Clustered Regularly Interspaced Short Palindromic Repeat/CRISPR-Associated Protein and Its Utility All at Sea: Status, Challenges, and Prospects
Abstract
:1. Genome Editing Technology
2. The Origin of the CRISPR/Cas System
3. The Basic Structure and Function of the CRISPR/Cas System
4. The Development and Application of the CRISPR/Cas System as a Genome Editing Tool
5. Huge Inventory of CRISPR/Cas in the Ocean
| Phylum | Cas Type | Species | Prevalence * | References |
|---|---|---|---|---|
| Pseudomonadota | I-F and I-E | Moritella viscosa | Prevalent | [32,57,58,73,74,75,77,78,79,94,98,100,118] |
| Vibrio | Some species | [56,65,66,69,70,76] | ||
| Cyanobacteriota | I-D and III-B | Geitlerinema sp. FC II | Prevalent | [63,90,91] |
| Marine subclade Synechococcus and Prochlorococcus | Rare | [60] | ||
| Actinomycetota | I-E and I-U | Marine actinomycete Salinispora | Prevalent | [55] |
| Bacteroidota | II-C and VI-B1 | Marinilongibacter aquaticus | Prevalent | [54] |
| Thermotogota | III-A and III-B | Thermosipho spp. | Limited study | [116] |
| Aquificota | unclassified | Aquificaceae | Prevalent | [115] |
| Thermodesulfobacteriota | III-B and I-B | Desulfobacterium | Prevalent | [53,81] |
| Bacillota | III-B | Bacillus pumilus 64-1 | Limited study | [96] |
| Planctomycetota | unclassified | Thalassoroseus pseudoceratinae | Limited study | [97] |
| Mycoplasmatota | II | Candidatus Mycoplasma liparidae | Limited study | [95] |
| Nitrososphaerota | unclassified | Candidatus Nitrosopumilus koreensis AR1 | Limited study | [82] |
| Nanoarchaeota | I-B | Nanoarchaeum equitans | Limited study | [83] |
| Euryarchaeota | I-A and III-B | Pyrococcus furiosus | Prevalent | [86,87,88,89] |
| Asgard group | I-A and III-B | Candidatus Thorarchaeota archaeon | Metagenome-assembled genomes | [85] |
6. Applications on Marine Prokaryotic Microbes
| Taxonomy | Species | CRISPR Systems | Application | References |
|---|---|---|---|---|
| Euryarchaeota | Methanococcus maripaludis | CRISPR/Cas9 | Deleted multiple genes across different loci; in situ genome modifications | [138] |
| Actinomycetota | Verrucosispora sp. MS100137 | CRISPR/Cas9 | Deleted orange-pigmented carotenoid gene cluster and abyssomicin gene cluster | [130] |
| Pseudomonadota | Shewanella algae | CRISPR/Cas9 | Deleted potential carbapenem resistance genes | [131] |
| Vibrio natriegens | NT-CRISPR (CRISPR/Cas9) | Deletions, integrations and single-base modifications | [135] | |
| Vibrio natriegens | CRISPR/dCas9 | Genome-wide CRISPR interference | [134] | |
| Vibrio fluvialis | CRISPR/dCas9 | CRISPR interference torA gene | [133] | |
| Pseudoalteromonas luteoviolacea | CRISPR/dCas9 | CRISPR interference the vioA gene and macB gene | [132] | |
| Roseovarius nubinhibens | CRISPR/dCas9 | PobA, pcaH, and pcaG in the β-ketoadipate pathway, and a transcription activator pcaQ | [136] | |
| Cyanobacteriota | Synechococcus sp. strain PCC 7002 | CRISPR/dCas9 | Achieved conditional and titratable repression of gene | [137] |
7. CRISPR/Cas in Marine Algal Research
| Application | Algal Species | Target Genes | References |
|---|---|---|---|
| Nutrient regulation | Thalassiosira pseudonana | Urease; nitrate reductase (NR); silacidin; flavodoxin | [161,162,173,175] |
| Chlamydomonas reinhardtii | Spermidine synthase (SPD1) | [164] | |
| Phaeodactylum tricornutum | Trypsin; alkaline phosphatase (AP); phosphate starvation response regulator (PHR); SPX; ferrisiderophore acquisition system (FBP1, FRE1, FRE2) | [165,166,168,169,172,174] | |
| Photosynthesis and pigment biosynthesis | Chlamydomonas reinhardtii | Zeaxanthin epoxidase (ZEP); chloroplast signal recognition particle (CpSRP) receptor (CpFTSY) | [158,176] |
| Dunaliella salina CCAP19/18 | β-carotene hydroxylase | [186] | |
| Phaeodactylum tricornutum | Light-harvesting complex (LHC); chloroplast signal recognition particle 54 kDa (CpSRP54); CpFTSY; cryptochrome; violaxanthin de-epoxidase (VDE); pyruvate orthophosphate dikinase (PPDK); Chl c synthase (CHLC) | [178,179,180,181,182,183,184,185,188] | |
| Porphyridium sp. | Chlorophyll synthase (CHS) | [187] | |
| Lipid production and fatty acid metabolism | Chlamydomonas reinhardtii | Phosphoenolpyruvate carboxylase (PEPC1); esterase lipase thioesterase (ELT); phospholipase A2 | [194,195,196] |
| Chlorella vulgaris FSP-E | Omega-3 fatty acid desaturase (Fad3) | [197] | |
| Tetraselmis sp. | ADP-glucose pyrophosphorylase (AGP) | [205] | |
| Parachlorella kessleri | Plastidic ATP/ADP translocase (AATP); duplicated mannanases 1 (DMAN1) | [206] | |
| Phaeodactylum tricornutum | Acyl-ACP D9-desaturase; long-chain acyl-CoA synthetases (LACS); monogalactosyldiacylglycerol synthase (MGD); stramenopile-type lipid droplet protein (StLDP); NR; glutamine synthetase 2 (GS2); chloroplast localized glutamate synthase(cGOGAT); AP; a novel gene Pt2015 | [41,168,198,200,201,203,204,207,208] | |
| Others | Chlamydomonas reinhardtii | Acetolactate synthase (ALS) | [157] |
| Volvox carteri | GlsA; regA; invA | [216] | |
| Picochlorum celeri | NR; carotenoid isomerase | [217] | |
| Ulva prolifera | Adenine phosphoribosyl transferase (APT) | [218] | |
| Thalassiosira pseudonana | Putative θ-carbonic anhydrase (θ-CA); bestrophin-like protein (BST2) | [209,210] | |
| Phaeodactylum tricornutum | Metacaspase (MCA); single-domain voltage-gated channel (EUKCATA); HMP-P synthase (THIC); thiamine-related proteins SSSP | [212,214,215] | |
| Saccharina japonica | APT | [220] | |
| Ectocarpus siliculosus (Ectocarpus 7) | APT | [219] | |
| Gracilariopsis lemaneiformis | CA; γ subunits of phycoerythrin (γpe) | [221] |
8. CRISPR/Cas in Marine Zooplankton Research
| Species | Target Genes | References |
|---|---|---|
| Clytia hemisphaerica | GFP; CheRfx123; Opsin9 | [226,227] |
| Daphnia magna | Eyeless; tryptophan hydroxylase (TRH); EcRE-mCherry; Dsx1; DNA methyltransferase 3.1 (DNMT3.1); Dma-ey | [228,229,231,232,233,234] |
| Daphnia pulex | Distal-less (DLL) | [230] |
| Brachionus manjavacas | Mlh3 | [235] |
9. CRISPR/Cas Application on Other Marine Animals
| Class | Species | Target Genes | References |
|---|---|---|---|
| Tentaculata | Mnemiopsis leidyi | Brachyury | [237] |
| Hydrozoa | Hydractinia echinata | Notch | [236] |
| Anthozoa | Acropora millepora | Fibroblast growth factor 1a (FGF1a); green fluorescent protein (GFP); red fluorescent protein (RFP); heat shock transcription factor 1 (HSF1); SLC4γ | [238,239,240] |
| Nematostella vectensis | NvFP-7R; brachyury | [241,242] | |
| Polychaeta | Capitella teleta | Rhabdomeric opsin (Ct-r-opsin1) | [243] |
| Gastropoda | Crepidula fornicata | β-catenin | [244] |
| Bivalvia | Crassostrea gigas | MSTN; Twist; myosin essential light chain gene (MELC) | [245,246] |
| Parhyale hawaiensis | Hox | [248,249] | |
| Exopalaemon carinicauda | EcChi4; EcMIH; EcNinaB-X1; EcBCO2 | [59,250,251,252] | |
| Echinoidea | Strongylocentrotus purpuratus | Nodal | [253] |
| Tunicata | Ciona robusta | Hox3; Hox5; Hox12; Ebf | [254,255] |
| Petromyzonti | Petromyzon marinus | Tyrosinase (Tyr); FGF8/17/18; golden (gol); kctd10; wee1; soxe2; wnt7b; LcHsp70A | [257,258,259] |
| Teleostei | Salmo salar | Tyr; solute carrier family 45 member 2 (slc45a2) | [264,265] |
| Pagrus major | Myostatin (mstn) | [266] | |
| Paralichthys olivaceus | PoMSTN | [267] | |
| Engraulis japonicus | Myostatin-2 (MSTN-2) | [268] | |
| Oryzias melastigma | SLC45a2 | [269] |
10. Challenges as Roadblocks
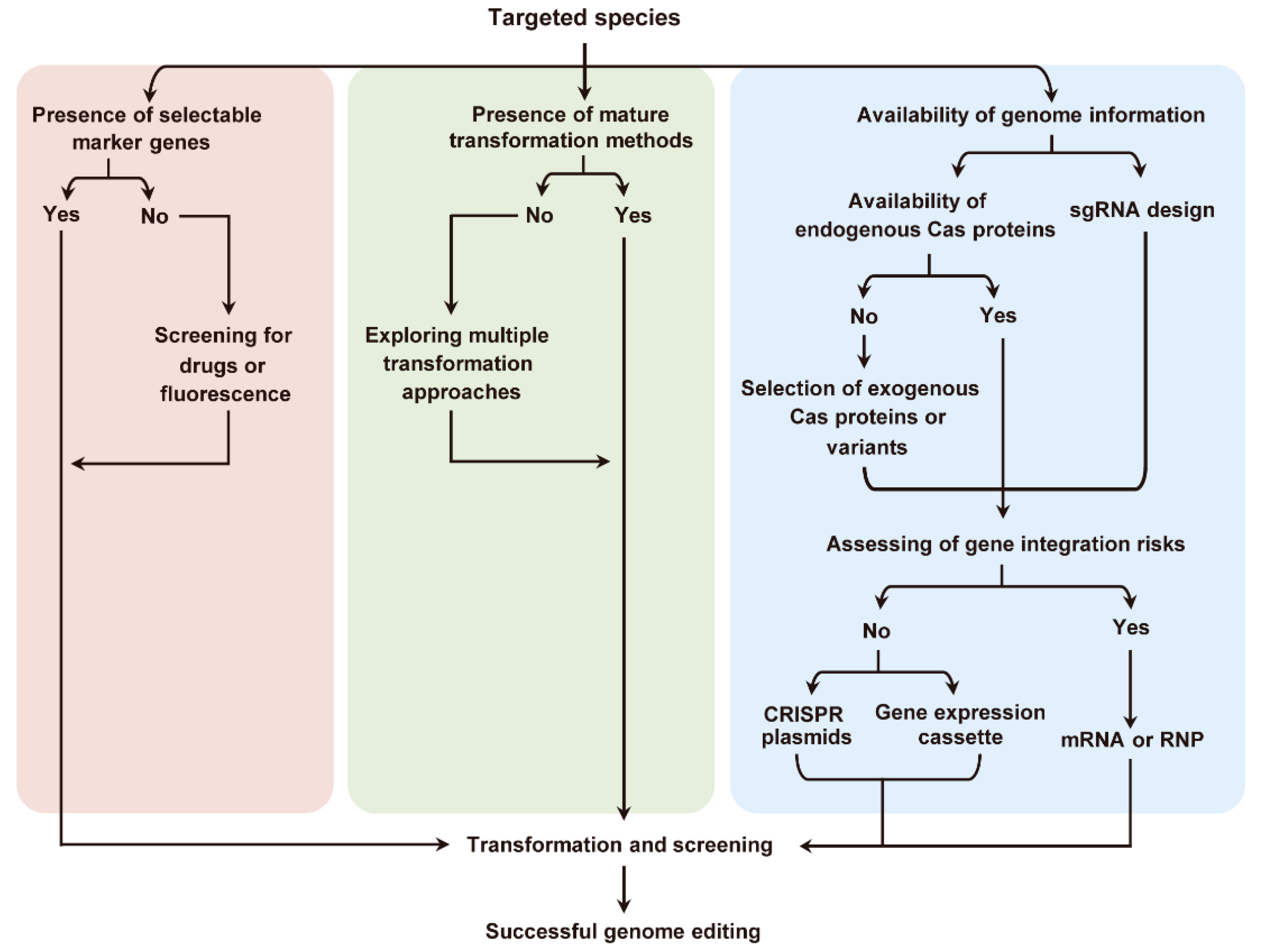
11. Prospects and a Roadmap
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA–guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Mali, P.; Yang, L.; Esvelt, K.M.; Aach, J.; Guell, M.; DiCarlo, J.E.; Norville, J.E.; Church, G.M. RNA-guided human genome engineering via Cas9. Science 2013, 339, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Bibikova, M.; Beumer, K.; Trautman, J.K.; Carroll, D. Enhancing gene targeting with designed zinc finger nucleases. Science 2003, 300, 764. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-G.; Cha, J.; Chandrasegaran, S. Hybrid restriction enzymes: Zinc finger fusions to Fok I cleavage domain. Proc. Natl. Acad. Sci. USA 1996, 93, 1156–1160. [Google Scholar] [CrossRef] [PubMed]
- Moscou, M.J.; Bogdanove, A.J. A simple cipher governs DNA recognition by TAL effectors. Science 2009, 326, 1501. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Lee, Y.-K.; Schaefer, E.A.; Peters, D.T.; Veres, A.; Kim, K.; Kuperwasser, N.; Motola, D.L.; Meissner, T.B.; Hendriks, W.T. A TALEN genome-editing system for generating human stem cell-based disease models. Cell Stem Cell 2013, 12, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Adli, M. The CRISPR tool kit for genome editing and beyond. Nat. Commun. 2018, 9, 1911. [Google Scholar] [CrossRef]
- Ishino, Y.; Shinagawa, H.; Makino, K.; Amemura, M.; Nakata, A. Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J. Bacteriol. 1987, 169, 5429–5433. [Google Scholar] [CrossRef]
- Mojica, F.; Ferrer, C.; Juez, G.; Rodríguez-Valera, F. Long stretches of short tandem repeats are present in the largest replicons of the Archaea Haloferax mediterranei and Haloferax volcanii and could be involved in replicon partitioning. Mol. Microbiol. 1995, 17, 85–93. [Google Scholar] [CrossRef]
- Mojica, F.J.; Díez-Villaseñor, C.; Soria, E.; Juez, G. Biological significance of a family of regularly spaced repeats in the genomes of Archaea, Bacteria and mitochondria. Mol. Microbiol. 2000, 36, 244–246. [Google Scholar] [CrossRef]
- Jansen, R.; Embden, J.D.V.; Gaastra, W.; Schouls, L.M. Identification of genes that are associated with DNA repeats in prokaryotes. Mol. Microbiol. 2002, 43, 1565–1575. [Google Scholar] [CrossRef]
- Bolotin, A.; Quinquis, B.; Sorokin, A.; Ehrlich, S.D. Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin. Microbiology 2005, 151, 2551–2561. [Google Scholar] [CrossRef] [PubMed]
- Barrangou, R.; Fremaux, C.; Deveau, H.; Richards, M.; Boyaval, P.; Moineau, S.; Romero, D.A.; Horvath, P. CRISPR provides acquired resistance against viruses in prokaryotes. Science 2007, 315, 1709–1712. [Google Scholar] [CrossRef] [PubMed]
- Garst, A.D.; Bassalo, M.C.; Pines, G.; Lynch, S.A.; Halweg-Edwards, A.L.; Liu, R.; Liang, L.; Wang, Z.; Zeitoun, R.; Alexander, W.G. Genome-wide mapping of mutations at single-nucleotide resolution for protein, metabolic and genome engineering. Nat. Biotechnol. 2017, 35, 48–55. [Google Scholar] [CrossRef]
- Wang, T.; Guan, C.; Guo, J.; Liu, B.; Wu, Y.; Xie, Z.; Zhang, C.; Xing, X.-H. Pooled CRISPR interference screening enables genome-scale functional genomics study in bacteria with superior performance. Nat. Commun. 2018, 9, 2475. [Google Scholar] [CrossRef] [PubMed]
- Bhaya, D.; Davison, M.; Barrangou, R. CRISPR-Cas systems in bacteria and archaea: Versatile small RNAs for adaptive defense and regulation. Annu. Rev. Genet. 2011, 45, 273–297. [Google Scholar] [CrossRef] [PubMed]
- Van Der Oost, J.; Westra, E.R.; Jackson, R.N.; Wiedenheft, B. Unravelling the structural and mechanistic basis of CRISPR–Cas systems. Nat. Rev. Microbiol. 2014, 12, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Rath, D.; Amlinger, L.; Rath, A.; Lundgren, M. The CRISPR-Cas immune system: Biology, mechanisms and applications. Biochimie 2015, 117, 119–128. [Google Scholar] [CrossRef]
- Makarova, K.S.; Wolf, Y.I.; Alkhnbashi, O.S.; Costa, F.; Shah, S.A.; Saunders, S.J.; Barrangou, R.; Brouns, S.J.; Charpentier, E.; Haft, D.H.; et al. An updated evolutionary classification of CRISPR–Cas systems. Nat. Rev. Microbiol. 2015, 13, 722–736. [Google Scholar] [CrossRef]
- Haft, D.H.; Selengut, J.; Mongodin, E.F.; Nelson, K.E. A guild of 45 CRISPR-associated (Cas) protein families and multiple CRISPR/Cas subtypes exist in prokaryotic genomes. PLoS Comput. Biol. 2005, 1, e60. [Google Scholar] [CrossRef]
- Koonin, E.V.; Makarova, K.S. Origins and evolution of CRISPR-Cas systems. Philos. Trans. R. Soc. B 2019, 374, 20180087. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, S.H.; Haurwitz, R.E.; Doudna, J.A. Mechanism of substrate selection by a highly specific CRISPR endoribonuclease. Rna 2012, 18, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Nishimasu, H.; Ran, F.A.; Hsu, P.D.; Konermann, S.; Shehata, S.I.; Dohmae, N.; Ishitani, R.; Zhang, F.; Nureki, O. Crystal structure of Cas9 in complex with guide RNA and target DNA. Cell 2014, 156, 935–949. [Google Scholar] [CrossRef]
- Kleinstiver, B.P.; Pattanayak, V.; Prew, M.S.; Tsai, S.Q.; Nguyen, N.T.; Zheng, Z.; Joung, J.K. High-fidelity CRISPR–Cas9 nucleases with no detectable genome-wide off-target effects. Nature 2016, 529, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Slaymaker, I.M.; Gao, L.; Zetsche, B.; Scott, D.A.; Yan, W.X.; Zhang, F. Rationally engineered Cas9 nucleases with improved specificity. Science 2016, 351, 84–88. [Google Scholar] [CrossRef]
- Chen, J.S.; Dagdas, Y.S.; Kleinstiver, B.P.; Welch, M.M.; Sousa, A.A.; Harrington, L.B.; Sternberg, S.H.; Joung, J.K.; Yildiz, A.; Doudna, J.A. Enhanced proofreading governs CRISPR–Cas9 targeting accuracy. Nature 2017, 550, 407–410. [Google Scholar] [CrossRef]
- Zong, Y.; Song, Q.; Li, C.; Jin, S.; Zhang, D.; Wang, Y.; Qiu, J.-L.; Gao, C. Efficient C-to-T base editing in plants using a fusion of nCas9 and human APOBEC3A. Nat. Biotechnol. 2018, 36, 950–953. [Google Scholar] [CrossRef]
- Guilinger, J.P.; Thompson, D.B.; Liu, D.R. Fusion of catalytically inactive Cas9 to FokI nuclease improves the specificity of genome modification. Nat. Biotechnol. 2014, 32, 577–582. [Google Scholar] [CrossRef]
- Fonfara, I.; Richter, H.; Bratovič, M.; Le Rhun, A.; Charpentier, E. The CRISPR-associated DNA-cleaving enzyme Cpf1 also processes precursor CRISPR RNA. Nature 2016, 532, 517–521. [Google Scholar] [CrossRef]
- Zetsche, B.; Gootenberg, J.S.; Abudayyeh, O.O.; Slaymaker, I.M.; Makarova, K.S.; Essletzbichler, P.; Volz, S.E.; Joung, J.; Van Der Oost, J.; Regev, A. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell 2015, 163, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Yakimov, M.M.; Crisafi, F.; Messina, E.; Smedile, F.; Lopatina, A.; Denaro, R.; Pieper, D.H.; Golyshin, P.N.; Giuliano, L. Analysis of defence systems and a conjugative IncP-1 plasmid in the marine polyaromatic hydrocarbons-degrading bacterium Cycloclasticus sp. 78-ME. Environ. Microbiol. Rep. 2016, 8, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gonçalves, M.A. Engineered viruses as genome editing devices. Mol. Ther. 2016, 24, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Burger, A.; Lindsay, H.; Felker, A.; Hess, C.; Anders, C.; Chiavacci, E.; Zaugg, J.; Weber, L.M.; Catena, R.; Jinek, M. Maximizing mutagenesis with solubilized CRISPR-Cas9 ribonucleoprotein complexes. Development 2016, 143, 2025–2037. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.W.; Kim, J.; Kwon, S.I.; Corvalán, C.; Cho, S.W.; Kim, H.; Kim, S.-G.; Kim, S.-T.; Choe, S.; Kim, J.-S. DNA-free genome editing in plants with preassembled CRISPR-Cas9 ribonucleoproteins. Nat. Biotechnol. 2015, 33, 1162–1164. [Google Scholar] [CrossRef]
- Kim, S.; Kim, D.; Cho, S.W.; Kim, J.; Kim, J.-S. Highly efficient RNA-guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Res. 2014, 24, 1012–1019. [Google Scholar] [CrossRef]
- Jinek, M.; Jiang, F.; Taylor, D.W.; Sternberg, S.H.; Kaya, E.; Ma, E.; Anders, C.; Hauer, M.; Zhou, K.; Lin, S. Structures of Cas9 endonucleases reveal RNA-mediated conformational activation. Science 2014, 343, 1247997. [Google Scholar] [CrossRef]
- Shan, Q.; Wang, Y.; Li, J.; Gao, C. Genome editing in rice and wheat using the CRISPR/Cas system. Nat. Protoc. 2014, 9, 2395–2410. [Google Scholar] [CrossRef]
- Qin, W.; Dion, S.L.; Kutny, P.M.; Zhang, Y.; Cheng, A.W.; Jillette, N.L.; Malhotra, A.; Geurts, A.M.; Chen, Y.-G.; Wang, H. Efficient CRISPR/Cas9-mediated genome editing in mice by zygote electroporation of nuclease. Genetics 2015, 200, 423–430. [Google Scholar] [CrossRef]
- Zuris, J.A.; Thompson, D.B.; Shu, Y.; Guilinger, J.P.; Bessen, J.L.; Hu, J.H.; Maeder, M.L.; Joung, J.K.; Chen, Z.-Y.; Liu, D.R. Cationic lipid-mediated delivery of proteins enables efficient protein-based genome editing in vitro and in vivo. Nat. Biotechnol. 2015, 33, 73–80. [Google Scholar] [CrossRef]
- Moosburner, M.; Allen, A.E.; Daboussi, F. Genetic engineering in marine diatoms: Current practices and emerging technologies. In The Molecular Life of Diatoms; Springer Nature: Berlin/Heidelberg, Germany, 2022; pp. 743–773. [Google Scholar]
- Gilbert, L.A.; Horlbeck, M.A.; Adamson, B.; Villalta, J.E.; Chen, Y.; Whitehead, E.H.; Guimaraes, C.; Panning, B.; Ploegh, H.L.; Bassik, M.C. Genome-scale CRISPR-mediated control of gene repression and activation. Cell 2014, 159, 647–661. [Google Scholar] [CrossRef]
- Xu, X.; Qi, L.S. A CRISPR–dCas toolbox for genetic engineering and synthetic biology. J. Mol. Biol. 2019, 431, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Gao, Y.; Dominguez, A.A.; Qi, L.S. CRISPR technologies for precise epigenome editing. Nat. Cell Biol. 2021, 23, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Kampmann, M. CRISPRi and CRISPRa screens in mammalian cells for precision biology and medicine. ACS Chem. Biol. 2018, 13, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Pickar-Oliver, A.; Gersbach, C.A. The next generation of CRISPR-Cas technologies and applications. Nat. Rev. Mol. Cell Biol. 2019, 20, 490–507. [Google Scholar] [CrossRef]
- Katti, A.; Diaz, B.J.; Caragine, C.M.; Sanjana, N.E.; Dow, L.E. CRISPR in cancer biology and therapy. Nat. Rev. Cancer 2022, 22, 259–279. [Google Scholar] [CrossRef] [PubMed]
- van Diemen, F.R.; Lebbink, R.J. CRISPR/Cas9, a powerful tool to target human herpesviruses. Cell. Microbiol. 2017, 19, e12694. [Google Scholar] [CrossRef]
- Cox, D.B.; Platt, R.J.; Zhang, F. Therapeutic genome editing: Prospects and challenges. Nat. Med. 2015, 21, 121–131. [Google Scholar] [CrossRef]
- Zhu, H.; Li, C.; Gao, C. Applications of CRISPR-Cas in agriculture and plant biotechnology. Nat. Rev. Mol. Cell Biol. 2020, 21, 661–677. [Google Scholar] [CrossRef]
- Zubkov, M.V. Photoheterotrophy in marine prokaryotes. J. Plankton Res. 2009, 31, 933–938. [Google Scholar] [CrossRef]
- Vincent, F.; Vardi, A. Viral infection in the ocean—A journey across scales. PLoS Biol. 2023, 21, e3001966. [Google Scholar] [CrossRef] [PubMed]
- Orellana, R.; Arancibia, A.; Badilla, L.; Acosta, J.; Arancibia, G.; Escar, R.; Ferrada, G.; Seeger, M. Ecophysiological features shape the distribution of prophages and CRISPR in sulfate reducing prokaryotes. Microorganisms 2021, 9, 931. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-F.; Yao, Y.-F.; Xue, H.-P.; Fu, Z.-Y.; Zhang, X.-M.; Shao, Z. Characterization of Marinilongibacter aquaticus gen. nov., sp. nov., a unique marine bacterium harboring four CRISPR-Cas systems in the phylum Bacteroidota. J. Microbiol. 2022, 60, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Wietz, M.; Millán-Aguiñaga, N.; Jensen, P.R. CRISPR-Cas systems in the marine actinomycete Salinispora: Linkages with phage defense, microdiversity and biogeography. BMC Genom. 2014, 15, 936. [Google Scholar] [CrossRef] [PubMed]
- Terceti, M.S.; Vences, A.; Matanza, X.M.; Dalsgaard, I.; Pedersen, K.; Osorio, C.R. Molecular epidemiology of Photobacterium damselae subsp. damselae outbreaks in marine rainbow trout farms reveals extensive horizontal gene transfer and high genetic diversity. Front. Microbiol. 2018, 9, 2155. [Google Scholar] [CrossRef] [PubMed]
- López-Pérez, M.; Gonzaga, A.; Martin-Cuadrado, A.-B.; Onyshchenko, O.; Ghavidel, A.; Ghai, R.; Rodriguez-Valera, F. Genomes of surface isolates of Alteromonas macleodii: The life of a widespread marine opportunistic copiotroph. Sci. Rep. 2012, 2, 696. [Google Scholar] [CrossRef]
- Huang, Z.; Yu, K.; Fu, S.; Xiao, Y.; Wei, Q.; Wang, D. Genomic analysis reveals high intra-species diversity of Shewanella algae. Microb. Genom. 2022, 8, 000786. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yan, C.; Liu, M.; Liu, Y.; Wang, W.; Cheng, W.; Yang, F.; Zhang, J. CRISPR/Cas9-mediated deletion of one carotenoid isomerooxygenase gene (EcNinaB-X1) from Exopalaemon carinicauda. Fish Shellfish Immunol. 2020, 97, 421–431. [Google Scholar] [CrossRef]
- Cai, F.; Axen, S.D.; Kerfeld, C.A. Evidence for the widespread distribution of CRISPR-Cas system in the Phylum Cyanobacteria. RNA Biol. 2013, 10, 687–693. [Google Scholar] [CrossRef]
- Hou, S.; Brenes-Álvarez, M.; Reimann, V.; Alkhnbashi, O.S.; Backofen, R.; Muro-Pastor, A.M.; Hess, W.R. CRISPR-Cas systems in multicellular cyanobacteria. RNA Biol. 2019, 16, 518–529. [Google Scholar] [CrossRef]
- Chandrababunaidu, M.M.; Sen, D.; Tripathy, S. Draft genome sequence of filamentous marine cyanobacterium Lyngbya confervoides strain BDU141951. Genome Announc. 2015, 3, e00066-15. [Google Scholar] [CrossRef] [PubMed]
- Batchu, N.K.; Khater, S.; Patil, S.; Nagle, V.; Das, G.; Bhadra, B.; Sapre, A.; Dasgupta, S. Whole genome sequence analysis of Geitlerinema sp. FC II unveils competitive edge of the strain in marine cultivation system for biofuel production. Genomics 2019, 111, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Webb, E.A.; Held, N.A.; Zhao, Y.; Graham, E.D.; Conover, A.E.; Semones, J.; Lee, M.D.; Feng, Y.; Fu, F.X.; Saito, M.A.; et al. Importance of mobile genetic element immunity in numerically abundant Trichodesmium clades. ISME Commun. 2023, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Alex, A.; Antunes, A. Genus-wide comparison of Pseudovibrio bacterial genomes reveal diverse adaptations to different marine invertebrate hosts. PLoS ONE 2018, 13, e0194368. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Yu, M.; Wang, X.; Zhang, X.-H. Comparative genomic analysis reveals the evolution and environmental adaptation strategies of vibrios. BMC Genom. 2018, 19, 135. [Google Scholar] [CrossRef] [PubMed]
- Kalatzis, P.G.; Castillo, D.; Katharios, P.; Middelboe, M. Bacteriophage interactions with marine pathogenic vibrios: Implications for phage therapy. Antibiotics 2018, 7, 15. [Google Scholar] [CrossRef]
- Baliga, P.; Shekar, M.; Venugopal, M.N. Investigation of direct repeats, spacers and proteins associated with clustered regularly interspaced short palindromic repeat (CRISPR) system of Vibrio parahaemolyticus. Mol. Genet. Genom. 2019, 294, 253–262. [Google Scholar] [CrossRef]
- Machado, H.; Gram, L. Comparative genomics reveals high genomic diversity in the genus Photobacterium. Front. Microbiol. 2017, 8, 1204. [Google Scholar] [CrossRef]
- McDonald, N.D.; Regmi, A.; Morreale, D.P.; Borowski, J.D.; Boyd, E.F. CRISPR-Cas systems are present predominantly on mobile genetic elements in Vibrio species. BMC Genom. 2019, 20, 105. [Google Scholar] [CrossRef]
- Stoddard, L.I.; Martiny, J.B.; Marston, M.F. Selection and characterization of cyanophage resistance in marine Synechococcus strains. Appl. Environ. Microb. 2007, 73, 5516–5522. [Google Scholar] [CrossRef]
- Avrani, S.; Wurtzel, O.; Sharon, I.; Sorek, R.; Lindell, D. Genomic island variability facilitates Prochlorococcus–virus coexistence. Nature 2011, 474, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-W.; Yang, S.-H.; Hwangbo, M.; Chu, K.-H. Analysis of Zobellella denitrificans ZD1 draft genome: Genes and gene clusters responsible for high polyhydroxybutyrate (PHB) production from glycerol under saline conditions and its CRISPR-Cas system. PLoS ONE 2019, 14, e0222143. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, X.; Zhao, C.; Chen, S.; Yang, S. Comparative genome analysis of marine purple sulfur bacterium Marichromatium gracile YL28 reveals the diverse nitrogen cycle mechanisms and habitat-specific traits. Sci. Rep. 2018, 8, 17803. [Google Scholar] [CrossRef] [PubMed]
- Math, R.K.; Jin, H.M.; Kim, J.M.; Hahn, Y.; Park, W.; Madsen, E.L.; Jeon, C.O. Comparative genomics reveals adaptation by Alteromonas sp. SN2 to marine tidal-flat conditions: Cold tolerance and aromatic hydrocarbon metabolism. PLoS ONE 2012, 7, e35784. [Google Scholar] [CrossRef] [PubMed]
- Baby, B.; Vijay, D.; Alnuaimi, A.A.; Vijayan, R.; Akhtar, M.K. Complete genome sequence of V. gazogenes PB1: An estuarine bacterium capable of producing prodigiosin from starch or cellulose. Front. Mar. Sci. 2023, 10, 1028319. [Google Scholar] [CrossRef]
- Fonseca, A.; Ishoey, T.; Espinoza, C.; Perez-Pantoja, D.; Manghisi, A.; Morabito, M.; Salas-Burgos, A.; Gallardo, V.A. Genomic features of “Candidatus Venteria ishoeyi”, a new sulfur-oxidizing macrobacterium from the Humboldt Sulfuretum off Chile. PLoS ONE 2017, 12, e0188371. [Google Scholar] [CrossRef]
- Zhang, D.-F.; Cui, X.-W.; Li, W.-J.; Zhang, X.-M.; Xue, H.-P.; Huang, J.-K.; Zhang, A.-H. Description of Salinimonas profundi sp. nov., a deep-sea bacterium harboring a transposon Tn6333. Antonie Leeuwenhoek 2021, 114, 69–81. [Google Scholar] [CrossRef]
- Patwardhan, S.; Phan, J.; Smedile, F.; Vetriani, C. The genome of Varunaivibrio sulfuroxidans Strain TC8T, a metabolically versatile alphaproteobacterium from the tor caldara gas vents in the Tyrrhenian Sea. Microorganisms 2023, 11, 1366. [Google Scholar] [CrossRef]
- Skoog, E.J.; Huber, J.A.; Serres, M.H.; Levesque, A.; Zeigler Allen, L. Draft genome sequence of Desulfurobacterium sp. Strain AV08, a thermophilic chemolithoautotroph Isolated from a deep-sea hydrothermal vent. Microbiol. Resour. Announc. 2021, 10, e00615-21. [Google Scholar] [CrossRef]
- Allioux, M.; Yvenou, S.; Godfroy, A.; Shao, Z.; Jebbar, M.; Alain, K. Genome analysis of a new sulphur disproportionating species Thermosulfurimonas strain F29 and comparative genomics of sulfur-disproportionating bacteria from marine hydrothermal vents. Microb. Genom. 2022, 8, mgen000865. [Google Scholar] [CrossRef]
- Luo, H.; Sun, Y.; Hollibaugh, J.T.; Moran, M.A. Low genome content diversity of marine planktonic Thaumarchaeota. Environ. Microbiol. Rep. 2016, 8, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Kelley, J.F. Expanding Metabolic Diversity of Two Archaeal Phyla: Nanoarchaeota and Korarchaeota. Ph.D. Thesis, Portland State University, Portland, Oregon, 2017. [Google Scholar]
- Wang, P.; Li, M.; Dong, L.; Zhang, C.; Xie, W. Comparative genomics of Thaumarchaeota from deep-sea sponges reveal their niche adaptation. Front. Microbiol. 2022, 13, 869834. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Gao, Z.-M.; Li, W.-L.; Wei, Z.-F.; Wei, T.-S.; Huang, J.-M.; Li, M.; Tao, J.; Wang, H.-B.; Wang, Y. Asgard archaea in the haima cold seep: Spatial distribution and genomic insights. Deep Sea Res. Part I Oceanogr. Res. Pap. 2021, 170, 103489. [Google Scholar] [CrossRef]
- Maslać, N.; Sidhu, C.; Teeling, H.; Wagner, T. Comparative transcriptomics sheds light on remodeling of gene expression during diazotrophy in the thermophilic methanogen Methanothermococcus thermolithotrophicus. mBio 2022, 13, e02443-22. [Google Scholar] [CrossRef] [PubMed]
- Norais, C.; Moisan, A.; Gaspin, C.; Clouet-d’Orval, B. Diversity of CRISPR systems in the euryarchaeal Pyrococcales. RNA Biol. 2013, 10, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-B.; Hou, X.-J.; Wu, C.; Zhao, Z.; Ju, Z.; Zhang, R.; Cui, H.-L.; Keen, L.J.; Xu, L.; Wu, M. Complete genome sequence of Salinigranum rubrum GX10T, an extremely halophilic archaeon isolated from a marine solar saltern. Mar. Genom. 2019, 44, 57–60. [Google Scholar] [CrossRef]
- Ding, Y.; Han, D.; Cui, H.-L. Halorussus halophilus sp. nov., a novel halophilic archaeon isolated from a marine solar saltern. Curr. Microbiol. 2020, 77, 1321–1327. [Google Scholar] [CrossRef]
- Jungblut, A.D.; Raymond, F.; Dion, M.B.; Moineau, S.; Mohit, V.; Nguyen, G.Q.; Déraspe, M.; Francovic-Fontaine, É.; Lovejoy, C.; Culley, A.I. Genomic diversity and CRISPR-Cas systems in the cyanobacterium Nostoc in the High Arctic. Environ. Microbiol. 2021, 23, 2955–2968. [Google Scholar] [CrossRef]
- Teikari, J.E.; Hou, S.; Wahlsten, M.; Hess, W.R.; Sivonen, K. Comparative genomics of the Baltic Sea toxic cyanobacteria Nodularia spumigena UHCC 0039 and its response to varying salinity. Front. Microbiol. 2018, 9, 356. [Google Scholar] [CrossRef]
- Saw, J.H.; Yuryev, A.; Kanbe, M.; Hou, S.; Young, A.G.; Aizawa, S.-I.; Alam, M. Complete genome sequencing and analysis of Saprospira grandis str. Lewin, a predatory marine bacterium. Stand. Genom. Sci. 2012, 6, 84–93. [Google Scholar]
- Zhang, B.-C.; Zhang, J.; Sun, L. Streptococcus iniae SF1: Complete genome sequence, proteomic profile, and immunoprotective antigens. PLoS ONE 2014, 9, e91324. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, C.; Hjerde, E.; Klemetsen, T.; Willassen, N.P. Pan genome and CRISPR analyses of the bacterial fish pathogen Moritella viscosa. BMC Genom. 2017, 18, 313. [Google Scholar] [CrossRef] [PubMed]
- Lian, C.-A.; Yan, G.-Y.; Huang, J.-M.; Danchin, A.; Wang, Y.; He, L.-S. Genomic characterization of a novel gut symbiont from the hadal snailfish. Front. Microbiol. 2020, 10, 2978. [Google Scholar] [CrossRef] [PubMed]
- Freitas-Silva, J.; de Oliveira, B.F.R.; Vigoder, F.d.M.; Muricy, G.; Dobson, A.D.; Laport, M.S. Peeling the layers away: The genomic characterization of Bacillus pumilus 64-1, an isolate with antimicrobial activity from the marine sponge Plakina cyanorosea (Porifera, Homoscleromorpha). Front. Microbiol. 2021, 11, 592735. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Jagadeeshwari, U.; Sreya, P.; Shabbir, A.; Sasikala, C.; Ramana, C.V. A genomic overview including polyphasic taxonomy of Thalassoroseus pseudoceratinae gen. nov., sp. nov. isolated from a marine sponge, Pseudoceratina sp. Antonie Leeuwenhoek 2022, 115, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Fang, Y.-C.; Li, H.; Chen, J.; Ye, Y.-L.; Ni, L.-F.; Xu, L.; Han, B.-N.; Wu, M.; Wang, C.-S. Complete genome sequence of marine Roseobacter lineage member Monaibacterium sp. ALG8 with six plasmids isolated from seawater around brown algae. Mar. Genom. 2021, 60, 100878. [Google Scholar] [CrossRef]
- Chan, Y.W.; Millard, A.D.; Wheatley, P.J.; Holmes, A.B.; Mohr, R.; Whitworth, A.L.; Mann, N.H.; Larkum, A.W.; Hess, W.R.; Scanlan, D.J. Genomic and proteomic characterization of two novel siphovirus infecting the sedentary facultative epibiont cyanobacterium Acaryochloris marina. Environ. Microbiol. 2015, 17, 4239–4252. [Google Scholar] [CrossRef]
- De Oliveira, A.L.; Srivastava, A.; Espada-Hinojosa, S.; Bright, M. The complete and closed genome of the facultative generalist Candidatus Endoriftia persephone from deep-sea hydrothermal vents. Mol. Ecol. Resour. 2022, 22, 3106–3123. [Google Scholar] [CrossRef]
- Dahle, H.; Roalkvam, I.; Thorseth, I.H.; Pedersen, R.B.; Steen, I.H. The versatile in situ gene expression of an Epsilonproteobacteria-dominated biofilm from a hydrothermal chimney. Environ. Microbiol. Rep. 2013, 5, 282–290. [Google Scholar] [CrossRef]
- Zhang, W.; Ding, W.; Li, Y.-X.; Tam, C.; Bougouffa, S.; Wang, R.; Pei, B.; Chiang, H.; Leung, P.; Lu, Y. Marine biofilms constitute a bank of hidden microbial diversity and functional potential. Nat. Commun. 2019, 10, 517. [Google Scholar] [CrossRef]
- Mion, S.; Plener, L.; Rémy, B.; Daudé, D.; Chabrière, É. Lactonase SsoPox modulates CRISPR-Cas expression in gram-negative proteobacteria using AHL-based quorum sensing systems. Res. Microbiol. 2019, 170, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Jaiani, E.; Kusradze, I.; Kokashvili, T.; Geliashvili, N.; Janelidze, N.; Kotorashvili, A.; Kotaria, N.; Guchmanidze, A.; Tediashvili, M.; Prangishvili, D. Microbial diversity and phage–host Interactions in the georgian coastal area of the Black Sea revealed by whole genome metagenomic sequencing. Mar. Drugs 2020, 18, 558. [Google Scholar] [CrossRef] [PubMed]
- De Menezes, T.A.; De Freitas, M.A.; Lima, M.S.; Soares, A.C.; Leal, C.; Busch, M.d.S.; Tschoeke, D.A.; Vidal, L.d.O.; Atella, G.C.; Kruger, R.H. Fluxes of the Amazon River plume nutrients and microbes into marine sponges. Sci. Total Environ. 2022, 847, 157474. [Google Scholar] [CrossRef] [PubMed]
- Robbins, S.; Song, W.; Engelberts, J.; Glasl, B.; Slaby, B.M.; Boyd, J.; Marangon, E.; Botté, E.; Laffy, P.; Thomas, T. A genomic view of the microbiome of coral reef demosponges. ISME J. 2021, 15, 1641–1654. [Google Scholar] [CrossRef] [PubMed]
- Karimi, E.; Ramos, M.; Gonçalves, J.M.; Xavier, J.R.; Reis, M.P.; Costa, R. Comparative metagenomics reveals the distinctive adaptive features of the Spongia officinalis endosymbiotic consortium. Front. Microbiol. 2017, 8, 2499. [Google Scholar] [CrossRef]
- Gauthier, M.-E.A.; Watson, J.R.; Degnan, S.M. Draft genomes shed light on the dual bacterial symbiosis that dominates the microbiome of the coral reef sponge Amphimedon queenslandica. Front. Mar. Sci. 2016, 3, 196. [Google Scholar] [CrossRef]
- Wei, T.-S.; Gao, Z.-M.; Gong, L.; Li, Q.-M.; Zhou, Y.-L.; Chen, H.-G.; He, L.-S.; Wang, Y. Genome-centric view of the microbiome in a new deep-sea glass sponge species Bathydorus sp. Front. Microbiol. 2023, 14, 1078171. [Google Scholar] [CrossRef]
- Huescas, C.; Pereira, R.; Prichula, J.; Azevedo, P.; Frazzon, J.; Frazzon, A. Frequency of clustered regularly interspaced short palindromic repeats (CRISPRs) in non-clinical Enterococcus faecalis and Enterococcus faecium strains. Braz. J. Biol. 2018, 79, 460–465. [Google Scholar] [CrossRef]
- Lian, C.-A.; Zhu, F.-C.; Wei, Z.-F.; He, L.-S. Composition and potential functions of the dominant microbiota in deep-sea hagfish gut from the South China Sea. Deep Sea Res. Part I Oceanogr. Res. Pap. 2021, 169, 103488. [Google Scholar] [CrossRef]
- Weinberger, A.D.; Wolf, Y.I.; Lobkovsky, A.E.; Gilmore, M.S.; Koonin, E.V. Viral diversity threshold for adaptive immunity in prokaryotes. mBio 2012, 3, e00456-12. [Google Scholar] [CrossRef]
- Colangelo-Lillis, J.R.; Deming, J.W. Genomic analysis of cold-active Colwelliaphage 9A and psychrophilic phage–host interactions. Extremophiles 2013, 17, 99–114. [Google Scholar] [CrossRef]
- Shtratnikova, V.Y.; Belalov, I.; Kasianov, A.S.; Schelkunov, M.I.; Maria, D.L.; Novikov, A.D.; Shatalov, A.A.; Gerasimova, T.V.; Yanenko, A.S.; Makeev, V.J. The complete genome of the oil emulsifying strain Thalassolituus oleivorans K-188 from the Barents Sea. Mar. Genom. 2018, 37, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Lechner, M.; Nickel, A.I.; Wehner, S.; Riege, K.; Wieseke, N.; Beckmann, B.M.; Hartmann, R.K.; Marz, M. Genomewide comparison and novel ncRNAs of Aquificales. BMC Genom. 2014, 15, 522. [Google Scholar] [CrossRef] [PubMed]
- Haverkamp, T.H.; Geslin, C.; Lossouarn, J.; Podosokorskaya, O.A.; Kublanov, I.; Nesbø, C.L. Thermosipho spp. immune system differences affect variation in genome size and geographical distributions. Genome Biol. Evol. 2018, 10, 2853–2866. [Google Scholar] [CrossRef] [PubMed]
- Prangishvili, D.; Bamford, D.H.; Forterre, P.; Iranzo, J.; Koonin, E.V.; Krupovic, M. The enigmatic archaeal virosphere. Nat. Rev. Microbiol. 2017, 15, 724–739. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, R.A.; Fu, G.Y.; Zhang, R.; Nie, Y.F.; Sun, C.; Wu, M. The Complete Genome of Emcibacter congregatus ZYL(T), a Marine Bacterium Encoding a CRISPR-Cas 9 Immune System. Curr. Microbiol. 2020, 77, 762–768. [Google Scholar] [CrossRef]
- Dudek, N.K.; Sun, C.L.; Burstein, D.; Kantor, R.S.; Goltsman, D.S.A.; Bik, E.M.; Thomas, B.C.; Banfield, J.F.; Relman, D.A. Novel microbial diversity and functional potential in the marine mammal oral microbiome. Curr. Biol. 2017, 27, 3752–3762. [Google Scholar] [CrossRef]
- Silas, S.; Mohr, G.; Sidote, D.J.; Markham, L.M.; Sanchez-Amat, A.; Bhaya, D.; Lambowitz, A.M.; Fire, A.Z. Direct CRISPR spacer acquisition from RNA by a natural reverse transcriptase–Cas1 fusion protein. Science 2016, 351, aad4234. [Google Scholar] [CrossRef]
- Nasko, D.J.; Ferrell, B.D.; Moore, R.M.; Bhavsar, J.D.; Polson, S.W.; Wommack, K.E. CRISPR spacers indicate preferential matching of specific virioplankton genes. mBio 2019, 10, e02651-18. [Google Scholar] [CrossRef]
- Šulčius, S.; Šimoliūnas, E.; Alzbutas, G.; Gasiūnas, G.; Jauniškis, V.; Kuznecova, J.; Miettinen, S.; Nilsson, E.; Meškys, R.; Roine, E. Genomic characterization of cyanophage vB_AphaS-CL131 infecting filamentous diazotrophic cyanobacterium Aphanizomenon flos-aquae reveals novel insights into virus-bacterium interactions. Appl. Environ. Microbiol. 2019, 85, e01311-18. [Google Scholar] [CrossRef]
- Silas, S.; Lucas-Elio, P.; Jackson, S.A.; Aroca-Crevillen, A.; Hansen, L.L.; Fineran, P.C.; Fire, A.Z.; Sanchez-Amat, A. Type III CRISPR-Cas systems can provide redundancy to counteract viral escape from type I systems. eLife 2017, 6, e27601. [Google Scholar] [CrossRef]
- Al-Shayeb, B.; Sachdeva, R.; Chen, L.-X.; Ward, F.; Munk, P.; Devoto, A.; Castelle, C.J.; Olm, M.R.; Bouma-Gregson, K.; Amano, Y. Clades of huge phages from across Earth’s ecosystems. Nature 2020, 578, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Bellas, C.M.; Anesio, A.M.; Barker, G. Analysis of virus genomes from glacial environments reveals novel virus groups with unusual host interactions. Front. Microbiol. 2015, 6, 656. [Google Scholar] [CrossRef] [PubMed]
- Kosmopoulos, J.C.; Campbell, D.E.; Whitaker, R.; Wilbanks, E. Horizontal gene transfer and CRISPR targeting drive phage-bacterial host interactions and co-evolution in pink berry marine microbial aggregates. bioRxiv 2023. [Google Scholar] [CrossRef]
- Kopfmann, S.; Hess, W.R. Toxin-antitoxin systems on the large defense plasmid pSYSA of Synechocystis sp. PCC 6803. J. Biol. Chem. 2013, 288, 7399–7409. [Google Scholar] [CrossRef] [PubMed]
- Niu, T.-C.; Lin, G.-M.; Xie, L.-R.; Wang, Z.-Q.; Xing, W.-Y.; Zhang, J.-Y.; Zhang, C.-C. Expanding the potential of CRISPR-Cpf1-based genome editing technology in the cyanobacterium Anabaena PCC 7120. ACS Synth. Biol. 2018, 8, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Braesel, J.; Lee, J.-H.; Arnould, B.; Murphy, B.T.; Eustáquio, A.S. Diazaquinomycin biosynthetic gene clusters from marine and freshwater actinomycetes. J. Nat. Prod. 2019, 82, 937–946. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, F.; Tong, Y.; Habisch, R.; Yang, B.; Zhang, L.; Müller, R.; Fu, C. Dual-function chromogenic screening-based CRISPR/Cas9 genome editing system for actinomycetes. Appl. Microbiol. Biotechnol. 2020, 104, 225–239. [Google Scholar] [CrossRef]
- Wu, Z.-Y.; Huang, Y.-T.; Chao, W.-C.; Ho, S.-P.; Cheng, J.-F.; Liu, P.-Y. Reversal of carbapenem-resistance in Shewanella algae by CRISPR/Cas9 genome editing. J. Adv. Res. 2019, 18, 61–69. [Google Scholar] [CrossRef]
- Alker, A.T.; Farrell, M.V.; Aspiras, A.E.; Dunbar, T.L.; Fedoriouk, A.; Jones, J.E.; Mikhail, S.R.; Salcedo, G.Y.; Moore, B.S.; Shikuma, N.J. A modular plasmid toolkit applied in marine bacteria reveals functional insights during bacteria-stimulated metamorphosis. mBio 2023, 14, e01502-23. [Google Scholar] [CrossRef]
- Yin, Q.-J.; Zhang, W.-J.; Qi, X.-Q.; Zhang, S.-D.; Jiang, T.; Li, X.-G.; Chen, Y.; Santini, C.-L.; Zhou, H.; Chou, I.-M. High hydrostatic pressure inducible trimethylamine N-oxide reductase improves the pressure tolerance of piezosensitive bacteria Vibrio fluvialis. Front. Microbiol. 2018, 8, 2646. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Ostrov, N.; Wong, B.G.; Gold, M.A.; Khalil, A.S.; Church, G.M. Functional genomics of the rapidly replicating bacterium Vibrio natriegens by CRISPRi. Nat. Microbiol. 2019, 4, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Stukenberg, D.; Hoff, J.; Faber, A.; Becker, A. NT-CRISPR, combining natural transformation and CRISPR-Cas9 counterselection for markerless and scarless genome editing in Vibrio natriegens. Commun. Biol. 2022, 5, 265. [Google Scholar] [CrossRef]
- Wei, Y.; Feng, L.-J.; Yuan, X.-Z.; Wang, S.-G.; Xia, P.-F. Developing a base editing system for marine Roseobacter clade bacteria. ACS Synth. Biol. 2023, 12, 2178–2186. [Google Scholar] [CrossRef] [PubMed]
- Gordon, G.C.; Korosh, T.C.; Cameron, J.C.; Markley, A.L.; Begemann, M.B.; Pfleger, B.F. CRISPR interference as a titratable, trans-acting regulatory tool for metabolic engineering in the cyanobacterium Synechococcus sp. strain PCC 7002. Metab. Eng. 2016, 38, 170–179. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Xu, Q.; Zhang, W.; Li, Z.; Chen, L.; Dong, X. CRISPR-Cas9 toolkit for genome editing in an autotrophic CO2-fixing methanogenic archaeon. Microbiol. Spectr. 2022, 10, e01165-22. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.E.; Brazelton, W.J.; Baross, J.A. Using CRISPRs as a metagenomic tool to identify microbial hosts of a diffuse flow hydrothermal vent viral assemblage. FEMS Microbiol. Ecol. 2011, 77, 120–133. [Google Scholar] [CrossRef]
- Palmer, M.; Hedlund, B.P.; Roux, S.; Tsourkas, P.K.; Doss, R.K.; Stamereilers, C.; Mehta, A.; Dodsworth, J.A.; Lodes, M.; Monsma, S. Diversity and distribution of a novel genus of hyperthermophilic aquificae viruses encoding a proof-reading family-A DNA polymerase. Front. Microbiol. 2020, 11, 583361. [Google Scholar] [CrossRef]
- Yoshida, M.; Yoshida-Takashima, Y.; Nunoura, T.; Takai, K. Identification and genomic analysis of temperate Pseudomonas bacteriophage PstS-1 from the Japan trench at a depth of 7000 m. Res. Microbiol. 2015, 166, 668–676. [Google Scholar] [CrossRef]
- Rusanova, A.; Fedorchuk, V.; Toshchakov, S.; Dubiley, S.; Sutormin, D. An interplay between viruses and bacteria associated with the White Sea sponges revealed by metagenomics. Life 2021, 12, 25. [Google Scholar] [CrossRef]
- Luo, E.; Leu, A.O.; Eppley, J.M.; Karl, D.M.; DeLong, E.F. Diversity and origins of bacterial and archaeal viruses on sinking particles reaching the abyssal ocean. ISME J. 2022, 16, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Kalatzis, P.G.; Rørbo, N.; Castillo, D.; Mauritzen, J.J.; Jørgensen, J.; Kokkari, C.; Zhang, F.; Katharios, P.; Middelboe, M. Stumbling across the same phage: Comparative genomics of widespread temperate phages infecting the fish pathogen Vibrio anguillarum. Viruses 2017, 9, 122. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhou, L.; Zhou, Y.; Wang, H.; Xiao, J.; Yan, S.; Wang, Y. Diverse and unique viruses discovered in the surface water of the East China Sea. BMC Genom. 2020, 21, 441. [Google Scholar] [CrossRef] [PubMed]
- Hoffert, M.; Anderson, R.E.; Reveillaud, J.; Murphy, L.G.; Stepanauskas, R.; Huber, J.A. Genomic variation influences Methanothermococcus fitness in marine hydrothermal systems. Front. Microbiol. 2021, 12, 714920. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zeng, H.; Zhang, J.; Luo, D.; Chen, M.; Lei, T.; Yang, X.; Wu, H.; Cai, S.; Ye, Y. Cronobacter spp. isolated from aquatic products in China: Incidence, antibiotic resistance, molecular characteristic and CRISPR diversity. Int. J. Food Microbiol. 2020, 335, 108857. [Google Scholar] [CrossRef] [PubMed]
- Steinum, T.; Turgay, E.; Yardımcı, R.; Småge, S.; Karataş, S. Tenacibaculum maritimum CRISPR loci analysis and evaluation of isolate spoligotyping. J. Appl. Microbiol. 2021, 131, 1848–1857. [Google Scholar] [CrossRef]
- Jingjit, N.; Preeprem, S.; Surachat, K.; Mittraparp-Arthorn, P. Characterization and analysis of clustered regularly interspaced short palindromic repeats (CRISPRs) in pandemic and non-pandemic Vibrio parahaemolyticus isolates from seafood sources. Microorganisms 2021, 9, 1220. [Google Scholar] [CrossRef]
- Sorokin, V.A.; Gelfand, M.S.; Artamonova, I.I. Evolutionary dynamics of clustered irregularly interspaced short palindromic repeat systems in the ocean metagenome. Appl. Environ. Microb. 2010, 76, 2136–2144. [Google Scholar] [CrossRef]
- White, R.A., III; Wong, H.L.; Ruvindy, R.; Neilan, B.A.; Burns, B.P. Viral communities of Shark Bay modern stromatolites. Front. Microbiol. 2018, 9, 1223. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, L.; Yan, S.; Chen, L.; Krupovic, M.; Wang, Y. Diverse viruses of marine archaea discovered using metagenomics. Environ. Microbiol. 2023, 25, 367–382. [Google Scholar] [CrossRef]
- Bartlau, N.; Wichels, A.; Krohne, G.; Adriaenssens, E.M.; Heins, A.; Fuchs, B.M.; Amann, R.; Moraru, C. Highly diverse flavobacterial phages isolated from North Sea spring blooms. ISME J. 2022, 16, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Kindler, G.S.; Wong, H.L.; Larkum, A.W.; Johnson, M.; MacLeod, F.I.; Burns, B.P. Genome-resolved metagenomics provides insights into the functional complexity of microbial mats in Blue Holes, Shark Bay. FEMS Microbiol. Ecol. 2022, 98, fiab158. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.; Angers, B.; Young, C.R.; Juniper, S.K. Shining light on a deep-sea bacterial symbiont population structure with CRISPR. Microb. Genom. 2021, 7, 000625. [Google Scholar] [CrossRef]
- Jiang, W.; Brueggeman, A.J.; Horken, K.M.; Plucinak, T.M.; Weeks, D.P. Successful transient expression of Cas9 and single guide RNA genes in Chlamydomonas reinhardtii. Eukaryot. Cell 2014, 13, 1465–1469. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.Z.; Weeks, D.P. A gene-within-a-gene Cas9/sgRNA hybrid construct enables gene editing and gene replacement strategies in Chlamydomonas reinhardtii. Algal Res. 2017, 26, 474–480. [Google Scholar] [CrossRef]
- Baek, K.; Kim, D.H.; Jeong, J.; Sim, S.J.; Melis, A.; Kim, J.-S.; Jin, E.; Bae, S. DNA-free two-gene knockout in Chlamydomonas reinhardtii via CRISPR-Cas9 ribonucleoproteins. Sci. Rep. 2016, 6, 30620. [Google Scholar] [CrossRef]
- Shin, S.E.; Lim, J.M.; Koh, H.G.; Kim, E.K.; Kang, N.K.; Jeon, S.; Kwon, S.; Shin, W.S.; Lee, B.; Hwangbo, K.; et al. CRISPR/Cas9-induced knockout and knock-in mutations in Chlamydomonas reinhardtii. Sci. Rep. 2016, 6, 27810. [Google Scholar] [CrossRef]
- Nymark, M.; Sharma, A.K.; Sparstad, T.; Bones, A.M.; Winge, P. A CRISPR/Cas9 system adapted for gene editing in marine algae. Sci. Rep. 2016, 6, 24951. [Google Scholar] [CrossRef]
- Hopes, A.; Nekrasov, V.; Kamoun, S.; Mock, T. Editing of the urease gene by CRISPR-Cas in the diatom Thalassiosira pseudonana. Plant Methods 2016, 12, 49. [Google Scholar] [CrossRef]
- Belshaw, N.; Grouneva, I.; Aram, L.; Gal, A.; Hopes, A.; Mock, T. Efficient gene replacement by CRISPR/Cas-mediated homologous recombination in the model diatom Thalassiosira pseudonana. New Phytol. 2023, 238, 438–452. [Google Scholar] [CrossRef]
- Yin, W.; Hu, H. CRISPR/Cas9-mediated genome editing via homologous recombination in a centric diatom Chaetoceros muelleri. ACS Synth. Biol. 2023, 12, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Freudenberg, R.A.; Wittemeier, L.; Einhaus, A.; Baier, T.; Kruse, O. The spermidine synthase gene SPD1: A novel auxotrophic marker for Chlamydomonas reinhardtii designed by enhanced CRISPR/Cas9 gene editing. Cells 2022, 11, 837. [Google Scholar] [CrossRef] [PubMed]
- Kumar Sharma, A.; Muhlroth, A.; Jouhet, J.; Marechal, E.; Alipanah, L.; Kissen, R.; Brembu, T.; Bones, A.M.; Winge, P. The Myb-like transcription factor phosphorus starvation response (PtPSR) controls conditional P acquisition and remodelling in marine microalgae. New Phytol. 2020, 225, 2380–2395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhou, Z.; Li, J.; Wang, J.; Yu, L.; Lin, S. SPX-related genes regulate phosphorus homeostasis in the marine phytoplankton, Phaeodactylum tricornutum. Commun. Biol. 2021, 4, 797. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, J.; Wang, J.; Lin, X.; Li, L.; You, Y.; Wu, X.; Zhou, Z.; Lin, S. Functional differentiation and complementation of alkaline phosphatases and choreography of DOP scavenging in a marine diatom. Mol. Ecol. 2022, 31, 3389–3399. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, K.; Lin, X.; Li, L.; Lin, S. Unsuspected functions of alkaline phosphatase PhoD in the diatom Phaeodactylum tricornutum. Algal Res. 2022, 68, 102873. [Google Scholar] [CrossRef]
- Zhang, K.; Li, J.; Zhou, Z.; Huang, R.; Lin, S. Roles of alkaline phosphatase PhoA in algal metabolic regulation under phosphorus-replete conditions. J. Phycol. 2021, 57, 703–707. [Google Scholar] [CrossRef]
- Karl, D.M. Microbially mediated transformations of phosphorus in the sea: New views of an old cycle. Annu. Rev. Mar. Sci. 2014, 6, 279–337. [Google Scholar] [CrossRef]
- Lin, S.; Litaker, R.W.; Sunda, W.G. Phosphorus physiological ecology and molecular mechanisms in marine phytoplankton. J. Phycol. 2016, 52, 10–36. [Google Scholar] [CrossRef]
- You, Y.; Sun, X.; Ma, M.; He, J.; Li, L.; Porto, F.W.; Lin, S. Trypsin is a coordinate regulator of N and P nutrients in marine phytoplankton. Nat. Commun. 2022, 13, 4022. [Google Scholar] [CrossRef]
- Görlich, S.; Pawolski, D.; Zlotnikov, I.; Kröger, N. Control of biosilica morphology and mechanical performance by the conserved diatom gene Silicanin-1. Commun. Biol. 2019, 2, 245. [Google Scholar] [CrossRef] [PubMed]
- Coale, T.H.; Moosburner, M.; Horák, A.; Oborník, M.; Barbeau, K.A.; Allen, A.E. Reduction-dependent siderophore assimilation in a model pennate diatom. Proc. Natl. Acad. Sci. USA 2019, 116, 23609–23617. [Google Scholar] [CrossRef] [PubMed]
- Graff van Creveld, S.; Coesel, S.N.; Blaskowski, S.; Groussman, R.D.; Schatz, M.J.; Armbrust, E.V. Divergent functions of two clades of flavodoxin in diatoms mitigate oxidative stress and iron limitation. Elife 2023, 12, e84392. [Google Scholar] [CrossRef] [PubMed]
- Baek, K.; Yu, J.; Jeong, J.; Sim, S.J.; Bae, S.; Jin, E. Photoautotrophic production of macular pigment in a Chlamydomonas reinhardtii strain generated by using DNA-free CRISPR-Cas9 RNP-mediated mutagenesis. Biotechnol. Bioeng. 2018, 115, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Kirst, H.; Garcia-Cerdan, J.G.; Zurbriggen, A.; Ruehle, T.; Melis, A. Truncated photosystem chlorophyll antenna size in the green microalga Chlamydomonas reinhardtii upon deletion of the TLA3-CpSRP43 gene. Plant Physiol. 2012, 160, 2251–2260. [Google Scholar] [CrossRef] [PubMed]
- Nymark, M.; Gronbech Hafskjold, M.C.; Volpe, C.; Fonseca, D.M.; Sharma, A.; Tsirvouli, E.; Serif, M.; Winge, P.; Finazzi, G.; Bones, A.M. Functional studies of CpSRP54 in diatoms show that the mechanism of thylakoid protein insertion differs from that in plants and green algae. Plant J. 2021, 106, 113–132. [Google Scholar] [CrossRef]
- Nymark, M.; Finazzi, G.; Volpe, C.; Serif, M.; Fonseca, D.M.; Sharma, A.; Sanchez, N.; Sharma, A.K.; Ashcroft, F.; Kissen, R.; et al. Loss of CpFTSY reduces photosynthetic performance and affects insertion of PsaC of PSI in diatoms. Plant Cell Physiol. 2023, 64, 583–603. [Google Scholar] [CrossRef]
- Sharma, A.K.; Nymark, M.; Flo, S.; Sparstad, T.; Bones, A.M.; Winge, P. Simultaneous knockout of multiple LHCF genes using single sgRNAs and engineering of a high-fidelity Cas9 for precise genome editing in marine algae. Plant Biotechnol. J. 2021, 19, 1658–1669. [Google Scholar] [CrossRef]
- Buck, J.M.; Wünsch, M.; Schober, A.F.; Kroth, P.G.; Lepetit, B. Impact of Lhcx2 on acclimation to low iron conditions in the diatom Phaeodactylum tricornutum. Front. Plant Sci. 2022, 13, 841058. [Google Scholar] [CrossRef]
- Wang, L.; Xie, X.; Gu, W.; Zheng, Z.; Chen, M.; Wang, G. LHCF15 facilitates the absorption of longer wavelength light and promotes growth of Phaeodactylum tricornutum under red light. Algal Res. 2023, 75, 103249. [Google Scholar] [CrossRef]
- Jiang, Y.; Cao, T.; Yang, Y.; Zhang, H.; Zhang, J.; Li, X. A chlorophyll c synthase widely co-opted by phytoplankton. Science 2023, 382, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhou, L.; Wang, J.; Wang, L.; Gao, S.; Wang, G. Knockout of a diatom cryptochrome by CRISPR/Cas9 causes an increase in light-harvesting protein levels and accumulation of fucoxanthin. Algal Res. 2022, 66, 102822. [Google Scholar] [CrossRef]
- Bai, Y.; Cao, T.; Dautermann, O.; Buschbeck, P.; Cantrell, M.B.; Chen, Y.; Lein, C.D.; Shi, X.; Ware, M.A.; Yang, F. Green diatom mutants reveal an intricate biosynthetic pathway of fucoxanthin. Proc. Natl. Acad. Sci. USA 2022, 119, e2203708119. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Feng, S.; Liang, G.; Du, J.; Li, A.; Niu, C. CRISPR/Cas9-induced beta-carotene hydroxylase mutation in Dunaliella salina CCAP19/18. AMB Express 2021, 11, 83. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.S.; Han, S.I.; Jeon, M.; Choi, Y.E. Enhancement of phycoerythrin productivity in Porphyridium purpureum using the clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9 ribonucleoprotein system. Bioresour. Technol. 2021, 330, 124974. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Hu, F.; Pan, Y.; Li, C.; Hu, H. Pyruvate orthophosphate dikinase is required for the acclimation to high bicarbonate concentrations in Phaeodactylum tricornutum. Algal Res. 2023, 72, 103131. [Google Scholar] [CrossRef]
- Rodolfi, L.; Chini Zittelli, G.; Bassi, N.; Padovani, G.; Biondi, N.; Bonini, G.; Tredici, M.R. Microalgae for oil: Strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng. 2009, 102, 100–112. [Google Scholar] [CrossRef]
- Schenk, P.M.; Thomas-Hall, S.R.; Stephens, E.; Marx, U.C.; Mussgnug, J.H.; Posten, C.; Kruse, O.; Hankamer, B. Second generation biofuels: High-efficiency microalgae for biodiesel production. BioEnergy Res. 2008, 1, 20–43. [Google Scholar] [CrossRef]
- Halim, R.; Danquah, M.K.; Webley, P.A. Extraction of oil from microalgae for biodiesel production: A review. Biotechnol. Adv. 2012, 30, 709–732. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Ranjbar, S.; Malcata, F.X. Challenges and prospects for sustainable microalga-based oil: A comprehensive review, with a focus on metabolic and genetic engineering. Fuel 2022, 324, 124567. [Google Scholar] [CrossRef]
- Kao, P.-H.; Ng, I.S. CRISPRi mediated phosphoenolpyruvate carboxylase regulation to enhance the production of lipid in Chlamydomonas reinhardtii. Bioresour. Technol. 2017, 245, 1527–1537. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.S.; Jeong, J.; Nguyen, T.H.T.; Kim, J.Y.H.; Jin, E.; Sim, S.J. Targeted knockout of phospholipase A2 to increase lipid productivity in Chlamydomonas reinhardtii for biodiesel production. Bioresour. Technol. 2019, 271, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.T.; Park, S.; Jeong, J.; Shin, Y.S.; Sim, S.J.; Jin, E. Increasing lipid productivity in Chlamydomonas by engineering lipid catabolism using the CRISPR-Cas9 system. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Lin, W.R.; Ng, I.S. Development of CRISPR/Cas9 system in Chlorella vulgaris FSP-E to enhance lipid accumulation. Enzym. Microb. Technol. 2020, 133, 109458. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Jouhet, J.; Gandini, C.; Nekrasov, V.; Marechal, E.; Napier, J.A.; Sayanova, O. Plastidial acyl carrier protein Delta9-desaturase modulates eicosapentaenoic acid biosynthesis and triacylglycerol accumulation in Phaeodactylum tricornutum. Plant J. 2021, 106, 1247–1259. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, M.; Pan, Y.; Hu, H.; Liu, J. Δ6 fatty acid elongase is involved in eicosapentaenoic acid biosynthesis via the ω6 pathway in the marine alga Nannochloropsis oceanica. J. Agric. Food Chem. 2021, 69, 9837–9848. [Google Scholar] [CrossRef]
- Hao, X.; Chen, W.; Amato, A.; Jouhet, J.; Marechal, E.; Moog, D.; Hu, H.; Jin, H.; You, L.; Huang, F.; et al. Multiplex CRISPR/Cas9 editing of the long-chain acyl-CoA synthetase family in the diatom Phaeodactylum tricornutum reveals that mitochondrial ptACSL3 is involved in the synthesis of storage lipids. New Phytol. 2022, 233, 1797–1812. [Google Scholar] [CrossRef]
- Shang, S.; Liu, R.; Luo, L.; Li, X.; Zhang, S.; Zhang, Y.; Zheng, P.; Chen, Z.; Wang, B. Functional characterization of the monogalactosyldiacylglycerol synthase gene ptMGD2 in the diatom Phaeodactylum tricornutum. Front. Mar. Sci. 2022, 9, 874448. [Google Scholar] [CrossRef]
- Pol, A.; Gross, S.P.; Parton, R.G. Review: Biogenesis of the multifunctional lipid droplet: Lipids, proteins, and sites. J. Cell Biol. 2014, 204, 635–646. [Google Scholar] [CrossRef]
- Taparia, Y.; Dolui, A.K.; Boussiba, S.; Khozin-Goldberg, I. Multiplexed genome editing via an RNA polymerase II promoter-driven sgRNA array in the diatom Phaeodactylum tricornutum: Insights into the role of StLDP. Front. Plant Sci. 2021, 12, 784780. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, K.; Oishi, R.; Yoshida, M.; Matsuda, Y.; Suzuki, I. Stramenopile-type lipid droplet protein functions as a lipid droplet scaffold protein in the marine diatom Phaeodactylum tricornutum. Plant Cell Physiol. 2023, 64, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.S.; Kim, J.; Park, H.; Hong, S.-J.; Lee, C.-G.; Jin, E. Enhanced lipid productivity in AGP knockout marine microalga Tetraselmis sp. using a DNA-free CRISPR-Cas9 RNP method. Bioresour. Technol. 2020, 303, 122932. [Google Scholar] [CrossRef] [PubMed]
- Kasai, Y.; Takagi, S.; Ota, S.; Ishii, K.; Takeshita, T.; Kawano, S.; Harayama, S. Development of efficient genetic-transformation-and genome-editing systems, and the isolation of aCRISPR/Cas9-mediated high-oil mutant in theunicellular green alga Parachlorella kessleri strain NIES-2152. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Moosburner, M. Uncoupling Nitrogen Limitation and Lipid Accumulation in the Marine Diatom Phaeodactylum tricornutum by CRISPR-Cas9 Genetic Engineering. Ph.D. Thesis, University of California, San Diego, CA, USA, 2021. [Google Scholar]
- Gao, S.; Zhou, L.; Yang, W.; Wang, L.; Liu, X.; Gong, Y.; Hu, Q.; Wang, G. Overexpression of a novel gene (Pt2015) endows the commercial diatom Phaeodactylum tricornutum high lipid content and grazing resistance. Biotechnol. Biofuels Bioprod. 2022, 15, 131. [Google Scholar] [CrossRef] [PubMed]
- Nawaly, H.; Tsuji, Y.; Matsuda, Y. Rapid and precise genome editing in a marine diatom, Thalassiosira pseudonana by Cas9 nickase (D10A). Algal Res. 2020, 47, 101855. [Google Scholar] [CrossRef]
- Nam, O.; Grouneva, I.; Mackinder, L.C.M. Endogenous GFP tagging in the diatom Thalassiosira pseudonana. bioRxiv 2022. [Google Scholar] [CrossRef]
- Hoguin, A.; Yang, F.; Groisillier, A.; Bowler, C.; Genovesio, A.; Ait-Mohamed, O.; Vieira, F.R.J.; Tirichine, L. The model diatom Phaeodactylum tricornutum provides insights into the diversity and function of microeukaryotic DNA methyltransferases. Commun. Biol. 2023, 6, 253. [Google Scholar] [CrossRef]
- Graff van Creveld, S.; Ben-Dor, S.; Mizrachi, A.; Alcolombri, U.; Hopes, A.; Mock, T.; Rosenwasser, S.; Vardi, A. Biochemical characterization of a novel redox-regulated metacaspase in a marine diatom. Front. Microbiol. 2021, 12, 2578. [Google Scholar] [CrossRef]
- Uren, A.G.; O’Rourke, K.; Aravind, L.; Pisabarro, M.T.; Seshagiri, S.; Koonin, E.V.; Dixit, V.M. Identification of paracaspases and metacaspases: Two ancient families of caspase-like proteins, one of which plays a key role in MALT lymphoma. Mol. Cell 2000, 6, 961–967. [Google Scholar] [CrossRef]
- Helliwell, K.E.; Chrachri, A.; Koester, J.A.; Wharam, S.; Verret, F.; Taylor, A.R.; Wheeler, G.L.; Brownlee, C. Alternative mechanisms for fast Na+/Ca2+ signaling in eukaryotes via a novel class of single-domain voltage-gated channels. Curr. Biol. 2019, 29, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Llavero-Pasquina, M.; Geisler, K.; Holzer, A.; Mehrshahi, P.; Mendoza-Ochoa, G.I.; Newsad, S.A.; Davey, M.P.; Smith, A.G. Thiamine metabolism genes in diatoms are not regulated by thiamine despite the presence of predicted riboswitches. New Phytol. 2022, 235, 1853–1867. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Escalante, J.A.; Jasper, R.; Miller, S.M. CRISPR/Cas9 mutagenesis in Volvox carteri. Plant J. 2019, 97, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.; Cano, M.; Burch, T.A.; Weissman, J.C.; Posewitz, M.C. Genome editing using Cas9-RNA ribonucleoprotein complexes in the high-productivity marine alga Picochlorum celeri. Algal Res. 2020, 49, 101944. [Google Scholar] [CrossRef]
- Ichihara, K.; Yamazaki, T.; Kawano, S. Genome editing using a DNA-free clustered regularly interspaced short palindromic repeats-Cas9 system in green seaweed Ulva prolifera. Phycol. Res. 2021, 70, 50–56. [Google Scholar] [CrossRef]
- Badis, Y.; Scornet, D.; Harada, M.; Caillard, C.; Godfroy, O.; Raphalen, M.; Gachon, C.M.M.; Coelho, S.M.; Motomura, T.; Nagasato, C. Targeted CRISPR-Cas9-based gene knockouts in the model brown alga Ectocarpus. New Phytol. 2021, 231, 2077–2091. [Google Scholar] [CrossRef]
- Shen, Y.; Motomura, T.; Ichihara, K.; Matsuda, Y.; Yoshimura, K.; Kosugi, C.; Nagasato, C. Application of CRISPR-Cas9 genome editing by microinjection of gametophytes of Saccharina japonica (Laminariales, Phaeophyceae). J. Appl. Phycol. 2023, 35, 1431–1441. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Q.; Eleouet, M.; Chen, R.; Chen, H.; Zhang, N.; Hu, Y.; Sui, Z. CRISPR/LbCas12a-mediated targeted mutation of Gracilariopsis lemaneiformis (Rhodophyta). Plant Biotechnol. J. 2023, 21, 235–237. [Google Scholar] [CrossRef]
- Gruber, H. Development of a Vector Construct for the Transformation of the Coccolithophore Emiliania huxleyi. Master. Thesis, Hochschule Bremerhaven, Bremerhaven, Germany, 2009. [Google Scholar]
- Prasad, B.; Vadakedath, N.; Jeong, H.-J.; General, T.; Cho, M.-G.; Lein, W. Agrobacterium tumefaciens-mediated genetic transformation of haptophytes (Isochrysis species). Appl. Microbiol. Biotechnol. 2014, 98, 8629–8639. [Google Scholar] [CrossRef]
- Endo, H.; Yoshida, M.; Uji, T.; Saga, N.; Inoue, K.; Nagasawa, H. Stable nuclear transformation system for the coccolithophorid alga Pleurochrysis carterae. Sci. Rep. 2016, 6, 22252. [Google Scholar] [CrossRef]
- Prasad, B. Agrobacterium-mediated nuclear transformation of haptophyte and rhodophyte species. Ph.D. Thesis, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Erlangen, Germany, 2017. [Google Scholar]
- Momose, T.; De Cian, A.; Shiba, K.; Inaba, K.; Giovannangeli, C.; Concordet, J.-P. High doses of CRISPR/Cas9 ribonucleoprotein efficiently induce gene knockout with low mosaicism in the hydrozoan Clytia hemisphaerica through microhomology-mediated deletion. Sci. Rep. 2018, 8, 11734. [Google Scholar] [CrossRef]
- Quiroga Artigas, G.; Lapebie, P.; Leclere, L.; Takeda, N.; Deguchi, R.; Jekely, G.; Momose, T.; Houliston, E. A gonad-expressed opsin mediates light-induced spawning in the jellyfish Clytia. eLife 2018, 7, e29555. [Google Scholar] [CrossRef]
- Nakanishi, T.; Kato, Y.; Matsuura, T.; Watanabe, H. CRISPR/Cas-mediated targeted mutagenesis in Daphnia magna. PLoS ONE 2014, 9, e98363. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, H.; Nakanishi, T.; Matsuura, T.; Kato, Y.; Watanabe, H. CRISPR/Cas-mediated knock-in via non-homologous end-joining in the crustacean Daphnia magna. PLoS ONE 2017, 12, e0186112. [Google Scholar] [CrossRef] [PubMed]
- Hiruta, C.; Kakui, K.; Tollefsen, K.E.; Iguchi, T. Targeted gene disruption by use of CRISPR/Cas9 ribonucleoprotein complexes in the water flea Daphnia pulex. Genes Cells 2018, 23, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Rivetti, C.; Campos, B.; Pina, B.; Raldua, D.; Kato, Y.; Watanabe, H.; Barata, C. Tryptophan hydroxylase (TRH) loss of function mutations induce growth and behavioral defects in Daphnia magna. Sci. Rep. 2018, 8, 1518. [Google Scholar] [CrossRef]
- Adhitama, N.; Matsuura, T.; Kato, Y.; Watanabe, H. Monitoring ecdysteroid activities using genetically encoded reporter gene in Daphnia magna. Mar. Environ. Res. 2018, 140, 375–381. [Google Scholar] [CrossRef]
- Mohamad Ishak, N.S.; Nong, Q.D.; Matsuura, T.; Kato, Y.; Watanabe, H. Co-option of the bZIP transcription factor Vrille as the activator of Doublesex1 in environmental sex determination of the crustacean Daphnia magna. PLoS Genet. 2017, 13, e1006953. [Google Scholar] [CrossRef]
- Nguyen, N.D.; Matsuura, T.; Kato, Y.; Watanabe, H. DNMT3.1 controls trade-offs between growth, reproduction, and life span under starved conditions in Daphnia magna. Sci. Rep. 2021, 11, 7326. [Google Scholar] [CrossRef]
- Feng, H.; Bavister, G.; Gribble, K.E.; Mark Welch, D.B. Highly efficient CRISPR-mediated gene editing in a rotifer. PLoS Biol. 2023, 21, e3001888. [Google Scholar] [CrossRef]
- Gahan, J.M.; Schnitzler, C.E.; DuBuc, T.Q.; Doonan, L.B.; Kanska, J.; Gornik, S.G.; Barreira, S.; Thompson, K.; Schiffer, P.; Baxevanis, A.D.; et al. Functional studies on the role of Notch signaling in Hydractinia development. Dev. Biol. 2017, 428, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Presnell, J.S.; Bubel, M.; Knowles, T.; Patry, W.; Browne, W.E. Multigenerational laboratory culture of pelagic ctenophores and CRISPR–Cas9 genome editing in the lobate Mnemiopsis leidyi. Nat. Protoc. 2022, 17, 1868–1900. [Google Scholar] [CrossRef]
- Cleves, P.A.; Strader, M.E.; Bay, L.K.; Pringle, J.R.; Matz, M.V. CRISPR/Cas9-mediated genome editing in a reef-building coral. Proc. Natl. Acad. Sci. USA 2018, 115, 5235–5240. [Google Scholar] [CrossRef] [PubMed]
- Cleves, P.A.; Tinoco, A.I.; Bradford, J.; Perrin, D.; Bay, L.K.; Pringle, J.R. Reduced thermal tolerance in a coral carrying CRISPR-induced mutations in the gene for a heat-shock transcription factor. Proc. Natl. Acad. Sci. USA 2020, 117, 28899–28905. [Google Scholar] [CrossRef] [PubMed]
- Tinoco, A.I.; Mitchison-Field, L.M.Y.; Bradford, J.; Renicke, C.; Perrin, D.; Bay, L.K.; Pringle, J.R.; Cleves, P.A. Role of the bicarbonate transporter SLC4gamma in stony-coral skeleton formation and evolution. Proc. Natl. Acad. Sci. USA 2023, 120, e2216144120. [Google Scholar] [CrossRef]
- Ikmi, A.; McKinney, S.A.; Delventhal, K.M.; Gibson, M.C. TALEN and CRISPR/Cas9-mediated genome editing in the early-branching metazoan Nematostella vectensis. Nat. Commun. 2014, 5, 5486. [Google Scholar] [CrossRef]
- Servetnick, M.D.; Steinworth, B.; Babonis, L.S.; Simmons, D.; Salinas-Saavedra, M.; Martindale, M.Q. Cas9-mediated excision of Nematostella brachyury disrupts endoderm development, pharynx formation, and oral-aboral patterning. Development 2017, 144, 2951–2960. [Google Scholar] [CrossRef]
- Neal, S.; de Jong, D.M.; Seaver, E.C. CRISPR/CAS9 mutagenesis of a single r-opsin gene blocks phototaxis in a marine larva. Proc. R. Soc. B Biol. Sci. 2019, 286, 20182491. [Google Scholar] [CrossRef]
- Perry, K.J.; Henry, J.Q. CRISPR/Cas9-mediated genome modification in the mollusc, Crepidula fornicata. Genesis 2015, 53, 237–244. [Google Scholar] [CrossRef]
- Yu, H.; Li, H.; Li, Q.; Xu, R.; Yue, C.; Du, S. Targeted gene disruption in pacific oyster based on CRISPR/Cas9 ribonucleoprotein complexes. Mar. Biotechnol. 2019, 21, 301–309. [Google Scholar] [CrossRef]
- Li, H.; Yu, H.; Du, S.; Li, Q. CRISPR/Cas9 mediated high efficiency knockout of myosin essential light chain gene in the pacific oyster (Crassostrea Gigas). Mar. Biotechnol. 2021, 23, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Zhang, B.; Jin, Q.; Cai, Z.; Wei, L.; Wang, X.; Zheng, Y.; Huang, B.; Zhang, M.; Qi, Y.; et al. CRISPR/Cas9 system-mediated gene editing in the Fujian oysters (Crassostrea angulate) by electroporation. Front. Mar. Sci. 2021, 8, 763470. [Google Scholar] [CrossRef]
- Martin, A.; Serano, J.M.; Jarvis, E.; Bruce, H.S.; Wang, J.; Ray, S.; Barker, C.A.; O’Connell, L.C.; Patel, N.H. CRISPR/Cas9 mutagenesis reveals versatile roles of Hox genes in crustacean limb specification and evolution. Curr. Biol. 2016, 26, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Serano, J.M.; Martin, A.; Liubicich, D.M.; Jarvis, E.; Bruce, H.S.; La, K.; Browne, W.E.; Grimwood, J.; Patel, N.H. Comprehensive analysis of Hox gene expression in the amphipod crustacean Parhyale hawaiensis. Dev. Biol. 2016, 409, 297–309. [Google Scholar] [CrossRef]
- Gui, T.; Zhang, J.; Song, F.; Sun, Y.; Xie, S.; Yu, K.; Xiang, J. CRISPR/Cas9-mediated genome editing and mutagenesis of EcChi4 in Exopalaemon carinicauda. G3 Genes|Genomes|Genet. 2016, 6, 3757–3764. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, J.; Xiang, J. A CRISPR/Cas9-mediated mutation in chitinase changes immune response to bacteria in Exopalaemon carinicauda. Fish Shellfish. Immunol. 2017, 71, 43–49. [Google Scholar] [CrossRef]
- Zhang, J.; Song, F.; Sun, Y.; Yu, K.; Xiang, J. CRISPR/Cas9-mediated deletion of EcMIH shortens metamorphosis time from mysis larva to postlarva of Exopalaemon carinicauda. Fish Shellfish Immunol. 2018, 77, 244–251. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Su, Y.-H. Genome editing in sea urchin embryos by using a CRISPR/Cas9 system. Dev. Biol. 2016, 409, 420–428. [Google Scholar] [CrossRef]
- Sasaki, H.; Yoshida, K.; Hozumi, A.; Sasakura, Y. CRISPR/Cas9-mediated gene knockout in the ascidian Ciona intestinalis. Dev. Growth Differ. 2014, 56, 499–510. [Google Scholar] [CrossRef]
- Stolfi, A.; Gandhi, S.; Salek, F.; Christiaen, L. Tissue-specific genome editing in Ciona embryos by CRISPR/Cas9. Development 2014, 141, 4115–4120. [Google Scholar] [CrossRef]
- Gandhi, S.; Haeussler, M.; Razy-Krajka, F.; Christiaen, L.; Stolfi, A. Evaluation and rational design of guide RNAs for efficient CRISPR/Cas9-mediated mutagenesis in Ciona. Dev. Biol. 2017, 425, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Square, T.; Romasek, M.; Jandzik, D.; Cattell, M.V.; Klymkowsky, M.; Medeiros, D.M. CRISPR/Cas9-mediated mutagenesis in the sea lamprey Petromyzon marinus: A powerful tool for understanding ancestral gene functions in vertebrates. Development 2015, 142, 4180–4187. [Google Scholar] [CrossRef] [PubMed]
- Zu, Y.; Zhang, X.; Ren, J.; Dong, X.; Zhu, Z.; Jia, L.; Zhang, Q.; Li, W. Biallelic editing of a lamprey genome using the CRISPR/Cas9 system. Sci. Rep. 2016, 6, 23496. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, D.G.; Wada, H.; Higashijima, S.-I. Generation of knock-in lampreys by CRISPR-Cas9-mediated genome engineering. Sci. Rep. 2021, 11, 19836. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.Y.; Fu, Y.; Reyon, D.; Maeder, M.L.; Tsai, S.Q.; Sander, J.D.; Peterson, R.T.; Yeh, J.R.; Joung, J.K. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat. Biotechnol. 2013, 31, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.Y.; Fu, Y.; Reyon, D.; Maeder, M.L.; Kaini, P.; Sander, J.D.; Joung, J.K.; Peterson, R.T.; Yeh, J.-R.J. Heritable and precise zebrafish genome editing using a CRISPR-Cas system. PLoS ONE 2013, 8, e68708. [Google Scholar] [CrossRef]
- Jao, L.-E.; Wente, S.R.; Chen, W. Efficient multiplex biallelic zebrafish genome editing using a CRISPR nuclease system. Proc. Natl. Acad. Sci. USA 2013, 110, 13904–13909. [Google Scholar] [CrossRef]
- Chang, N.; Sun, C.; Gao, L.; Zhu, D.; Xu, X.; Zhu, X.; Xiong, J.-W.; Xi, J.J. Genome editing with RNA-guided Cas9 nuclease in zebrafish embryos. Cell Res. 2013, 23, 465–472. [Google Scholar] [CrossRef]
- Fraidenraich, D.; Edvardsen, R.B.; Leininger, S.; Kleppe, L.; Skaftnesmo, K.O.; Wargelius, A. Targeted mutagenesis in Atlantic salmon (Salmo salar L.) using the CRISPR/Cas9 system induces complete knockout individuals in the F0 generation. PLoS ONE 2014, 9, e108622. [Google Scholar] [CrossRef]
- Straume, A.H.; Kjærner-Semb, E.; Ove Skaftnesmo, K.; Güralp, H.; Kleppe, L.; Wargelius, A.; Edvardsen, R.B. Indel locations are determined by template polarity in highly efficient in vivo CRISPR/Cas9-mediated HDR in Atlantic salmon. Sci. Rep. 2020, 10, 409. [Google Scholar] [CrossRef]
- Kishimoto, K.; Washio, Y.; Yoshiura, Y.; Toyoda, A.; Ueno, T.; Fukuyama, H.; Kato, K.; Kinoshita, M. Production of a breed of red sea bream Pagrus major with an increase of skeletal muscle mass and reduced body length by genome editing with CRISPR/Cas9. Aquaculture 2018, 495, 415–427. [Google Scholar] [CrossRef]
- Kim, J.; Cho, J.Y.; Kim, J.-W.; Kim, H.-C.; Noh, J.K.; Kim, Y.-O.; Hwang, H.-K.; Kim, W.-J.; Yeo, S.-Y.; An, C.M.; et al. CRISPR/Cas9-mediated myostatin disruption enhances muscle mass in the olive flounder Paralichthys olivaceus. Aquaculture 2019, 512, 734336. [Google Scholar] [CrossRef]
- Sakaguchi, K.; Yoneda, M.; Sakai, N.; Nakashima, K.; Kitano, H.; Matsuyama, M. Comprehensive experimental system for a promising model organism candidate for marine teleosts. Sci. Rep. 2019, 9, 4948. [Google Scholar] [CrossRef]
- Jeong, C.-B.; Kang, H.-M.; Hong, S.-A.; Byeon, E.; Lee, J.-S.; Lee, Y.H.; Choi, I.-Y.; Bae, S.; Lee, J.-S. Generation of albino via SLC45a2 gene targeting by CRISPR/Cas9 in the marine medaka Oryzias melastigma. Mar. Pollut. Bull. 2020, 154, 111038. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, C.; Zhang, F.; Mendez, J.; Lozano, Y.; Chatpar, K.; Irish, V.F.; Jacob, Y. Increased efficiency of targeted mutagenesis by CRISPR/Cas9 in plants using heat stress. Plant J. 2018, 93, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Faktorova, D.; Nisbet, R.E.R.; Fernandez Robledo, J.A.; Casacuberta, E.; Sudek, L.; Allen, A.E.; Ares, M., Jr.; Areste, C.; Balestreri, C.; Barbrook, A.C.; et al. Genetic tool development in marine protists: Emerging model organisms for experimental cell biology. Nat. Methods 2020, 17, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Bortesi, L.; Zhu, C.; Zischewski, J.; Perez, L.; Bassié, L.; Nadi, R.; Forni, G.; Lade, S.B.; Soto, E.; Jin, X. Patterns of CRISPR/Cas9 activity in plants, animals and microbes. Plant Biotechnol. J. 2016, 14, 2203–2216. [Google Scholar] [CrossRef]
- Wilbie, D.; Walther, J.; Mastrobattista, E. Delivery aspects of CRISPR/Cas for in vivo genome editing. Acc. Chem. Res. 2019, 52, 1555–1564. [Google Scholar] [CrossRef]
- Sprecher, B.N.; Zhang, H.; Lin, S. Nuclear gene transformation in the dinoflagellate Oxyrrhis marina. Microorganisms 2020, 8, 126. [Google Scholar] [CrossRef]
- Nimmo, I.C.; Barbrook, A.C.; Lassadi, I.; Chen, J.E.; Geisler, K.; Smith, A.G.; Aranda, M.; Purton, S.; Waller, R.F.; Nisbet, R.E.R.; et al. Genetic transformation of the dinoflagellate chloroplast. eLife 2019, 8, e45292. [Google Scholar] [CrossRef]
- Gornik, S.G.; Maegele, I.; Hambleton, E.A.; Voss, P.A.; Waller, R.F.; Guse, A. Nuclear transformation of a dinoflagellate symbiont of corals. Front. Mar. Sci. 2022, 9, 1035413. [Google Scholar] [CrossRef]
- Lin, S. Genomic understanding of dinoflagellates. Res. Microbiol. 2011, 162, 551–569. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Sui, T.; Liu, D.; Liu, T.; Chen, M.; Deng, J.; Xu, Y.; Li, Z. Multiple homologous genes knockout (KO) by CRISPR/Cas9 system in rabbit. Gene 2018, 647, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Y.; Li, T.; Qiu, H.; Xia, Z.; Dong, Y. Target-specific mutations efficiency at multiple loci of CRISPR/Cas9 system using one sgRNA in soybean. Transgenic Res. 2021, 30, 51–62. [Google Scholar] [CrossRef]
- Du, L.-L. Resurrection from lethal knockouts: Bypass of gene essentiality. Biochem. Biophys. Res. Commun. 2020, 528, 405–412. [Google Scholar] [CrossRef]
- Saito, M.; Xu, P.Y.; Faure, G.; Maguire, S.; Kannan, S.; Altae-Tran, H.; Vo, S.; Desimone, A.; Macrae, R.K.; Zhang, F. Fanzor is a eukaryotic programmable RNA-guided endonuclease. Nature 2023, 620, 660–668. [Google Scholar] [CrossRef]
- Pal, P.; Anand, U.; Saha, S.C.; Sundaramurthy, S.; Okeke, E.S.; Kumar, M.; Bontempi, E.; Albertini, E.; Dey, A.; Di Maria, F. Novel CRISPR/Cas technology in the realm of algal bloom biomonitoring: Recent trends and future perspectives. Environ. Res. 2023, 231, 115989. [Google Scholar] [CrossRef]
- Wang, L.; Chen, X.; Pan, F.; Yao, G.; Chen, J. Development of a rapid detection method for Karenia mikimotoi by using CRISPR-Cas12a. Front. Microbiol. 2023, 14, 1205765. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wu, S.; Zhang, K.; Sun, X.; Lin, W.; Wang, C.; Lin, S. Clustered Regularly Interspaced Short Palindromic Repeat/CRISPR-Associated Protein and Its Utility All at Sea: Status, Challenges, and Prospects. Microorganisms 2024, 12, 118. https://doi.org/10.3390/microorganisms12010118
Li J, Wu S, Zhang K, Sun X, Lin W, Wang C, Lin S. Clustered Regularly Interspaced Short Palindromic Repeat/CRISPR-Associated Protein and Its Utility All at Sea: Status, Challenges, and Prospects. Microorganisms. 2024; 12(1):118. https://doi.org/10.3390/microorganisms12010118
Chicago/Turabian StyleLi, Jiashun, Shuaishuai Wu, Kaidian Zhang, Xueqiong Sun, Wenwen Lin, Cong Wang, and Senjie Lin. 2024. "Clustered Regularly Interspaced Short Palindromic Repeat/CRISPR-Associated Protein and Its Utility All at Sea: Status, Challenges, and Prospects" Microorganisms 12, no. 1: 118. https://doi.org/10.3390/microorganisms12010118
APA StyleLi, J., Wu, S., Zhang, K., Sun, X., Lin, W., Wang, C., & Lin, S. (2024). Clustered Regularly Interspaced Short Palindromic Repeat/CRISPR-Associated Protein and Its Utility All at Sea: Status, Challenges, and Prospects. Microorganisms, 12(1), 118. https://doi.org/10.3390/microorganisms12010118







