Comparison of Structural Features of CRISPR-Cas Systems in Thermophilic Bacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. CRISPR/Cas System Identification
2.3. Bioinformatics Analysis of CRISPR/Cas System
3. Results
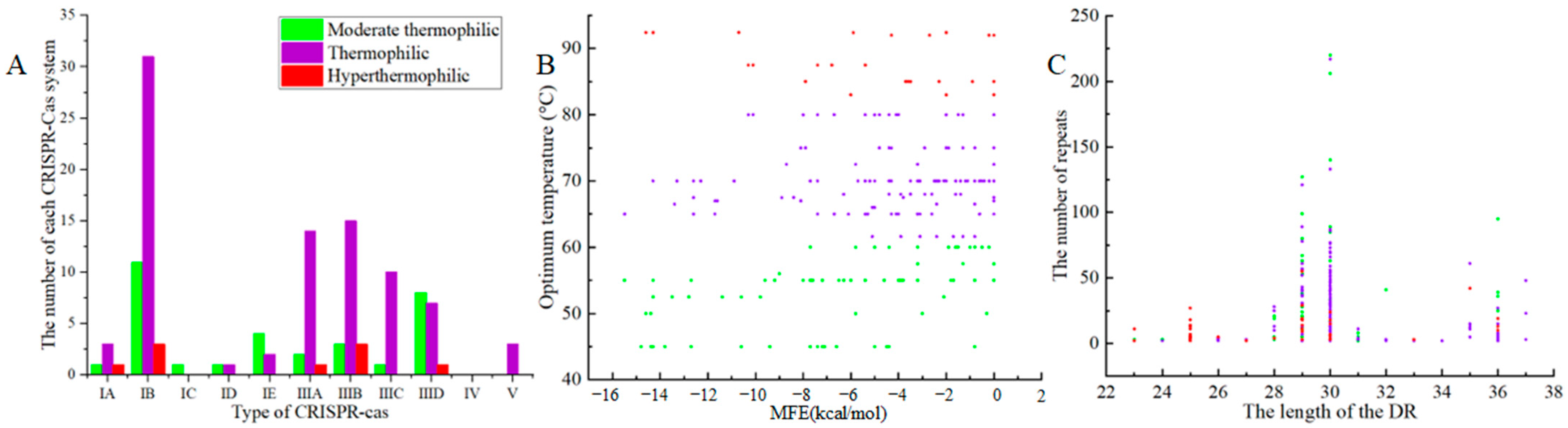
3.1. Genomic Distribution of CRISPR Structural Loci in Thermophilic Bacteria
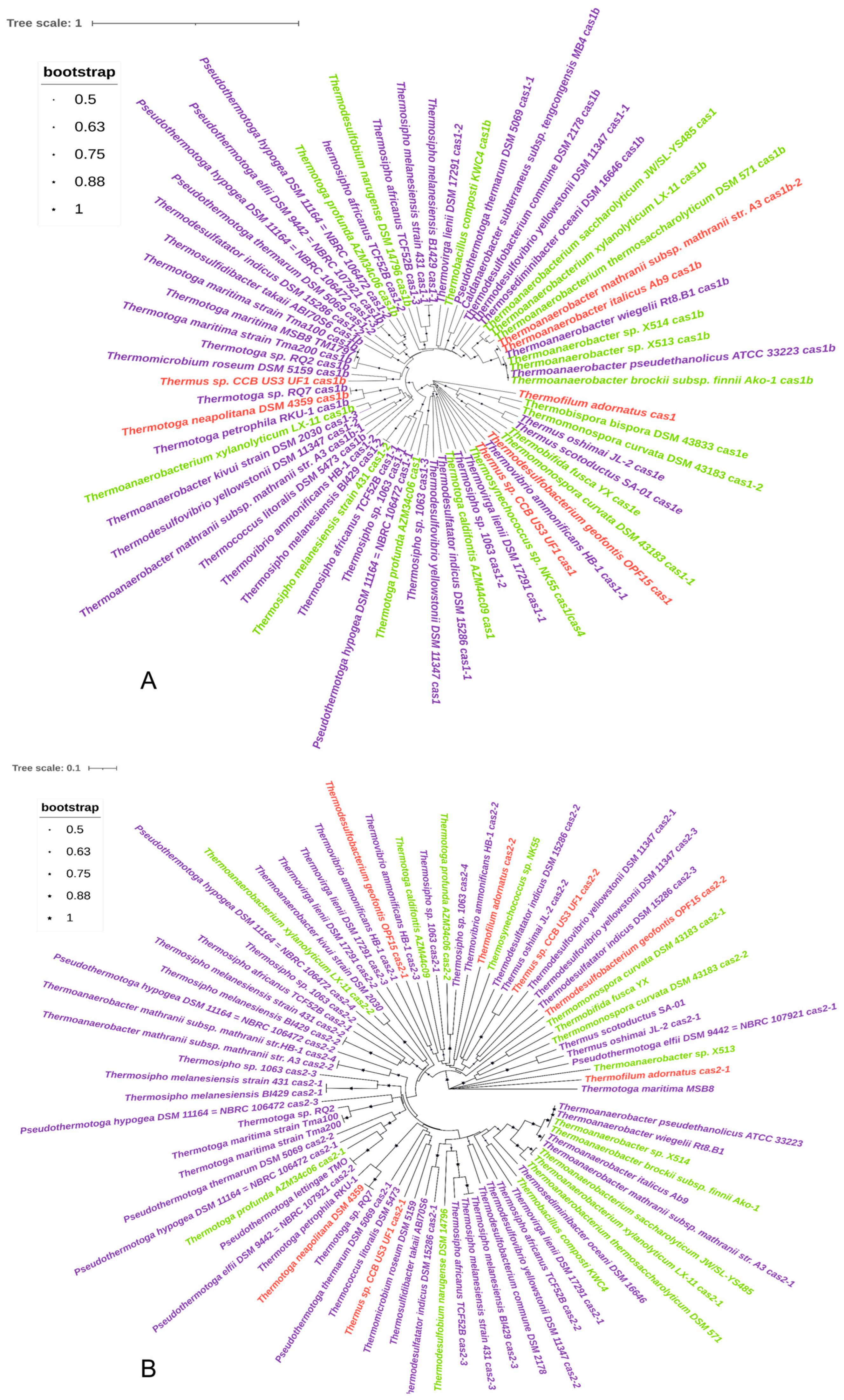
3.2. Distribution and Type of Cas Genes
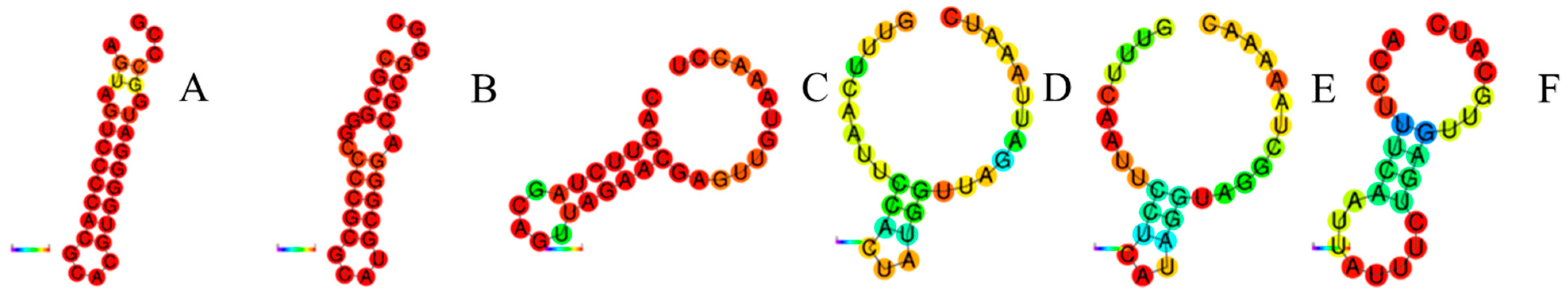
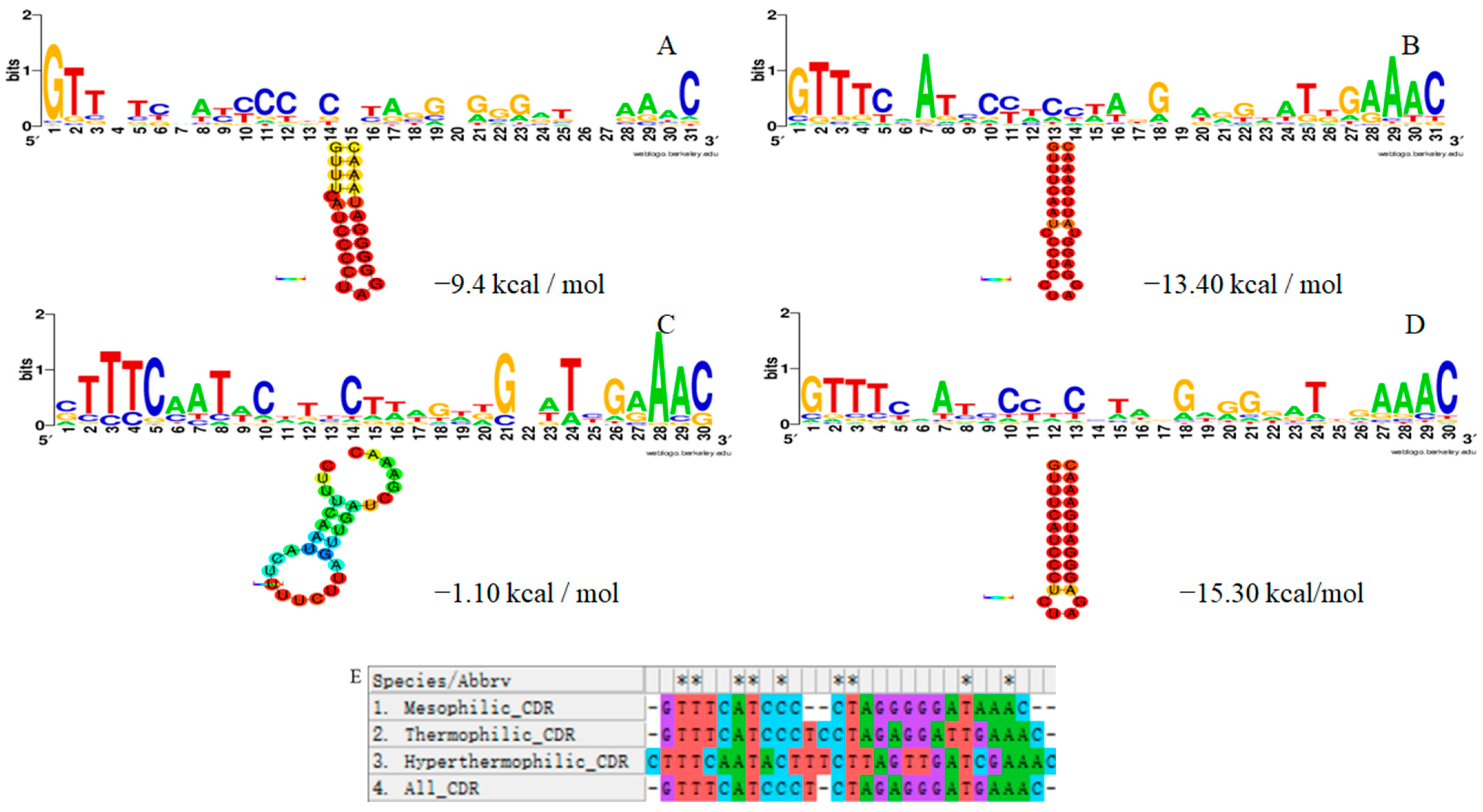
3.3. Repeat Sequence
3.4. Spacer Sequence Analysis
3.5. The Relationship between the CRISPR-Cas System of Thermophilic Bacteria and the Optimum Growth Temperature
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ishino, Y.; Shinagawa, H.; Makino, K.; Amemura, M.; Nakata, A. Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J. Bacteriol. 1987, 169, 5429–5433. [Google Scholar] [CrossRef]
- Jansen, R.; Embden, J.D.; Gaastra, W.; Schouls, L.M. Identification of genes that are associated with DNA repeats in prokaryotes. Mol. Microbiol. 2002, 43, 1565–1575. [Google Scholar] [CrossRef] [PubMed]
- Barrangou, R.; Fremaux, C.; Deveau, H.; Richards, M.; Boyaval, P.; Moineau, S.; Romero, D.A.; Horvath, P. CRISPR provides acquired resistance against viruses in prokaryotes. Science 2007, 315, 1709–1712. [Google Scholar] [CrossRef] [PubMed]
- Karginov, F.V.; Hannon, G.J. The CRISPR system: Small RNA-guided defense in bacteria and archaea. Mol. Cell 2010, 37, 7–19. [Google Scholar] [CrossRef]
- Kunin, V.; Sorek, R.; Hugenholtz, P. Evolutionary conservation of sequence and secondary structures in CRISPR repeats. Genome Biol. 2007, 8, R61. [Google Scholar] [CrossRef]
- Bolotin, A.; Quinquis, B.; Sorokin, A.; Ehrlich, S.D. Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin. Microbiology 2005, 151 Pt 8, 2551–2561. [Google Scholar] [CrossRef] [PubMed]
- Westra, E.R.; Semenova, E.; Datsenko, K.A.; Jackson, R.N.; Wiedenheft, B.; Severinov, K.; Brouns, S.J. Type I-E CRISPR-cas systems discriminate target from non-target DNA through base pairing-independent PAM recognition. PLoS Genet. 2013, 9, e1003742. [Google Scholar] [CrossRef]
- Vicencio, J.; Sánchez-Bolaños, C.; Moreno-Sánchez, I.; Brena, D.; Vejnar, C.E.; Kukhtar, D.; Ruiz-López, M.; Cots-Ponjoan, M.; Rubio, A.; Melero, N.R.; et al. Genome editing in animals with minimal PAM CRISPR-Cas9 enzymes. Nat. Commun. 2022, 13, 2601. [Google Scholar] [CrossRef]
- Makarova, K.S.; Koonin, E.V. Annotation and Classification of CRISPR-Cas Systems. Methods Mol. Biol. 2015, 1311, 47–75. [Google Scholar]
- Haft, D.H.; Selengut, J.; Mongodin, E.F.; Nelson, K.E. A guild of 45 CRISPR-associated (Cas) protein families and multiple CRISPR/Cas subtypes exist in prokaryotic genomes. PLoS Comput. Biol. 2005, 1, e60. [Google Scholar] [CrossRef]
- Makarova, K.S.; Wolf, Y.I.; Iranzo, J.; Shmakov, S.A.; Alkhnbashi, O.S.; Brouns, S.J.J.; Charpentier, E.; Cheng, D.; Haft, D.H.; Horvath, P.; et al. Evolutionary classification of CRISPR-Cas systems: A burst of class 2 and derived variants. Nat. Rev. Microbiol. 2020, 18, 67–83. [Google Scholar] [CrossRef]
- van der Oost, J.; Jore, M.M.; Westra, E.R.; Lundgren, M.; Brouns, S.J. CRISPR-based adaptive and heritable immunity in prokaryotes. Trends Biochem. Sci. 2009, 34, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef]
- Bhattacharjee, R.; Nandi, A.; Mitra, P.; Saha, K.; Patel, P.; Jha, E.; Panda, P.K.; Singh, S.K.; Dutt, A.; Mishra, Y.K.; et al. Theragnostic application of nanoparticle and CRISPR against food-borne multi-drug resistant pathogens. Mater. Today Bio 2022, 15, 100291. [Google Scholar] [CrossRef] [PubMed]
- Berezovsky, I.N.; Shakhnovich, E.I. Physics and evolution of thermophilic adaptation. Proc. Natl. Acad. Sci. USA 2005, 102, 12742–12747. [Google Scholar] [CrossRef] [PubMed]
- Ishino, Y.; Krupovic, M.; Forterre, P. History of CRISPR-Cas from Encounter with a Mysterious Repeated Sequence to Genome Editing Technology. J. Bacteriol. 2018, 200, e00580-17. [Google Scholar] [CrossRef] [PubMed]
- Grissa, I.; Vergnaud, G.; Pourcel, C. The CRISPRdb database and tools to display CRISPRs and to generate dictionaries of spacers and repeats. BMC Bioinform. 2007, 8, 172. [Google Scholar] [CrossRef]
- Couvin, D.; Bernheim, A.; Toffano-Nioche, C.; Touchon, M.; Michalik, J.; Néron, B.; Rocha, E.P.C.; Vergnaud, G.; Gautheret, D.; Pourcel, C. CRISPRCasFinder, an update of CRISRFinder, includes a portable version, enhanced performance and integrates search for Cas proteins. Nucleic Acids Res. 2018, 46, W246–W251. [Google Scholar] [CrossRef]
- Brininger, C.; Spradlin, S.; Cobani, L.; Evilia, C. The more adaptive to change, the more likely you are to survive: Protein adaptation in extremophiles. Semin. Cell Dev. Biol. 2018, 84, 158–169. [Google Scholar] [CrossRef]
- Wang, Q.; Cen, Z.; Zhao, J. The survival mechanisms of thermophiles at high temperatures: An angle of omics. Physiology 2015, 30, 97–106. [Google Scholar] [CrossRef]
- Kates, M. Structural analysis of phospholipids and glycolipids in extremely halophilic archaebacteria. J. Microbiol. Methods 1996, 25, 113–128. [Google Scholar] [CrossRef]
- Yano, Y.; Nakayama, A.; Ishihara, K.; Saito, H. Adaptive changes in membrane lipids of barophilic bacteria in response to changes in growth pressure. Appl. Environ. Microbiol. 1998, 64, 479–785. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, A.D.; Wolf, Y.I.; Lobkovsky, A.E.; Gilmore, M.S.; Koonin, E.V. Viral diversity threshold for adaptive immunity in prokaryotes. mBio 2012, 3, e00456-12. [Google Scholar] [CrossRef] [PubMed]
- Makarova, K.S.; Wolf, Y.I.; Alkhnbashi, O.S.; Costa, F.; Shah, S.A.; Saunders, S.J.; Barrangou, R.; Brouns, S.J.; Charpentier, E.; Haft, D.H.; et al. An updated evolutionary classification of CRISPR-Cas systems. Nat. Rev. Microbiol. 2015, 13, 722–736. [Google Scholar] [CrossRef]
- Chylinski, K.; Makarova, K.S.; Charpentier, E.; Koonin, E.V. Classification and evolution of type II CRISPR-Cas systems. Nucleic Acids Res. 2014, 42, 6091–6105. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Jin, C.; Zhang, J.; Bao, Q.; Liu, B.; Tan, H. Transcriptomic analysis of Thermoanaerobacter tengcongensis grown at different temperatures by RNA sequencing. J. Genet. Genom. 2015, 42, 335–338. [Google Scholar] [CrossRef]
- Mojica, F.J.; Ferrer, C.; Juez, G.; Rodríguez-Valera, F. Long stretches of short tandem repeats are present in the largest replicons of the Archaea Haloferax mediterranei and Haloferax volcanii and could be involved in replicon partitioning. Mol. Microbiol. 1995, 17, 85–93. [Google Scholar] [CrossRef]
- Godde, J.S.; Bickerton, A. The repetitive DNA elements called CRISPRs and their associated genes: Evidence of horizontal transfer among prokaryotes. J. Mol. Evol. 2006, 62, 718–729. [Google Scholar] [CrossRef]
- Kim, K.S.; Manasherob, R.; Cohen, S.N. YmdB: A stress-responsive ribonuclease-binding regulator of E. coli RNase III activity. Genes Dev. 2008, 22, 3497–3508. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.C.; Susko, E.; Roger, A.J. On the correlation between genomic G+C content and optimal growth temperature in prokaryotes: Data quality and confounding factors. Biochem. Biophys. Res. Commun. 2006, 342, 681–684. [Google Scholar] [CrossRef]
- Brown, J.W.; Haas, E.S.; Pace, N.R. Characterization of ribonuclease P RNAs from thermophilic bacteria. Nucleic Acids Res. 1993, 21, 671–679. [Google Scholar] [CrossRef]
- Galtier, N.; Lobry, J.R. Relationships between genomic G+C content, RNA secondary structures, and optimal growth temperature in prokaryotes. J. Mol. Evol. 1997, 44, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Hagedoorn, P.L. Steady-state kinetics of the tungsten containing aldehyde: Ferredoxin oxidoreductases from the hyperthermophilic archaeon Pyrococcus furiosus. J. Biotechnol. 2019, 306, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Kletzin, A.; Adams, M.W. Tungsten in biological systems. FEMS Microbiol. Rev. 1996, 18, 5–63. [Google Scholar] [CrossRef]
- Salzer, R.; Kern, T.; Joos, F.; Averhoff, B. The Thermus thermophilus comEA/comEC operon is associated with DNA binding and regulation of the DNA translocator and type IV pili. Environ. Microbiol. 2016, 18, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Andrews, G.; Lewis, D.; Notey, J.; Kelly, R.; Muddiman, D. Part II: Defining and quantifying individual and co-cultured intracellular proteomes of two thermophilic microorganisms by GeLC-MS2 and spectral counting. Anal. Bioanal. Chem. 2010, 398, 391–404. [Google Scholar] [CrossRef]

| Type | Subtype | Moderate Thermophiles | Total (%) | Thermophiles | Total | Hyperthermophiles | Total (%) |
|---|---|---|---|---|---|---|---|
| I | IA | 1 (0.76%) | 18 (13.74%) | 3 (2.29%) | 37 (28.24%) | 1 (0.76%) | 4 (3.05%) |
| IB | 11 (8.40%) | 31 (23.66%) | 3 (2.29%) | ||||
| IC | 1 (0.76%) | 0 | 0 | ||||
| ID | 1 (0.76%) | 1 (0.76%) | 0 | ||||
| IE | 4 (3.05%) | 2 (1.53%) | 0 | ||||
| III | IIIA | 2 (1.53%) | 14 (10.69%) | 14 (10.69%) | 46 (35.11%) | 1 (0.76%) | 5 (3.82%) |
| IIIB | 3 (2.29%) | 15 (11.45%) | 3 (2.29%) | ||||
| IIIC | 1 (0.76%) | 10 (7.63%) | 0 | ||||
| IIID | 8 (6.11%) | 7 (5.34%) | 1 (0.76%) | ||||
| IV | 1 (0.76%) 3 (2.29%) | 0 | 0 | ||||
| V | 3 (2.29%) | 0 | |||||
| total | 36 | 86 | 9 | ||||
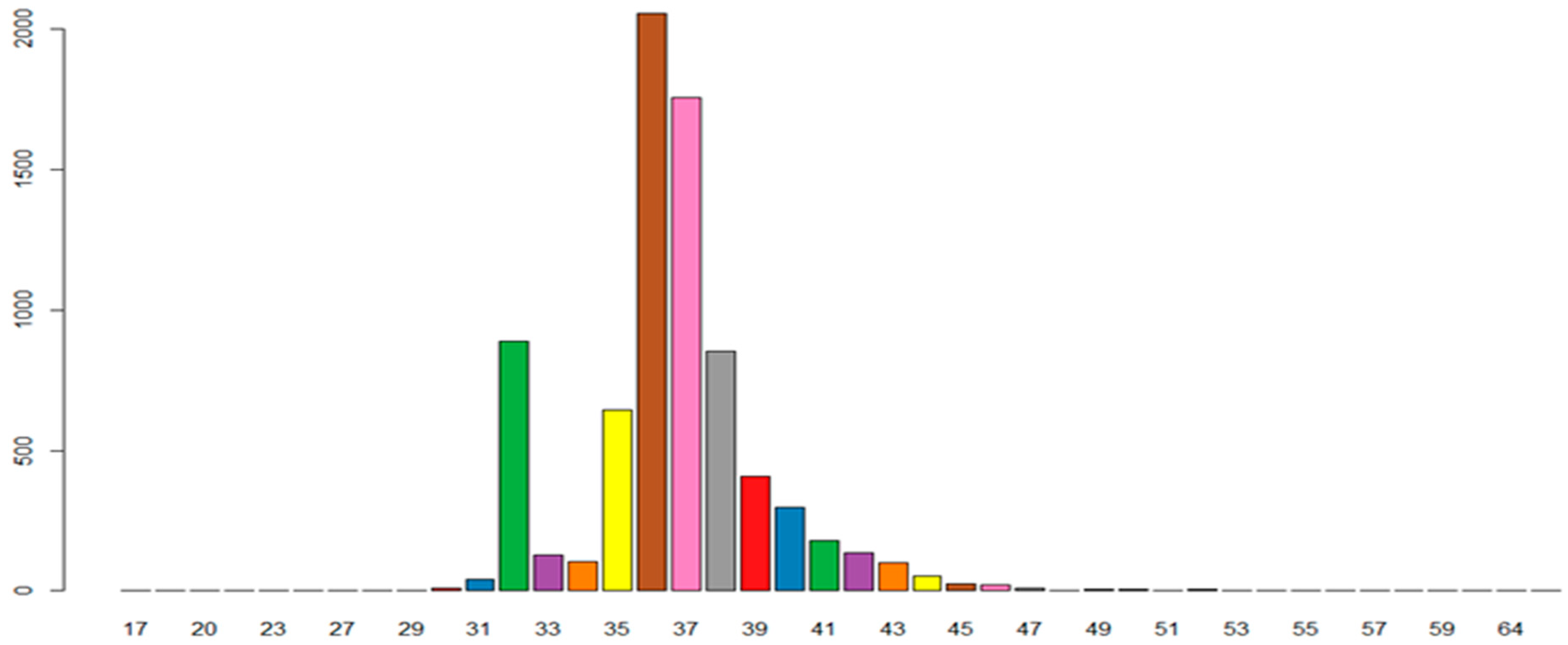
| Category Repetitive Sequence Length | Moderate Thermophiles | Thermophiles | Hyperthermophiles | Total |
|---|---|---|---|---|
| 23 | 8 (2.21%) | 2 (0.55%) | 2 (0.55%) | 12 (3.31%) |
| 24 | 6 (1.66%) | 3 (0.83%) | 0 (0.00%) | 9 (2.49%) |
| 25 | 4 (1.66%) | 4 (1.10%) | 17 (4.70%) | 25 (6.91%) |
| 26 | 3 (0.83%) | 3 (0.83%) | 1 (0.28%) | 7 (1.93%) |
| 27 | 1 (0.28%) | 1 (0.28%) | 1 (0.28%) | 3 (0.83%) |
| 28 | 6 (1.66%) | 6 (1.66%) | 1 (0.28%) | 13 (3.59%) |
| 29 | 27 (7.46%) | 22 (6.08%) | 8 (2.21%) | 57 (15.75%) |
| 30 | 44 (12.15%) | 130 (35.91%) | 10 (2.76%) | 184 (50.83%) |
| 31 | 4 (1.10%) | 6 (1.66%) | 0 (0.00%) | 10 (2.76%) |
| 32 | 3 (0.83%) | 5 (1.38%) | 0 (0.00%) | 8 (2.21%) |
| 33 | 0 (0.00%) | 2 (0.55%) | 1 (0.28%) | 3 (0.83%) |
| 34 | 0 (0.00%) | 1 (0.28%) | 0 (0.00%) | 1 (0.28%) |
| 35 | 0 (0.00%) | 5 (1.38%) | 1 (0.28%) | 6 (1.66%) |
| 36 | 5 (1.38%) | 13 (3.59%) | 3 (0.83%) | 21 (5.80) |
| 37 | 0 (0.00%) | 3 (0.83%) | 0 (0.00%) | 3 (0.83%) |
| total | 111 (30.66%) | 206 (56.91%) | 45 (12.43%) | 362 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Yang, Y.; Tang, S.; Liu, Y.; Wei, Y.; Wan, X.; Liu, Y.; Zhang, Z.; Sunkang, Y. Comparison of Structural Features of CRISPR-Cas Systems in Thermophilic Bacteria. Microorganisms 2023, 11, 2275. https://doi.org/10.3390/microorganisms11092275
Wang C, Yang Y, Tang S, Liu Y, Wei Y, Wan X, Liu Y, Zhang Z, Sunkang Y. Comparison of Structural Features of CRISPR-Cas Systems in Thermophilic Bacteria. Microorganisms. 2023; 11(9):2275. https://doi.org/10.3390/microorganisms11092275
Chicago/Turabian StyleWang, Chuan, Yuze Yang, Shaoqing Tang, Yuanzi Liu, Yaqin Wei, Xuerui Wan, Yajuan Liu, Zhao Zhang, and Yongjie Sunkang. 2023. "Comparison of Structural Features of CRISPR-Cas Systems in Thermophilic Bacteria" Microorganisms 11, no. 9: 2275. https://doi.org/10.3390/microorganisms11092275





