Rapid Responses of Greenhouse Gas Emissions and Microbial Communities to Carbon and Nitrogen Addition in Sediments
Abstract
1. Introduction
2. Materials and Methods
2.1. Sediments Sampling
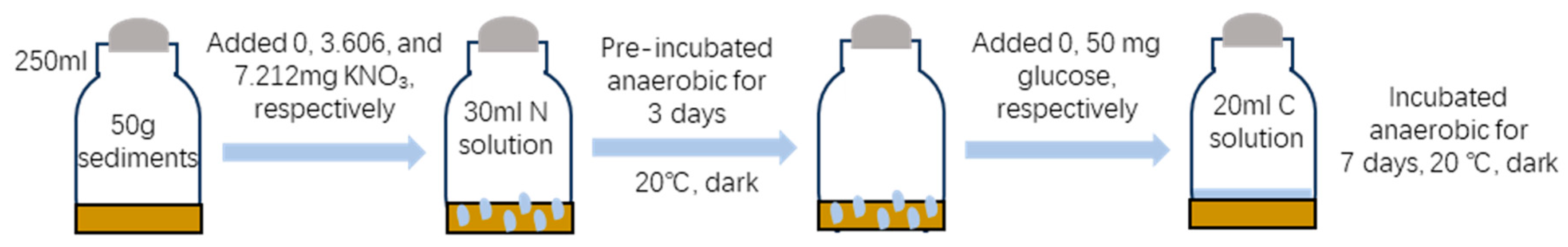
2.2. Experimental Design and Incubation
2.3. Measurements
2.3.1. Greenhouse Gas Collection and Measurement
2.3.2. DNA Extraction, PCR Amplification, and Metagenomic Sequencing
2.3.3. Sequence Quality Control and Genome Assembly
2.3.4. Processing of Sequencing Data
2.4. Statistical Analysis
3. Results
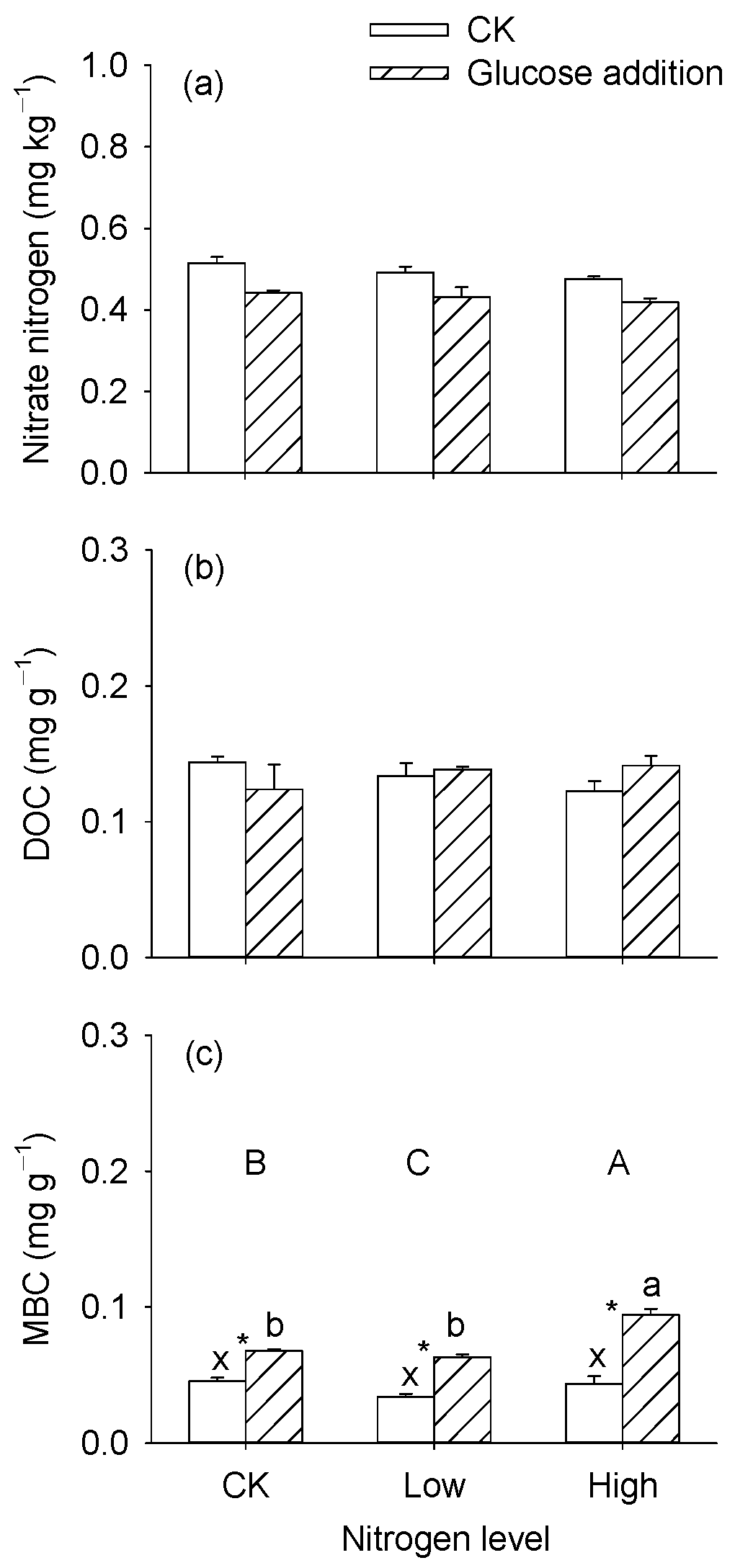
3.1. Nitrogen and Carbon Contents in the Sediments
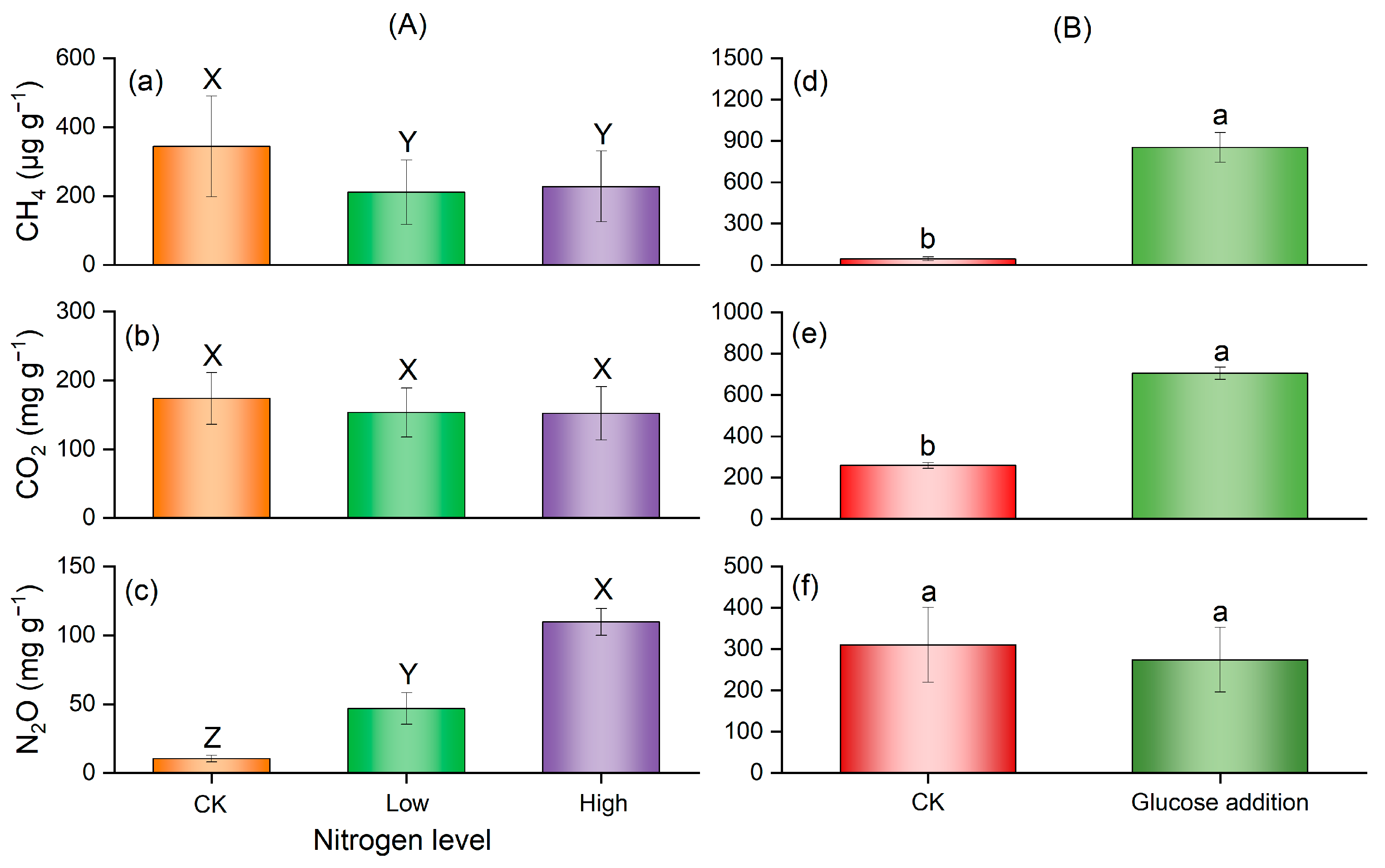
3.2. Greenhouse Gas Emission after Glucose Addition
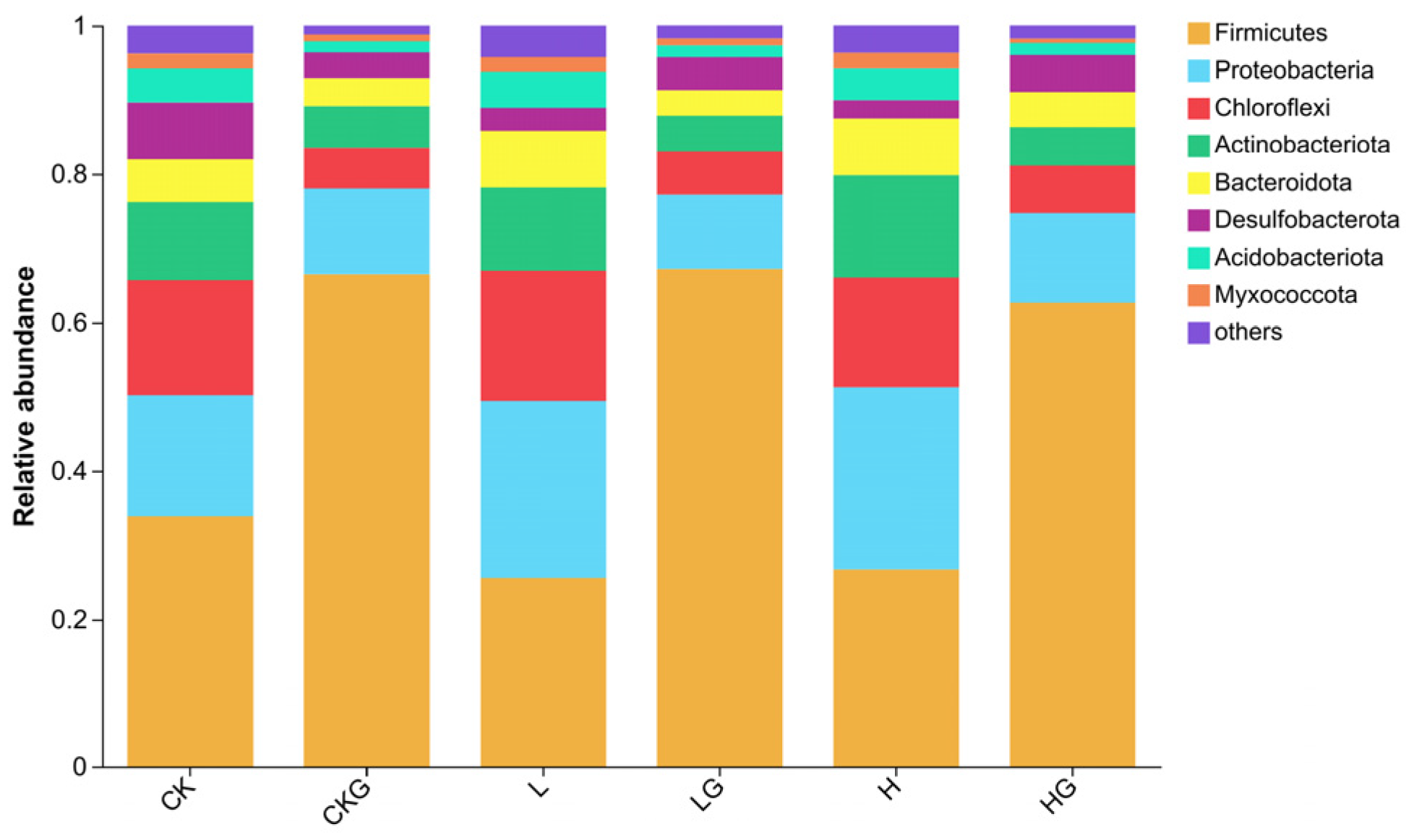
3.3. Bacterial Community Structure and Related Associated Function Genes in the Sediments
3.4. Relationships among Greenhouse Gas, Soil Nutrients, and Microbes
4. Discussion
4.1. Impact of Labile Carbon and Nitrogen Inputs on Greenhouse Gas Emissions
4.2. The Changes in Soil Microbial Structure after External Nutrient Addition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zheng, Y.L.; Hou, L.J.; Liu, M.; Yin, G.Y. Dynamics and environmental importance of anaerobic ammonium oxidation (anammox) bacteria in urban river networks. Environ. Pollut. 2019, 254, 112998. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.; Zou, W.B.; Jiang, X.L.; Wan, X.H.; Hsu, T.C.; Zheng, Z.Z.; Chen, L.; Xu, M.; Dai, M.H.; Kao, S.J. Organic matter decomposition sustains sedimentary nitrogen loss in the Pearl River Estuary, China. Sci. Total Environ. 2019, 648, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Craig, H.; Antwis, R.E.; Cordero, I.; Ashworth, D.; Robinson, C.H.; Osborne, T.Z.; Bardgett, R.D.; Rowntree, J.K.; Simpson, L.T. Nitrogen addition alters composition, diversity, and functioning of microbial communities in mangrove soils: An incubation experiment. Soil Biol. Biochem. 2021, 153, 108076. [Google Scholar] [CrossRef]
- Goldberg, S.J.; Nelson, C.E.; Viviani, D.A.; Shulse, C.N.; Church, M.J. Cascading influence of inorganic nitrogen sources on DOM production, composition, lability and microbial community structure in the open ocean. Environ. Microbiol. 2017, 19, 3450–3464. [Google Scholar] [CrossRef] [PubMed]
- Doroski, A.A.; Helton, A.M.; Vadas, T.M. Greenhouse gas fluxes from coastal wetlands at the intersection of urban pollution and saltwater intrusion: A soil core experiment. Soil Biol. Biochem. 2019, 1131, 44–53. [Google Scholar] [CrossRef]
- Kathiravan, M.; Niel, V.; Johannes, I.; Lucia, F. Individual and interactive effects of warming and nitrogen supply on CO2 fluxes and carbon allocation in subarctic grassland. Global Chang. Biol. 2023, 29, 5276–5291. [Google Scholar]
- Qiu, L.J.; Gou, X.; Kong, Y.M.; Tu, F.Y.; Peng, X. Nitrogen addition stimulates N2O emissions via changes in denitrification community composition in a subtropical nitrogen-rich forest. J. Environ. Manag. 2023, 348, 119274. [Google Scholar] [CrossRef]
- Aronson, E.L.; Dubinsky, E.A.; Helliker, B.R. Effects of nitrogen addition on soil microbial diversity and methane cycling capacity depend on drainage conditions in a pine forest soil. Soil Biol. Biochem. 2013, 62, 119–128. [Google Scholar] [CrossRef]
- Xu, G.M.; Li, Y.; Hou, W.H.; Wang, S.; Kong, F.L. Effects of substrate type on enhancing pollutant removal performance and reducing greenhouse gas emission in vertical subsurface flow constructed wetland. J. Environ. Manag. 2021, 280, 111674. [Google Scholar] [CrossRef]
- Pang, Y.M.; Ji, G.D. Biotic factors drive distinct DNRA potential rates and contributions in typical Chinese shallow lake sediments. Environ. Pollut. 2019, 254, 112903. [Google Scholar] [CrossRef]
- Bernard-Jannin, L.; Sun, X.; Teissier, S.; Sauvage, S.; Sánchez-Pérez, J.M. Spatio-temporal analysis of factors controlling nitrate dynamics and potential denitrification hot spots and hot moments in groundwater of an alluvial floodplain. Ecol. Eng. 2017, 103, 372–384. [Google Scholar] [CrossRef]
- Dai, H.T.; Zhu, R.B.; Sun, B.W.; Che, C.S.; Hou, L.J. Effects of sea animal activities on tundra soil denitrification and nirS- and nirK-encoding denitrifier community in maritime antarctica. Front. Microbiol. 2020, 11, 573302. [Google Scholar] [CrossRef]
- Minor, E.C.; Oyler, A.R. Dissolved organic matter in large lakes: A key but understudied component of the carbon cycle. Biogeochemistry 2023, 164, 295–318. [Google Scholar] [CrossRef]
- Liu, S.S.; Hou, J.W.; Suo, C.Y.; Chen, J.Y.; Liu, X.H.; Fu, R.; Wu, F.C. Molecular-level composition of dissolved organic matter in distinct trophic states in Chinese lakes: Implications for eutrophic lake management and the global carbon cycle. Water Res. 2022, 217, 118438. [Google Scholar] [CrossRef]
- Murray, R.H.; Erler, D.V.; Eyre, B.D. Nitrous oxide fluxes in estuarine environments: Response to global change. Global Chang. Biol. 2015, 21, 3219–3245. [Google Scholar] [CrossRef]
- Surey, R.; Schimpf, C.M.; Sauheitl, L.; Mueller, C.W.; Rummel, P.S.; Dittert, K.; Kaiser, K.; Böttcher, J.; Mikutta, R. Potential denitrification stimulated by water-soluble organic carbon from plant residues during initial decomposition. Soil Biol. Biochem. 2020, 147, 107841. [Google Scholar] [CrossRef]
- Zhao, S.Y.; Wang, Q.; Zhou, J.M.; Yuan, D.B.; Zhu, G.B. Linking abundance and community of microbial N2O-producers and N2O-reducers with enzymatic N2O production potential in a riparian zone. Sci. Total Environ. 2018, 642, 1090–1099. [Google Scholar] [CrossRef]
- Yang, H.L.; Chen, X.C.; Tang, J.W.; Zhang, L.; Zhang, C.S.; Perry, D.C.; You, W.H. External carbon addition increases nitrate removal and decreases nitrous oxide emission in a restored wetland. Ecol. Eng. 2019, 138, 200–208. [Google Scholar] [CrossRef]
- Xu, P.; Zhou, W.; Jiang, M.; Khan, I.; Wu, T.; Zhou, M.; Zhu, B.; Hu, R. Methane emission from rice cultivation regulated by soil hydrothermal condition and available carbon and nitrogen under a rice–wheat rotation system. Plant Soil 2022, 480, 283–294. [Google Scholar] [CrossRef]
- Li, X.W.; Song, C.L.; Zhou, Z.J.; Xiao, J.; Wang, S.Y.; Yang, L.; Cao, X.Y.; Zhou, Y.Y. Comparison of community and function of dissimilatory nitrate reduction to ammonium (DNRA) bacteria in Chinese shallow lakes with different eutrophication degrees. Water 2020, 12, 174. [Google Scholar] [CrossRef]
- Lv, S.C.; Wang, F.; Yan, W.J.; Wang, Y.C.; Yu, Q.B.; Li, Y.Q. DOC fluorescence properties and degradation in the Changjiang River Network, China: Implications for estimating in-stream DOC removal. Biogeochemistry 2019, 145, 255–273. [Google Scholar] [CrossRef]
- Hellman, M.; Bonilla-Rosso, G.; Widerlund, A.; Juhanson, J.; Hallin, S. External carbon addition for enhancing denitrification modifies bacterial community composition and affects CH4 and N2O production in sub-arctic mining pond sediments. Water Res. 2019, 158, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, Y.; Zhang, H.J.; Wang, L.; Zhang, W.F.; Niu, L.H.; Wang, P.F.; Wang, C. The responses of bacterial community and N2O emission to nitrogen input in lake sediment: Estrogen as a co-pollutant. Environ. Res. 2019, 179, 108769. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.M.; Mao, L.J.; Chen, B.L. Driving forces linking microbial community structure and functions to enhanced carbon stability in biochar-amended soil. Environ. Int. 2019, 133, 105211. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.L.; Chang, L.; Zhang, J.M.; Guo, F.C.; Vymazal, J.; He, Q.; Chen, Y. Global nitrogen input on wetland ecosystem: The driving mechanism of soil labile carbon and nitrogen on greenhouse gas emissions. Environ. Sci. Ecotechnol. 2020, 4, 100063. [Google Scholar] [CrossRef] [PubMed]
- You, L.C.; Ros, G.H.; Chen, Y.L.; Yang, X.; Cui, Z.L.; Liu, X.J.; Jiang, R.F.; Zhang, F.S.; de Vries, W. Global meta-analysis of terrestrial nitrous oxide emissions and associated functional genes under nitrogen addition. Soil Biol. Biochem. 2022, 165, 108523. [Google Scholar] [CrossRef]
- Lin, Y.X.; Yuan, J.J.; Liu, D.Y.; Kang, H.; Freeman, C.; Hu, H.-W.; Ye, G.P.; Ding, W.X. Divergent responses of wetland methane emissions to elevated atmospheric CO2 dependent on water table. Water Res. 2021, 205, 117682. [Google Scholar] [CrossRef]
- Luo, D.; Li, Y.; Yao, H.; Chapman, S.J. Effects of different carbon sources on methane production and the methanogenic communities in iron rich flooded paddy soil. Sci. Total Environ. 2022, 823, 153636. [Google Scholar] [CrossRef]
- Bonetti, G.; Limpert, K.E.; Brodersen, K.E.; Trevathan-Tackett, S.M.; Carnell, P.E.; Macreadie, P.I. The combined effect of short-term hydrological and N-fertilization manipulation of wetlands on CO2, CH4, and N2O emissions. Environ. Pollut. 2022, 294, 118637. [Google Scholar] [CrossRef]
- Lu, Z.Y.; Wang, F.F.; Xiao, K.; Wang, Y.; Yu, Q.B.; Cheng, P. Carbon dynamics and greenhouse gas outgassing in an estuarine mangrove wetland with high input of riverine nitrogen. Biogeochemistry 2023, 162, 221–235. [Google Scholar] [CrossRef]
- Sun, F.; Deng, Q.H.; Li, X.W.; Tang, M.J.; Ma, X.F.; Cao, X.Y.; Zhou, Y.Y.; Song, C.L. Organic carbon quantity and quality jointly triggered the switch between dissimilatory nitrate reduction to ammonium (DNRA) and denitrification in biofilters. Chemosphere 2021, 280, 130917. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Gao, G.; Zhang, L.; Tang, X.; Shao, K.; Hu, Y. Denitrification and dissimilatory nitrate reduction to ammonium in freshwater lakes of the Eastern Plain, China: Influences of organic carbon and algal bloom. Sci. Total Environ. 2020, 710, 136303. [Google Scholar] [CrossRef]
- Liang, J.F.; Li, Q.W.; Gao, J.Q.; Feng, J.G.; Zhang, X.Y.; Hao, Y.J.; Yu, F.H. Biochar-compost addition benefits Phragmites australis growth and soil property in coastal wetlands. Sci. Total Environ. 2021, 769, 145166. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.T.; He, C.; Yuan, X.; Zhang, Q.; Wang, S.L.; Wang, B.H.; Guo, X.M.; Zhang, L. Rice straw biochar mitigated more N2O emissions from fertilized paddy soil with higher water content than that derived from ex situ biowaste. Environ. Pollut. 2020, 263, 114477. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Ding, Y.; Yue, Y.; Zhang, X.Y.; Song, M.H.; Gao, J.Q.; Yu, F.H. Litter affects CO2 emission from alpine wetland soils experiencing drying-rewetting cycles with different intensities and frequencies. Catena 2021, 198, 105025. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, W.K.; Li, X.C.; Gao, G.; Jiang, J.H. Linking bacterial community shifts with changes in the dissolved organic matter pool in a eutrophic lake. Sci. Total Environ. 2020, 719, 137387. [Google Scholar] [CrossRef]
- Li, Y.; Shang, J.; Zhang, C.; Zhang, W.; Niu, L.; Wang, L.; Zhang, H. The role of freshwater eutrophication in greenhouse gas emissions: A review. Sci. Total Environ. 2021, 768, 144582. [Google Scholar] [CrossRef]
- Giblin, A.E.; Tobias, C.R.; Song, B.; Weston, N.; Banta, G.T.; Rivera-Monroy, V.H. The importance of dissimilatory nitrate reduction to ammonium (DNRA) in the nitrogen cycle of coastal ecosystems. Oceanography 2013, 26, 124–131. [Google Scholar] [CrossRef]
- Yang, S.H.; Xiao, Y.N.; Sun, X.; Ding, J.; Jiang, Z.W.; Xu, J.Z. Biochar improved rice yield and mitigated CH4 and N2O emissions from paddy field under controlled irrigation in the Taihu Lake Region of China. Atmos. Environ. 2019, 200, 69–77. [Google Scholar] [CrossRef]
- Bodelier, P.L.E.; Roslev, P.; Henckel, T.; Frenzel, P.J.N. Stimulation by ammonium-based fertilizers of methane oxidation in soil around rice roots. Nature 2000, 403, 421–424. [Google Scholar] [CrossRef]
- Wu, J.W.; Zhang, Q.S.; Guo, C.Y.; Li, Q.K.; Hu, Y.W.; Jiang, X.M.; Zhao, Y.C.; Wang, J.; Zhao, Q. Effects of aeration on pollution load and greenhouse gas emissions from agricultural drainage ditches. Water 2022, 14, 3783. [Google Scholar] [CrossRef]
- Deng, S.P.; Parham, J.A.; Hattey, J.A.; Babu, D. Animal manure and anhydrous ammonia amendment alter microbial carbon use efficiency, microbial biomass, and activities of dehydrogenase and amidohydrolasess in semiarid agroecosystems. Appl. Soil Ecol. 2006, 33, 258–268. [Google Scholar] [CrossRef]
- Guan, B.; Xie, B.H.; Yang, S.S.; Hou, A.X.; Chen, M.; Han, G.X. Effects of five years’ nitrogen deposition on soil properties and plant growth in a salinized reed wetland of the Yellow River Delta. Ecol. Eng. 2019, 136, 160–166. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.S.; He, L.X.; Dou, J.X.; Zhao, H.M. Responses of carbon dynamics to nitrogen deposition in typical freshwater wetland of Sanjiang plain. J. Chem. 2014, 2014, 603948. [Google Scholar] [CrossRef]
- Jia, J.; Bai, J.H.; Wang, W.; Yin, S.; Zhang, G.G.; Zhao, Q.Q.; Wang, X.; Liu, X.H.; Cui, B.S. Salt stress alters the short-term responses of nitrous oxide emissions to the nitrogen addition in salt-affected coastal soils. Sci. Total Environ. 2020, 742, 140124. [Google Scholar] [CrossRef]
- Gillam, K.M.; Zebarth, B.J.; Burton, D.L. Nitrous oxide emissions from denitrification and the partitioning of gaseous losses as affected by nitrate and carbon addition and soil aeration. Can. J. Soil Sci. 2008, 88, 133–143. [Google Scholar] [CrossRef]
- Peng, B.; Sun, J.; Liu, J.; Xia, Z.; Dai, W. Relative contributions of different substrates to soil N2O emission and their responses to N addition in a temperate forest. Sci. Total Environ. 2021, 767, 144126. [Google Scholar] [CrossRef]
- Zheng, J.F.; Chen, J.H.; Pan, G.X.; Liu, X.Y.; Zhang, X.H.; Li, L.Q.; Bian, R.J.; Cheng, K.; Zheng, J.W. Biochar decreased microbial metabolic quotient and shifted community composition four years after a single incorporation in a slightly acid rice paddy from southwest China. Sci. Total Environ. 2016, 571, 206–217. [Google Scholar] [CrossRef]
- Chen, C.J.; Wang, Y.Q.; Jiang, Y.; Guo, M.L.; Cui, M.H.; Zhang, T.C. Effects of organic-matter-induced short-term stresses on performance and population dynamics of anammox systems. J. Environ. Eng. 2020, 146, 04020120. [Google Scholar] [CrossRef]
- Pishgar, R.; Dominic, J.A.; Sheng, Z.; Tay, J.H. Denitrification performance and microbial versatility in response to different selection pressures. Bioresour. Technol. 2019, 281, 72–83. [Google Scholar] [CrossRef]
- Hug, L.A.; Castelle, C.J.; Wrighton, K.C.; Thomas, B.C.; Sharon, I.; Frischkorn, K.R.; Williams, K.H.; Tringe, S.G.; Banfield, J.F. Community genomic analyses constrain the distribution of metabolic traits across the Chloroflexi phylum and indicate roles in sediment carbon cycling. Microbiome 2013, 1, 22. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Lauber, C.L.; Ramirez, K.S.; Zaneveld, J.; Bradford, M.A.; Knight, R. Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. ISME J. 2012, 6, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Qu, Q.; Wang, M.G.; Xu, H.W.; Yan, Z.R.; Liu, G.B.; Xue, S. Role of soil biotic and abiotic properties in plant community composition in response to nitrogen addition. Land Degrad. Dev. 2022, 33, 904–915. [Google Scholar] [CrossRef]
- Yang, Z.; Lu, L.; Cheng, Z.; Xian, J.; Yang, Y.; Liu, L.; Xu, X. Dissimilatory nitrate reduction in urban lake ecosystems: A comparison study between closed and open lakes in Chengdu, China. Water Res. 2022, 214, 118218. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, F.; Abalos, D.; Luo, Y.Q.; Hui, D.F.; Hungate, B.A.; García-Palacios, P.; Kuzyakov, Y.; Olesen, J.E.; Jørgensen, U.; et al. Stimulation of ammonia oxidizer and denitrifier abundances by nitrogen loading: Poor predictability for increased soil N2O emission. Global Chang. Biol. 2022, 22, 2158–2168. [Google Scholar] [CrossRef]
- Freitag, T.E.; Toet, S.; Ineson, P.; Prosser, J.I. Links between methane flux and transcriptional activities of methanogens and methane oxidizers in a blanket peat bog. FEMS Microbiol. Ecol. 2010, 73, 157–165. [Google Scholar] [CrossRef]
- Wang, L.; Qin, T.; Liu, T.; Guo, L.; Li, C.; Zhai, Z. Inclusion of microbial inoculants with straw mulch enhances grain yields from rice fields in central China. Food Energy Secur. 2020, 9, e230. [Google Scholar] [CrossRef]




| Variable | Sediments |
|---|---|
| Ammonia nitrogen (mg kg−1) | 12.00 ± 6.01 |
| Nitrate nitrogen (mg kg−1) | 2.82 ± 1.26 |
| Total nitrogen (mg g−1) | 15.30 ± 0.31 |
| Total carbon (mg g−1) | 0.50 ± 0.02 |
| Dissolved organic carbon (mg g−1) | 0.11 ± 0.03 |
| pH | 7.62 ± 0.39 |
| EC (μS cm−1) | 560.33 ± 133.16 |
| Clay (%) | 3.74 ± 0.08 |
| Silt (%) | 29.74 ± 0.54 |
| Gravel (%) | 66.49 ± 0.62 |
| Variables | Nitrogen (N) | Glucose (G) | N × G | |||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| Nitrate nitrogen (NO3−-N) | 2.42 | 0.131 | 29.01 | <0.001 | 0.35 | 0.713 |
| Dissolved organic carbon (DOC) | 0.09 | 0.917 | 0.02 | 0.883 | 1.70 | 0.228 |
| Microbial biomass carbon (MBC) | 18.94 | <0.001 | 157.97 | <0.001 | 9.89 | 0.003 |
| Variables | Nitrogen (N) | Glucose (G) | N × G | |||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| (a) Emission rate | ||||||
| CH4 | 1.21 | 0.302 | 46.92 | <0.001 | 0.76 | 0.471 |
| CO2 | 1.32 | 0.271 | 148.78 | <0.001 | 0.141 | 0.869 |
| N2O | 18.14 | <0.001 | 0.26 | 0.610 | 0.46 | 0.630 |
| (b) Cumulative amount | ||||||
| CH4 | 4.01 | 0.046 | 85.00 | <0.001 | 2.32 | 0.140 |
| CO2 | 2.15 | 0.159 | 196.51 | <0.001 | 0.17 | 0.845 |
| N2O | 35.60 | <0.001 | 0.48 | 0.502 | 0.99 | 0.397 |
| Variables | Nitrogen (N) | Glucose (G) | N × G | |||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| nirS | 1.50 | 0.262 | 7.40 | 0.019 | 3.86 | 0.051 |
| nirK | 2.75 | 0.104 | 37.44 | <0.001 | 0.40 | 0.680 |
| nosZ | 1.34 | 0.298 | 19.84 | 0.001 | 0.19 | 0.827 |
| mcrA1 | 0.51 | 0.613 | 11.07 | 0.006 | 2.18 | 0.156 |
| mcrA2 | 1.74 | 0.218 | 0.72 | 0.414 | 0.04 | 0.959 |
| pmoA | 1.55 | 0.252 | 0.01 | 0.935 | 3.96 | 0.048 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, J.-F.; Yao, B.; Zhang, X.-Y.; Hu, Q.-W. Rapid Responses of Greenhouse Gas Emissions and Microbial Communities to Carbon and Nitrogen Addition in Sediments. Microorganisms 2024, 12, 1940. https://doi.org/10.3390/microorganisms12101940
Liang J-F, Yao B, Zhang X-Y, Hu Q-W. Rapid Responses of Greenhouse Gas Emissions and Microbial Communities to Carbon and Nitrogen Addition in Sediments. Microorganisms. 2024; 12(10):1940. https://doi.org/10.3390/microorganisms12101940
Chicago/Turabian StyleLiang, Jin-Feng, Bo Yao, Xiao-Ya Zhang, and Qi-Wu Hu. 2024. "Rapid Responses of Greenhouse Gas Emissions and Microbial Communities to Carbon and Nitrogen Addition in Sediments" Microorganisms 12, no. 10: 1940. https://doi.org/10.3390/microorganisms12101940
APA StyleLiang, J.-F., Yao, B., Zhang, X.-Y., & Hu, Q.-W. (2024). Rapid Responses of Greenhouse Gas Emissions and Microbial Communities to Carbon and Nitrogen Addition in Sediments. Microorganisms, 12(10), 1940. https://doi.org/10.3390/microorganisms12101940





