Dupilumab Alters Both the Bacterial and Fungal Skin Microbiomes of Patients with Atopic Dermatitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and Sample Collection
2.2. Scale Sample Collection and Analysis of the Skin Microbiome
2.3. Fungal 28S rRNA Gene Sequencing and Taxonomic Assignment
2.4. Bacterial 16S rRNA Gene Sequencing and Taxonomic Assignment
2.5. Degree of Skin Colonization by Malassezia spp. and S. aureus Determined Using qPCR
2.6. Statistical Analysis
3. Results
3.1. Patients
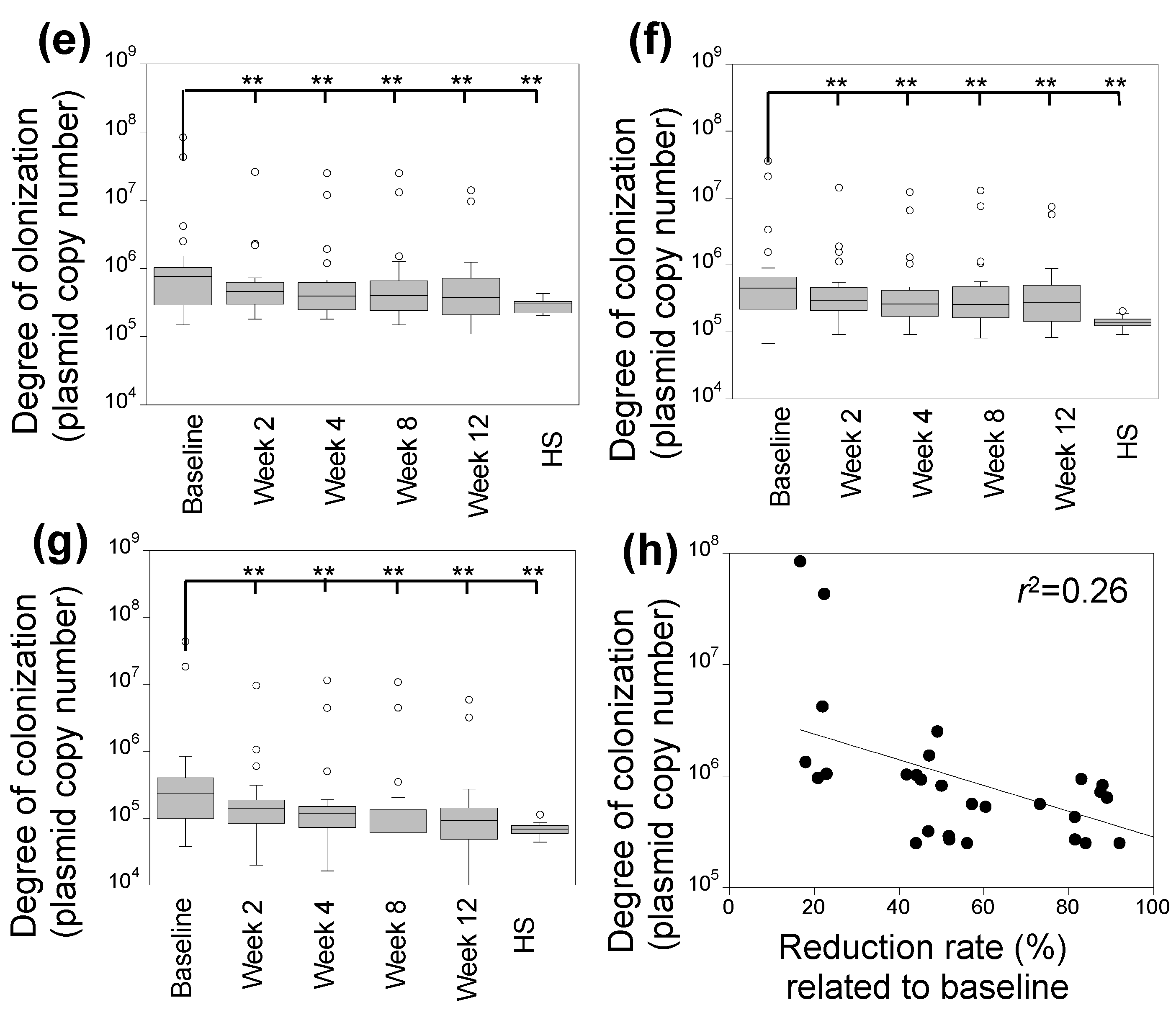
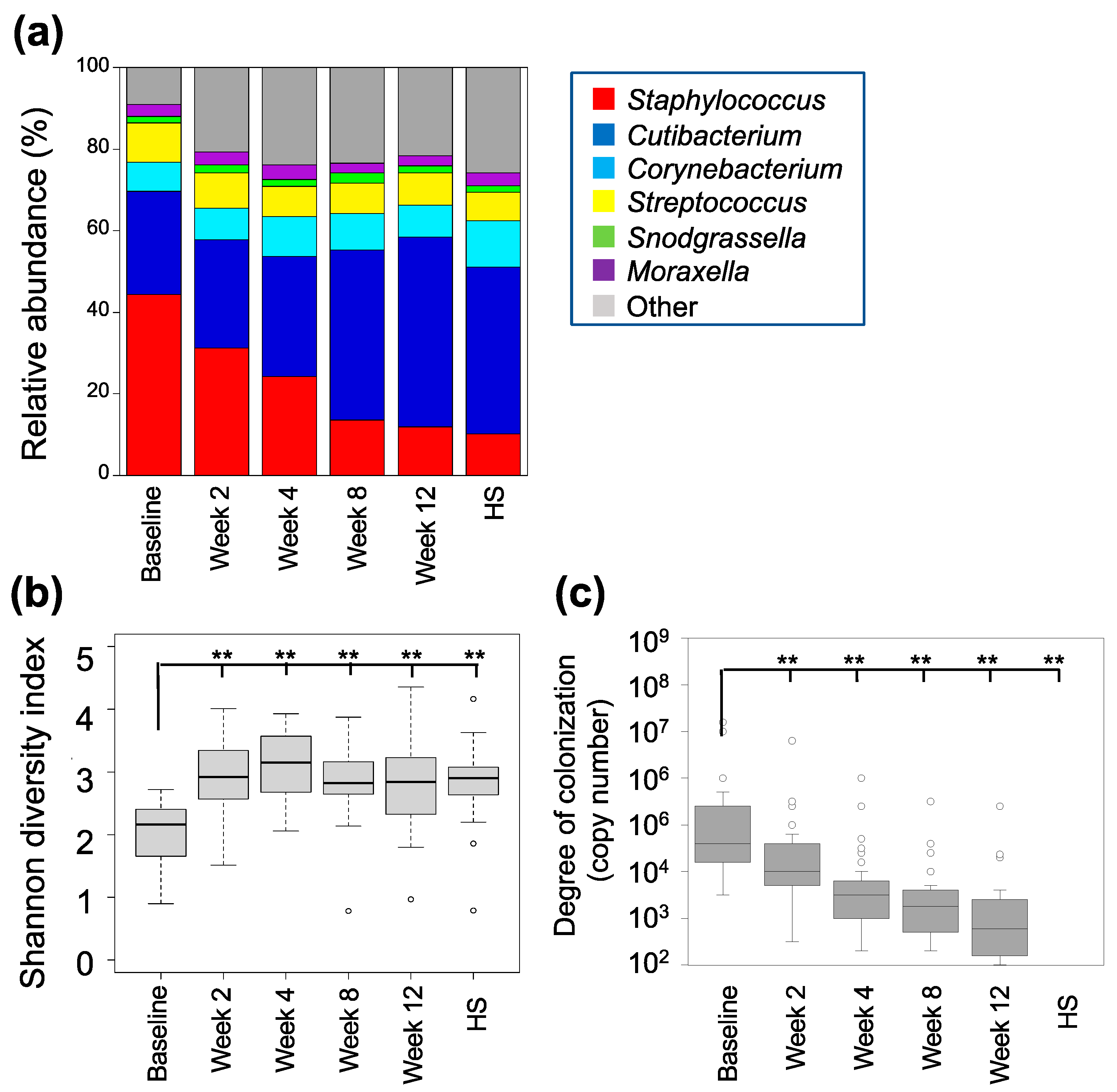
3.2. Characterization of Skin Fungal Microbiome
3.3. Characterization of the Skin Bacterial Microbiome
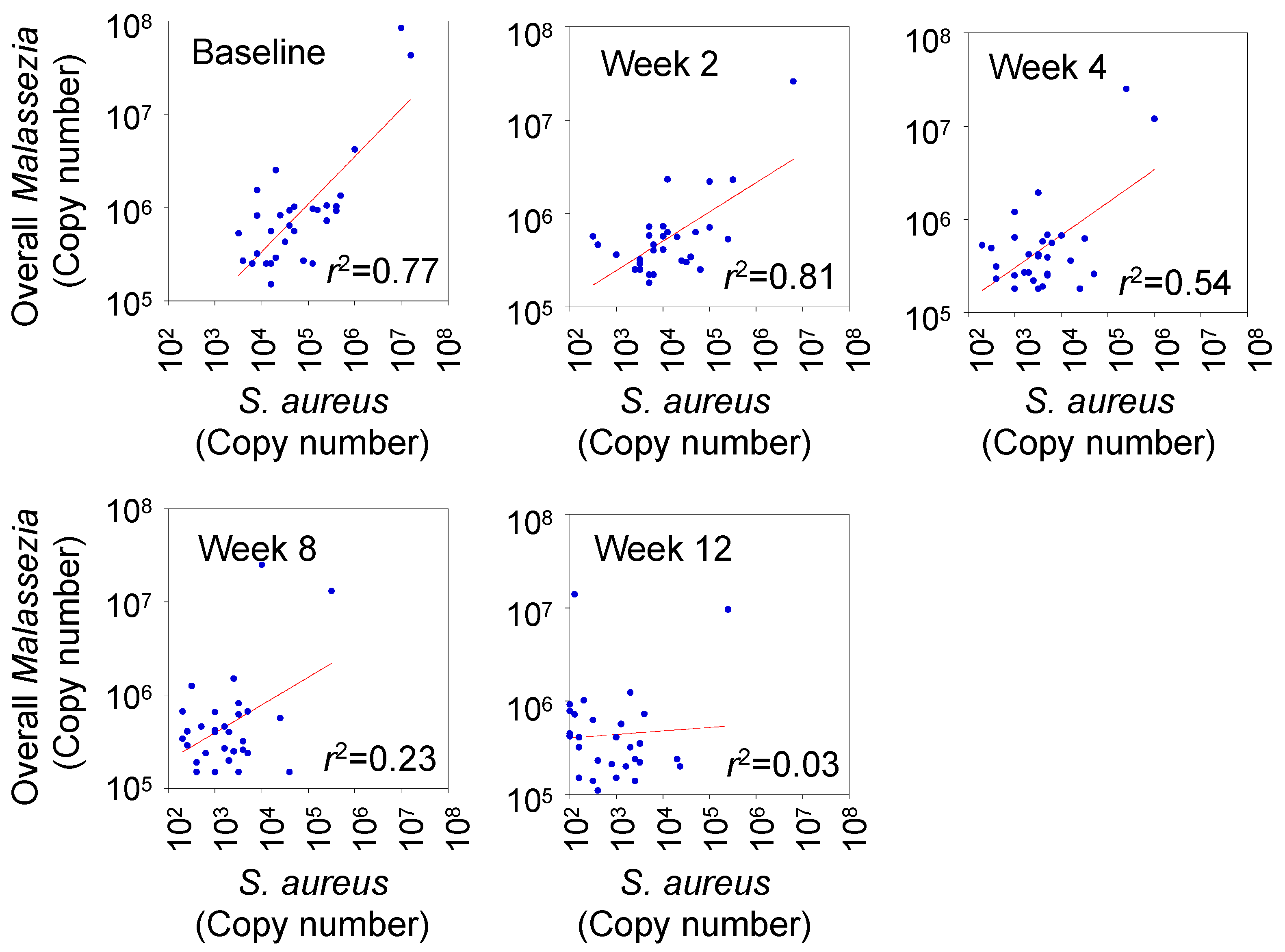
3.4. Correlation between Degree of Skin Colonization by Malassezia and S. aureus
3.5. Correlation between Clinical Score and Abundance of Skin Microbes
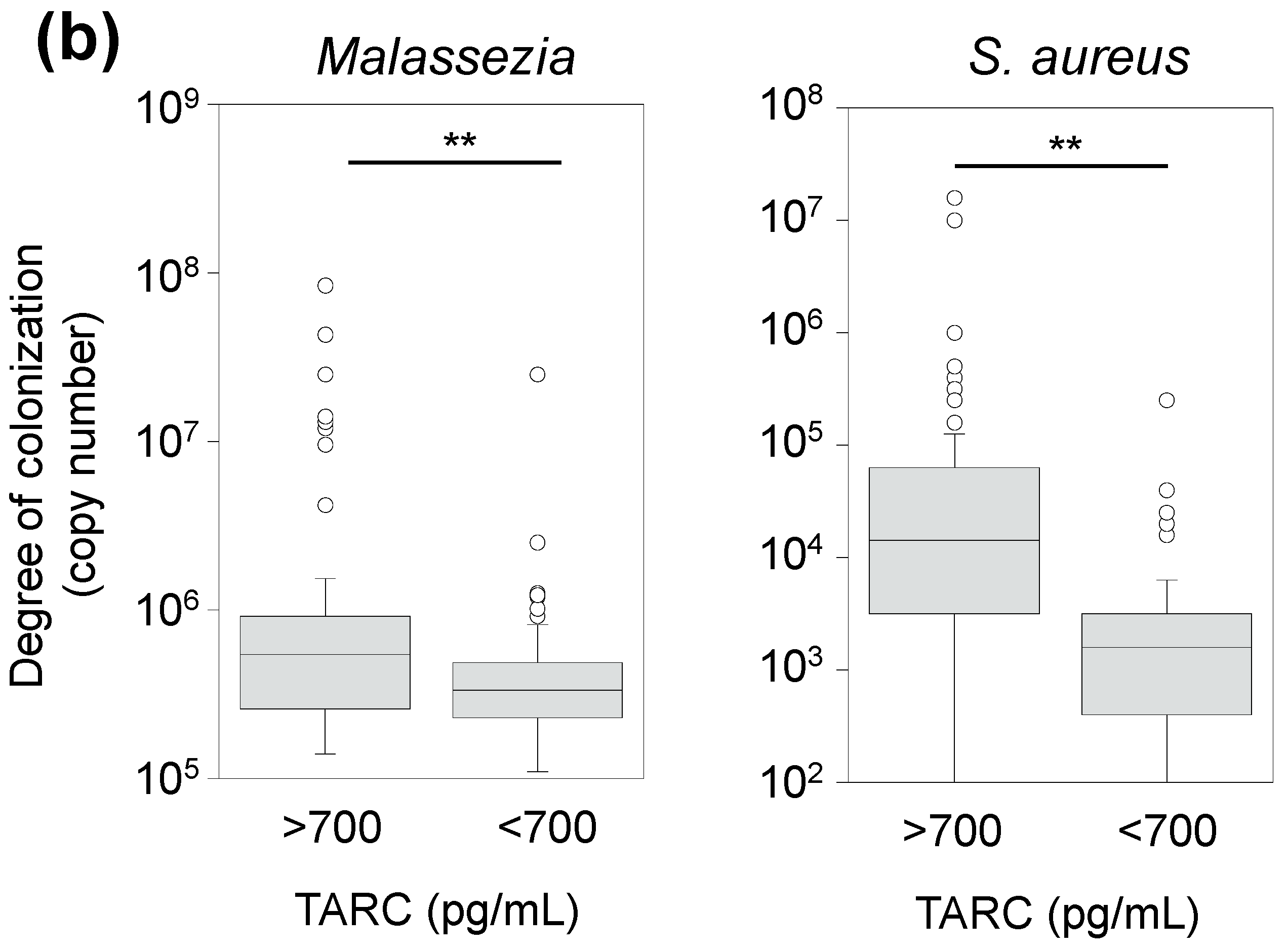
3.6. Correlation between TARC Concentration and Abundance of Skin Microbes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Luger, T.; Amagai, M.; Dreno, B.; Dagnelie, M.A.; Liao, W.; Kabashima, K.; Schikowski, T.; Proksch, E.; Elias, P.M.; Simon, M.; et al. Atopic dermatitis: Role of the skin barrier, environment, microbiome, and therapeutic agents. J. Dermatol. Sci. 2021, 102, 142–157. [Google Scholar] [CrossRef] [PubMed]
- Furue, M. Regulation of filaggrin, loricrin, and involucrin by IL-4, IL-13, IL-17A, IL-22, AHR, and NRF2: Pathogenic implications in atopic dermatitis. Int. J. Mol. Sci. 2020, 21, 5382. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.H.; Oh, J.; Deming, C.; Conlan, S.; Grice, E.A.; Beatson, M.A.; Nomicos, E.; Polley, E.C.; Komarow, H.D.; NISC Comparative Sequence Program; et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012, 22, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Edslev, S.M.; Olesen, C.M.; Nørreslet, L.B.; Ingham, A.C.; Iversen, S.; Lilje, B.; Clausen, M.L.; Jensen, J.S.; Stegger, M.; Agner, T.; et al. Staphylococcal communities on skin are associated with atopic dermatitis and disease severity. Microorganisms 2021, 9, 432. [Google Scholar] [CrossRef]
- Rauer, L.; Reiger, M.; Bhattacharyya, M.; Brunner, P.M.; Krueger, J.G.; Guttman-Yassky, E.; Traidl-Hoffmann, C.; Neumann, A.U. Skin microbiome and its association with host cofactors in determining atopic dermatitis severity. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 772–782. [Google Scholar] [CrossRef] [PubMed]
- Dinges, M.M.; Orwin, P.M.; Schlievert, P.M. Exotoxins of Staphylococcus aureus. Clin. Microbiol. Rev. 2000, 13, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Geoghegan, J.A.; Irvine, A.D.; Foster, T.J. Staphylococcus aureus and atopic dermatitis: A complex and evolving relationship. Trends Microbiol. 2018, 26, 484–497. [Google Scholar] [CrossRef]
- Demessant-Flavigny, A.L.; Connétable, S.; Kerob, D.; Moreau, M.; Aguilar, L.; Wollenberg, A. Skin microbiome dysbiosis and the role of Staphylococcus aureus in atopic dermatitis in adults and children: A narrative review. J. Eur. Acad. Dermatol. Venereol. 2023, 37 (Suppl. S5), 3–17. [Google Scholar] [CrossRef]
- Harb, H.; Chatila, T.A. Mechanisms of dupilumab. Clin. Exp. Allergy 2020, 50, 5–14. [Google Scholar] [CrossRef]
- Traidl, S.; Roesner, L.; Zeitvogel, J.; Werfel, T. Eczema herpeticum in atopic dermatitis. Allergy 2021, 76, 3017–3027. [Google Scholar] [CrossRef]
- Chun, P.I.F.; Lehman, H. Current and future monoclonal antibodies in the treatment of atopic dermatitis. Clin. Rev. Allergy Immunol. 2020, 59, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Callewaert, C.; Nakatsuji, T.; Knight, R.; Kosciolek, T.; Vrbanac, A.; Kotol, P.; Ardeleanu, M.; Hultsch, T.; Guttman-Yassky, E.; Bissonnette, R.; et al. IL-4Rα blockade by Dupilumab decreases Staphylococcus aureus colonization and increases microbial diversity in atopic dermatitis. J. Investig. Dermatol. 2020, 140, 191–202.e7. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.Q.; Lin, L.; Lin, T.; Hao, F.; Zeng, F.Q.; Bi, Z.G.; Yi, D.; Zhao, B. Skin colonization by Staphylococcus aureus in patients with eczema and atopic dermatitis and relevant combined topical therapy: A double-blind multicentre randomized controlled trial. Br. J. Dermatol. 2006, 155, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Findley, K.; Oh, J.; Yang, J.; Conlan, S.; Deming, C.; Meyer, J.A.; Schoenfeld, D.; Nomicos, E.; Park, M.; NIH Intramural Sequencing Center Comparative Sequencing Program; et al. Topographic diversity of fungal and bacterial communities in human skin. Nature 2013, 498, 367–370. [Google Scholar] [CrossRef]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef]
- Dawson, T.L., Jr. Malassezia globosa and restricta: Breakthrough understanding of the etiology and treatment of dandruff and seborrheic dermatitis through whole-genome analysis. J. Investig. Dermatol. Symp. Proc. 2007, 12, 15–19. [Google Scholar] [CrossRef]
- Scheynius, A.; Johansson, C.; Buentke, E.; Zargari, A.; Linder, M.T. Atopic eczema/dermatitis syndrome and Malassezia. Int. Arch. Allergy Immunol. 2002, 127, 161–169. [Google Scholar] [CrossRef]
- Zhang, E.; Tanaka, T.; Tajima, M.; Tsuboi, R.; Kato, H.; Nishikawa, A.; Sugita, T. Anti-Malassezia-specific IgE antibodies production in Japanese patients with head and neck atopic dermatitis: Relationship between the level of specific IgE antibody and the colonization frequency of cutaneous Malassezia species and clinical severity. J. Allergy 2011, 2011, 645670. [Google Scholar] [CrossRef]
- Glatz, M.; Buchner, M.; von Bartenwerffer, W.; Schmid-Grendelmeier, P.; Worm, M.; Hedderich, J.; Fölster-Holst, R. Malassezia spp.-specific immunoglobulin E level is a marker for severity of atopic dermatitis in adults. Acta Derm. Venereol. 2015, 95, 191–196. [Google Scholar] [CrossRef]
- Jo, C.E.; Finstad, A.; Georgakopoulos, J.R.; Piguet, V.; Yeung, J.; Drucker, A.M. Facial and neck erythema associated with dupilumab treatment: A systematic review. J. Am. Acad. Dermatol. 2021, 84, 1339–1347. [Google Scholar] [CrossRef]
- Vittrup, I.; Krogh, N.S.; Larsen, H.H.P.; Elberling, J.; Skov, L.; Ibler, K.S.; Jemec, G.B.E.; Mortz, C.G.; Bach, R.O.; Bindslev-Jensen, C.; et al. A nationwide 104 weeks real-world study of dupilumab in adults with atopic dermatitis: Ineffectiveness in head-and-neck dermatitis. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Kozera, E.; Stewart, T.; Gill, K.; De La Vega, M.A.; Frew, J.W. Dupilumab-associated head and neck dermatitis is associated with elevated pretreatment serum Malassezia-specific IgE: A multicentre, prospective cohort study. Br. J. Dermatol. 2022, 186, 1050–1052. [Google Scholar] [CrossRef] [PubMed]
- Muzumdar, S.; Skudalski, L.; Sharp, K.; Waldman, R.A. Dupilumab facial redness/dupilumab facial dermatitis: A guide for clinicians. Am. J. Clin. Dermatol. 2022, 23, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Ordóñez-Rubiano, M.F.; Casas, M.; Balaguera-Orjuela, V. Dupilumab facial redness: Clinical characteristics and proposed treatment in a cohort. Dermatol. Ther. 2021, 34, e15140. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Triviño, F.J.; Ayén-Rodríguez, Á. Study of hypersensitivity to Malassezia furfur in patients with atopic dermatitis with head and neck pattern: Is it useful as a biomarker and therapeutic indicator in these patients? Life 2022, 12, 299. [Google Scholar] [CrossRef] [PubMed]
- Saeki, H.; Ohya, Y.; Furuta, J.; Arakawa, H.; Ichiyama, S.; Katsunuma, T.; Katoh, N.; Tanaka, A.; Tsunemi, Y.; Nakahara, T.; et al. English Version of Clinical Practice Guidelines for the Management of Atopic Dermatitis 2021. J. Dermatol. Engl. Version 2022, 49, e315–e375. [Google Scholar] [CrossRef] [PubMed]
- Sugita, T.; Suto, H.; Unno, T.; Tsuboi, R.; Ogawa, H.; Shinoda, T.; Nishikawa, A. Molecular analysis of Malassezia microflora on the skin of atopic dermatitis patients and healthy subjects. J. Clin. Microbiol. 2001, 39, 3486–3490. [Google Scholar] [CrossRef]
- Suzuki, K.; Inoue, M.; Cho, O.; Mizutani, R.; Shimizu, Y.; Nagahama, T.; Sugita, T. Scalp microbiome and sebum composition in Japanese male individuals with and without androgenetic alopecia. Microorganisms 2021, 9, 2132. [Google Scholar] [CrossRef]
- Yang, S.; Lin, S.; Kelen, G.D.; Quinn, T.C.; Dick, J.D.; Gaydos, C.A.; Rothman, R.E. Quantitative multiprobe PCR assay for simultaneous detection and identification to species level of bacterial pathogens. J. Clin. Microbiol. 2002, 40, 3449–3454. [Google Scholar] [CrossRef]
- Sugita, T.; Tajima, M.; Tsubuku, H.; Tsuboi, R.; Nishikawa, A. Quantitative analysis of cutaneous Malassezia in atopic dermatitis patients using real-time PCR. Microbiol. Immunol. 2006, 50, 549–552. [Google Scholar] [CrossRef]
- Simpson, E.L.; Schlievert, P.M.; Yoshida, T.; Lussier, S.; Boguniewicz, M.; Hata, T.; Fuxench, Z.; De Benedetto, A.; Ong, P.Y.; Ko, J.; et al. Rapid reduction in Staphylococcus aureus in atopic dermatitis subjects following dupilumab treatment. J. Allergy Clin. Immunol. 2023, 152, 1179–1195. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, J.; Moitinho-Silva, L.; Sander, N.; Harder, I.; Häsler, R.; Rodriguez, E.; Haufe, E.; Kleinheinz, A.; Abraham, S.; Heratizadeh, A.; et al. Dupilumab but not cyclosporine treatment shifts the microbiome toward a healthy skin flora in patients with moderate-to-severe atopic dermatitis. Allergy 2023, 78, 2290–2300. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, S.E.; Shin, K.O.; Park, K.; Lee, S.E. Dupilumab therapy improves stratum corneum hydration and skin dysbiosis in patients with atopic dermatitis. Allergy Asthma Immunol. Res. 2021, 13, 762–775. [Google Scholar] [CrossRef] [PubMed]
- Olesen, C.M.; Ingham, A.C.; Thomsen, S.F.; Clausen, M.L.; Andersen, P.S.; Edslev, S.M.; Yüksel, Y.T.; Guttman-Yassky, E.; Agner, T. Changes in skin and nasal microbiome and Staphylococcal species following treatment of atopic dermatitis with dupilumab. Microorganisms 2021, 9, 1487. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Saunders, C.W.; Hu, P.; Grant, R.A.; Boekhout, T.; Kuramae, E.E.; Kronstad, J.W.; Deangelis, Y.M.; Reeder, N.L.; Johnstone, K.R.; et al. Dandruff-associated Malassezia genomes reveal convergent and divergent virulence traits shared with plant and human fungal pathogens. Proc. Natl Acad. Sci. USA 2007, 104, 18730–18735. [Google Scholar] [CrossRef] [PubMed]
- Tajima, M.; Sugita, T.; Nishikawa, A.; Tsuboi, R. Molecular analysis of Malassezia microflora in seborrheic dermatitis patients: Comparison with other diseases and healthy subjects. J. Investig. Dermatol. 2008, 128, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Aiba, S.; Takahashi, K.; Tagami, H. Reduced proliferative responses of peripheral blood mononuclear cells specifically to Candida albicans antigen in patients with atopic dermatitis—Comparison with their normal reactivity to bacterial superantigens. Arch. Dermatol. Res. 1996, 288, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Romani, L.; Bistoni, F.; Puccetti, P. Initiation of T-helper cell immunity to 4 by IL-12: The role of neutrophils. Chem. Immunol. 1997, 68, 110–135. [Google Scholar] [CrossRef]
- Kaga, M.; Sugita, T.; Nishikawa, A.; Wada, Y.; Hiruma, M.; Ikeda, S. Molecular analysis of the cutaneous Malassezia microbiota from the skin of patients with atopic dermatitis of different severities. Mycoses 2011, 54, e24–e28. [Google Scholar] [CrossRef]
- Homey, B.; Meller, S.; Savinko, T.; Alenius, H.; Lauerma, A. Modulation of chemokines by staphylococcal superantigen in atopic dermatitis. Chem. Immunol. Allergy 2007, 93, 181–194. [Google Scholar] [CrossRef]
- Li, H.; Goh, B.N.; Teh, W.K.; Jiang, Z.; Goh, J.P.Z.; Goh, A.; Wu, G.; Hoon, S.S.; Raida, M.; Camattari, A.; et al. Skin commensal Malassezia globosa secreted protease attenuates Staphylococcus aureus biofilm formation. J. Investig. Dermatol. 2018, 138, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Ianiri, G.; Heitman, J.; Scheynius, A. The skin commensal yeast Malassezia globosa thwarts bacterial biofilms to benefit the host. J. Investig. Dermatol. 2018, 138, 1026–1029. [Google Scholar] [CrossRef] [PubMed]




| Characteristics | Patients with Atopic Dermatitis | Healthy Individuals | ||||
|---|---|---|---|---|---|---|
| Baseline | Follow-Up | |||||
| Week 2 | Week 4 | Week 8 | Week 12 | |||
| Age, | 42.1 ± 13.2 | 40.9 ± 12.0 | ||||
| mean + SD (range) | (17–64) | (21–60) | ||||
| Female sex no. (%) | 10 (33%) | 2 (20%) | ||||
| EASI score, | 27.0 ± 11.1 | 21.6 ± 10.6 | 17.3 ± 9.8 | 14.9 ± 9.3 | 12.6 ± 8.6 | |
| mean + SD (range) | (9.6–57.8) | (5.6–50.3) | (0.5–39.8) | (2.0–40.2) | (1.7–31.8) | |
| Chamge in EASI score from baseline,% | 21.2 | 36.4 | 47 | 53.3 | ||
| EASI-75, no. (%) | 0 (0%) | 3 (10%) | 5 (16.7%) | 9 (30%) | ||
| Serum total IgE, | 13,875 ± 8920 | 7883 ± 4042 | ||||
| mean + SD (range) | (610–25,000) | (210–19,000) | ||||
| TARC (pg/mL), | 5274 ± 5279 | 1002 ± 910 | 715 ± 526 | 622 ± 407 | ||
| mean + SD (range) | (331–24,200) | (168–4656) | (196–2176) | (132–1722) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umemoto, N.; Kakurai, M.; Matsumoto, T.; Mizuno, K.; Cho, O.; Sugita, T.; Demitsu, T. Dupilumab Alters Both the Bacterial and Fungal Skin Microbiomes of Patients with Atopic Dermatitis. Microorganisms 2024, 12, 224. https://doi.org/10.3390/microorganisms12010224
Umemoto N, Kakurai M, Matsumoto T, Mizuno K, Cho O, Sugita T, Demitsu T. Dupilumab Alters Both the Bacterial and Fungal Skin Microbiomes of Patients with Atopic Dermatitis. Microorganisms. 2024; 12(1):224. https://doi.org/10.3390/microorganisms12010224
Chicago/Turabian StyleUmemoto, Naoka, Maki Kakurai, Takanao Matsumoto, Kenta Mizuno, Otomi Cho, Takashi Sugita, and Toshio Demitsu. 2024. "Dupilumab Alters Both the Bacterial and Fungal Skin Microbiomes of Patients with Atopic Dermatitis" Microorganisms 12, no. 1: 224. https://doi.org/10.3390/microorganisms12010224
APA StyleUmemoto, N., Kakurai, M., Matsumoto, T., Mizuno, K., Cho, O., Sugita, T., & Demitsu, T. (2024). Dupilumab Alters Both the Bacterial and Fungal Skin Microbiomes of Patients with Atopic Dermatitis. Microorganisms, 12(1), 224. https://doi.org/10.3390/microorganisms12010224


_Di_Marco.png)



