaffe, David Maimoun, Sharon Amit, and Maya Davidovich-Cohen. 2023. "Invasive Disease Due to Neisseria meningitidis: Surveillance and Trends in Israel Prior to and during the COVID-19 Pandemic" Microorganisms 11, no. 9: 2212. https://doi.org/10.3390/microorganisms11092212
APA StyleStein-Zamir, C., Shoob, H., Abramson, N., Valinsky, L., Jaffe, J., Maimoun, D., Amit, S., & Davidovich-Cohen, M. (2023). Invasive Disease Due to Neisseria meningitidis: Surveillance and Trends in Israel Prior to and during the COVID-19 Pandemic. Microorganisms, 11(9), 2212. https://doi.org/10.3390/microorganisms11092212





