Biogenic Sulfide-Mediated Iron Reduction at Low pH †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site Description
2.2. Sample Collection
2.3. Microcosms Preparation and Analysis
2.4. Characterization of Precipitate
2.5. Sulfate Reduction Rate (SRR)
2.6. Quantification of Sulfate-Reducing Bacteria (SRB)
2.7. DAPI Staining for Total Cell Count
3. Results
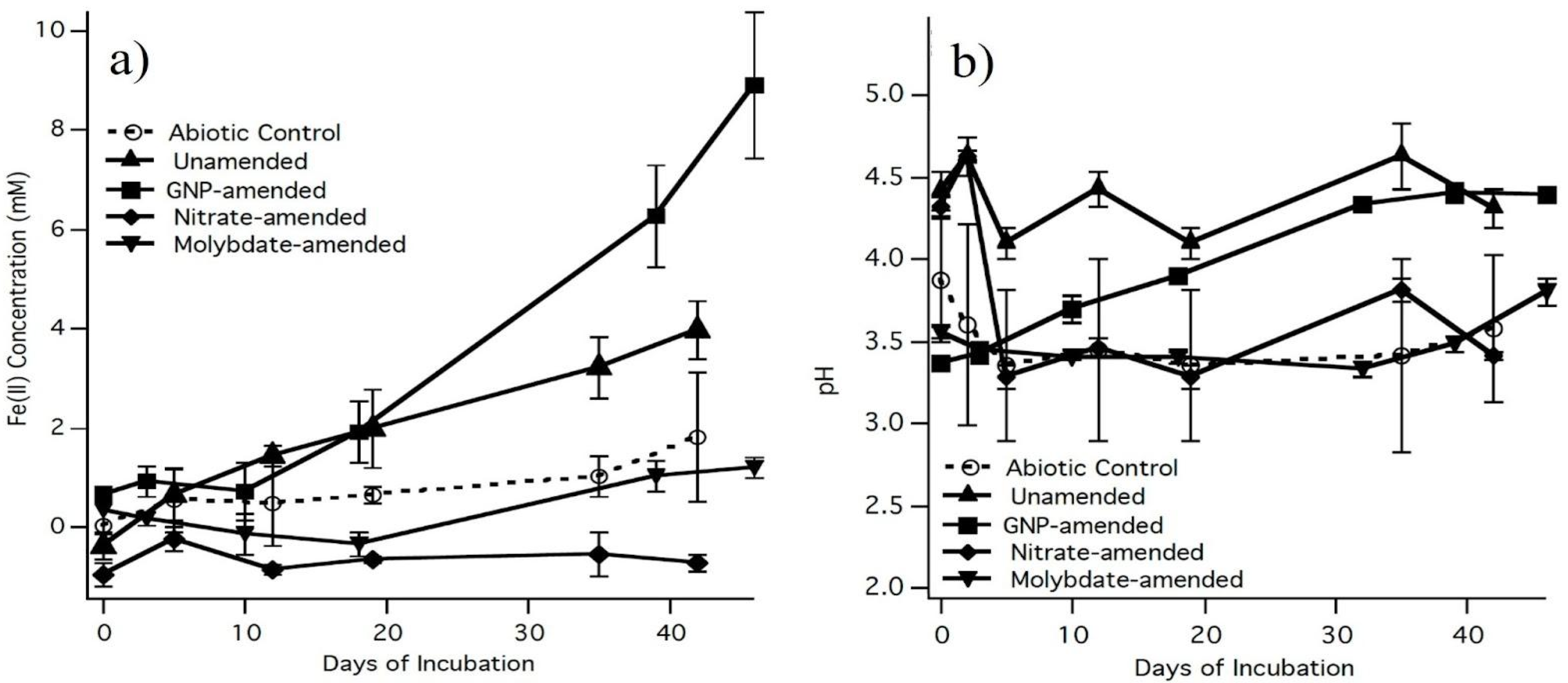
3.1. Inhibition and Stimulation of Sulfate-Reducing Bacteria (SRB) in Microcosms
3.2. Sulfate Reduction Rates
3.3. Abundance and Diversity of SRB
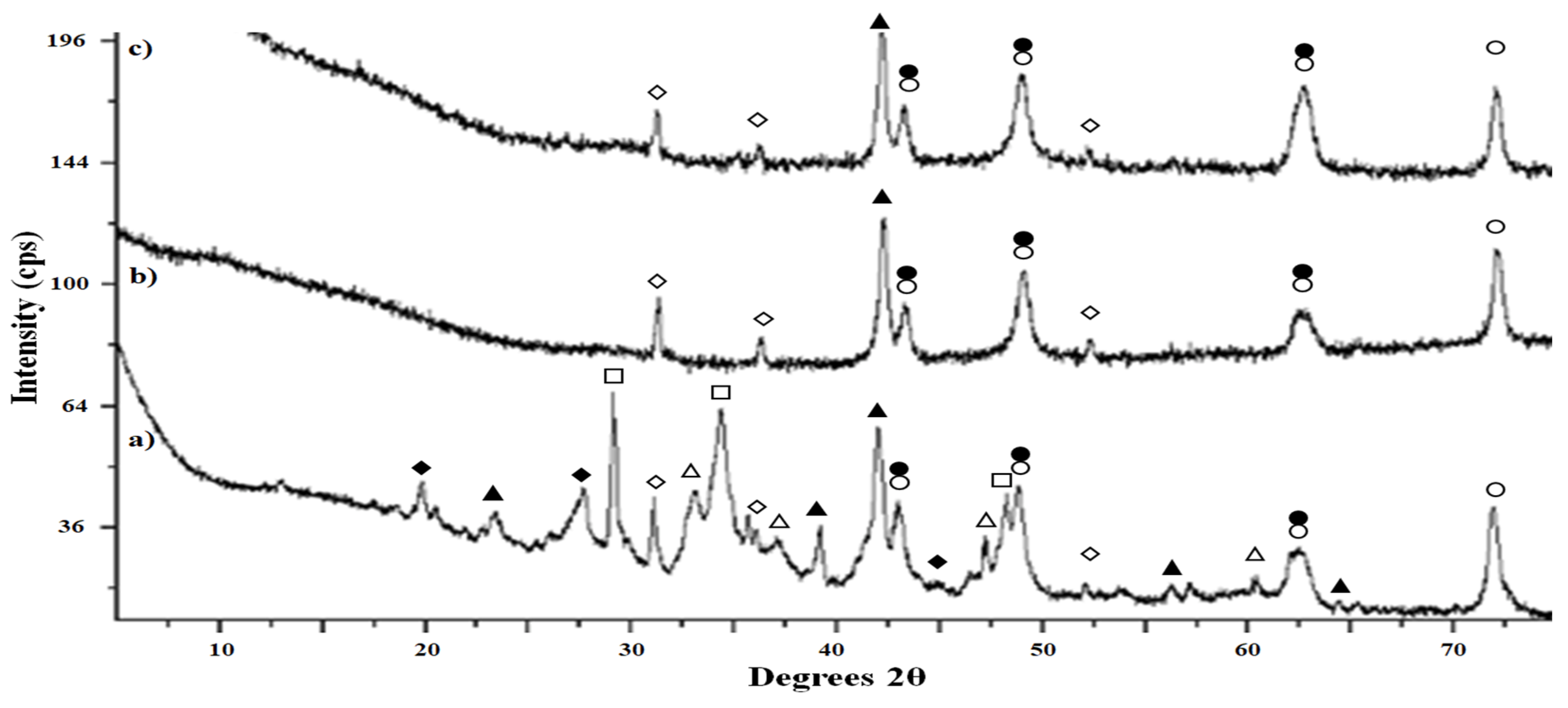
3.4. Origin and Characterization of Precipitate
4. Discussion
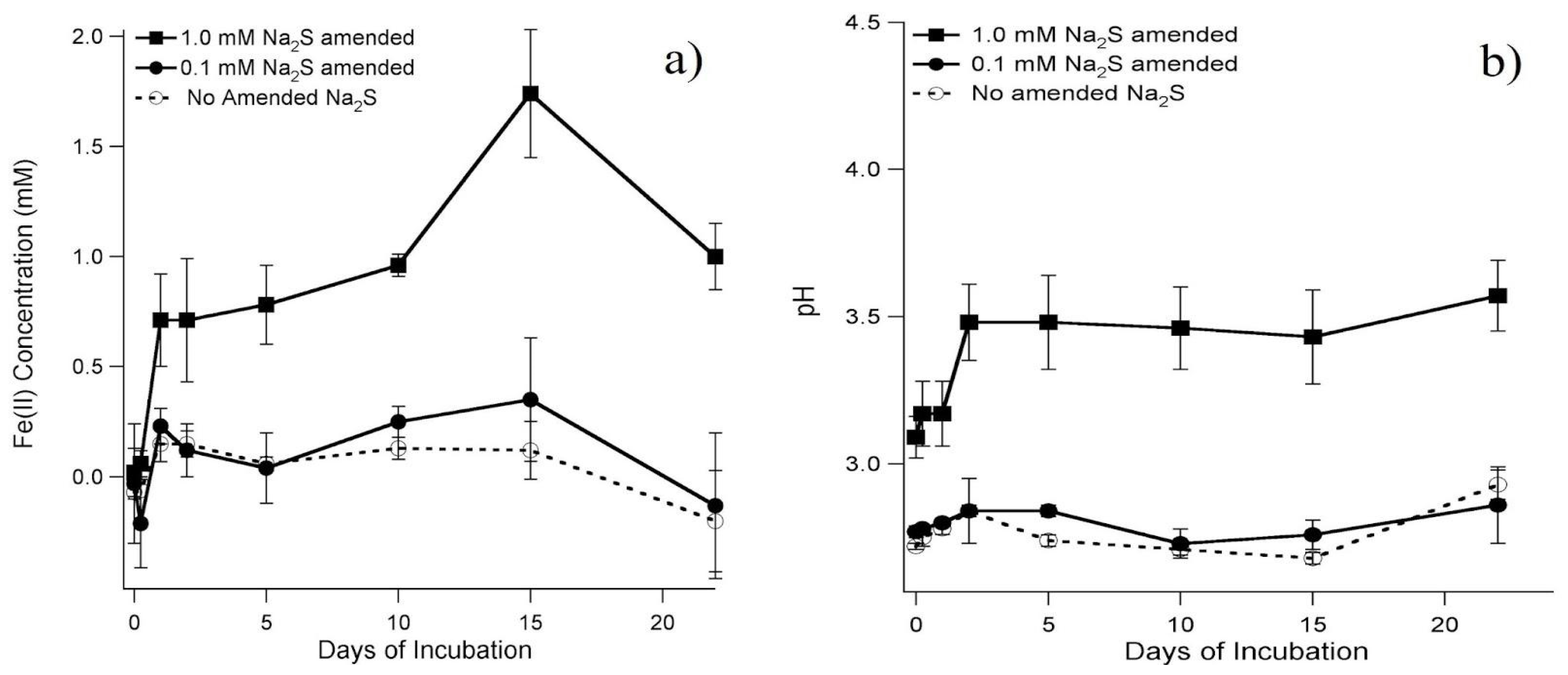
4.1. Iron Reduction Mediated by Sulfate-Reducing Bacteria
4.2. Abundance and Diversity of SRB
4.3. Origin and Characterization of the Sulfide Precipitate
4.4. Coupling Sulfur and Iron Cycles
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, D.B.; Hallberg, K.B. The microbiology of acid mine waters. Res. Microbiol. 2003, 154, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Bloom, J.; Yuretich, R.F.; Gál, N.E. Environmental consequences of acid mine-drainage from Davis pyrite mine, Rowe, Massachusetts. Northeast. Geol. Environ. Sci. 2007, 29, 108–121. [Google Scholar]
- Aguinaga, O.E.; Wakelin, J.F.T.; White, K.N.; Dean, A.P.; Pittman, J.K. The association of microbial activity with Fe, S and trace element distribution in sediment cores within a natural wetland polluted by acid mine drainage. Chemosphere 2019, 231, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Benner, S.G.; Gould, W.D.; Blowes, D.W. Microbial populations associated with the generation and treatment of acid mine drainage. Chem. Geol. 2000, 169, 435–448. [Google Scholar] [CrossRef]
- Gould, W.; Kapoor, A. The microbiology of acid mine drainage. In Environmental Aspects of Mine Wastes; Jambor, J.L., Blowes, D.W., Ritchie, A.I.M., Eds.; Mineralogical Association of Canada: Ottawa, ON, Canada, 2003; pp. 203–226. [Google Scholar]
- Ayala-Parra, P.; Sierra-Alvarez, R.; Field, J.A. Treatment of acid rock drainage using a sulfate-reducing bioreactor with zero-valent iron. J. Hazard. Mater. 2016, 308, 97–105. [Google Scholar] [CrossRef]
- Dore, E.; Fancello, D.; Rigonat, N.; Medas, D.; Cidu, R.; Da Pelo, S.; Frau, F.; Lattanzi, P.; Marras, P.A.; Meneghini, C.; et al. Natural attenuation can lead to environmental resilience in mine environment. Appl. Geochem. 2020, 117, 104597. [Google Scholar] [CrossRef]
- Ergas, S.J.; Harrison, J.; Bloom, J.; Forloney, K.; Ahlfeld, D.P.; Nüsslein, K.; Yuretich, R.F. Natural attenuation of acid mine drainage by acidophilic and acidotolerant Fe(III)- and sulfate-reducing bacteria. remediation of hazardous waste in the subsurface: Bridging flask and field studies. In American Chemical Society Symposium Series; Clark, C., II, Lindner, A., Eds.; ACS Publications: Washington, DC, USA, 2006; pp. 105–127. [Google Scholar]
- Neal, A.L.; Techkarnjanarauk, K.; Dohnalkova, A.; McCready, D.; Peyton, B.M.; Geesey, G. Iron sulfides and sulfur species produced at hematite surfaces in the presence of sulfate-reducing bacteria. Geochim. Cosmochim. Acta 2001, 65, 223–235. [Google Scholar] [CrossRef]
- Neculita, C.M.; Zagury, G.J.; Bussière, B. Passive treatment of acid mine drainage in bioreactors using sulfate-reducing bacteria: Critical review and research needs. J. Environ. Qual. 2007, 36, 1–16. [Google Scholar] [CrossRef]
- Church, C.D.; Wilkin, R.T.; Alpers, C.N.; Rye, R.O.; McCleskey, R.B. Microbial sulfate reduction and metal attenuation in pH 4 acid mine water. Geochem. Trans. 2007, 8, 10. [Google Scholar] [CrossRef]
- Paganin, P.; Alisi, C.; Dore, E.; Fancello, D.; Marras, P.A.; Medas, D.; Montereali, R.; Naitza, S.; Rigonat, N.; Sporacati, A.R.; et al. Microbial diversity of bacteria involved in biomineralization processes in mine-impacted freshwaters. Front. Microbiol. 2021, 12, 778199. [Google Scholar] [CrossRef]
- Becerra, C.A.; López-Luna, E.L.; Ergas, S.J.; Nüsslein, K. Microcosm-based study of the attenuation of an acid mine drainage-impacted site through biological sulfate and iron reduction. Geomicrobiol. J. 2009, 26, 9–20. [Google Scholar] [CrossRef]
- Coleman, M.L.; Hedrick, D.B.; Lovley, D.R.; White, D.C.; Pye, K. Reduction of Fe(III) in sediments by sulphate-reducing bacteria. Nature 1993, 361, 436–438. [Google Scholar] [CrossRef]
- Dalla Vecchia, E.; Suvorova, E.I.; Maillard, J.; Bernier-Latmani, R. Fe(III) reduction during pyruvate fermentation by Desulfotomaculum reducens strain MI-1. Geobiology 2013, 12, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R.; Roden, E.E.; Phillips, E.J.P.; Woodward, J.C. Enzymatic iron and uranium reduction by sulfate-reducing bacteria. Mar. Geol. 1993, 113, 41–53. [Google Scholar] [CrossRef]
- Li, Y.L.; Vali, H.; Sears, S.K.; Yang, J.; Deng, B.; Zhang, C.L. Iron reduction and alteration of nontronite NAu-2 by a sulfate-reducing bacterium. Geochim. Cosmochim. Acta 2004, 68, 3251–3260. [Google Scholar] [CrossRef]
- dos Santos Afonso, M.; Stumm, W. Reductive dissolution of iron(III) (hydr)oxide by hydrogen sulfide. Langmuir 1992, 8, 1671–1675. [Google Scholar] [CrossRef]
- Canfield, D.E.; Raiswell, R.; Bottrell, S. The reactivity of sedimentary iron minerals toward sulfide. Am. J. Sci. 1992, 292, 659–683. [Google Scholar] [CrossRef]
- Li, Y.L.; Valli, J.; Yang, J.; Phelps, T.J.; Zhang, C.L. Reduction of iron oxides by a sulfate-reducing bacterium and biogenic H2S. Geomicrobiol. J. 2006, 23, 103–117. [Google Scholar] [CrossRef]
- Roberts, M.; Srivastava, P.; Webster, G.; Weightman, A.J.; Sapsford, D.J. Biostimulation of jarosite and iron oxide-bearing mine waste enhances subsequent metal recovery. J. Hazard. Mater. 2023, 445, 130498. [Google Scholar] [CrossRef]
- Brown, P.W.; Williams, I.N. The History of Rowe, Massachusetts, 4th ed.; Rowe Historical Society: Rowe, MA, USA, 2006. [Google Scholar]
- Stookey, L.L. Ferrozine—A new spectrophotometric reagent for iron. Anal. Chem. 1970, 42, 779–781. [Google Scholar] [CrossRef]
- Lovley, D.R.; Phillips, E.J.P. Organic matter mineralization with the reduction of ferric iron in anaerobic sediments. Appl. Environ. Microbiol. 1996, 51, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Coggon, M.; Becerra, C.A.; Nüsslein, K.; Miller, K.; Yuretich, R.; Ergas, S.J. Bioavailability of jarosite for stimulating acid mine drainage attenuation. Geochim. Cosmochim. Acta 2012, 78, 65–76. [Google Scholar] [CrossRef]
- Jercinovic, M.J.; Williams, M.L. Analytical perils (and progress) in electron microprobe trace element analysis applied to geochronology: Background acquisition, interferences, and beam irradiation effects. Am. Mineral. 2005, 90, 526–546. [Google Scholar] [CrossRef]
- Kallmeyer, J.; Ferdelman, T.G.; Weber, A.; Fossing, H.; Jorgensen, B.B. A cold chromium distillation procedure for radiolabeled applied to sulfate reduction measurements. Limnol. Oceanogr. Methods 2004, 2, 171–180. [Google Scholar] [CrossRef]
- Chin, K.J.; Sharma, M.I.; Russel, L.A.; O’Neill, K.R.; Lovely, D.R. Quantifying expression of a dissimilatory bi(sulfite) reductase gene in petroleum-contaminated marine harbor sediments. Microb. Ecol. 2008, 55, 489–499. [Google Scholar] [CrossRef]
- Daly, K.; Sharp, R.J.; McCarthy, A.J. Development of oligonucleotide probes and PCR primers for detecting phylogenetic subgroups of sulfate-reducing bacteria. Microbiology 2000, 146, 1693–1705. [Google Scholar] [CrossRef]
- Gramp, J.P.; Bigham, J.M.; Jones, F.S.; Tuovinen, O.H. Formation of Fe-sulfides in cultures of sulfate-reducing bacteria. J. Hazard. Mater. 2010, 175, 1062–1067. [Google Scholar] [CrossRef]
- Guinier, A. X-ray diffraction. In Crystals, Imperfect Crystals, and Amorphous Bodies; Dover Publications: New York, NY, USA, 1994. [Google Scholar]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N. The power of databases: The RRUFF project. In Highlights in Mineralogical Crystallography; Armbruster, T., Danisi, R.M., Eds.; W. De Gruyter: Berlin, Germany, 2015; pp. 1–30. [Google Scholar]
- The RRUFF ™ Project. RRUFF ID’s: Hematite R110013.9, Magnetite R061111.9, Greigite R120103, Troilite R070242.9, (Pseudomorphic) Pyrite R080030 and R070692.9, Pyrite R100166.9, Chalcopyrite R050559.1. Available online: https://rruff.info/ (accessed on 8 August 2024).
- Sakala, E.; Fourie, F.; Gomo, M.; Madzivire, G. Natural attenuation of acid mine drainage by various rocks in the Witbank, Ermelo and Highveld Coalfields, South Africa. Nat. Resour. Res. 2020, 30, 557–570. [Google Scholar] [CrossRef]
- Tum, S.; Toda, K.; Matsui, T.; Kikuchi, R.; Kong, S.; Meas, P.; Ear, U.; Ohtomo, Y.; Otake, T.; Sato, T. Seasonal effects of natural attenuation on drainage contamination from artisanal gold mining, Cambodia: Implication for passive treatment. Sci. Total Environ. 2022, 806, 150398. [Google Scholar] [CrossRef]
- Lovley, D.R.; Phillips, E.J.P. Rapid assay for microbially reducible ferric iron in aquatic sediments. Appl. Environ. Microb. 1987, 53, 1536–1540. [Google Scholar] [CrossRef]
- Coetser, S.E.; Pulles, W.; Heath, R.G.M.; Cloete, T.E. Chemical characteristics of organic electron donors for sulfate reduction for potential use in acid mine drainage treatment. Biodegradation 2006, 17, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Bilgin, A.A.; Harrington, J.M. Enhancement of bacterial iron and sulfate respiration for in situ bioremediation of acid mine drainage sites: A case study. Miner. Metall. Proc. 2007, 24, 139–144. [Google Scholar] [CrossRef]
- Gyure, R.A.; Konopka, A.; Brooks, A.; Doemel, W. Microbial sulfate reduction in acidic (pH 3) strip-mine lakes. FEMS Microbiol. Ecol. 1990, 73, 193–201. [Google Scholar] [CrossRef]
- Lovley, D.R.; Phillips, E.J.P.; Lonergan, D.J. Enzymatic versus nonenzymatic mechanisms for Fe(III) reduction in aquatic sediments. Environ. Sci. Technol. 1991, 25, 1062–1067. [Google Scholar] [CrossRef]
- Poulton, S.W.; Krom, M.D.; Raiswell, R. A revised scheme for the reactivity of iron (oxyhydr)oxide minerals towards dissolved sulfide. Geochim. Cosmochim. Acta 2004, 68, 3703–3715. [Google Scholar] [CrossRef]
- Senko, J.M.; Zhang, G.; McDonough, J.T.; Bruns, M.A.; Burgos, W.D. Metal reduction at low pH by Desulfosporosinus species: Implications for the biological treatment of acidic mine drainage. Geomicrobiol. J. 2009, 26, 71–82. [Google Scholar] [CrossRef]
- Tebo, B.M.; Obraztsova, A.Y. Sulfate-reducing bacterium grows with Cr(VI), U(VI), Mn(IV), and Fe(III) as electron acceptors. FEMS Microbiol. Ecol. 1998, 162, 193–198. [Google Scholar] [CrossRef]
- Weber, K.A.; Achenbach, L.A.; Coates, J.D. Microorganisms pumping iron: Anaerobic microbial iron oxidation and reduction. Nature 2006, 4, 752–764. [Google Scholar] [CrossRef]
- Paper, J.M.; Flynn, T.M.; Boyanov, M.I.; Kemner, K.M.; Haller, B.R.; Crank, K.; Lower, A.M.; Jin, Q.; Kirk, M.F. Influences of pH and substrate supply on the ratio of iron to sulfate reduction. Geobiology 2021, 19, 405–420. [Google Scholar] [CrossRef]
- Stögbauer, A.; Koydon, S.; Berner, Z.; Winter, J.; Stüben, D. Effect of molybdate and cell growth on S-isotope fractionation during bacterial sulfate reduction. Geomicrobiol. J. 2004, 21, 207–219. [Google Scholar] [CrossRef]
- DiChristina, T.J. Effects of nitrate and nitrite on dissimilatory iron reduction by Shewanella putrefaciens 200. J. Bacteriol. 1992, 174, 1891–1896. [Google Scholar] [CrossRef] [PubMed]
- Finneran, K.T.; Housewright, M.E.; Lovley, D.R. Multiple influences of nitrate on uranium solubility during bioremediation of uranium-contaminated subsurface sediments. Environ. Microbiol. 2002, 4, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Akob, D.M.; Mills, H.J.; Gihring, T.M.; Kerkhof, L.; Stucki, J.W.; Anastacio, A.S.; Chin, K.J.; Küsel, K.; Palumbo, A.V.; Watson, D.B.; et al. Functional diversity and electron donor dependence of microbial populations capable of U(VI) reduction in radionuclide-contaminated subsurface sediments. Appl. Environ. Microb. 2008, 74, 3159–3170. [Google Scholar] [CrossRef] [PubMed]
- Moura, J.J.G.; Gonzalez, P.; Moura, I.; Faque, G. Dissimilatory nitrate and nitrite ammonification by sulphate-reducing eubacteria. In Sulphate-Reducing Bacteria: Environmental and Engineered Systems; Barton, L.L., Hamilton, W.A., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 241–264. [Google Scholar]
- Greene, E.A.; Brunelle, V.; Jenneman, G.E.; Voordouw, G. Synergistic inhibition of microbial sulfide production by combinations of the metabolic inhibitor nitrite and biocides. Appl. Environ. Microb. 2006, 72, 7897–7901. [Google Scholar] [CrossRef]
- Mangalo, M.; Einsiedl, F.; Meckenstock, R.U.; Stichler, W. Influence of the enzyme dissimilatory sulfite reductase on stable isotope fractionation during sulfate reduction. Geochim. Cosmochim. Acta 2008, 72, 1513–1520. [Google Scholar] [CrossRef]
- Vile, M.A.; Wieder, R.K. Alkalinity generation by Fe(III) reduction versus sulfate reduction in wetlands constructed for acid mine drainage treatment. Water Air Soil. Poll. 1993, 69, 425–441. [Google Scholar] [CrossRef]
- Küsel, K.; Dorsch, T. Effect of supplemental electron donors on the microbial reduction of Fe(III), sulfate, and CO2 in coal mining-impacted freshwater lake sediments. Microb. Ecol. 2000, 40, 238–249. [Google Scholar] [CrossRef]
- Gibert, O.; de Pablo, J.; Cortina, J.L.; Ayora, C. Chemical characterization of natural organic substrates for biological mitigation of acid mine drainage. Water Res. 2004, 38, 4186–4196. [Google Scholar] [CrossRef]
- Karnachuk, O.V.; Kurochkina, S.Y.; Tuovinen, O.H. Growth of sulfate-reducing bacteria with solid-phase electron acceptors. Appl. Microbiol. Biot. 2002, 58, 482–486. [Google Scholar] [CrossRef]
- Harrison, J. Ferric Iron and Sulfate Reduction in the Attenuation of Acid Mine Drainage: A Microcosm Study. Master’s Thesis, University of Massachusetts, Amherst, MA, USA, 2005. [Google Scholar]
- Gilmore, A. Attenuation of Acid Mine Drainage Enhanced by Organic Carbon and Limestone Addition: A Process Characterization. Master’s Thesis, University of Massachusetts, Amherst, MA, USA, 2011. [Google Scholar]
- Fortin, D.; Davis, B.; Beveridge, T.J. Role of Thiobacillus and sulfate-reducing bacteria in iron biocycling in oxic and acidic mine tailings. FEMS Microbiol. Ecol. 1996, 21, 11–24. [Google Scholar] [CrossRef]
- Johnson, D.B. Minireview: Biodiversity and ecology of acidophilic microorganisms. FEMS Microbiol. Ecol. 1998, 27, 307–317. [Google Scholar] [CrossRef]
- Frederico, T.D.; Nancucheo, I.; Santos, W.C.B.; Oliveira, R.R.M.; Buzzi, D.C.; Pires, E.S.; Silva, P.M.P.; Lucheta, A.R.; Alves, J.O.; de Oliveira, G.C.; et al. Comparison of two acidophilic sulfidogenic consortia for the treatment of acidic mine water. Front. Bioeng. Biotechnol. 2022, 29, 1048412. [Google Scholar] [CrossRef] [PubMed]
- Koschorreck, M.; Wedt-Potthoff, K.; Geller, W. Microbial sulfate reduction at low pH in sediments of an acidic lake in Argentina. Environ. Sci. Technol. 2003, 37, 1159–1162. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, S.; Piotrowski, J.S.; Langner, H.W.; Holben, W.E. Ecology of sulfate-reducing bacteria in an iron-dominated, mining-impacted freshwater sediment. J. Environ. Qual. 2009, 38, 675–684. [Google Scholar] [CrossRef]
- Anantharaman, K.; Hausmann, B.; Jungbluth, S.P.; Kantor, R.S.; Lavy, A.; Warren, L.A.; Rappe, M.S.; Pester, M.; Loy, A.; Thomas, B.C.; et al. Expanded diversity of microbial groups that shape the dissimilatory sulfur cycle. ISME J. 2018, 12, 1715–1728. [Google Scholar] [CrossRef]
- Valdez-Nunez, L.F.; Ayala-Munoz, D.; Sanchez-Espana, J.; Sanchez-Andrea, I. Microbial communities in Peruvian acid mine drainages: Low-abundance sulfate-reducing bacteria with high metabolic activity. Geomicrobiol. J. 2022, 39, 867–883. [Google Scholar] [CrossRef]
- Rambabu, K.; Banat, F.; Pham, Q.M.; Ho, S.H.; Ren, N.Q.; Show, P.L. Biological remediation of acid mine drainage: A review of past trends and current outlook. Environ. Sci. Ecotechnol. 2020, 2, 100024. [Google Scholar] [CrossRef]
- Sánchez-Andrea, I.; Sanz, J.L.; Bijmans, M.F.M.; Stams, A.J.M. Sulfate reduction at low pH to remediate acid mine drainage. J. Hazard. Mater. 2014, 269, 98–109. [Google Scholar] [CrossRef]
- Bruneel, O.; Duran, R.; Koffie, K.; Casiot, C.; Fourçans, A.; Elbaz-Poulichet, F.; Personne, J.-C. Microbial diversity in a pyrite-rich tailings impoundment (Carnoulès, France). Geomicrobiol. J. 2005, 22, 249–257. [Google Scholar] [CrossRef]
- Praharaj, T.; Fortin, D. Indicators of microbial sulfate reduction in acidic sulfide-rich mine tailings. Geomicrobiol. J. 2004, 21, 457–467. [Google Scholar] [CrossRef]
- Roychoudhury, A.N. Sulfate respiration in extreme environments: A kinetic study. Geomicrobiol. J. 2004, 21, 33–43. [Google Scholar] [CrossRef]
- Rowe, O.F.; Sánchez-España, J.; Hallberg, K.B.; Johnson, D.B. Microbial communities and geochemical dynamics in an extremely acidic, metal-rich stream at an abandoned sulfide mine (Huelva, Spain) underpinned by two functional primary production systems. Environ. Microbiol. 2007, 9, 1761–1771. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Hamai, T.; Hori, T.; Aoyagi, T.; Inaba, T.; Kobayashi, M.; Have, H.; Sakata, T. Desulfosporosinus spp. were the most predominant sulfate-reducing bacteria in pilot- and laboratory-scale passive bioreactors for acid mine drainage treatment. Appl. Microbiol. Biotech. 2019, 103, 7783–7793. [Google Scholar] [CrossRef] [PubMed]
- van der Maas, P.; Peng, S.; Klapwijk, B.; Lens, P. Enzymatic versus nonenzymatic conversions during the reduction of EDTA-chelated Fe(III) in BioDeNOx reactors. Environ. Sci. Technol. 2005, 39, 2616–2623. [Google Scholar] [CrossRef] [PubMed]
- Diez-Ercilla, M.; Sánchez-España, J.; Yusta, I.; Wendt-Potthoff, K.; Koschorreck, M. Formation of biogenic sulphides in the water column of an acidic pit lake: Biogeochemical controls and effects on trace metal dynamics. Biogeochemistry 2014, 121, 519–536. [Google Scholar] [CrossRef]
- An, W.; Hu, X.; Chen, H.; Wang, Q.; Zheng, Y.; Wang, J.; Di, J. Experimental study on the treatment of AMD by SRB immobilized particles containing “active iron” system. PLoS ONE 2023, 18, 20161. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.; Zhao, D.; Zhuang, L.; Yang, G.; Gong, Y. Immobilization of mercury by iron sulfide nanoparticles alters mercury speciation an dmicrobial methylation in contaminated groundwater. Chem. Eng. J. 2020, 381, 122664. [Google Scholar] [CrossRef]
- Osorio, H.; Mangold, S.; Denis, Y.; Nancucheo, I.; Esparza, M.; Johnson, D.B.; Bonnefoy, V.; Dopson, M.; Holmes, D.S. Anaerobic sulfur metabolism coupled to dissimilatory iron reduction in the extremophile Acidithiobacillus ferrooxidans. Appl. Environ. Microbiol. 2013, 79, 2172–2181. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, X.; Sun, S.; Wang, Y. Arsenic transformation and redistribution in groundwater induced by the complex geochemical cycling of iron and sulfur. Sci. Total Environ. 2023, 894, 164941. [Google Scholar] [CrossRef]
- Jorgensen, B.B.; Bak, F. Pathways and microbiology of thiosulfate transformations and sulfate reduction in a marine sediment (Kattegat, Denmark). Appl. Environ. Microbiol. 1991, 57, 847–856. [Google Scholar] [CrossRef]
- van der Graff, C.M.; Sanchez-Espana, J.; Yusta, I.; Ilin, A.; Shetty, S.A.; Bale, N.J.; Villanueva, L.; Stams, A.J.M.; Sanchez-Andrea, I. Biosulfidogenesis mediates natural attenuation in acidic mine pit lakes. Microorganisms 2020, 8, 1275. [Google Scholar] [CrossRef] [PubMed]
- Straub, K.L.; Benz, M.; Schink, B. Iron metabolism in anoxic environments at near neutral pH. FEMS Microbiol. Ecol. 2001, 34, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Blöthe, M.; Akob, D.M.; Kostka, J.E.; Göschel, K.; Drake, H.L.; Küsel, K. pH gradient-induced heterogeneity of Fe(III)-reducing microorganisms in coal mining-associated lake sediments. Appl. Environ. Microbiol. 2008, 74, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Blodau, C. A review of acidity generation and consumption in acidic coal mine lakes and their watersheds. Sci. Total Environ. 2006, 369, 307–332. [Google Scholar] [CrossRef]


| Microcosms | SRR (nmol/(cm3/d)) | pH | ORP (mV) | DOC (mg/L) | Sulfate Conc. (mM) | Fe2+ (mM) |
|---|---|---|---|---|---|---|
| Unamended | 19 ± 2 | 5.6 ± 0.1 | 43 ± 5 | 8.4 ± 0.5 | 5.1 ± 0.3 | 1.6 ± 0.1 |
| Glycerol + N + P | 22 ± 2 | 5.5 ± 0.3 | −25 ± 9 | 13.6 ± 0.9 | 4.5 ± 0.1 | 1.0 ± 0.1 |
| Abiotic control | 0.3 ± 0.01 | 3.0 ± 0.1 | 375 ± 7 | 12.8 ± 0.35 | NA | BDL |
| Groundwater | NA | 5.3 ± 0.2 | 235 ± 32 | NA | 9.5 ± 0.8 | BDL |
| Microcosms | SRB/mL (Initial) | SRB/mL (Final) | Cells/mL (Initial) | Cells/mL (Final) |
|---|---|---|---|---|
| Unamended | 2.5 ± 0.8 × 104 | 2.75 ± 0.01 × 106 | 1.52 × 107 | 1.89 × 107 |
| Glycerol + N + P | 2.2 ± 0.7 × 104 | 6.59 ± 0.06 × 106 | 1.83 × 107 | 2.84 × 107 |
| SRB Group | Unamended Microcosms | Glycerol + N + P Amended Microcosms |
|---|---|---|
| Desulfotomaculum (dfm) | + | + |
| Desulfobacter (dsb) | − | + |
| Desulfococcus–Desulfonema–Desulfosarcina (dcc) | − | − |
| Desulfovibrio (dsv) | + | + |
| Desulfobulbus (dbb) | + | + |
| Desulfobacterium (dbm) | − | − |
| Precipitate Samples | Fe | S |
|---|---|---|
| GNP Microcosms | 55.2 ± 18.0 | 39.0 ± 11.8 |
| Davis Mine | 64.9 ± 4.8 | 35.1 ± 4.8 |
| FeCl2 + Na2S | 47.5 ± 1.9 | 37.1 ± 1.4 |
| FeCl3 + Na2S | 45.5 ± 0.7 | 37.4 ± 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becerra, C.A.; Murphy, B.; Veldman, B.V.; Nüsslein, K. Biogenic Sulfide-Mediated Iron Reduction at Low pH. Microorganisms 2024, 12, 1939. https://doi.org/10.3390/microorganisms12101939
Becerra CA, Murphy B, Veldman BV, Nüsslein K. Biogenic Sulfide-Mediated Iron Reduction at Low pH. Microorganisms. 2024; 12(10):1939. https://doi.org/10.3390/microorganisms12101939
Chicago/Turabian StyleBecerra, Caryl Ann, Brendan Murphy, Brittnee V. Veldman, and Klaus Nüsslein. 2024. "Biogenic Sulfide-Mediated Iron Reduction at Low pH" Microorganisms 12, no. 10: 1939. https://doi.org/10.3390/microorganisms12101939
APA StyleBecerra, C. A., Murphy, B., Veldman, B. V., & Nüsslein, K. (2024). Biogenic Sulfide-Mediated Iron Reduction at Low pH. Microorganisms, 12(10), 1939. https://doi.org/10.3390/microorganisms12101939






