Optimizing Diagnosis and Management of Ventilator-Associated Pneumonia: A Systematic Evaluation of Biofilm Detection Methods and Bacterial Colonization on Endotracheal Tubes
Abstract
1. Introduction
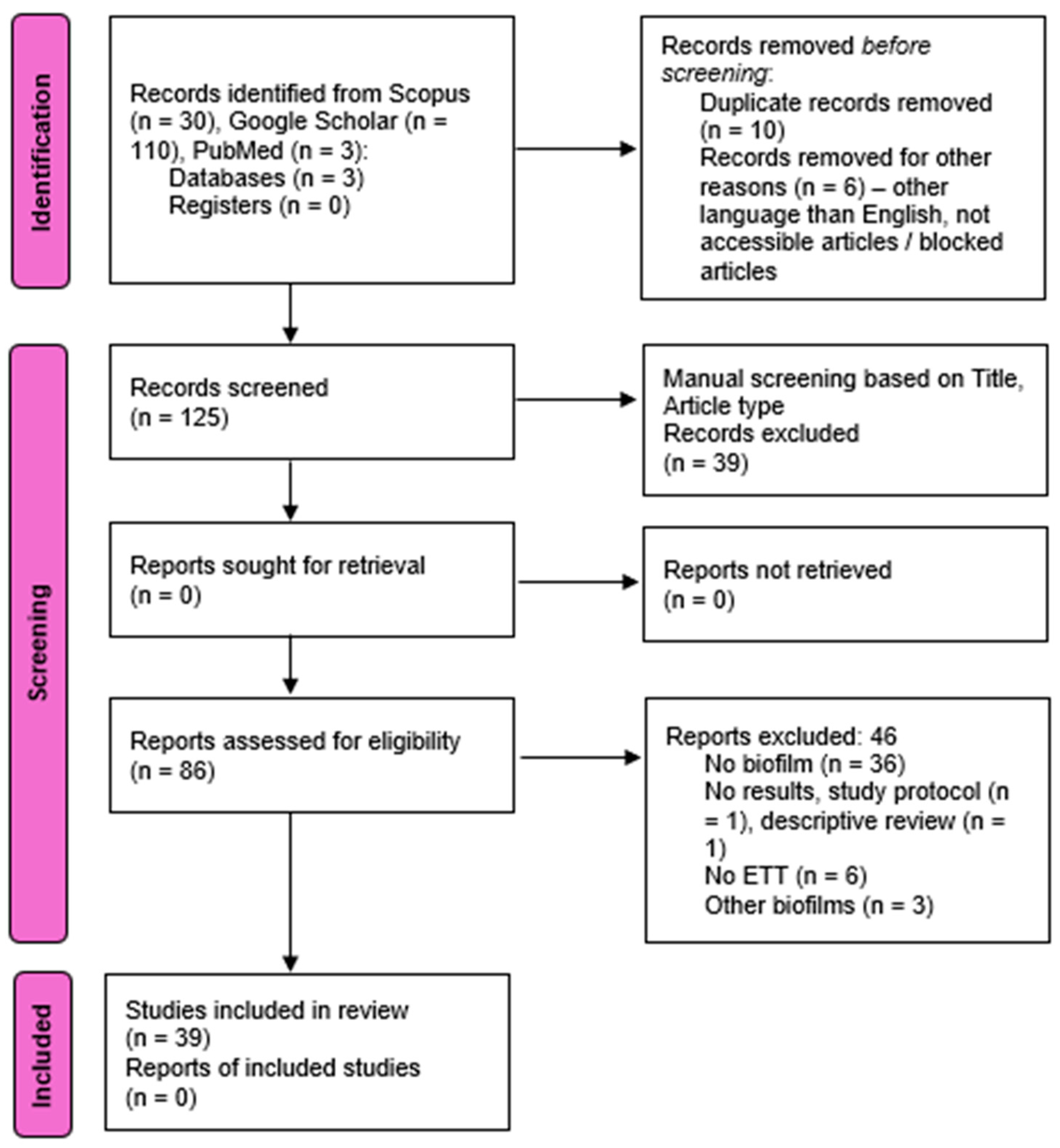
2. Materials and Methods
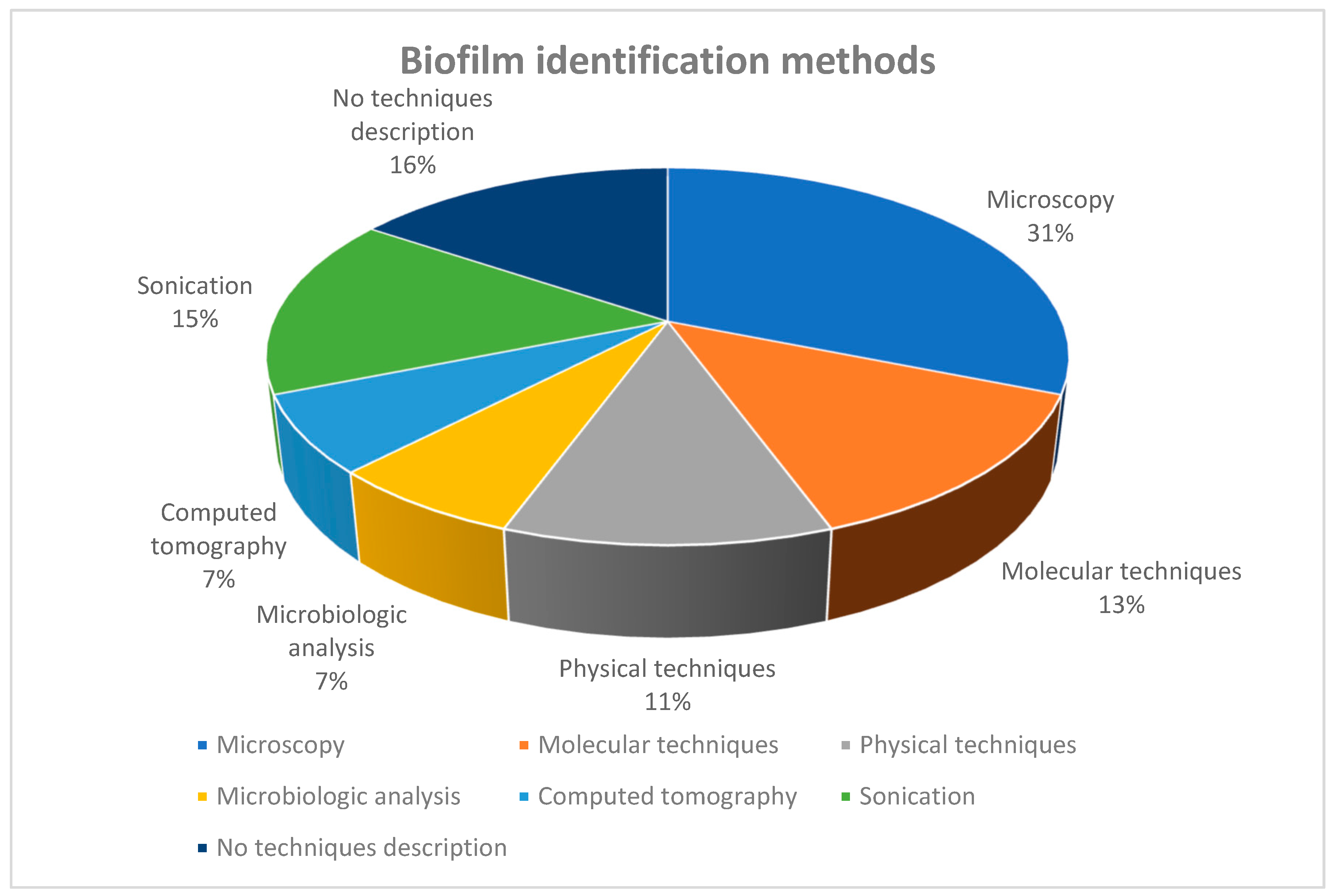
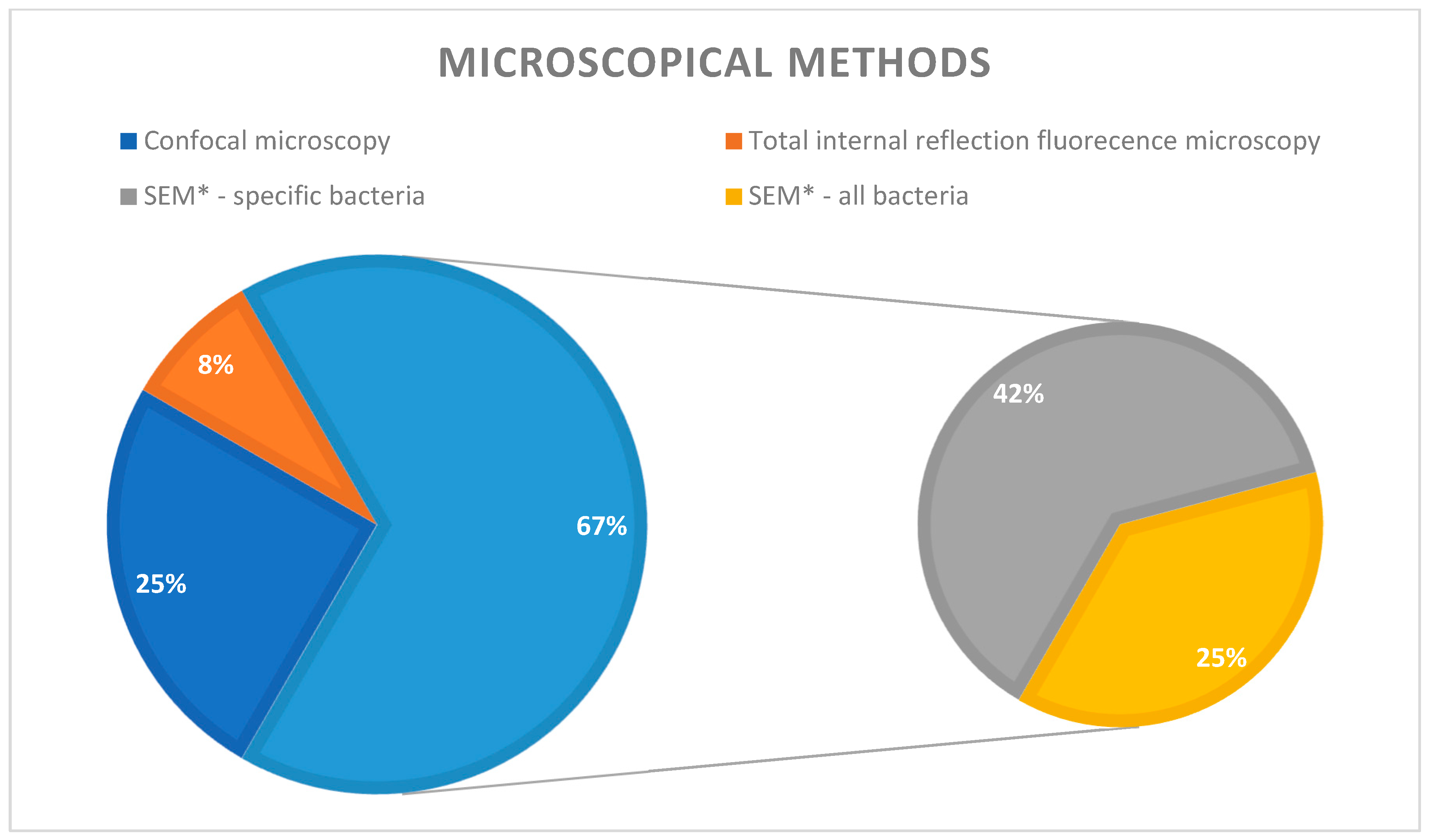
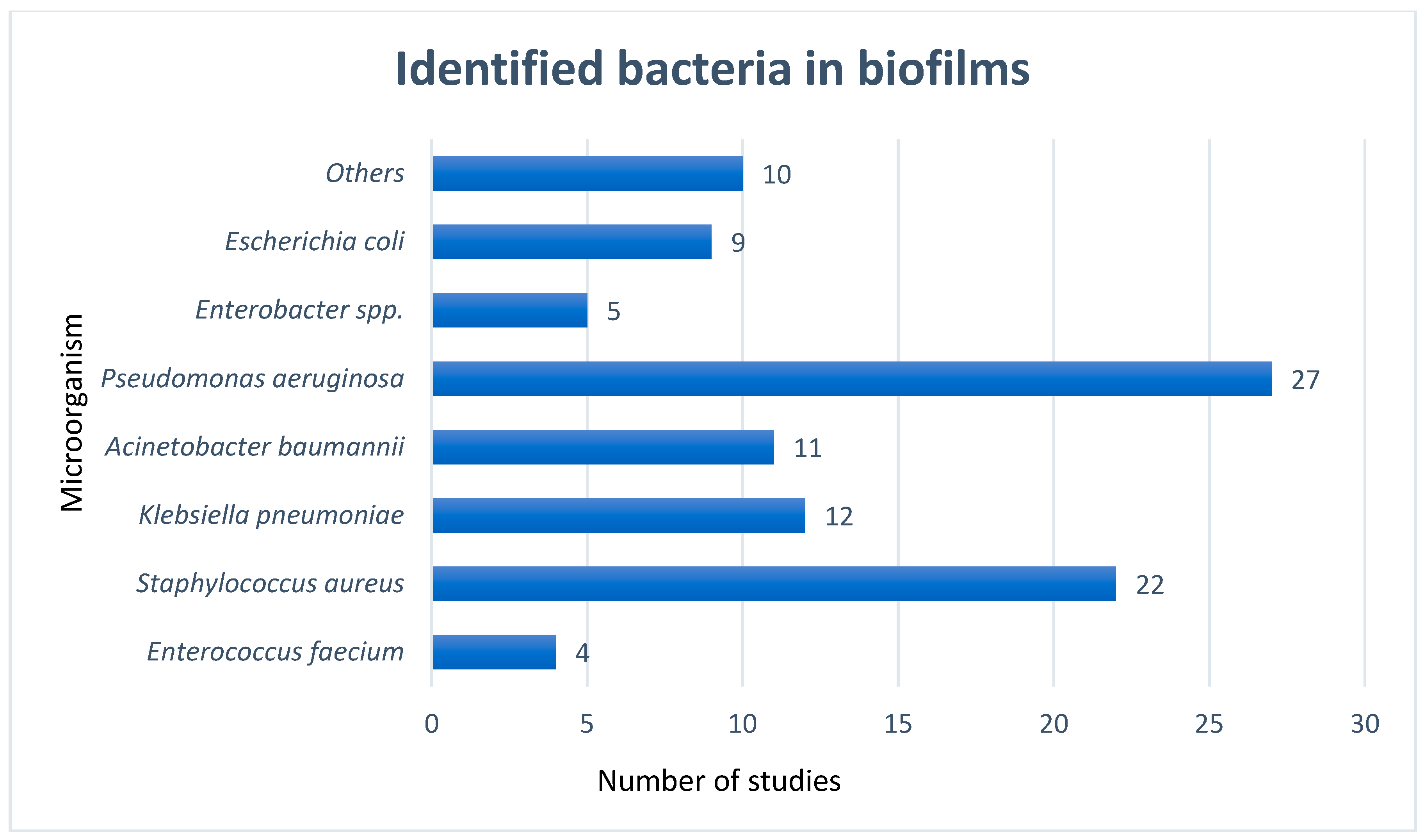
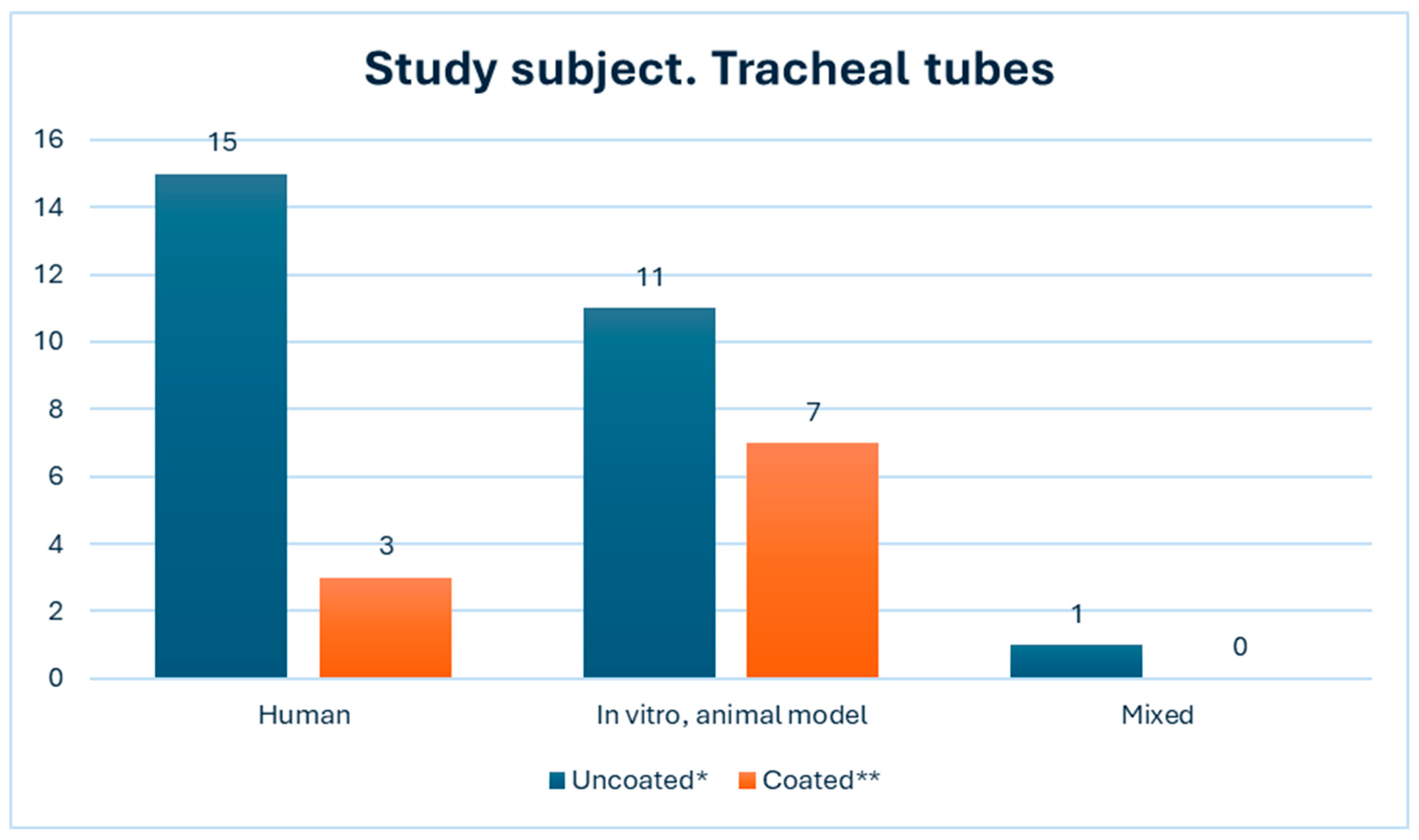
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BF | biofilm |
| CAMPs | cationic antimicrobial peptides |
| CIP/CHX | Ciprofloxacin/Chlorhexidine |
| Cryo-SEM | cryo-scanning electron microscopy |
| DRI | device-related infections |
| DTT | dithiothetriol |
| EPS | extracellular polymeric substances |
| ESKAPE | Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, Enterobacter species |
| ETT | endotracheal tube |
| FT-IR | Fourier Transform InfraRed |
| ICU | intensive care unit |
| OCT | optical coherence tomography |
| TiO2 | titanium dioxide |
| TST | tracheostomy tube |
| VAP | ventilator-associated pneumonia |
References
- Boicean, A.; Neamtu, B.; Birsan, S.; Batar, F.; Tanasescu, C.; Dura, H.; Roman, M.D.; Hașegan, A.; Bratu, D.; Mihetiu, A.; et al. Fecal Microbiota Transplantation in Patients Co-Infected with SARS-CoV2 and Clostridioides Difficile. Biomedicines 2022, 11, 7. [Google Scholar] [CrossRef]
- Vintila, B.I.; Arseniu, A.M.; Morgovan, C.; Butuca, A.; Sava, M.; Bîrluțiu, V.; Rus, L.L.; Ghibu, S.; Bereanu, A.S.; Roxana Codru, I.; et al. A Pharmacovigilance Study Regarding the Risk of Antibiotic-Associated Clostridioides Difficile Infection Based on Reports from the EudraVigilance Database: Analysis of Some of the Most Used Antibiotics in Intensive Care Units. Pharmaceuticals 2023, 16, 1585. [Google Scholar] [CrossRef] [PubMed]
- Bereanu, A.S.; Bereanu, R.; Mohor, C.; Vintilă, B.I.; Codru, I.R.; Olteanu, C.; Sava, M. Prevalence of Infections and Antimicrobial Resistance of ESKAPE Group Bacteria Isolated from Patients Admitted to the Intensive Care Unit of a County Emergency Hospital in Romania. Antibiotics 2024, 13, 400. [Google Scholar] [CrossRef] [PubMed]
- Papazian, L.; Klompas, M.; Luyt, C.-E. Ventilator-Associated Pneumonia in Adults: A Narrative Review. Intensiv. Care Med. 2020, 46, 888–906. [Google Scholar] [CrossRef] [PubMed]
- Thorarinsdottir, H.R.; Kander, T.; Holmberg, A.; Petronis, S.; Klarin, B. Biofilm Formation on Three Different Endotracheal Tube s: A Prospective Clinical Trial. Crit. Care 2020, 24, 382. [Google Scholar] [CrossRef] [PubMed]
- Diaconu, O.; Siriopol, I.; Polosanu, L.I.; Grigoras, I. Endotracheal Tube Biofilm and Its Impact on the Pathogenesis of Ventilator-Associated Pneumonia. J. Crit. Care Med. 2018, 4, 50–55. [Google Scholar] [CrossRef]
- Zimlichman, E.; Henderson, D.; Tamir, O.; Franz, C.; Song, P.; Yamin, C.K.; Keohane, C.; Denham, C.R.; Bates, D.W. Health Care–Associated Infections. JAMA Intern. Med. 2013, 173, 2039–2046. [Google Scholar] [CrossRef]
- Codru, I.R.; Sava, M.; Vintilă, B.I.; Bereanu, A.S.; Bîrluțiu, V. A Study on the Contributions of Sonication to the Identification of Bacteria Associated with Intubation Cannula Biofilm and the Risk of Ventilator-Associated Pneumonia. Medicina 2023, 59, 1058. [Google Scholar] [CrossRef]
- Marcut, L.; Manescu, V.; Antoniac, A.; Paltanea, G.; Robu, A.; Mohan, A.G.; Grosu, E.; Corneschi, I.; Bodog, A.D. Antimicrobial Solutions for Endotracheal Tubes in Prevention of Ventilator-Associated Pneumonia. Materials 2023, 16, 5034. [Google Scholar] [CrossRef]
- Kang, X.; Yang, X.; He, Y.; Guo, C.; Li, Y.; Ji, H.; Qin, Y.; Wu, L. Strategies and Materials for the Prevention and Treatment of Biofilms. Mater. Today Bio 2023, 23, 100827. [Google Scholar] [CrossRef]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef] [PubMed]
- Birlutiu, V.; Birlutiu, R.M. Endocarditis Due to Abiotrophia Defectiva, a Biofilm-Related Infection Associated with the Presence of Fixed Braces: A Case Report. Medicine 2017, 96, e8756. [Google Scholar] [CrossRef] [PubMed]
- Rather, M.A.; Gupta, K.; Mandal, M. Microbial Biofilm: Formation, Architecture, Antibiotic Resistance, and Control Strategies. Braz. J. Microbiol. 2021, 52, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Bjerkan, G.; Witso, E.; Bergh, K. Sonication Is Superior to Scraping for Retrieval of Bacteria in Biofilm on Titanium and Steel Surfaces in Vitro. Acta Orthop. 2009, 80, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, J.; Azevedo, N.F.; Briandet, R.; Cerca, N.; Coenye, T.; Costa, A.R.; Desvaux, M.; Di Bonaventura, G.; Hébraud, M.; Jaglic, Z.; et al. Critical Review on Biofilm Methods. Crit. Rev. Microbiol. 2017, 43, 313–351. [Google Scholar] [CrossRef]
- Birlutiu, R.M.; Roman, M.D.; Cismasiu, R.S.; Fleaca, S.R.; Popa, C.M.; Mihalache, M.; Birlutiu, V. Sonication Contribution to Identifying Prosthetic Joint Infection with Ralstonia Pickettii: A Case Report and Review of the Literature. BMC Musculoskelet. Disord. 2017, 18, 311. [Google Scholar] [CrossRef]
- Maurice, N.M.; Bedi, B.; Sadikot, R.T. Pseudomonas Aeruginosa Biofilms: Host Response and Clinical Implications in Lung Infections. Am. J. Respir. Cell Mol. Biol. 2018, 58, 428–439. [Google Scholar] [CrossRef]
- Alves, D.; Lopes, H.; Machado, I.; Pereira, M.O. Colistin Conditioning Surfaces Combined with Antimicrobial Treatment to Prevent Ventilator-Associated Infections. Biofouling 2022, 38, 547–557. [Google Scholar] [CrossRef]
- Amar, A.K.; Ramakrishnan, K.; Sawant, A.R.; Karamveer, K.; Menon, J.; Tiwary, B.K.; Prashanth, K. Investigations on Microbiome of the Used Clinical Device Revealed Many Uncultivable Newer Bacterial Species Associated with Persistent Chronic Infections. Microbes Infect. Chemother. 2022, 2, e1542. [Google Scholar] [CrossRef]
- Azmi, A.; Mojtabavi, S.; Fakhrmousavi, S.A.A.; Faizi, M.; Forootanfar, H.; Samadi, N.; Faramarzi, M.A. Surface Functionalization of Endotracheal Tubes Coated with Laccase–Gadolinium Phosphate Hybrid Nanoparticles for Antibiofilm Activity and Contrasting Properties. Biomater. Sci. 2024, 12, 674–690. [Google Scholar] [CrossRef]
- Bereanu, A.S.; Vintilă, B.I.; Bereanu, R.; Codru, I.R.; Hașegan, A.; Olteanu, C.; Săceleanu, V.; Sava, M. TiO2 Nanocomposite Coatings and Inactivation of Carbapenemase-Producing Klebsiella Pneumoniae Biofilm—Opportunities and Challenges. Microorganisms 2024, 12, 684. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, E.A.; Sierra, M.A.; Yepes, A.F.; Baldión, A.M.; Rojas, J.A.; Álvarez-Moreno, C.A.; Anzola, J.M.; Zambrano, M.M.; Huertas, M.G. Endotracheal Tube Microbiome in Hospitalized Patients Defined Largely by Hospital Environment. Respir. Res. 2022, 23, 168. [Google Scholar] [CrossRef] [PubMed]
- Daengngam, C.; Lethongkam, S.; Srisamran, P.; Paosen, S.; Wintachai, P.; Anantravanit, B.; Vattanavanit, V.; Voravuthikunchai, S. Green Fabrication of Anti-Bacterial Biofilm Layer on Endotracheal Tubing Using Silver Nanoparticles Embedded in Polyelectrolyte Multilayered Film. Mater. Sci. Eng. C 2019, 101, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Dewi, F.H.; Purwanto, B.; Wasita, B. Quantitative Biofilm for Bacterial Pathogens of Ventilator-Associated Pneumonia. Anaesthesia Pain Intensiv. Care 2021, 25, 132–137. [Google Scholar] [CrossRef]
- Drago, L.; Fidanza, A.; Giannetti, A.; Ciuffoletti, A.; Logroscino, G.; Romanò, C.L. Bacteria Living in Biofilms in Fluids: Could Chemical Antibiofilm Pretreatment of Culture Represent a Paradigm Shift in Diagnostics? Microorganisms 2024, 12, 259. [Google Scholar] [CrossRef]
- Dsouza, R.; Spillman, D.R.; Barkalifa, R.; Monroy, G.L.; Chaney, E.J.; White, K.C.; Boppart, S.A. In Vivo Detection of Endotracheal Tube Biofilms in Intubated Critical Care Patients Using Catheter-Based Optical Coherence Tomography. J. Biophotonics 2019, 12, e201800307. [Google Scholar] [CrossRef]
- Dsouza, R.; Spillman, D.R.; Barkalifa, R.; Monroy, G.L.; Chaney, E.J.; Johnson, M.A.; White, K.C.; Boppart, S.A. Efficacy of Endotracheal Tube Suctioning in Intubated Intensive Care Unit Patients Determined by In Vivo Catheter-Based Optical Coherence Tomography—A Pilot Study. Quant. Imaging Med. Surg. 2021, 11, 1–8. [Google Scholar] [CrossRef]
- Fady, M.; Rizwana, H.; Alarjani, K.M.; Alghamdi, M.A.; Ibrahim, S.S.; Geyer, J.; Abbas, A. Evaluation of Antibiofilm and Cytotoxicity Effect of Rumex Vesicarius Methanol Extract. Open Chem. 2023, 21, 20220286. [Google Scholar] [CrossRef]
- Fernández-Barat, L.; Ciofu, O.; Kragh, K.N.; Pressler, T.; Johansen, U.; Motos, A.; Torres, A.; Hoiby, N. Phenotypic Shift in Pseudomonas Aeruginosa Populations from Cystic Fibrosis Lungs after 2-Week Antipseudomonal Treatment. J. Cyst. Fibros. 2017, 16, 222–229. [Google Scholar] [CrossRef]
- Fernández-Barat, L.; Motos, A.; Panigada, M.; Álvarez-Lerma, F.; Viña, L.; Lopez-Aladid, R.; Ceccato, A.; Bassi, G.L.; Nicolau, D.P.; Lopez, Y.; et al. Comparative Efficacy of Linezolid and Vancomycin for Endotracheal Tube MRSA Biofilms from ICU Patients. Crit. Care 2019, 23, 251. [Google Scholar] [CrossRef]
- Fernández-Barat, L.; López-Aladid, R.; Vázquez, N.; Cabrera, R.; Vila, J.; Ferrer, M.; Torres, A. Bacterial Adaptive Memory in Methicillin-Resistant Staphylococcus Aureus from Endotracheal Tubes. Pathogens 2024, 13, 144. [Google Scholar] [CrossRef] [PubMed]
- Gasparetto, J.; Jurkonis, L.B.; Dantas, L.R.; Suss, P.H.; Tuon, F.F. Antiseptic-Impregnated Tracheostomy Tube for the Prevention of Ventilator-Associated Pneumonia Caused by Multidrug-Resistant Bacteria: In-Vitro and Pilot Study in Humans. Authorea Preprints 2022. [Google Scholar] [CrossRef]
- Silveira, G.G.O.S.; Torres, M.D.T.; Ribeiro, C.F.A.; Meneguetti, B.T.; Carvalho, C.M.E.; De La Fuente-Nunez, C.; Franco, O.L.; Cardoso, M.H. Antibiofilm Peptides: Relevant Preclinical Animal Infection Models and Translational Potential. ACS Pharmacol. Transl. Sci. 2021, 4, 55–73. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.J.; Grotewold, N.; Wozniak, D.J.; Gloag, E.S. Pseudomonas Aeruginosa Initiates a Rapid and Specific Transcriptional Response during Surface Attachment. J. Bacteriol. 2022, 204, e0008622. [Google Scholar] [CrossRef] [PubMed]
- Khazaal, S.S.; Al-Saryi, N.; Ibrahim, S.A. Immunomodulation by Acinetobacter Baumannii of Endotracheal Tube Biofilm in Ventilator-Associated Pneumonia. Meta Gene 2020, 24, 100672. [Google Scholar] [CrossRef]
- Kiarostami, K.; Fernández-Barat, L.; Battaglini, D.; Motos, A.; Bueno-Freire, L.; Soler-Comas, A.; Bassi, G.L.; Torres, A. The Efficacy of Telavancin in Comparison with Linezolid on Endotracheal Tube Biofilm in Pigs with Methicillin-Resistant Staphylococcus Aureus Pneumonia. Int. J. Antimicrob. Agents 2024, 63, 107052. [Google Scholar] [CrossRef]
- Latorre, M.C.; Pérez-Granda, M.J.; Savage, P.B.; Alonso, B.; Martín-Rabadán, P.; Samaniego, R.; Bouza, E.; Muñoz, P.; Guembe, M. Endotracheal Tubes Coated with a Broad-Spectrum Antibacterial Ceragenin Reduce Bacterial Biofilm in an In Vitro Bench Top Model. J. Antimicrob. Chemother. 2021, 76, 1168–1173. [Google Scholar] [CrossRef]
- Lethongkam, S.; Sunghan, J.; Wangdee, C.; Durongphongtorn, S.; Siri, R.; Wunnoo, S.; Paosen, S.; Voravuthikunchai, S.P.; Dejyong, K.; Daengngam, C. Biogenic Nanosilver-Fabricated Endotracheal Tube to Prevent Microbial Colonization in a Veterinary Hospital. Appl. Microbiol. Biotechnol. 2023, 107, 623–638. [Google Scholar] [CrossRef]
- Luo, Y.; McAuley, D.F.; Fulton, C.R.; Pessoa, J.S.; McMullan, R.; Lundy, F.T. Targeting Candida Albicans in Dual-Species Biofilms with Antifungal Treatment Reduces Staphylococcus Aureus and MRSA In Vitro. PLoS ONE 2021, 16, e0249547. [Google Scholar] [CrossRef]
- Maldiney, T.; Pineau, V.; Neuwirth, C.; Ouzen, L.; Eberl, I.; Jeudy, G.; Dalac, S.; Piroth, L.; Blot, M.; Sautour, M.; et al. Endotracheal Tube Biofilm in Critically Ill Patients during the COVID-19 Pandemic: Description of an Underestimated Microbiological Compartment. Sci. Rep. 2022, 12, 1–13. [Google Scholar] [CrossRef]
- Mazzolini, R.; Rodríguez-Arce, I.; Fernández-Barat, L.; Piñero-Lambea, C.; Garrido, V.; Rebollada-Merino, A.; Motos, A.; Torres, A.; Grilló, M.J.; Serrano, L.; et al. Engineered Live Bacteria Suppress Pseudomonas Aeruginosa Infection in Mouse Lung and Dissolve Endotracheal-Tube Biofilms. Nat. Biotechnol. 2023, 41, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Baidya, S.; Bhattarai, A.; Shrestha, S.; Homagain, S.; Rayamajhee, B.; Hui, A.; Willcox, M. Bacteriology of Endotracheal Tube Biofilms and Antibiotic Resistance: A Systematic Review. J. Hosp. Infect. 2024, 147, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.C.; Bim, F.L.; Monteiro, R.M.; Macedo, A.P.; Santos, E.S.; Silva-Lovato, C.H.; Paranhos, H.F.O.; Melo, L.D.R.; Santos, S.B.; Watanabe, E. Identification and Characterization of New Bacteriophages to Control Multidrug-Resistant Pseudomonas Aeruginosa Biofilm on Endotracheal Tubes. Front. Microbiol. 2020, 11, 580779. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.C.; Macedo, A.P.; Melo, L.D.R.; Santos, S.B.; Hermann, P.R.S.; Silva-Lovato, C.H.; Paranhos, H.F.O.; Andrade, D.; Watanabe, E. Bacteriophage Cocktail-Mediated Inhibition of Pseudomonas Aeruginosa Biofilm on Endotracheal Tube Surface. Antibiotics 2021, 10, 78. [Google Scholar] [CrossRef]
- Ozcelik, B.; Pasic, P.; Sangwan, P.; Be, C.L.; Glattauer, V.; Thissen, H.; Boulos, R.A. Evaluation of the Novel Antimicrobial BCP3 in a Coating for Endotracheal Tubes. ACS Omega 2020, 5, 10288–10296. [Google Scholar] [CrossRef]
- Pérez-Granda, M.J.; Alonso, B.; Zavala, R.; Latorre, M.C.; Hortal, J.; Samaniego, R.; Bouza, E.; Muñoz, P.; Guembe, M. Selective Digestive Decontamination Solution Used as “Lock Therapy” Prevents and Eradicates Bacterial Biofilm in an In Vitro Bench-Top Model. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 44. [Google Scholar] [CrossRef]
- Rangel, K.; De-Simone, S.G. Treatment and Management of Acinetobacter Pneumonia: Lessons Learned from Recent World Event. Infect. Drug Resist. 2024, 17, 507–529. [Google Scholar] [CrossRef]
- Rao, H.; Choo, S.; Mahalingam, S.R.R.; Adisuri, D.S.; Madhavan, P.; Akim, A.M.; Chong, P.P. Approaches for Mitigating Microbial Biofilm-Related Drug Resistance: A Focus on Micro- and Nanotechnologies. Molecules 2021, 26, 1870. [Google Scholar] [CrossRef]
- Roy, S.; Chowdhury, G.; Mukhopadhyay, A.K.; Dutta, S.; Basu, S. Convergence of Biofilm Formation and Antibiotic Resistance in Acinetobacter Baumannii Infection. Front. Med. 2022, 9, 793615. [Google Scholar] [CrossRef]
- Shaqour, B.; Aizawa, J.; Guarch-Pérez, C.; Górecka, Ż.; Christophersen, L.; Martinet, W.; Choińska, E.; Riool, M.; Verleije, B.; Beyers, K.; et al. Coupling Additive Manufacturing with Hot Melt Extrusion Technologies to Validate a Ventilator-Associated Pneumonia Mouse Model. Pharmaceutics 2021, 13, 772. [Google Scholar] [CrossRef]
- Soares, R.B.; Costa, D.H.; Miyakawa, W.; Delgado, M.G.T.; Garcez, A.S.; Yoshimura, T.M.; Ribeiro, M.S.; Nunez, S.C. Photodynamic Activity on Biofilm in Endotracheal Tubes of Patients Admitted to an Intensive Care Unit. Photochem. Photobiol. 2020, 96, 618–624. [Google Scholar] [CrossRef] [PubMed]
- van Charante, F.; Wieme, A.; Rigole, P.; De Canck, E.; Ostyn, L.; Grassi, L.; Deforce, D.; Crabbé, A.; Vandamme, P.; Joossens, M.; et al. Microbial Diversity and Antimicrobial Susceptibility in Endotracheal Tube Biofilms Recovered from Mechanically Ventilated COVID-19 Patients. Biofilm 2022, 4, 100079. [Google Scholar] [CrossRef] [PubMed]
- Alves, D.; Grainha, T.; Pereira, M.O.; Lopes, S.P. Antimicrobial Materials for Endotracheal Tubes: A Review on the Last Two Decades of Technological Progress. Acta Biomater. 2023, 158, 32–55. [Google Scholar] [CrossRef] [PubMed]
- Zangirolami, A.C.; Dias, L.D.; Blanco, K.C.; Vinagreiro, C.S.; Inada, N.M.; Arnaut, L.G.; Pereira, M.M.; Bagnato, V.S. Avoiding Ventilator-Associated Pneumonia: Curcumin-Functionalized Endotracheal Tube and Photodynamic Action. Proc. Natl. Acad. Sci. USA 2020, 117, 22967–22973. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.M.; Holden, B.S.; DurnaAA, B.; Buck, R.; Savage, P.B. Ceragenins as Mimics of Endogenous Antimicrobial Peptides. J. Antimicrob. Agents 2017, 3. [Google Scholar] [CrossRef]
- Mitchell, G.; Silvis, M.R.; Talkington, K.C.; Budzik, J.M.; Dodd, C.E.; Paluba, J.M.; Oki, E.A.; Trotta, K.L.; Licht, D.J.; Jimenez-Morales, D.; et al. Ceragenins and Antimicrobial Peptides Kill Bacteria through Distinct Mechanisms. mBio 2022, 13, e0272621. [Google Scholar] [CrossRef]
- Li, C.; Peters, A.S.; Meredith, E.L.; Allman, G.W.; Savage, P.B. Design and Synthesis of Potent Sensitizers of Gram-Negative Bacteria Based on a Cholic Acid Scaffolwding. J. Am. Chem. Soc. 1998, 120, 2961–2962. [Google Scholar] [CrossRef]
- Malacarne, P.; Corini, M.; Maremmani, P.; Viaggi, B.; Verdigi, S. Diagnostic Characteristics of Routine Surveillance Cultures of Endotracheal Aspirate Samples in Cases of Late-Onset Ventilator-Associated Pneumonia Due to Acinetobacter Baumannii. Infect. Control Hosp. Epidemiol. 2007, 28, 867–869. [Google Scholar] [CrossRef]
- Costa-Orlandi, C.; Sardi, J.; Pitangui, N.; De Oliveira, H.; Scorzoni, L.; Galeane, M.; Medina-Alarcón, K.; Melo, W.; Marcelino, M.; Braz, J.; et al. Fungal Biofilms and Polymicrobial Diseases. J. Fungi 2017, 3, 22. [Google Scholar] [CrossRef]
- Silva, N.B.S.; Marques, L.A.; Röder, D.D.B. Diagnosis of Biofilm Infections: Current Methods Used, Challenges and Perspectives for the Future. J. Appl. Microbiol. 2021, 131, 2148–2160. [Google Scholar] [CrossRef]
- Schlafer, S.; Meyer, R.L. Confocal Microscopy Imaging of the Biofilm Matrix. J. Microbiol. Methods 2017, 138, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vaquero, M.; Rajauria, G.; O’Doherty, J.V.; Sweeney, T. Polysaccharides from Macroalgae: Recent Advances, Innovative Technologies and Challenges in Extraction and Purification. Food Res. Int. 2017, 99, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Rondaan, C.; Maso, A.; Birlutiu, R.M.; Fernandez Sampedro, M.; Soriano, A.; Diaz de Brito, V.; Gómez Junyent, J.; Del Toro, M.D.; Hofstaetter, J.G.; Salles, M.J.; et al. Is an Isolated Positive Sonication Fluid Culture in Revision Arthroplasties Clinically Relevant? Clin. Microbiol. Infect. 2023, 29, 1431–1436. [Google Scholar] [CrossRef]
- Roman, M.D.; Bocea, B.A.; Ion, N.I.C.; Vorovenci, A.E.; Dragomirescu, D.; Birlutiu, R.M.; Birlutiu, V.; Fleaca, S.R. Are There Any Changes in the Causative Microorganisms Isolated in the Last Years from Hip and Knee Periprosthetic Joint Infections? Antimicrobial Susceptibility Test Results Analysis. Microorganisms 2023, 11, 116. [Google Scholar] [CrossRef]
- Moncada, M.; Aryana, K.J. Influence of “Mild” Sonication Conditions on the Characteristics of Streptococcus Thermophilus ST-M5. Adv. Microbiol. 2012, 2, 8–16. [Google Scholar] [CrossRef]
- Riesz, P.; Kondo, T. Free Radical Formation Induced by Ultrasound and Its Biological Implications. Free Radic. Biol. Med. 1992, 13, 247–270. [Google Scholar] [CrossRef]
- Birlutiu, R.M.; Birlutiu, V.; Cismasiu, R.S.; Mihalache, M. BbFISH-Ing in the Sonication Fluid. Medicine 2019, 98, e16501. [Google Scholar] [CrossRef]
- Sauer, K.; Stoodley, P.; Goeres, D.M.; Hall-Stoodley, L.; Burmølle, M.; Stewart, P.S.; Bjarnsholt, T. The Biofilm Life Cycle– Expanding the Conceptual Model of Biofilm Formation. Nat. Rev. Microbiol. 2022, 20, 608–620. [Google Scholar] [CrossRef]
- Nair, G.B.; Niederman, M.S. Ventilator-Associated Pneumonia: Present Understanding and Ongoing Debates. Intensiv. Care Med. 2015, 41, 34–48. [Google Scholar] [CrossRef]
| Authors | Biofilm Identification Method | Human | In Vitro | Animals | ETT | Microorganisms |
|---|---|---|---|---|---|---|
| Alves D. et al., 2023 [18] | Infrared spectroscopy | • | Double-coated ETT (ciprofloxacin and chlorhexidine) | Ps. Aeruginosa, A. baumanii, K. pneumoniae, S. aureus, S. epidermidis | ||
| Amar A.K. et al., 2022 [19] | 16SrRNA gene amplification followed by Sanger sequencing; NGS of the device metagenome | • | Invasive medical devices | S. infantis, Gemella haemolysans, Meiothermus silvanus, Schlegelella aquatica, Rothia mucilaginosa, Serratia nematodiphila, and Enterobacter asburiae, along with some known common nosocomial pathogens | ||
| Azmi A. et al., 2023 [20] | Computed tomography | • | Laccase@GdPO4 * HNP, Enzyme mediator (antibiofilm property) | E. coli S. aureus P. aeruginosa | ||
| Bereanu A.S. et al., 2024 [21] | Not the case | • | Medical devices, including ETT–TiO2 photocatalytic | K. pneumoniae | ||
| Cifuentes E.A. et al., 2022 [22] | Sonication Powersoil Kit for DNA extraction | • | Usual ETT | Proteobacteria (p = 0.01), Firmicutes, Bacteroidetes, Fusobacteria (p = 0.01), Actinobacteria (p = 0.02)—bacteria species with statistical significance difference between the 2 ICUs Biofilm bacteria, patients with VAP *: K. pneumoniae, E. coli, P. aeruginosa, A. baumanii, S. aureus | ||
| Daengngam C. et al., 2019 [23] | Scanning electron microscopy | • | Coated ETT ** | S. aureus, P. aeruginosa | ||
| Dewi F.H. et al., 2021 [24] | Quantitative biofilm measurement using a microtiter plate method, optical density | • | Usual PVC | 48 specimens were obtained; Gram–negative bacteria were more common cause of VAP than Gram–positive bacteria (81% vs. 17%). There was one unidentified microorganism (2%) | ||
| Drago L. et al., 2024 [25] | Sonication DTT | Not the case | A. baumanii, S. aureus, P. aeruginosa, Enterobacteriaceae, Citrobacter koseri, P. mirabilis, P. fluorescence | |||
| Dsouza et al., 2019 [26] | Catheter-based OCT | • | Usual PVC | |||
| Dsouza R. et al., 2021 [27] | OCT * | • | Usual PVC | Klebsiella spp. (mainly) | ||
| Fady M. et al., 2023 [28] | Scanning electron microscopy | • | Usual PVC | S. aureus, S. epidermidis, P. vulgaris, K. pneumoniae, and P. aeruginosa | ||
| Fernandez-Barat L. et al., 2017 [29] | Sonication | • | Not the case | P. aeruginosa, MRSA | ||
| Fernandez-Barat L. et al., 2019 [30] | Scanning electron microscopy | • | Usual PVC | MRSA | ||
| Fernandez-Barat L. et al., 2024 [31] | Real-time PCR for the assessment of genes in the biofilm | • | Usual PVC | MRSA | ||
| Gasparetto J. et al., 2022 [32] | No method for biofilm detection | • | • | Chlorhexidine-impregnated TST and violet-crystal-coated TST | Standard strains of S. aureus, P. aeruginosa, E. coli, and MDR bacteria (MRSA, carbapenem-resistant A. baumannii, P. aeruginosa, K. pneumoniae | |
| Guilhen C. et al., 2019 [33] | Confocal Microscopy Sonication Flow cytometry | • | • | Not the case | K. pneumoniae | |
| Jones C.J. et al., 2022 [34] | RNA-sequencing analysis | • | Usual PVC (and 2 other abiotic surfaces) | P. aeruginosa | ||
| Khazaal S.S. et al., 2020 [35] | No description of biofilm data collection | • | Usual PVC (bacteria was initially grown) | A. baumannii | ||
| Kiarostami K. et al., 2024 [36] | Scanning electron microscopy | • | Usual PVC | MRSA | ||
| Latorre M.C. et al., 2021 [37] | Cfu count by culture of sonicate and the total number of cells by confocal laser scanning microscopy | • | Ceragenin CSA-131 coated ETT and uncoated PVC ETT | P. aeruginosa, S. aureus, E. coli | ||
| Lethongkam S. et al., 2023 [38] | Energy dispersive X-ray spectroscopy | • | Eucalyptus-mediated synthesized silver nanoparticles (AgNPs) | P. aeruginosa | ||
| Luo Y. et al., 2021 [39] | Total internal reflection fluorescence microscopy | • | Usual PVC | P. aeruginosa, E. coli, S. aureus | ||
| Maldiney T. et al., 2022 [40] | Confocal microscopy MALDI-TOF MS * | • | Usual PVC | Mushroom-shaped BF *: S. aureus, S. haemolitycus, S. epidermidis, E. coli, K. oxytoca, P. aeruginosa, Serratia marcescens, E. cloacae (p = 0.002), E. xiangfangensis (p = 0.02), E. faecalis, E. faecium, S. pneumoniae (p = 0.009), S. oralis (p = 0.004), Hafnia alvei (p = 0.02) Ribbon-shaped BF *: S. aureus, S. haemolitycus, S. epidermidis, E. coli, K. oxytoca, P. aeruginosa, Serratia marcescens, E. cloacae, E. faecalis, E. faecium | ||
| Marcut L. et al., 2023 [9] | Scanning electron microscopy | • | • | Modified ETT *** | E. coli, P. aeruginosa, S. aureus (including MRSA), and B. subtilis, K. pneumoniae, A. baumannii | |
| Mazzolini R. et al., 2022 [41] | Crystal-violet assay, Alcian Blue | • | • | • | - | P. aeruginosa |
| Mishra S. et al., 2024 [42] | Multiple methods | • | Usual PVC | Specific bacteria from the EKAPE group | ||
| Oliveira V.C. et al., 2020 [43] | - | • | ETT with bacteriophages on the surface | P. aeruginosa | ||
| Oliveira V.C. et al., 2021 [44] | Sonication, Scanning Electron Microscopy | • | Phage cocktail adsorbed to ETT | P. aeruginosa | ||
| Ozcelik B. et al., 2020 [45] | No description | • | A novel styryl benzene-based antimicrobial (BCP3) coating | S. aureus, P. aeruginosa | ||
| Perez-Granda M.J. et al., 2020 [46] | Sonication Confocal laser scanning microscopy | • | Usual PVC | P. aeruginosa, S. aureus, E. coli | ||
| Rangel K. et al., 2024 [47] | Rapid molecular testing | • | Usual PVC | A. baumannii | ||
| Rao H. et al., 2021 [48] | - | • | Usual PVC (also venous catheter, urinary catheters) | ESKAPE group bacteria | ||
| Roy S. et al., 2022 [49] | Not the case | • | Usual PVC, coated ETT | A. baumannii | ||
| Shaqour B. et al., 2021 [50] | Scanning electron microscopy | • | TPU polymeric matrix with incorporated ciprofloxacin | S. aureus | ||
| Soares R.B. et al., 2020 [51] | Crystal violet absorbance | • | Methylene blue associated with external illumination | P. aeruginosa | ||
| Thorarinsdottir H.R. et al., 2020 [5] | Electron microscopy | • | Uncoated PVC Silicon-coated PVC PVC coated with noble metals | E. faecalis, E. faecium, S. aureus, Klebsiella spp., Stenotrophomonas maltophilia, P. aeruginosa | ||
| van Charante F. et al., 2022 [52] | Culture-dependent (MALDI-TOF mass spectrometry and biochemical tests) and culture-independent (16S and ITS1 rRNA amplicon sequencing) | • | Usual PVC | S. epidermidis, E. faecalis, P. aeruginosa | ||
| Walsh D. et al., 2024 [53] | Matrix-degrading enzymes and cryo-SEM | • | PVC ETT segments in the presence of synthetic ventilator airway mucus | P. aeruginosa, K. pneumoniae | ||
| Zangirolami A.C. et al., 2020 [54] | FT-IR* spectroscopy | • | PVC coated with curcumin-photosensitizer | P. aeruginosa, S. aureus, E. coli |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Codru, I.R.; Vintilă, B.I.; Sava, M.; Bereanu, A.S.; Neamțu, S.I.; Bădilă, R.M.; Bîrluțiu, V. Optimizing Diagnosis and Management of Ventilator-Associated Pneumonia: A Systematic Evaluation of Biofilm Detection Methods and Bacterial Colonization on Endotracheal Tubes. Microorganisms 2024, 12, 1966. https://doi.org/10.3390/microorganisms12101966
Codru IR, Vintilă BI, Sava M, Bereanu AS, Neamțu SI, Bădilă RM, Bîrluțiu V. Optimizing Diagnosis and Management of Ventilator-Associated Pneumonia: A Systematic Evaluation of Biofilm Detection Methods and Bacterial Colonization on Endotracheal Tubes. Microorganisms. 2024; 12(10):1966. https://doi.org/10.3390/microorganisms12101966
Chicago/Turabian StyleCodru, Ioana Roxana, Bogdan Ioan Vintilă, Mihai Sava, Alina Simona Bereanu, Sandra Ioana Neamțu, Raluca Maria Bădilă, and Victoria Bîrluțiu. 2024. "Optimizing Diagnosis and Management of Ventilator-Associated Pneumonia: A Systematic Evaluation of Biofilm Detection Methods and Bacterial Colonization on Endotracheal Tubes" Microorganisms 12, no. 10: 1966. https://doi.org/10.3390/microorganisms12101966
APA StyleCodru, I. R., Vintilă, B. I., Sava, M., Bereanu, A. S., Neamțu, S. I., Bădilă, R. M., & Bîrluțiu, V. (2024). Optimizing Diagnosis and Management of Ventilator-Associated Pneumonia: A Systematic Evaluation of Biofilm Detection Methods and Bacterial Colonization on Endotracheal Tubes. Microorganisms, 12(10), 1966. https://doi.org/10.3390/microorganisms12101966







