Impact of Additives and Packing Density on Fermentation Weight Loss, Microbial Diversity, and Fermentation Quality of Rape Straw Silage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Experimental Design
2.2. Fermentation Weight Loss (FWL) Analysis
2.3. Fermentation Quality Analysis
2.4. Microbial Community Structure Analysis
2.5. Nutritional Composition Analysis
3. Results
3.1. Fermentation Weight Loss
3.2. Fermentation Quality
3.3. Microbial Counts
3.4. Nutritional Composition
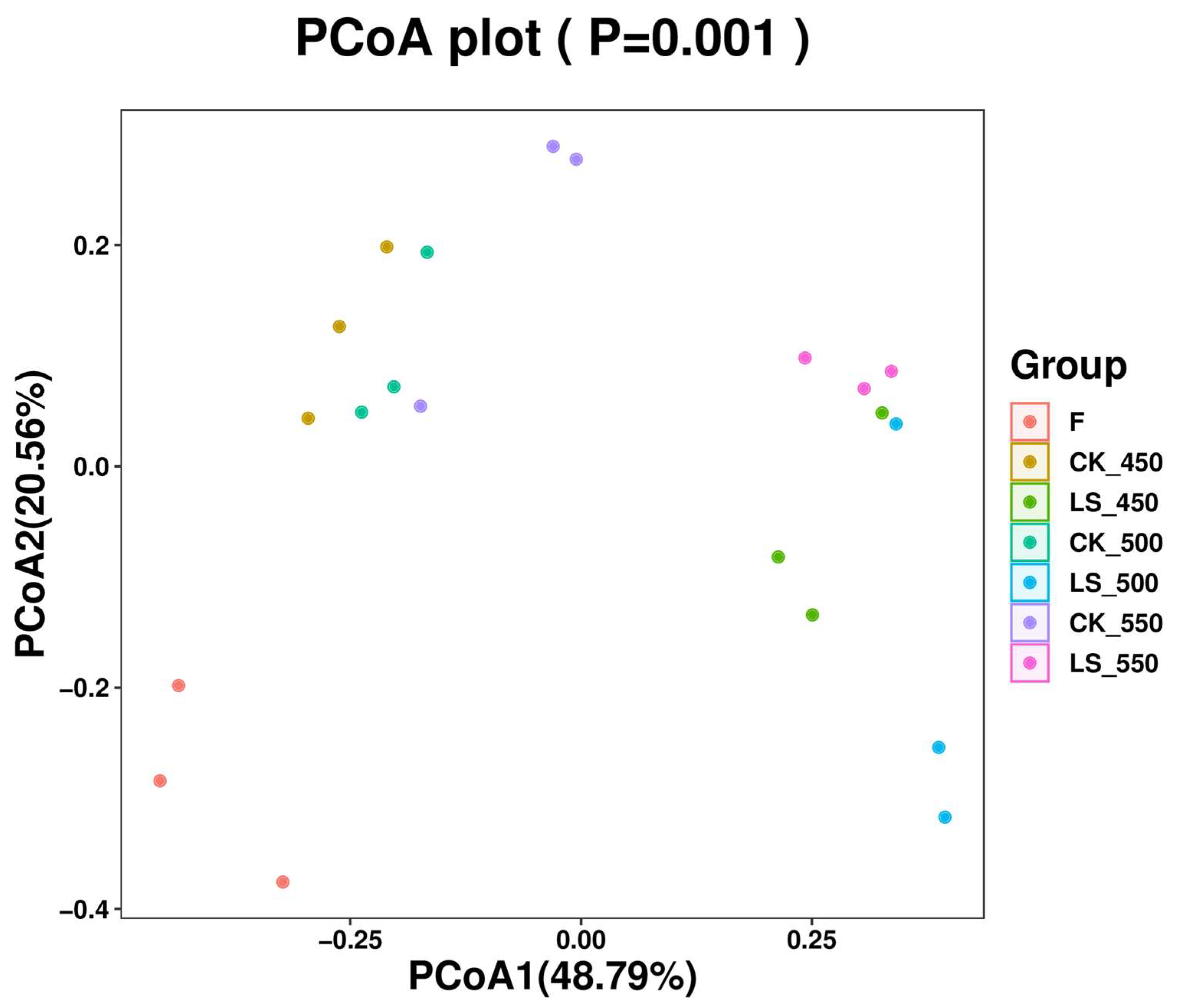
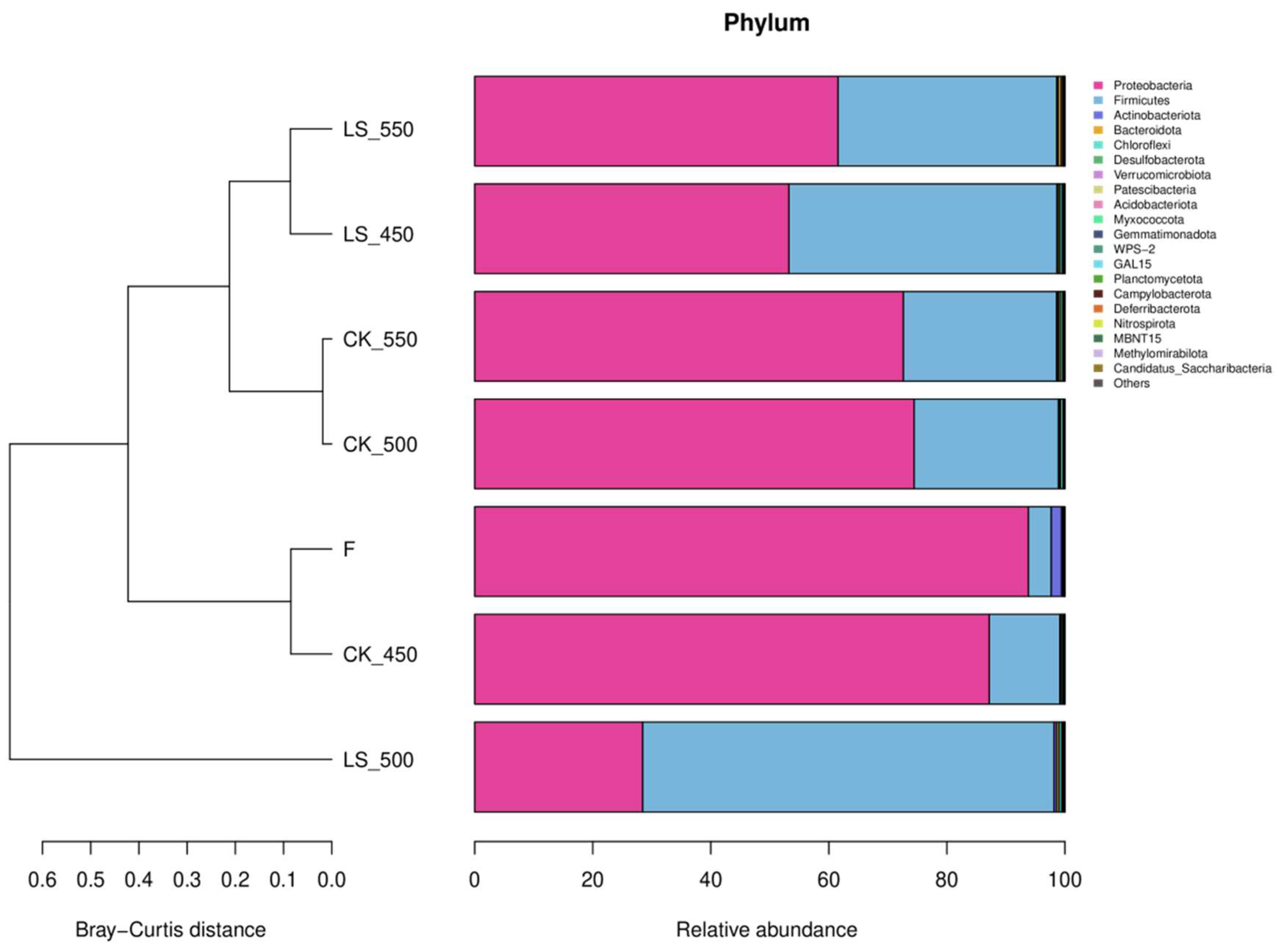
3.5. Bacterial Communities
4. Discussion
4.1. Effect of Additives and Packing Density on Fermentation Weight Loss during the Fermentation Process of Rape Straw Silage
4.2. Effect of Additives and Packing Density on Fermentation Quality of Rape Straw Silage
4.3. Effect of Additives and Packing Density on Nutritional Composition of Rape Straw Silage
4.4. Effect of Additives and Packing Density on Bacterial Communities of Rape Straw Silage
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, S.; Wan, C.; Ma, Y.; Zhang, K.; Wang, F.; Shen, S. Study on the quality of mixed silage of rapeseed with alfalfa or myriophyllum. Int. J. Environ. Res. Public Health 2023, 20, 3884. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, F.; Wang, Z.; Shi, H.; Wang, X.; Huang, Y.; Li, S. Effect of Anaerobic Calcium Oxide Alkalization on the Carbohydrate Molecular Structures, Chemical Profiles, and Ruminal Degradability of Rape Straw. Animals 2023, 13, 2421. [Google Scholar] [CrossRef]
- Barry, T. The feeding value of forage brassica plants for grazing ruminant livestock. Anim. Feed. Sci. Technol. 2013, 181, 15–25. [Google Scholar] [CrossRef]
- Li, L.; Liu, C.; Qu, M.; Zhang, W.; Pan, K.; Ouyang, K.; Song, X.; Zhao, X. Characteristics of a recombinant Lentinula edodes endoglucanase and its potential for application in silage of rape straw. Int. J. Biol. Macromol. 2019, 139, 49–56. [Google Scholar] [CrossRef]
- Yuan, L.; Gao, Y.; Mei, Y.; Liu, J.; Kalkhajeh, Y.K.; Hu, H.; Huang, J. Effects of continuous straw returning on bacterial community structure and enzyme activities in rape-rice soil aggregates. Sci. Rep. 2023, 13, 2357. [Google Scholar] [CrossRef]
- Banaszak, A.; Woźniak, M.; Dziurka, D.; Mirski, R. Annual Plants and Thermoplastics in the Production of Polymer and Lignocellulose Boards. Materials 2023, 16, 4400. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, Z.-Q.; Han, B.; Su, C.-L.; Han, Q.; Chen, W.-Z. Biosorption of copper ions from aqueous solution using rape straw powders: Optimization, equilibrium and kinetic studies. Ecotoxicol. Environ. Saf. 2018, 150, 251–259. [Google Scholar] [CrossRef]
- Van Pamel, E.; Verbeken, A.; Vlaemynck, G.; De Boever, J.; Daeseleire, E. Ultrahigh-performance liquid chromatographic–tandem mass spectrometric multimycotoxin method for quantitating 26 mycotoxins in maize silage. J. Agric. Food Chem. 2011, 59, 9747–9755. [Google Scholar] [CrossRef]
- Dunière, L.; Sindou, J.; Chaucheyras-Durand, F.; Chevallier, I.; Thévenot-Sergentet, D. Silage processing and strategies to prevent persistence of undesirable microorganisms. Anim. Feed Sci. Technol. 2013, 182, 1–15. [Google Scholar] [CrossRef]
- Guo, X.; Xu, D.; Li, F.; Bai, J.; Su, R. Current approaches on the roles of lactic acid bacteria in crop silage. Microb. Biotechnol. 2023, 16, 67–87. [Google Scholar] [CrossRef]
- Muck, R.; Nadeau, E.; McAllister, T.; Contreras-Govea, F.; Santos, M.; Kung, L., Jr. Silage review: Recent advances and future uses of silage additives. J. Dairy Sci. 2018, 101, 3980–4000. [Google Scholar] [CrossRef]
- Wang, Y.; He, L.; Xing, Y.; Zheng, Y.; Zhou, W.; Pian, R.; Yang, F.; Chen, X.; Zhang, Q. Dynamics of bacterial community and fermentation quality during ensiling of wilted and unwilted Moringa oleifera leaf silage with or without lactic acid bacterial inoculants. Msphere 2019, 4, 10–1128. [Google Scholar] [CrossRef]
- Muck, R.E. Factors Influencing Silage Quality and Their Implications for Management. J. Dairy Sci. 1988, 71, 2992–3002. [Google Scholar] [CrossRef]
- Xu, H.; Sun, L.; Na, N.; Wang, C.; Yin, G.; Liu, S.; Xue, Y. Dynamics of bacterial community and fermentation quality in Leymus chinensis silage treated with lactic acid bacteria and/or water. Front. Microbiol. 2021, 12, 717120. [Google Scholar] [CrossRef]
- Wang, C.; Sun, L.; Xu, H.; Na, N.; Yin, G.; Liu, S.; Jiang, Y.; Xue, Y. Microbial communities, metabolites, fermentation quality and aerobic stability of whole-plant corn silage collected from family farms in desert steppe of North China. Processes 2021, 9, 784. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef]
- Jansson, J.K.; Hofmockel, K.S. The soil microbiome—From metagenomics to metaphenomics. Curr. Opin. Microbiol. 2018, 43, 162–168. [Google Scholar] [CrossRef]
- McDonald, P.; Henderson, A. Determination of water-soluble carbohydrates in grass. J. Sci. Food Agric. 1964, 15, 395–398. [Google Scholar] [CrossRef]
- Randby, Å.T.; Bakken, A. Effect of acid based additive treatment of low dry matter grass crops on losses and silage quality in bunker silos. Anim. Feed. Sci. Technol. 2021, 275, 114869. [Google Scholar] [CrossRef]
- Samarasinghe, M.B.; Larsen, M.; Johansen, M.; Waldemar, P.; Weisbjerg, M.R. Effects of shredding on silage density and fermentation quality. Grass Forage Sci. 2019, 74, 244–253. [Google Scholar] [CrossRef]
- Borreani, G.; Tabacco, E.; Schmidt, R.; Holmes, B.; Muck, R. Silage review: Factors affecting dry matter and quality losses in silages. J. Dairy Sci. 2018, 101, 3952–3979. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Bai, C.; Xu, H.; Na, N.; Jiang, Y.; Yin, G.; Liu, S.; Xue, Y. Succession of bacterial community during the initial aerobic, intense fermentation, and stable phases of whole-plant corn silages treated with lactic acid bacteria suspensions prepared from other silages. Front. Microbiol. 2021, 12, 655095. [Google Scholar] [CrossRef]
- Xu, H.; Wu, N.; Na, N.; Sun, L.; Zhao, Y.; Ding, H.; Fang, Y.; Wang, T.; Xue, Y.; Zhong, J. Fermentation weight loss, fermentation quality, and bacterial community of ensiling of sweet sorghum with lactic acid bacteria at different silo densities. Front. Microbiol. 2022, 13, 1013913. [Google Scholar] [CrossRef]
- Jungbluth, K.H.; Trimborn, M.; Maack, G.-C.; Büscher, W.; Li, M.; Cheng, H.; Cheng, Q.; Sun, Y. Effects of three different additives and two different bulk densities on maize silage characteristics, temperature profiles, CO2 and O2–dynamics in small scale silos during aerobic exposure. Appl. Sci. 2017, 7, 545. [Google Scholar] [CrossRef]
- Muck, R.; Kung, L., Jr. Effects of silage additives on ensiling proceedings from the silage: Field to feedbunk. Effects of Silage Additives on Ensiling. In Effects of Silage Additives on Ensiling in Silage: Field to Feed Bunk; NRAES-99; Northeast Regional Agricultural Engineering Service: Hershey, PA, USA, 1997; pp. 187–199. [Google Scholar] [CrossRef]
- Kung, L., Jr.; Shaver, R.; Grant, R.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef]
- Herrmann, C.; Heiermann, M.; Idler, C. Effects of ensiling, silage additives and storage period on methane formation of biogas crops. Bioresour. Technol. 2011, 102, 5153–5161. [Google Scholar] [CrossRef] [PubMed]
- Blajman, J.E.; Paez, R.B.; Vinderola, C.G.; Lingua, M.S.; Signorini, M. A meta-analysis on the effectiveness of homofermentative and heterofermentative lactic acid bacteria for corn silage. J. Appl. Microbiol. 2018, 125, 1655–1669. [Google Scholar] [CrossRef]
- Xia, T.; Wang, T.; Sun, J.; Shi, W.; Liu, Y.; Huang, F.; Zhang, J.; Zhong, J. Modulation of fermentation quality and metabolome in co-ensiling of Sesbania cannabina and sweet sorghum by lactic acid bacterial inoculants. Front. Microbiol. 2022, 13, 851271. [Google Scholar] [CrossRef]
- Mehdizadeh Gohari, I.; Navarro, M.A.; Li, J.; Shrestha, A.; Uzal, F.; McClane, B.A. Pathogenicity and virulence of Clostridium perfringens. Virulence 2021, 12, 723–753. [Google Scholar] [CrossRef] [PubMed]
- Ruppel, K.; Pitt, R.; Chase, L.; Galton, D. Bunker silo management and its relationship to forage preservation on dairy farms. J. Dairy Sci. 1995, 78, 141–153. [Google Scholar] [CrossRef]
- McDonald, P.; Henderson, A.; Heron, S.J.E. The Biochemistry of Silage; Chalcombe Publications: Marlow, UK, 1991. [Google Scholar]
- Sucu, E.; Kalkan, H.; Canbolat, O.; Filya, I. Effects of ensiling density on nutritive value of maize and sorghum silages. Rev. Bras. De Zootec. 2016, 45, 596–603. [Google Scholar] [CrossRef]
- Pahlow, G.; Muck, R.E.; Driehuis, F.; Elferink, S.J.O.; Spoelstra, S.F. Microbiology of ensiling. Agronomy 2003, 42, 31–93. [Google Scholar] [CrossRef]
- Sun, L.; Na, N.; Li, X.; Li, Z.; Wang, C.; Wu, X.; Xiao, Y.; Yin, G.; Liu, S.; Liu, Z. Impact of packing density on the bacterial community, fermentation, and in vitro digestibility of whole-crop barley silage. Agriculture 2021, 11, 672. [Google Scholar] [CrossRef]
- Tian, J.; Xu, N.; Liu, B.; Huan, H.; Gu, H.; Dong, C.; Ding, C. Interaction effect of silo density and additives on the fermentation quality, microbial counts, chemical composition and in vitro degradability of rice straw silage. Bioresour. Technol. 2020, 297, 122412. [Google Scholar] [CrossRef]
- Xue, Y.; Wu, N.; Na, N.; Sun, J.; Sun, L.; Qili, M.; Li, D.; Li, E.; Yang, B. Dynamics of Gas and Greenhouse Gases of Ensiling Barley with Lactic Acid Bacteria during Fermentation. Chem. Biol. Technol. Agric. 2024, 11, 82. [Google Scholar] [CrossRef]
- Bai, C.; Pan, G.; Leng, R.; Ni, W.; Yang, J.; Sun, J.; Yu, Z.; Liu, Z.; Xue, Y. Effect of ensiling density and storage temperature on fermentation quality, bacterial community, and nitrate concentration of sorghum-sudangrass silage. Front. Microbiol. 2022, 13, 828320. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Zhou, W.; Zou, X.; Wu, S.; Chen, X.; Zhang, Q. Fermentation Quality and Bacterial Diversity of Broussonetia papyrifera Leaves Ensiled with Lactobacillus plantarum and Stored at Different Temperatures. Agronomy 2022, 12, 986. [Google Scholar] [CrossRef]
- Wong, M.T.; Zhang, D.; Li, J.; Hui, R.K.H.; Tun, H.M.; Brar, M.S.; Park, T.-J.; Chen, Y.; Leung, F.C. Towards a metagenomic understanding on enhanced biomethane production from waste activated sludge after pH 10 pretreatment. Biotechnol. Biofuels 2013, 6, 38. [Google Scholar] [CrossRef]
- Yuan, X.; Li, J.; Dong, Z.; Shao, T. The reconstitution mechanism of napier grass microiota during the ensiling of alfalfa and their contributions to fermentation quality of silage. Bioresour. Technol. 2020, 297, 122391. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, M.; Wu, S.; Zou, X.; Chen, X.; Ge, L.; Zhang, Q. Effects of gallic acid on fermentation parameters, protein fraction, and bacterial community of whole plant soybean silage. Front. Microbiol. 2021, 12, 662966. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Qin, G.; Tan, Z.; Li, Z.; Wang, Y.; Cai, Y. Natural populations of lactic acid bacteria associated with silage fermentation as determined by phenotype, 16S ribosomal RNA and recA gene analysis. Syst. Appl. Microbiol. 2011, 34, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Ogunade, I.; Jiang, Y.; Cervantes, A.P.; Kim, D.; Oliveira, A.; Vyas, D.; Weinberg, Z.; Jeong, K.; Adesogan, A. Bacterial diversity and composition of alfalfa silage as analyzed by Illumina MiSeq sequencing: Effects of Escherichia coli O157: H7 and silage additives. J. Dairy Sci. 2018, 101, 2048–2059. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Lv, H.; Xing, Y.; Chen, X.; Zhang, Q. Intrinsic tannins affect ensiling characteristics and proteolysis of Neolamarckia cadamba leaf silage by largely altering bacterial community. Bioresour. Technol. 2020, 311, 123496. [Google Scholar] [CrossRef]
- Liu, B.; Huan, H.; Gu, H.; Xu, N.; Shen, Q.; Ding, C. Dynamics of a microbial community during ensiling and upon aerobic exposure in lactic acid bacteria inoculation-treated and untreated barley silages. Bioresour. Technol. 2019, 273, 212–219. [Google Scholar] [CrossRef]
- RE, M. Inoculation of silage and its effects on silage quality. In Proceedings of the Informational Conference with Dairy and Forage Industries, Madison, WI, USA, 1 January 1996; pp. 43–51. [Google Scholar]


| Items | Ensiling Days (g/kg FW) | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| 1 d | 3 d | 6 d | 20 d | 45 d | |||
| CK 450 | 0.104 bcE | 0.376 cD | 0.576 bcC | 1.13 bB | 1.80 bA | 0.162 | <0.001 |
| LS 450 | 0.201 aD | 0.722 aC | 0.926 aC | 1.53 aB | 2.43 aA | 0.207 | <0.001 |
| CK 500 | 0.112 bcE | 0.388 cD | 0.595 bcC | 1.14 bB | 1.82 bA | 0.163 | <0.001 |
| LS 500 | 0.159 abE | 0.486 bD | 0.633 bC | 1.07 bB | 1.69 bcA | 0.142 | <0.001 |
| CK 550 | 0.080 cE | 0.352 cD | 0.524 cC | 1.01 bcB | 1.66 bcA | 0.149 | <0.001 |
| LS 550 | 0.100 cD | 0.402 cC | 0.527 cC | 0.918 cB | 1.40 cA | 0.122 | <0.001 |
| SEM | 0.012 | 0.031 | 0.035 | 0.050 | 0.082 | ||
| p-value | 0.003 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Internation | D | L | T | DxL | DxT | LxT | DxLxT |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| pH | LA (g/kg DM) | AA (g/kg DM) | LA/AA | PA (g/kg DM) | BA (g/kg DM) | AN (g/kg) | |
|---|---|---|---|---|---|---|---|
| CK 450 | 6.24 a | 4.34 c | 15.4 | 0.279 b | 2.76 a | 2.27 b | 0.736 a |
| LS 450 | 4.95 b | 30.7 b | 11.0 | 2.92 a | ND b | ND c | 0.338 c |
| CK 500 | 6.30 a | 2.01 c | 12.2 | 0.165 b | 1.63 ab | 2.42 b | 0.625 b |
| LS 500 | 4.90 bc | 38.3 ab | 11.1 | 3.76 a | ND b | ND c | 0.322 c |
| CK 550 | 6.25 a | ND c | 12.7 | ND b | 1.41 ab | 5.38 a | 0.633 b |
| LS 550 | 4.75 c | 46.3 a | 12.2 | 4.09 a | 0.643 b | ND c | 0.355 c |
| SEM | 0.171 | 4.67 | 0.623 | 0.475 | 0.292 | 0.500 | 0.042 |
| p-value | <0.001 | <0.001 | 0.384 | 0.001 | 0.013 | <0.001 | <0.001 |
| D | 0.124 | 0.245 | 0.599 | 0.747 | 0.533 | 0.008 | 0.094 |
| L | <0.001 | <0.001 | 0.130 | <0.001 | 0.001 | <0.001 | <0.001 |
| DxL | 0.158 | 0.026 | 0.384 | 0.504 | 0.172 | 0.008 | 0.101 |
| LAB (lg cfu/g FW) | Yeasts (lg cfu/g FW) | Coliforms (lg cfu/g FW) | Bacteria (lg cfu/g FW) | |
|---|---|---|---|---|
| CK 450 | 9.11 | 8.75 | 5.15 a | 8.78 a |
| LS 450 | 8.99 | 8.34 | 4.31 b | 8.01 a |
| CK 500 | 9.00 | 8.64 | 5.53 a | 8.69 a |
| LS 500 | 9.11 | 8.39 | <2.00 c | 6.62 b |
| CK 550 | 9.10 | 8.63 | 4.99 ab | 8.69 a |
| LS 550 | 9.04 | 7.31 | <2.00 c | 6.21 b |
| SEM | 0.039 | 0.194 | 0.584 | 0.294 |
| p-value | 0.932 | 0.293 | 0.107 | 0.003 |
| D | 0.983 | 0.382 | <0.001 | 0.121 |
| L | 0.801 | 0.097 | <0.001 | <0.001 |
| DxL | 0.577 | 0.458 | <0.001 | 0.176 |
| DM (g/kg) | WSC (g/kg DM) | CP (g/kg DM) | |
|---|---|---|---|
| CK 450 | 394 b | 0.560 c | 38.6 bc |
| LS 450 | 419 a | 1.12 b | 45.2 a |
| CK 500 | 398 b | 0.84 bc | 37.6 bc |
| LS 500 | 411 a | 0.89 bc | 43.1 a |
| CK 550 | 380 c | 0.85 b | 37.3 c |
| LS 550 | 414 a | 1.33 a | 40.3 b |
| SEM | 3.47 | 0.122 | 0.077 |
| p-value | <0.001 | <0.001 | <0.001 |
| D | 0.040 | <0.001 | 0.013 |
| L | <0.001 | 0.00139 | <0.001 |
| DxL | 0.032 | 0.012 | 0.160 |
| Raw Tags | Valid Tags | Observed Otus | Shannon | Simpson | Chao1 | Goods Coverage | Pielou_e | |
|---|---|---|---|---|---|---|---|---|
| freach | 84,063 | 65,144 b | 334 a | 5.46 a | 0.943 a | 338 a | 1 | 0.657 a |
| CK 450 | 84,289 | 69,917 ab | 160 b | 4.20 b | 0.880 ab | 160 b | 1 | 0.587 ab |
| LS 450 | 85,638 | 73,589 a | 196 ab | 3.44 bcd | 0.823 b | 199 ab | 1 | 0.453 cd |
| CK 500 | 82,711 | 69,278 ab | 171 b | 4.23 b | 0.893 a | 172 ab | 1 | 0.580 ab |
| LS 500 | 84,074 | 74,119 a | 206 ab | 2.61 d | 0.683 bc | 209 ab | 1 | 0.343 e |
| CK 550 | 83,181 | 68,542 ab | 205 ab | 3.80 bc | 0.803 b | 205 ab | 1 | 0.510 bc |
| LS 550 | 82,406 | 74,915 a | 184 ab | 3.05 cd | 0.773 bc | 188 ab | 1 | 0.407 de |
| SEM | 490.6 | 984.6 | 19.5 | 0.212 | 0.021 | 19.8 ab | — | 0.024 |
| p-value | 0.696 | 0.044 | 0.262 | <0.001 | 0.004 | 0.271ab | — | <0.001 |
| D | 0.330 | 1.00 | 0.937 | 0.333 | 0.218 | 0.938 | — | 0.083 |
| L | 0.592 | 0.018 | 0.654 | 0.001 | 0.009 | 0.621 | — | <0.001 |
| DxL | 0.701 | 0.831 | 0.780 | 0.287 | 0.084 | 0.790 | — | 0.080 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Na, N.; Wu, N.; Sun, L.; Li, Z.; Qili, M.; Han, H.; Xue, Y. Impact of Additives and Packing Density on Fermentation Weight Loss, Microbial Diversity, and Fermentation Quality of Rape Straw Silage. Microorganisms 2024, 12, 1985. https://doi.org/10.3390/microorganisms12101985
Yang B, Na N, Wu N, Sun L, Li Z, Qili M, Han H, Xue Y. Impact of Additives and Packing Density on Fermentation Weight Loss, Microbial Diversity, and Fermentation Quality of Rape Straw Silage. Microorganisms. 2024; 12(10):1985. https://doi.org/10.3390/microorganisms12101985
Chicago/Turabian StyleYang, Baozhu, Na Na, Nier Wu, Lin Sun, Ziqin Li, Moge Qili, Hongyan Han, and Yelin Xue. 2024. "Impact of Additives and Packing Density on Fermentation Weight Loss, Microbial Diversity, and Fermentation Quality of Rape Straw Silage" Microorganisms 12, no. 10: 1985. https://doi.org/10.3390/microorganisms12101985
APA StyleYang, B., Na, N., Wu, N., Sun, L., Li, Z., Qili, M., Han, H., & Xue, Y. (2024). Impact of Additives and Packing Density on Fermentation Weight Loss, Microbial Diversity, and Fermentation Quality of Rape Straw Silage. Microorganisms, 12(10), 1985. https://doi.org/10.3390/microorganisms12101985







