Effects of Music and White Noise Exposure on the Gut Microbiota, Oxidative Stress, and Immune-Related Gene Expression of Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model and Experimental Design
2.2. Measurement of Serum Oxidative Stress Indicators
2.3. Quantitative RT-PCR
2.4. 16s rRNA Gene Extraction and Sequencing
2.5. Bioinformatics and Statistical Analysis
3. Results
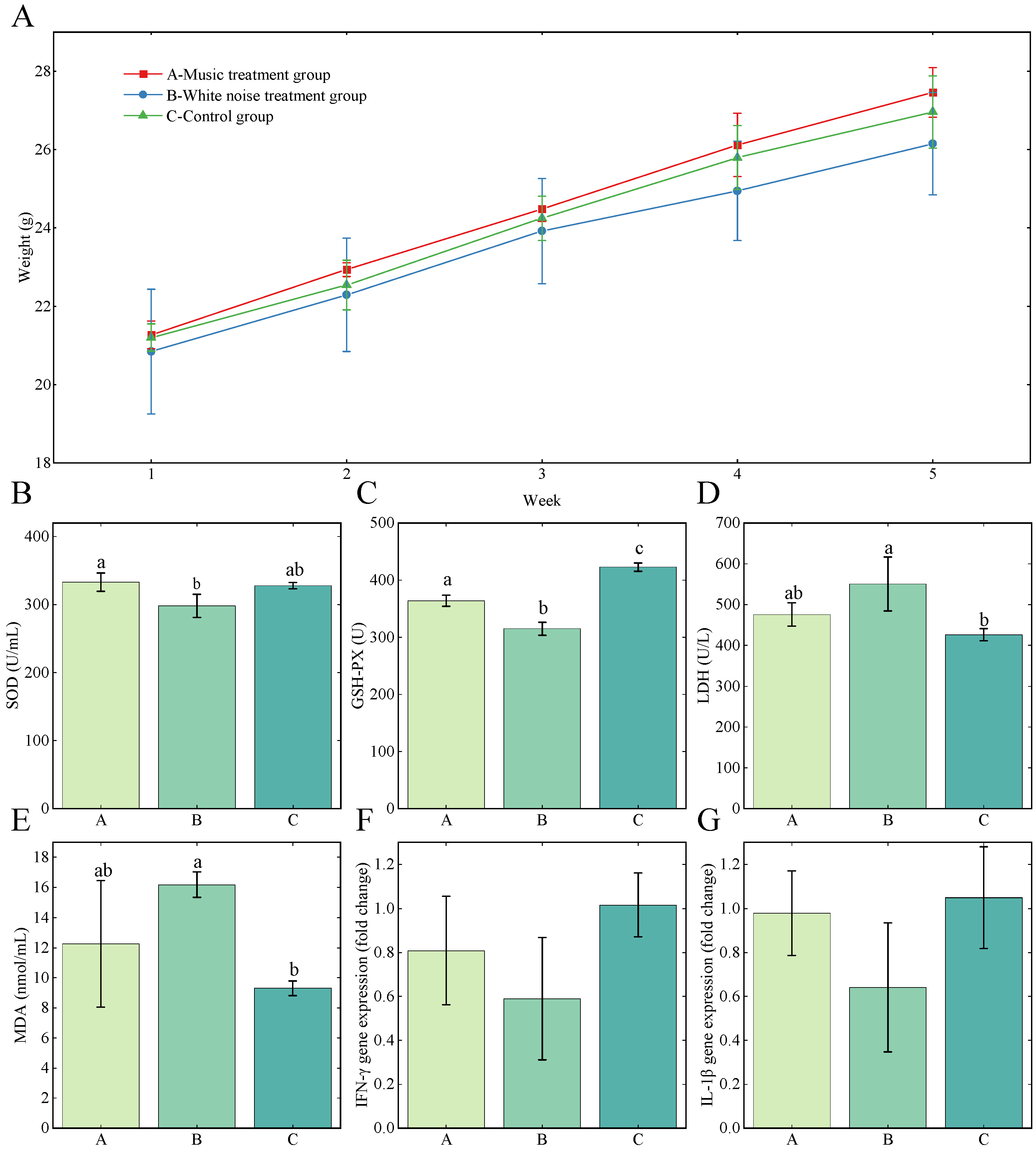
3.1. Animal Weight Change and Behavior
3.2. Serum Oxidation Status Indicators Levels
3.3. Relative Expression Level of IFN-γ and IL-1β
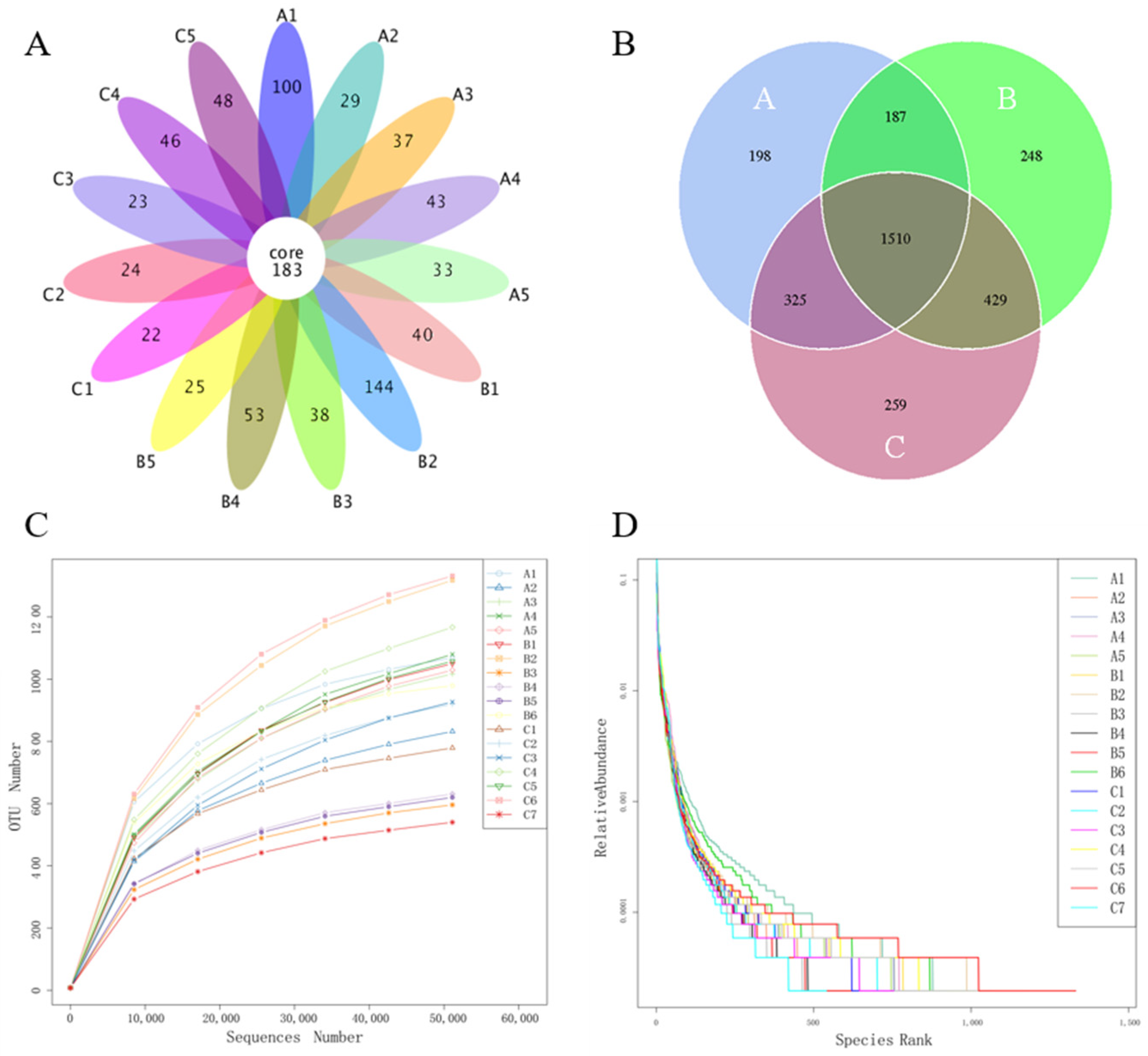
3.4. 16s rRNA Gene Sequencing Data
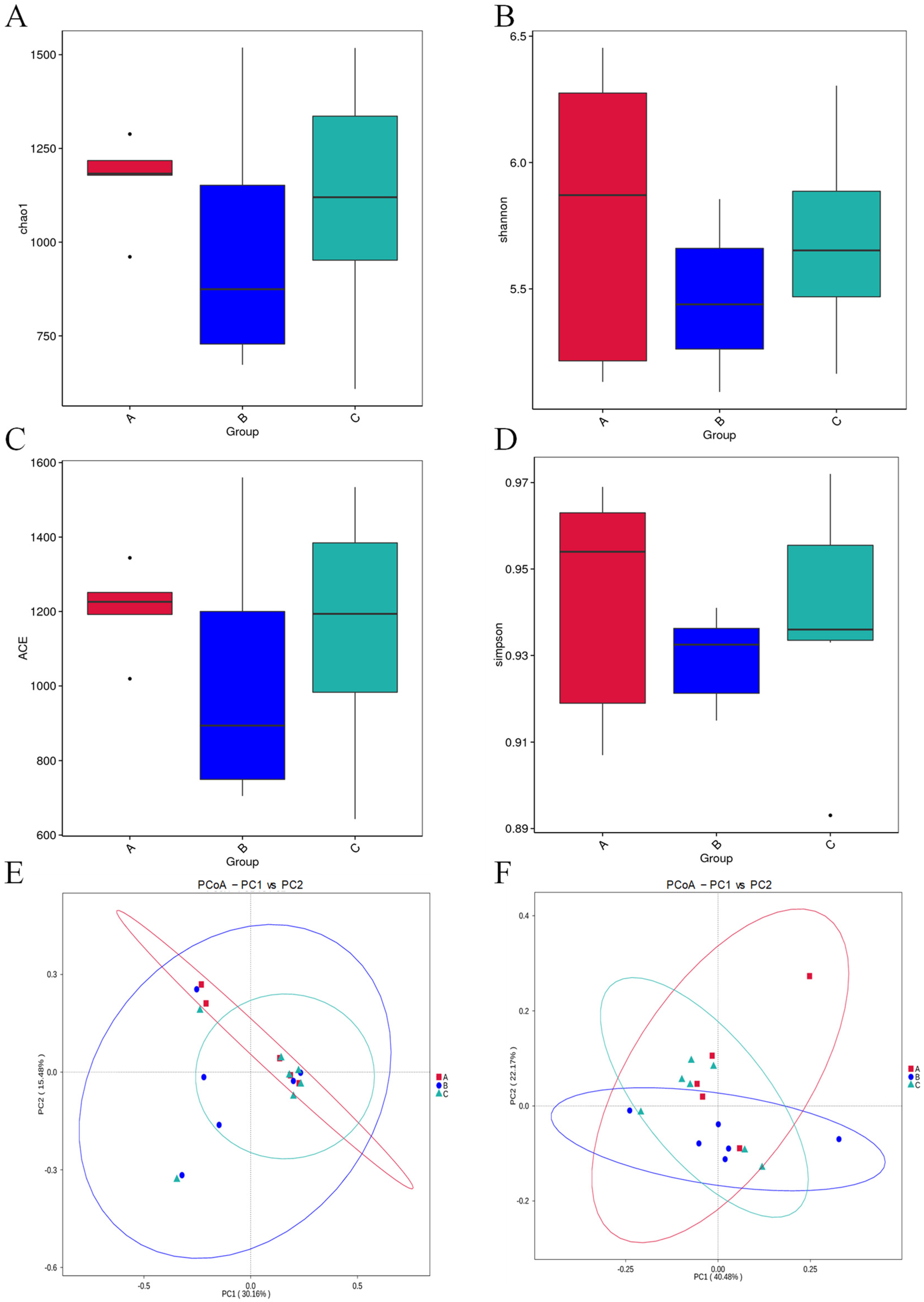
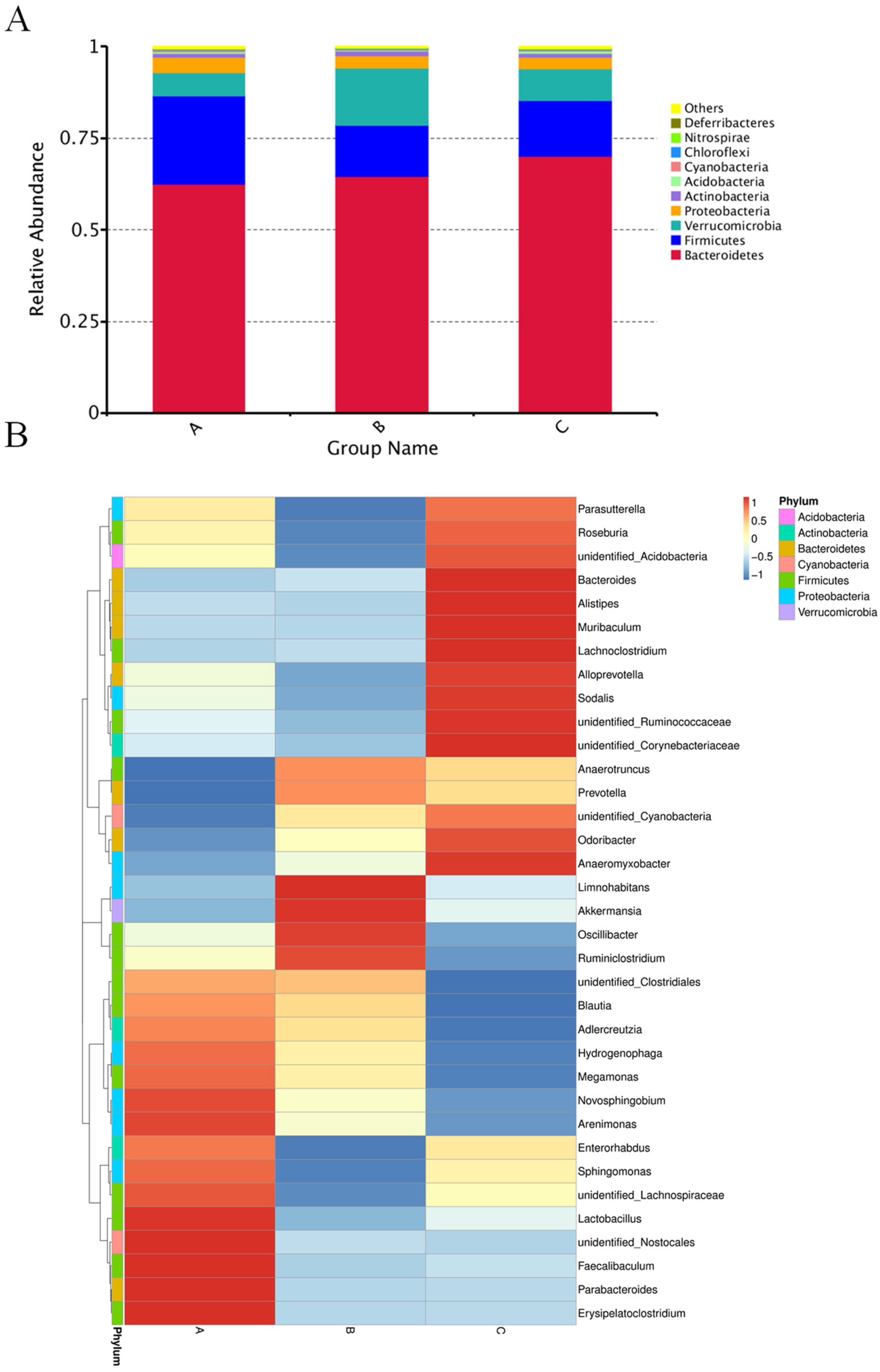
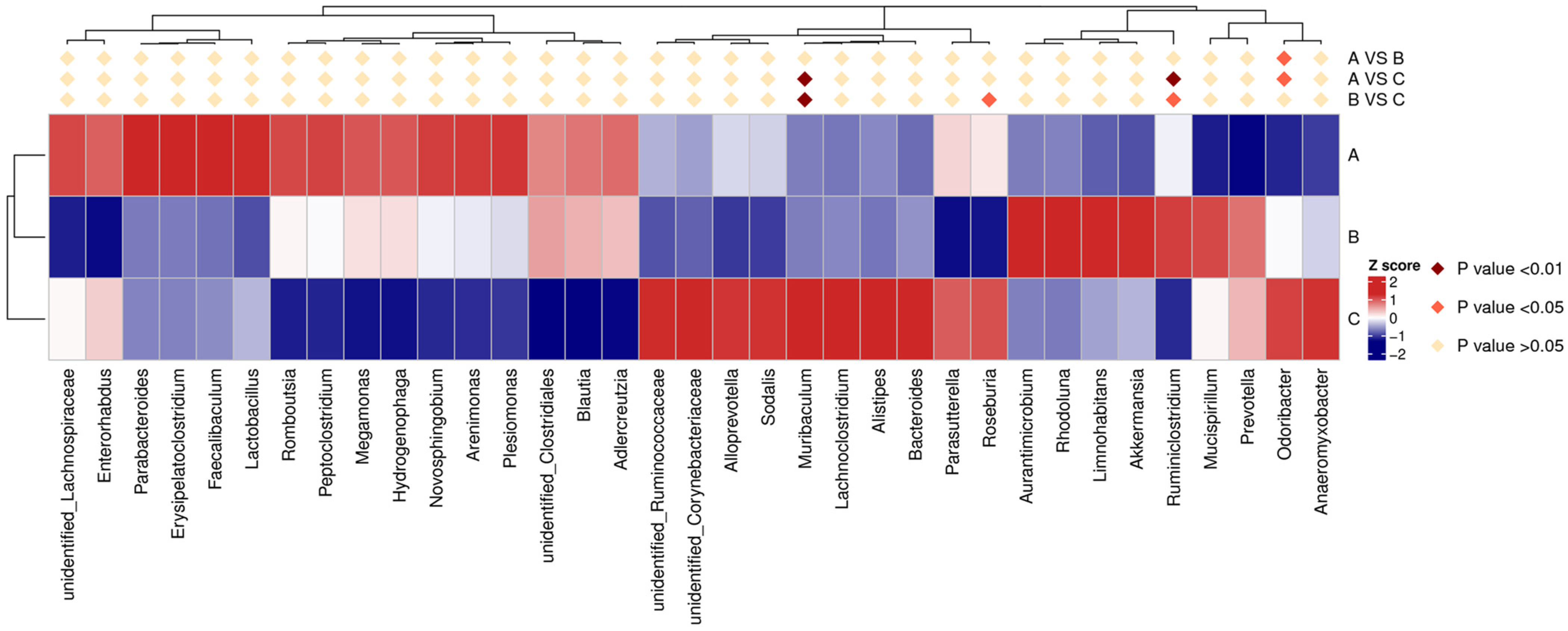
3.5. Microbial Diversity and Distribution
3.6. Comparison of the Functional Pathway of the Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reybrouck, M. Music as Environment: An Ecological and Biosemiotic Approach. Behav. Sci. 2014, 5, 1–26. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Liu, H.; Li, J.; Han, Q.; Wang, C.; Zeng, X.; Li, Y.; Ji, W.; Zhang, R.; et al. Effects of Music Stimulus on Behavior Response, Cortisol Level, and Horizontal Immunity of Growing Pigs. J. Anim. Sci. 2021, 99, skab043. [Google Scholar] [CrossRef]
- Lin, L.-C.; Juan, C.-T.; Chang, H.-W.; Chiang, C.-T.; Wei, R.-C.; Lee, M.-W.; Mok, H.-K.; Yang, R.-C. Mozart K.448 Attenuates Spontaneous Absence Seizure and Related High-Voltage Rhythmic Spike Discharges in Long Evans Rats. Epilepsy Res. 2013, 104, 234–240. [Google Scholar] [CrossRef]
- Rauscher, F.H.; Shaw, G.L.; Ky, C.N. Music and Spatial Task Performance. Nature 1993, 365, 611. [Google Scholar] [CrossRef]
- Lin, L.-C.; Lee, W.-T.; Wang, C.-H.; Chen, H.-L.; Wu, H.-C.; Tsai, C.-L.; Wei, R.-C.; Mok, H.-K.; Weng, C.-F.; Lee, M.-W.; et al. Mozart K.448 Acts as a Potential Add-on Therapy in Children with Refractory Epilepsy. Epilepsy Behav. 2011, 20, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Barcellos, H.H.A.; Koakoski, G.; Chaulet, F.; Kirsten, K.S.; Kreutz, L.C.; Kalueff, A.V.; Barcellos, L.J.G. The Effects of Auditory Enrichment on Zebrafish Behavior and Physiology. PeerJ 2018, 6, e5162. [Google Scholar] [CrossRef]
- Papoutsoglou, S.E.; Karakatsouli, N.; Skouradakis, C.; Papoutsoglou, E.S.; Batzina, A.; Leondaritis, G.; Sakellaridis, N. Effect of Musical Stimuli and White Noise on Rainbow Trout (Oncorhynchus Mykiss) Growth and Physiology in Recirculating Water Conditions. Aquacult. Eng. 2013, 55, 16–22. [Google Scholar] [CrossRef]
- Uetake, K.; Hurnik, J.F.; Johnson, L. Effect of Music on Voluntary Approach of Dairy Cows to an Automatic Milking System. Appl. Anim. Behav. Sci. 1997, 53, 175–182. [Google Scholar] [CrossRef]
- Ciborowska, P.; Michalczuk, M.; Bień, D. The Effect of Music on Livestock: Cattle, Poultry and Pigs. Animals 2021, 11, 3572. [Google Scholar] [CrossRef]
- Backus, B.L.; Sutherland, M.A.; Brooks, T.A. Relationship between Environmental Enrichment and the Response to Novelty in Laboratory-Housed Pigs. J. Am. Assoc. Lab. Anim. Sci. 2017, 56, 735–741. [Google Scholar]
- Zikic, D.; Uscebrka, G.; Gledic, D.; Lazarevic, M.; Stojanovic, S.; Kanacki, Z. The Influence of Long Term Sound Stress on Histological Structure of Broiler’s Adrenal Glands. Biotechnol. Anim. Husb. 2011, 27, 1613–1619. [Google Scholar] [CrossRef]
- Pope, D. Decibel Levels and Noise Generators on Four Medical/Surgical Nursing Units. J. Clin. Nurs. 2010, 19, 2463–2470. [Google Scholar] [CrossRef]
- Weeks, C.A.; Brown, S.N.; Warriss, P.D.; Lane, S.; Heasman, L.; Benson, T. Noise Levels in Lairages for Cattle, Sheep and Pigs in Abattoirs in England and Wales. Vet. Rec. 2009, 165, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Talling, J.C.; Lines, J.A.; Wathes, C.M.; Waran, N.K. The Acoustic Environment of the Domestic Pig. J. Agric. Eng. Res. 1998, 71, 1–12. [Google Scholar] [CrossRef]
- Gržinić, G.; Piotrowicz-Cieślak, A.; Klimkowicz-Pawlas, A.; Górny, R.L.; Ławniczek-Wałczyk, A.; Piechowicz, L.; Olkowska, E.; Potrykus, M.; Tankiewicz, M.; Krupka, M.; et al. Intensive Poultry Farming: A Review of the Impact on the Environment and Human Health. Sci. Total Environ. 2023, 858, 160014. [Google Scholar] [CrossRef]
- Morgan, K.N.; Tromborg, C.T. Sources of Stress in Captivity. Appl. Anim. Behav. Sci. 2007, 102, 262–302. [Google Scholar] [CrossRef]
- Broucek, J.; Kovalcikova, M.; Kovalcik, K. The Effect of Noise on the Biochemical Characteristics of Blood in Dairy Cows. Zivocisna Vyrob. 2021, 28, 261–267. [Google Scholar]
- Williams, R.; Erbe, C.; Duncan, A.; Nielsen, K.; Washburn, T.; Smith, C. Noise from Deep-Sea Mining May Span Vast Ocean Areas. Science 2022, 377, 157–158. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Zhao, X.; Liu, G. Noise in the Sea and Its Impacts on Marine Organisms. Int. J. Environ. Res. Public Health 2015, 12, 12304–12323. [Google Scholar] [CrossRef]
- Cao, L.; Chen, Y.; Dong, S.; Hanson, A.; Huang, B.; Leadbitter, D.; Little, D.C.; Pikitch, E.K.; Qiu, Y.; Sadovy De Mitcheson, Y.; et al. Opportunity for Marine Fisheries Reform in China. Proc. Natl. Acad. Sci. USA 2017, 114, 435–442. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Eddaikra, A.; Eddaikra, N. Endogenous Enzymatic Antioxidant Defense and Pathologies. In Antioxidants—Benefits, Sources, Mechanisms of Action; Waisundara, V., Ed.; IntechOpen: London, UK, 2021; ISBN 978-1-83968-864-5. [Google Scholar]
- Jovanović, P.; Žorić, L.; Stefanović, I.; Džunić, B.; Djordjević-Jocić, J.; Radenković, M.; Jovanovic, M. Lactate Dehydrogenase and Oxidative Stress Activity in Primary Open-Angle Glaucoma Aqueous Humour. Bosn. J. Basic Med. 2010, 10, 83–88. [Google Scholar] [CrossRef]
- Aliahmat, N.S.; Noor, M.R.M.; Yusof, W.J.W.; Makpol, S.; Ngah, W.Z.W.; Yusof, Y.A.M. Antioxidant Enzyme Activity and Malondialdehyde Levels Can Be Modulated by Piper Betle, Tocotrienol Rich Fraction and Chlorella Vulgaris in Aging C57BL/6 Mice. Clinics 2012, 67, 1447–1454. [Google Scholar] [CrossRef]
- Daiber, A.; Kröller-Schön, S.; Oelze, M.; Hahad, O.; Li, H.; Schulz, R.; Steven, S.; Münzel, T. Oxidative Stress and Inflammation Contribute to Traffic Noise-Induced Vascular and Cerebral Dysfunction via Uncoupling of Nitric Oxide Synthases. Redox Biol. 2020, 34, 101506. [Google Scholar] [CrossRef]
- Manikandan, S.; Padma, M.K.; Srikumar, R.; Jeya Parthasarathy, N.; Muthuvel, A.; Devi, R.S. Effects of Chronic Noise Stress on Spatial Memory of Rats in Relation to Neuronal Dendritic Alteration and Free Radical-Imbalance in Hippocampus and Medial Prefrontal Cortex. Neurosci. Lett. 2006, 399, 17–22. [Google Scholar] [CrossRef]
- Demirel, R.; Mollaoğlu, H.; Yeşilyurt, H.; Üçok, K.; Ayçiçek, A.; Akkaya, M.; Genç, A.; Uygur, R.; Doğan, M. Noise Induces Oxidative Stress in Rat. Electron. J. Gen. Med. 2009, 6, 20–24. [Google Scholar] [CrossRef]
- Hooper, L.V.; Gordon, J.I. Commensal Host-Bacterial Relationships in the Gut. Science 2001, 292, 1115–1118. [Google Scholar] [CrossRef]
- Cryan, J.F.; Dinan, T.G. Mind-Altering Microorganisms: The Impact of the Gut Microbiota on Brain and Behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Cui, B.; Su, D.; Li, W.; She, X.; Zhang, M.; Wang, R.; Zhai, Q. Effects of Chronic Noise Exposure on the Microbiome-Gut-Brain Axis in Senescence-Accelerated Prone Mice: Implications for Alzheimer’s Disease. J. Neuroinflamm. 2018, 15, 190. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Gai, Z.; She, X.; Wang, R.; Xi, Z. Effects of Chronic Noise on Glucose Metabolism and Gut Microbiota–Host Inflammatory Homeostasis in Rats. Sci. Rep. 2016, 6, 36693. [Google Scholar] [CrossRef] [PubMed]
- Schmedes, A.; Hølmer, G. A New Thiobarbituric Acid (TBA) Method for Determining Free Malondialdehyde (MDA) and Hydroperoxides Selectively as a Measure of Lipid Peroxidation. J. Am. Oil Chem. Soc. 1989, 66, 813–817. [Google Scholar] [CrossRef]
- Peskin, A.V.; Winterbourn, C.C. Assay of Superoxide Dismutase Activity in a Plate Assay Using WST-1. Free Radic. Biol. Med. 2017, 103, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Megraw, R.E. Reaction of Reduced Nicotinamide Adenine Dinucleotide with 2,4-Dinitrophenylhydrazine in Serum Lactate Dehydrogenase Assays. Am. J. Clin. Pathol. 1971, 56, 225–226. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for Quantitative Determination of Glutathione and Glutathione Disulfide Levels Using Enzymatic Recycling Method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Pruesse, E.; Quast, C.; Knittel, K.; Fuchs, B.M.; Ludwig, W.; Peplies, J.; Glockner, F.O. SILVA: A Comprehensive Online Resource for Quality Checked and Aligned Ribosomal RNA Sequence Data Compatible with ARB. Nucleic Acids Res. 2007, 35, 7188–7196. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and Clustering Orders of Magnitude Faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Colwell, R.K.; Coddington, J.A. Coddington Estimating Terrestrial Biodiversity through Extrapolation. Phil. Trans. R. Soc. Lond. B 1994, 345, 101–118. [Google Scholar] [CrossRef]
- Chao, A.; Bunge, J. Estimating the Number of Species in a Stochastic Abundance Model. Biometrics 2002, 58, 531–539. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Gotelli, N.J. Biodiversity in the Scales. Nature 2002, 419, 575–576. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple Sequence Alignment with High Accuracy and High Throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Lozupone, C.; Knight, R. UniFrac: A New Phylogenetic Method for Comparing Microbial Communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef] [PubMed]
- Aßhauer, K.P.; Wemheuer, B.; Daniel, R.; Meinicke, P. Tax4Fun: Predicting Functional Profiles from Metagenomic 16S RRNA Data. Bioinformatics 2015, 31, 2882–2884. [Google Scholar] [CrossRef]
- Darzi, Y.; Letunic, I.; Bork, P.; Yamada, T. IPath3.0: Interactive Pathways Explorer V3. Nucleic Acids Res. 2018, 46, W510–W513. [Google Scholar] [CrossRef]
- Broucek, J. Effects of Noise on Performance, Stress, and Behaviour of Animals: A Review. Slovak J. Anim. Sci. 2014, 47, 111–123. [Google Scholar]
- Talling, J.C.; Waran, N.K.; Wathes, C.M.; Lines, J.A. Behavioural and Physiological Responses of Pigs to Sound. Appl. Anim. Behav. Sci. 1996, 48, 187–201. [Google Scholar] [CrossRef]
- Ekachat, K.; Vajrabukka, C. Effect of Music Rhythm on Growth Performance of Growing Pigs. Agric. Nat. Resour. 1994, 28, 640–643. [Google Scholar]
- Li, X.; Zhao, J.N.; Zhao, P.; Zhang, X.; Bi, Y.J.; Li, J.H.; Liu, H.G.; Wang, C.; Bao, J. Behavioural Responses of Piglets to Different Types of Music. Animal 2019, 13, 2319–2326. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, H.-Y.; Wang, N.; Zhang, Z.-J.; Lao, L.; Wong, C.-W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef]
- Fancourt, D.; Ockelford, A.; Belai, A. The Psychoneuroimmunological Effects of Music: A Systematic Review and a New Model. Brain Behav. Immunol. 2014, 36, 15–26. [Google Scholar] [CrossRef]
- Wachi, M.; Utsuyama, M.; Bittman, B.; Hirokawa, K.; Kitagawa, M. Recreational Music-Making Modulates Immunological Responses and Mood States in Older Adults. J. Med. Dent. Sci. 2009, 56, 79–90. [Google Scholar]
- Zhang, A.; Zou, T.; Guo, D.; Wang, Q.; Shen, Y.; Hu, H.; Ye, B.; Xiang, M. The Immune System Can Hear Noise. Front. Immunol. 2021, 11, 619189. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in Health and Diseases. Sig. Transduct. Target Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Maynard, C.L.; Elson, C.O.; Hatton, R.D.; Weaver, C.T. Reciprocal Interactions of the Intestinal Microbiota and Immune System. Nature 2012, 489, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.M.; Cameron, R.; Parker, B. The Effects of Anthropogenic Sound and Artificial Light Exposure on Microbiomes: Ecological and Public Health Implications. Front. Ecol. Evol. 2021, 9. [Google Scholar] [CrossRef]
- Berlow, M.; Wada, H.; Derryberry, E.P. Experimental Exposure to Noise Alters Gut Microbiota in a Captive Songbird. Microb. Ecol. 2022, 84, 1264–1277. [Google Scholar] [CrossRef]
- Niu, J.; Xu, H.; Zeng, G.; Wang, P.; Raciheon, B.; Nawaz, S.; Zeng, Z.; Zhao, J. Music-Based Interventions in the Feeding Environment on the Gut Microbiota of Mice. Sci. Rep. 2023, 13, 6313. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut Microbiota in Human Metabolic Health and Disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, S.; Nie, Q.; He, H.; Tan, H.; Geng, F.; Ji, H.; Hu, J.; Nie, S. Gut Firmicutes: Relationship with Dietary Fiber and Role in Host Homeostasis. Crit. Rev. Food Sci. Nutr. 2022, 1–16. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- An, J.; Kwon, H.; Kim, Y.J. The Firmicutes/Bacteroidetes Ratio as a Risk Factor of Breast Cancer. J. Clin. Med. 2023, 12, 2216. [Google Scholar] [CrossRef]
- Koliada, A.; Syzenko, G.; Moseiko, V.; Budovska, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Dorofeyev, A.; Romanenko, M.; Tkach, S.; et al. Association between Body Mass Index and Firmicutes/Bacteroidetes Ratio in an Adult Ukrainian Population. BMC Microbiol. 2017, 17, 120. [Google Scholar] [CrossRef]
- Heinken, A.; Khan, M.T.; Paglia, G.; Rodionov, D.A.; Harmsen, H.J.M.; Thiele, I. Functional Metabolic Map of Faecalibacterium Prausnitzii, a Beneficial Human Gut Microbe. J. Bacteriol. 2014, 196, 3289–3302. [Google Scholar] [CrossRef] [PubMed]
- So, D.; Whelan, K.; Rossi, M.; Morrison, M.; Holtmann, G.; Kelly, J.T.; Shanahan, E.R.; Staudacher, H.M.; Campbell, K.L. Dietary Fiber Intervention on Gut Microbiota Composition in Healthy Adults: A Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2018, 107, 965–983. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Luo, Y. Bacterial Quorum-Sensing Systems and Their Role in Intestinal Bacteria-Host Crosstalk. Front. Microbiol. 2021, 12, 611413. [Google Scholar] [CrossRef] [PubMed]
- Markus, V.; Paul, A.A.; Teralı, K.; Özer, N.; Marks, R.S.; Golberg, K.; Kushmaro, A. Conversations in the Gut: The Role of Quorum Sensing in Normobiosis. Int. J. Mol. Sci. 2023, 24, 3722. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, B.; Li, R.; Yang, P.; Leng, P.; Huang, Y. Exploration of the Link between Gut Microbiota and Purinergic Signalling. Purinergic Signal. 2023, 19, 315–327. [Google Scholar] [CrossRef]
- Serpa, J. The Putative Role of Gut Microbiota in Cancer: Cysteine Is a Pivotal Coin. Front. Gastroenterol. 2022, 1, 966957. [Google Scholar] [CrossRef]
- Korsmo, H.W.; Jiang, X. One Carbon Metabolism and Early Development: A Diet-Dependent Destiny. Trends Endocrinol. Metab. 2021, 32, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.P.; Redinbo, M.R.; Bultman, S.J. The Role of the Microbiome in Cancer Development and Therapy. CA Cancer J. Clin. 2017, 67, 326–344. [Google Scholar] [CrossRef]
- Chi, H.; Cao, W.; Zhang, M.; Su, D.; Yang, H.; Li, Z.; Li, C.; She, X.; Wang, K.; Gao, X.; et al. Environmental Noise Stress Disturbs Commensal Microbiota Homeostasis and Induces Oxi-Inflammmation and AD-like Neuropathology through Epithelial Barrier Disruption in the EOAD Mouse Model. J. Neuroinflamm. 2021, 18, 9. [Google Scholar] [CrossRef]
- Kriengwatana, B.P.; Mott, R.; Ten Cate, C. Music for Animal Welfare: A Critical Review & Conceptual Framework. Appl. Anim. Behav. Sci. 2022, 251, 105641. [Google Scholar] [CrossRef]
- Snowdon, C.T. Animal Signals, Music and Emotional Well-Being. Animals 2021, 11, 2670. [Google Scholar] [CrossRef] [PubMed]
- Coppola, C.L.; Enns, R.M.; Grandin, T. Noise in the Animal Shelter Environment: Building Design and the Effects of Daily Noise Exposure. J. Appl. Anim. Welf. Sci. 2006, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Patterson-Kane, E.G.; Farnworth, M.J. Noise Exposure, Music, and Animals in the Laboratory: A Commentary Based on Laboratory Animal Refinement and Enrichment Forum (LAREF) Discussions. J. Appl. Anim. Welf. Sci. 2006, 9, 327–332. [Google Scholar] [CrossRef]
- Kraimi, N.; Dawkins, M.; Gebhardt-Henrich, S.G.; Velge, P.; Rychlik, I.; Volf, J.; Creach, P.; Smith, A.; Colles, F.; Leterrier, C. Influence of the Microbiota-Gut-Brain Axis on Behavior and Welfare in Farm Animals: A Review. Physiol. Behav. 2019, 210, 112658. [Google Scholar] [CrossRef]
- Diaz, J.; Reese, A.T. Possibilities and Limits for Using the Gut Microbiome to Improve Captive Animal Health. Anim. Microbiome 2021, 3, 89. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Wu, Y.; Zhou, S.; Fu, P.; Yan, H. Effects of Music and White Noise Exposure on the Gut Microbiota, Oxidative Stress, and Immune-Related Gene Expression of Mice. Microorganisms 2023, 11, 2272. https://doi.org/10.3390/microorganisms11092272
Zhang Z, Wu Y, Zhou S, Fu P, Yan H. Effects of Music and White Noise Exposure on the Gut Microbiota, Oxidative Stress, and Immune-Related Gene Expression of Mice. Microorganisms. 2023; 11(9):2272. https://doi.org/10.3390/microorganisms11092272
Chicago/Turabian StyleZhang, Zhenyu, Yinqiang Wu, Shizheng Zhou, Pengcheng Fu, and Hong Yan. 2023. "Effects of Music and White Noise Exposure on the Gut Microbiota, Oxidative Stress, and Immune-Related Gene Expression of Mice" Microorganisms 11, no. 9: 2272. https://doi.org/10.3390/microorganisms11092272
APA StyleZhang, Z., Wu, Y., Zhou, S., Fu, P., & Yan, H. (2023). Effects of Music and White Noise Exposure on the Gut Microbiota, Oxidative Stress, and Immune-Related Gene Expression of Mice. Microorganisms, 11(9), 2272. https://doi.org/10.3390/microorganisms11092272






