In Vitro Digestion of Vacuum-Impregnated Yam Bean Snacks: Pediococcus acidilactici Viability and Mango Seed Polyphenol Bioaccessibility
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mango Seed Polyphenol Extraction, Preparation of the Suspension of P. acidilactici and Yam Bean Conditioning
2.2. Evaluation of the In Vitro Antimicrobial Activity of Mango Seed Extract and P. acidilactici Suspension against Pathogenic Bacteria
2.3. Preparation of Impregnating Solutions and Vacuum Impregnation (VI) Process
2.4. Characterization of Vacuum-Impregnated Yam Bean Snacks
2.4.1. Quantification of Total Soluble Phenols (TSP) and Hydrolyzable Polyphenols (HP)
2.4.2. Antioxidant Capacity (AOX)
2.4.3. Viability of P. acidilactici after Vacuum Impregnation Process and Dehydration
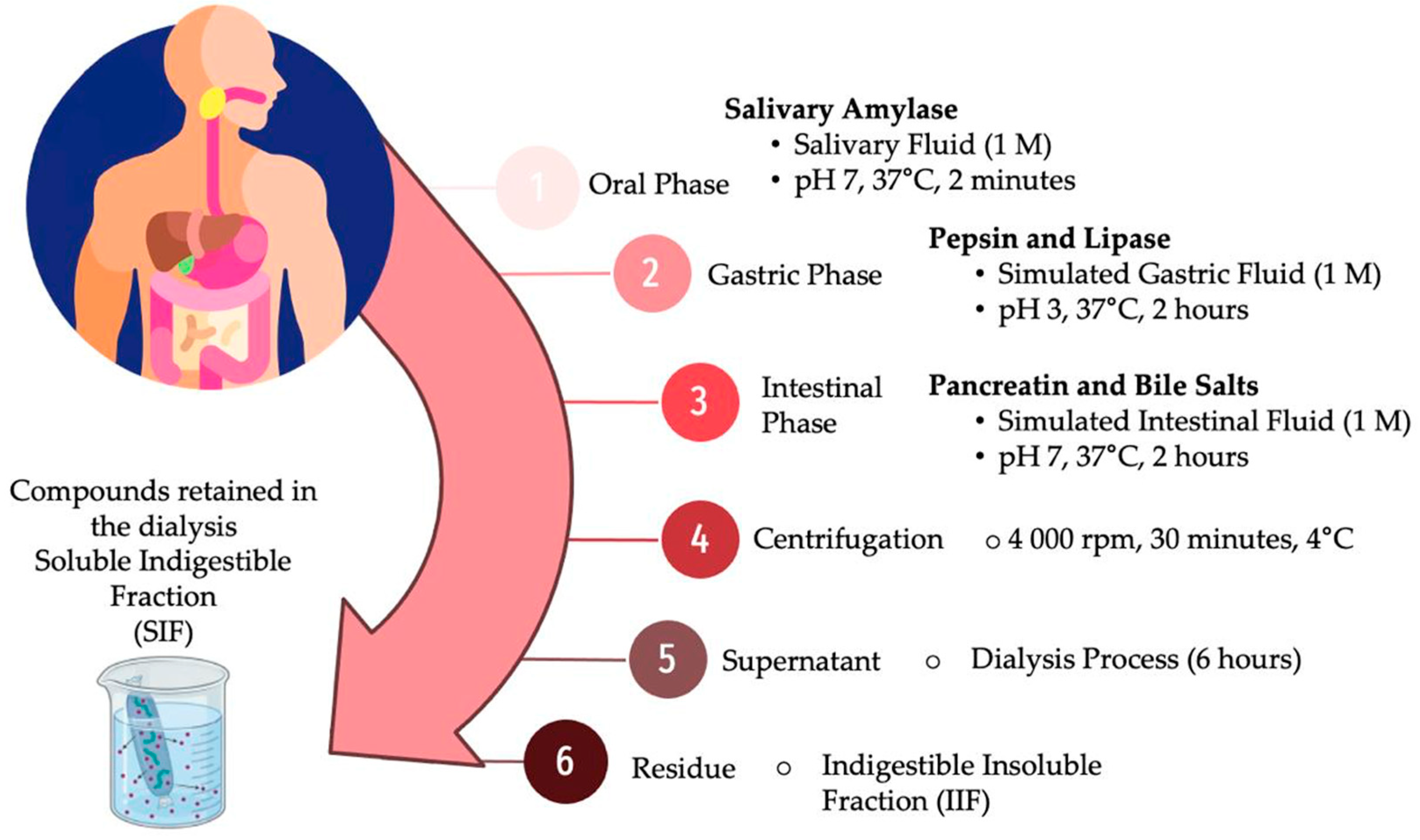
2.5. In Vitro Gastrointestinal Digestion of Vacuum-Impregnated Yam Bean Snacks
2.6. Statistical Analysis
3. Results and Discussion
3.1. Antimicrobial Activity of Mango Seed Extract and P. acidilactici Suspension
3.2. Total Soluble Phenol (TSP) Content, Hydrolyzable Polyphenols (HP) and Antioxidant Capacity (AOX) of Vacuum-Impregnated Yam Bean Snacks
3.3. Survival of P. acidilactici after Vacuum Impregnation and Dehydration
3.4. Data Associated with In Vitro Gastrointestinal Digestion of Vacuum-Impregnated Yam Bean Snacks and Bioaccessibility
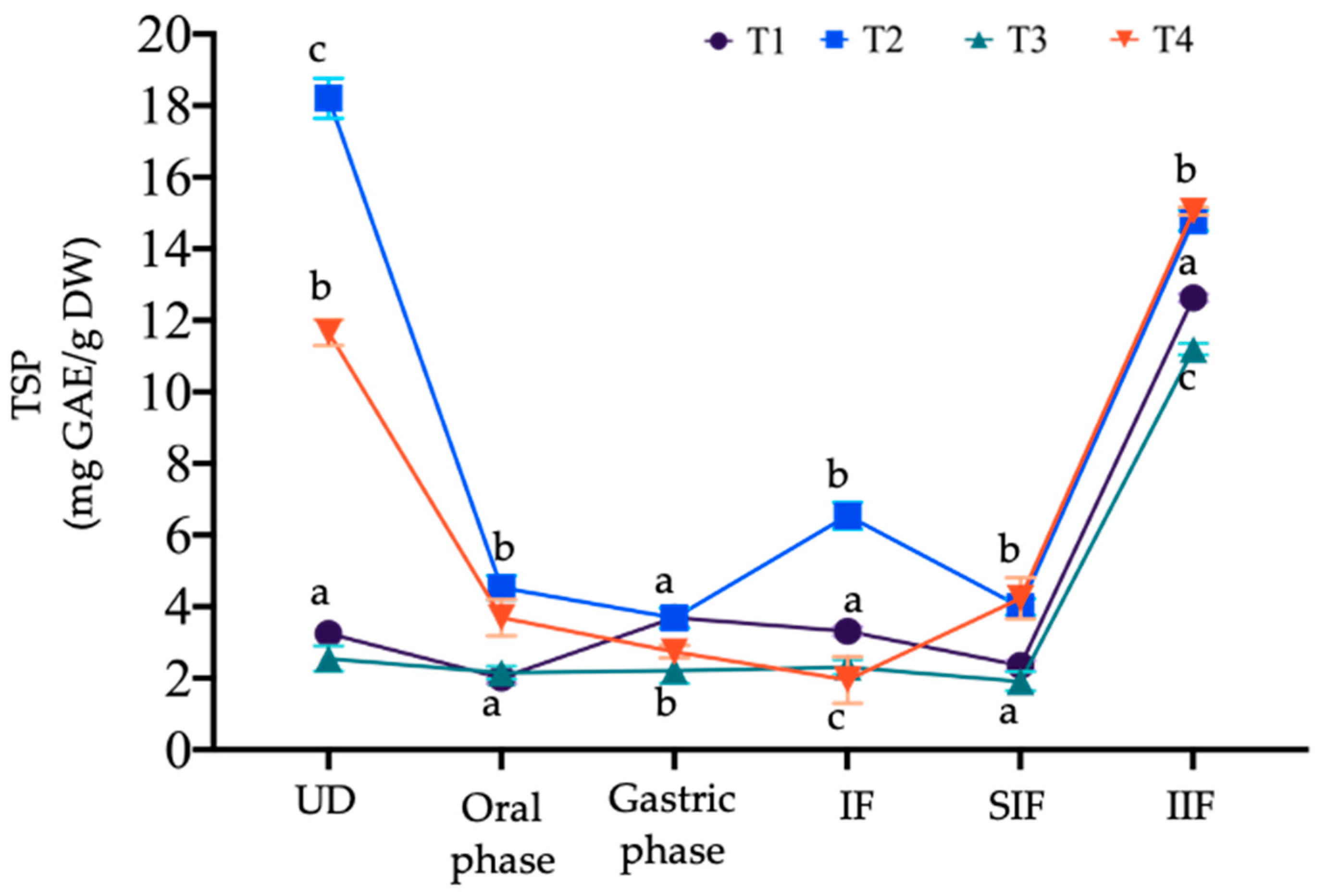
3.4.1. Changes in Total Soluble Phenol (TSP) Content and Bioaccessibility
3.4.2. Changes in Antioxidant Capacity (AOX)
3.4.3. Survival of P. acidilactici
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sasi, M.; Kumar, S.; Hasan, M.; S. R., A.; Garcia-Gutierrez, E.; Kumari, S.; Prakash, O.; Nain, L.; Sachdev, A.; Dahuja, A. Current Trends in the Development of Soy-Based Foods Containing Probiotics and Paving the Path for Soy-Synbiotics. Crit. Rev. Food Sci. Nutr. 2023, 63, 9995–10013. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M. The Future of Foods. Futur. Food 2024, 13, 506. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Jin, W.; Liu, S.J.; Jiao, Z.; Li, X. Probiotics, Prebiotics, and Postbiotics in Health and Disease. MedComm 2023, 4, e420. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Yeboah, P.J.; Ayivi, R.D.; Eddin, A.S.; Wijemanna, N.D.; Paidari, S.; Bakhshayesh, R.V. A Review and Comparative Perspective on Health Benefits of Probiotic and Fermented Foods. Int. J. Food Sci. Technol. 2023, 58, 4948–4964. [Google Scholar] [CrossRef]
- Yadav, M.K.; Kumari, I.; Singh, B.; Sharma, K.K.; Tiwari, S.K. Probiotics, Prebiotics and Synbiotics: Safe Options for next-Generation Therapeutics. Appl. Microbiol. Biotechnol. 2022, 106, 505–521. [Google Scholar] [CrossRef]
- Ma, T.; Shen, X.; Shi, X.; Sakandar, H.A.; Quan, K.; Li, Y.; Jin, H.; Kwok, L.Y.; Zhang, H.; Sun, Z. Targeting Gut Microbiota and Metabolism as the Major Probiotic Mechanism-An Evidence-Based Review. Trends Food Sci. Technol. 2023, 138, 178–198. [Google Scholar] [CrossRef]
- Attri, P.; Jodha, D.; Gandhi, D.; Chanalia, P.; Dhanda, S. In Vitro Evaluation of Pediococcus Acidilactici NCDC 252 for Its Probiotic Attributes. Int. J. Dairy Technol. 2015, 68, 533–542. [Google Scholar] [CrossRef]
- Fugaban, J.I.I.; Vazquez Bucheli, J.E.; Park, Y.J.; Suh, D.H.; Jung, E.S.; Franco, B.D.G.d.M.; Ivanova, I.V.; Holzapfel, W.H.; Todorov, S.D. Antimicrobial Properties of Pediococcus Acidilactici and Pediococcus Pentosaceus Isolated from Silage. J. Appl. Microbiol. 2022, 132, 311–330. [Google Scholar] [CrossRef]
- Durán-Castañeda, A.C.; Bueno-Durán, A.Y.; Girón-Pérez, M.I.; Ragazzo-Sánchez, J.A.; Sánchez-Burgos, J.A.; Sáyago-Ayerdi, S.G.; Zamora-Gasga, V.M. Effect of Pediococcus Acidilactici and Mango Seed Polyphenols on the Fermentative Profile of the Indigestible Fraction of Yam Bean. Food Res. Int. 2024, 178, 113970. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Catalkaya, G.; Venema, K.; Lucini, L.; Rocchetti, G.; Delmas, D.; Daglia, M.; De Filippis, A.; Xiao, H.; Quiles, J.L.; Xiao, J.; et al. Interaction of Dietary Polyphenols and Gut Microbiota: Microbial Metabolism of Polyphenols, Influence on the Gut Microbiota, and Implications on Host Health. Food Front. 2020, 1, 109–133. [Google Scholar] [CrossRef]
- Mithul Aravind, S.; Wichienchot, S.; Tsao, R.; Ramakrishnan, S.; Chakkaravarthi, S. Role of Dietary Polyphenols on Gut Microbiota, Their Metabolites and Health Benefits. Food Res. Int. 2021, 142, 110189. [Google Scholar] [CrossRef] [PubMed]
- López-Cobo, A.; Verardo, V.; Diaz-de-Cerio, E.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Gómez-Caravaca, A.M. Use of HPLC- and GC-QTOF to Determine Hydrophilic and Lipophilic Phenols in Mango Fruit (Mangifera indica L.) and Its by-Products. Food Res. Int. 2017, 100, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Olivo, A.O.; Zamora-Gasga, V.M.; Medina-Torres, L.; Pérez-Larios, A.; Sánchez-Burgos, J.A. Formulation of Double Emulsions of Mango Seed Extract (Mangifera indica L.) “Ataulfo” Incorporated into a Mango by-Product Flour Drink: Release Kinetics, Antioxidant Capacity, and Inhibition of Cyclooxygenases. Food Hydrocoll. Health 2023, 3, 100120. [Google Scholar] [CrossRef]
- Blancas-Benitez, F.J.; Mercado-Mercado, G.; Quirós-Sauceda, A.E.; Montalvo-González, E.; González-Aguilar, G.A.; Sáyago-Ayerdi, S.G. Bioaccessibility of Polyphenols Associated with Dietary Fiber and in Vitro Kinetics Release of Polyphenols in Mexican ‘Ataulfo’ Mango (Mangifera indica L.) by-Products. Food Funct. 2015, 6, 859–868. [Google Scholar] [CrossRef]
- Gao, J.; Li, X.; Zhang, G.; Sadiq, F.A.; Simal-Gandara, J.; Xiao, J.; Sang, Y. Probiotics in the Dairy Industry—Advances and Opportunities. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3937–3982. [Google Scholar] [CrossRef]
- Rasika, D.M.D.; Vidanarachchi, J.K.; Luiz, S.F.; Azeredo, D.R.P.; Cruz, A.G.; Ranadheera, C.S. Probiotic Delivery through Non-Dairy Plant-Based Food Matrices. Agriculture 2021, 11, 599. [Google Scholar] [CrossRef]
- Parra, K.; Ferrer, M.; Piñero, M.; Barboza, Y.; Medina, L.M. Use of Lactobacillus Acidophilus and Lactobacillus Casei for a Potential Probiotic Legume-Based Fermented Product Using Pigeon Pea (Cajanus cajan). J. Food Prot. 2013, 76, 265–271. [Google Scholar] [CrossRef]
- Demarinis, C.; Verni, M.; Pinto, L.; Rizzello, C.G.; Baruzzi, F. Use of Selected Lactic Acid Bacteria for the Fermentation of Legume-Based Water Extracts. Foods 2022, 11, 3346. [Google Scholar] [CrossRef]
- Abalos, R.A.; Naef, E.F.; Aviles, M.V.; Gómez, M.B. Vacuum Impregnation: A Methodology for the Preparation of a Ready-to-Eat Sweet Potato Enriched in Polyphenols. LWT-Food Sci. Technol. 2020, 131, 109773. [Google Scholar] [CrossRef]
- Ertek, G.; Taştan, Ö.; Baysal, T. Combined Use of Vacuum Impregnation and Encapsulation Technologies for Phenolic Enrichment of Strawberries. Food Chem. 2023, 398, 133853. [Google Scholar] [CrossRef] [PubMed]
- Fito, P.; Andrés, A.; Chiralt, A.; Pardo, P. Coupling of Hydrodynamic Mechanism and Deformation-Relaxation Phenomena during Vacuum Treatments in Solid Porous Food-Liquid Systems. J. Food Eng. 1996, 27, 229–240. [Google Scholar] [CrossRef]
- Durán-Castañeda, A.C.; González-Moya, S.; Sánchez-Burgos, J.A.; Sáyago-Ayerdi, S.G.; Zamora-Gasga, V.M. Applications of Vacuum Impregnation as a Technology to Incorporate Functional Components in Vegetal Matrices. Food Chem. Adv. 2024, 4, 100579. [Google Scholar] [CrossRef]
- de Oliveira, P.M.; Ramos, A.M.; Martins, E.M.F.; Vieira, É.N.R.; Soares, A.d.S.; de Noronha, M.C. Comparison of Vacuum Impregnation and Soaking Techniques for Addition of the Probiotic Lactobacillus acidophilus to Minimally Processed Melon. Int. J. Food Sci. Technol. 2017, 52, 2547–2554. [Google Scholar] [CrossRef]
- Burca-Busaga, C.G.; Betoret, N.; Seguí, L.; García-Hernández, J.; Hernández, M.; Barrera, C. Antioxidants Bioaccessibility and Lactobacillus Salivarius (CECT 4063) Survival Following the In Vitro Digestion of Vacuum Impregnated Apple Slices: Effect of the Drying Technique, the Addition of Trehalose, and High-Pressure Homogenization. Foods 2021, 10, 2155. [Google Scholar] [CrossRef]
- Barrera, C.; Burca, C.; Betoret, E.; García-Hernández, J.; Hernández, M.; Betoret, N. Improving Antioxidant Properties and Probiotic Effect of Clementine Juice Inoculated with Lactobacillus Salivarius Spp. Salivarius (CECT 4063) by Trehalose Addition and/or Sublethal Homogenisation. Int. J. Food Sci. Technol. 2019, 54, 2109–2122. [Google Scholar] [CrossRef]
- Sarkar, R.; Bhowmik, A.; Kundu, A.; Dutta, A.; Nain, L.; Chawla, G.; Saha, S. Inulin from Pachyrhizus erosus Root and Its Production Intensification Using Evolutionary Algorithm Approach and Response Surface Methodology. Carbohydr. Polym. 2021, 251, 117042. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, V.; Chauhan, S.; Lee, H.J. The Bioactivity and Phytochemicals of Pachyrhizus erosus (L.) Urb.: A Multifunctional Underutilized Crop Plant. Antioxidants 2022, 11, 58. [Google Scholar] [CrossRef]
- Liu, H.; Cui, S.W.; Chen, M.; Li, Y.; Liang, R.; Xu, F.; Zhong, F. Protective Approaches and Mechanisms of Microencapsulation to the Survival of Probiotic Bacteria during Processing, Storage and Gastrointestinal Digestion: A Review. Crit. Rev. Food Sci. Nutr. 2017, 59, 2863–2878. [Google Scholar] [CrossRef]
- Thakur, N.; Raigond, P.; Singh, Y.; Mishra, T.; Singh, B.; Lal, M.K.; Dutt, S. Recent Updates on Bioaccessibility of Phytonutrients. Trends Food Sci. Technol. 2020, 97, 366–380. [Google Scholar] [CrossRef]
- Andrés, A.; Heredia, A. Advanced Research in Food Digestion. Foods 2021, 10, 122. [Google Scholar] [CrossRef] [PubMed]
- Negara, B.F.S.P.; Tirtawijaya, G.; Cho, W.H.; Harwanto, D.; Sohn, J.H.; Kim, J.S.; Choi, J.S. Effects of Frying Processes on the Nutritional and Sensory Characteristics of Different Mackerel Products. Processes 2021, 9, 1645. [Google Scholar] [CrossRef]
- Pi, W.; Ryu, J.S.; Roh, J. Lactobacillus acidophilus Contributes to a Healthy Environment for Vaginal Epithelial Cells. Korean J. Parasitol. 2011, 49, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Gayathiri, E. Study of the Enumeration of Twelve Clinical Important Bacterial Populations at 0.5 McFarland Standard. Study of The Enumeration Of Twelve Clinical Important Bacterial Populations At 0.5 Mcfarland Standard. Int. J. Creat. Res. Thoughts 2018, 6, 2320–2882. [Google Scholar]
- Weinstein, M.P.; Lewis, J.S. The Clinical and Laboratory Standards Institute Subcommittee on Antimicrobial Susceptibility Testing: Background, Organization, Functions, and Processes. J. Clin. Microbiol. 2020, 58, 10–1128. [Google Scholar] [CrossRef]
- González-Moya, S.; Durán-Castañeda, A.C.; Velázquez-Estrada, R.M.; Sánchez-Burgos, J.A.; Sáyago-Ayerdi, S.G.; Zamora-Gasga, V.M. Vacuum Impregnation of Polyphenols in Yam Bean: Effect on Sensory Acceptability, Antioxidant Capacity, and Potential Absorption Ability. ACS Food Sci. Technol. 2023, 3, 1155–1164. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Arranz, S.; Tabernero, M.; Díaz-Rubio, M.E.; Serrano, J.; Goñi, I.; Saura-Calixto, F. Updated Methodology to Determine Antioxidant Capacity in Plant Foods, Oils and Beverages: Extraction, Measurement and Expression of Results. Food Res. Int. 2008, 41, 247–285. [Google Scholar] [CrossRef]
- Alvarez-Parrilla, E.; De-la-Rosa, L.A.; Armarowics, R.; Shahidi, F. Antioxidant Activity of Fresh and Processed Jalapeño and Serrano Peppers. J. Agric. Food Chem. 2011, 59, 163–173. [Google Scholar] [CrossRef]
- Hartzfeld, P.W.; Forkner, R.; Hunter, M.D.; Hagerman, A.E. Determination of Hydrolyzable Tannins (Gallotannins and Ellagitannins) after Reaction with Potassium Iodate. J. Agric. Food Chem. 2002, 50, 1785–1790. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay Medicine. Free Radic. Biol. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Parrilla, E.; De-la-Rosa, L.; Legarreta, P.; Saenz, L.; Rodrigo-García, J.; González-Aguilar, G. Daily Consumption of Apple, Pear and Orange Juice Differently Affects Plasma Lipids and Antioxidant Capacity of Smoking and Non-Smoking Adults. J. Food Sci. Nutr. 2010, 239, 369–380. [Google Scholar] [CrossRef]
- Akman, P.K.; Uysal, E.; Ozkaya, G.U.; Tornuk, F.; Durak, M.Z. Development of Probiotic Carrier Dried Apples for Consumption as Snack Food with the Impregnation of Lactobacillus paracasei. LWT-Food Sci. Technol. 2019, 103, 60–68. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Cruz-Trinidad, B.; Sánchez-Burgos, J.; Tovar, J.; Sáyago-Ayerdi, S.G.; Zamora-Gasga, V.M. In Vitro Gastrointestinal Digestion of Mango By-Product Snacks: Potential Absorption of Polyphenols and Antioxidant Capacity. Int. J. Food Sci. Technol. 2019, 54, 3091–3098. [Google Scholar] [CrossRef]
- Manso, T.; Lores, M.; de Miguel, T. Antimicrobial Activity of Polyphenols and Natural Polyphenolic Extracts on Clinical Isolates. Antibiotics 2022, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Valbuena, D.; Ayora-Talavera, T.; Luján-Hidalgo, C.; Álvarez-Gutiérrez, P.; Martínez-Galero, N.; Meza-Gordillo, R. Ultrasound Extraction Conditions Effect on Antioxidant Capacity of Mango By-Product Extracts. Food Bioprod. Process. 2021, 127, 212–224. [Google Scholar] [CrossRef]
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas Aeruginosa: Pathogenesis, Virulence Factors, Antibiotic Resistance, Interaction with Host, Technology Advances and Emerging Therapeutics. Signal Transduct. Target. Ther. 2022, 7, 199. [Google Scholar] [CrossRef]
- Yang, H.; Cheng, J.; Hu, L.; Zhu, Y.; Li, J. Mechanisms of Antimicrobial Resistance in Serratia Marcescens. Afr. J. Microbiol. Res. 2012, 6, 4427–4437. [Google Scholar] [CrossRef]
- Mousavi Khaneghah, A.; Abhari, K.; Eş, I.; Soares, M.B.; Oliveira, R.B.A.; Hosseini, H.; Rezaei, M.; Balthazar, C.F.; Silva, R.; Cruz, A.G.; et al. Interactions between Probiotics and Pathogenic Microorganisms in Hosts and Foods: A Review. Trends Food Sci. Technol. 2020, 95, 205–218. [Google Scholar] [CrossRef]
- Yadav, M.K.; Tiwari, S.K. Methods for Determination of Antimicrobial Activity of Bacteriocins of Lactic Acid Bacteria. Microbiology 2023, 92, 745–765. [Google Scholar] [CrossRef]
- Pacheco-Ordaz, R.; Wall-Medrano, A.; Goñi, M.G.; Ramos-Clamont-Montfort, G.; Ayala-Zavala, J.F.; González-Aguilar, G.A. Effect of Phenolic Compounds on the Growth of Selected Probiotic and Pathogenic Bacteria. Lett. Appl. Microbiol. 2018, 66, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Nawirska-Olszańska, A.; Pasławska, M.; Stępień, B.; Oziembłowski, M.; Sala, K.; Smorowska, A. Effect of Vacuum Impregnation with Apple-Pear Juice on Content of Bioactive Compounds and Antioxidant Activity of Dried Chokeberry Fruit. Foods 2020, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- de Souza, E.L.; de Albuquerque, T.M.R.; dos Santos, A.S.; Massa, N.M.L.; de Brito Alves, J.L. Potential Interactions among Phenolic Compounds and Probiotics for Mutual Boosting of Their Health-Promoting Properties and Food Functionalities–A Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1645–1659. [Google Scholar] [CrossRef]
- Zhao, Y.; Xie, J. Practical Applications of Vacuum Impregnation in Fruit and Vegetable Processing. Trends Food Sci. Technol. 2004, 15, 434–451. [Google Scholar] [CrossRef]
- Quirós-Sauceda, A.; Palafox-Carlos, H.; Sáyago-Ayerdi, S.; Ayála-Zavala, J.; Bello-Perez, L.; Álvarez-Parrilla, E.; González-Aguilar, G. Dietary Fiber and Phenolic Compounds as Functional Ingredients: Interaction and Possible Effect after Ingestion. Food Funct. 2014, 6, 1063–1067. [Google Scholar] [CrossRef]
- Christodoulou, M.C.; Orellana Palacios, J.C.; Hesami, G.; Jafarzadeh, S.; Lorenzo, J.M.; Domínguez, R.; Moreno, A.; Hadidi, M. Spectrophotometric Methods for Measurement of Antioxidant Activity in Food and Pharmaceuticals. Antioxidants 2022, 11, 2213. [Google Scholar] [CrossRef]
- Betoret, E.; Betoret, N.; Rocculi, P.; Dalla Rosa, M. Strategies to Improve Food Functionality: Structure-Property Relationships on High Pressures Homogenization, Vacuum Impregnation and Drying Technologies. Trends Food Sci. Technol. 2015, 46, 1–12. [Google Scholar] [CrossRef]
- Duarte-Correa, Y.; Díaz-Osorio, A.; Osorio-Arias, J.; Sobral, P.J.A.; Vega-Castro, O. Development of Fortified Low-Fat Potato Chips through Vacuum Impregnation and Microwave Vacuum Drying. Innov. Food Sci. Emerg. Technol. 2020, 64, 102437. [Google Scholar] [CrossRef]
- Freitas, D.; Le Feunteun, S.; Panouillé, M.; Souchon, I. The Important Role of Salivary α-Amylase in the Gastric Digestion of Wheat Bread Starch. Food Funct. 2018, 9, 200–208. [Google Scholar] [CrossRef]
- Durán-Castañeda, A.C.; Cardenas-Castro, A.P.; Pérez-Jiménez, J.; Pérez-Carvajal, A.M.; Sánchez-Burgos, J.A.; Mateos, R.; Sáyago-Ayerdi, S.G. Bioaccessibility of Phenolic Compounds in Psidium guajava L. Varieties and P. Friedrichsthalianum Nied. after Gastrointestinal Digestion. Food Chem. 2023, 400, 134046. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wichienchot, S.; He, X.; Fu, X.; Huang, Q.; Zhang, B. In Vitro Colonic Fermentation of Dietary Fibers: Fermentation Rate, Short-Chain Fatty Acid Production and Changes in Microbiota. Trends Food Sci. Technol. 2019, 88, 1–9. [Google Scholar] [CrossRef]
- Park, C.J.; Han, J.S. Hypoglycemic Effect of Jicama (Pachyrhizus erosus) Extract on Streptozotocin-Induced Diabetic Mice. Prev. Nutr. Food Sci. 2015, 20, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; Mazzacane, F.; Rizzello, C.G.; Vincentini, O.; Silano, M.; Giuliani, G.; De Angeli, M.; Gobbetti, M. Synthesis of Isoflavone Aglycones and Equol in Soy Milks Fermented by Food-Related Lactic Acid Bacteria and Their Effect on Human Intestinal Caco-2 Cells. J. Agric. Food Chem. 2010, 58, 10338–10346. [Google Scholar] [CrossRef]
- Duarte-Correa, Y.; Granda-Restrepo, D.; Cortés, M.; Vega-Castro, O. Potato Snacks Added with Active Components: Effects of the Vacuum Impregnation and Drying Processes. J. Food Sci. Technol. 2020, 57, 1523–1534. [Google Scholar] [CrossRef]
- Hernández-Maldonado, L.M.; Blancas-Benítez, F.J.; Zamora-Gasga, V.M.; Cárdenas-Castro, A.P.; Tovar, J.; Sáyago-Ayerdi, S.G. In Vitro Gastrointestinal Digestion and Colonic Fermentation of High Dietary Fiber and Antioxidant-Rich Mango (Mangifera indica L.) “Ataulfo”-Based Fruit Bars. Nutrients 2019, 11, 1564. [Google Scholar] [CrossRef]
- Cárdenas-Castro, A.P.; Zamora-Gasga, V.M.; Alvarez-Parrilla, E.; Ruíz-Valdiviezo, V.M.; Venema, K.; Sáyago-Ayerdi, S.G. In Vitro Gastrointestinal Digestion and Colonic Fermentation of Tomato (Solanum lycopersicum L.) and Husk Tomato (Physalis ixocarpa Brot.): Phenolic Compounds Released and Bioconverted by Gut Microbiota. Food Chem. 2021, 360, 130051. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Y.; Wang, A.; Wang, J.; Wu, X.; Wu, Y.; Fu, Y.; Sun, H. Insights into Interactions between Food Polyphenols and Proteins: An Updated Overview. J. Food Process. Preserv. 2022, 46, 16597. [Google Scholar] [CrossRef]
- Chandra, P.; Sharma, R.K.; Arora, D.S. Antioxidant Compounds from Microbial Sources: A Review; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; Volume 129, ISBN 0141399910. [Google Scholar]
- Zhu, W.; Lyu, F.; Naumovski, N.; Ajlouni, S.; Ranadheera, C.S. Functional Efficacy of Probiotic Lactobacillus Sanfranciscensis in Apple, Orange and Tomato Juices with Special Reference to Storage Stability and in Vitro Gastrointestinal Survival. Beverages 2020, 6, 13. [Google Scholar] [CrossRef]
- Minelli, E.B.; Benini, A. Relationship between Number of Bacteria and Their Probiotic Effects. Microb. Ecol. Health Dis. 2008, 20, 180–183. [Google Scholar] [CrossRef]


| Treatment | Pathogenic Bacteria (Zone Diameter Breakpoint, mm) | |||||||
|---|---|---|---|---|---|---|---|---|
| S. aureus | E. coli | P. aeruginosa | S. marcescens | |||||
| Mango seed extract (7 mg/mL) | 9 | R | 9 | R | 10 | R | 9 | R |
| P. acidilactici (106 cell/mL) | 0 | R | 0 | R | 0 | R | 0 | R |
| Combination (1:1, v/v) | 6 | R | 7 | R | 7 | R | 6 | R |
| Gentamicin (10 mg) a | 15 | S | - | 19 | S | - | ||
| Tetracycline (30 mg) b | - | 20 | S | - | - | |||
| Cefotaxime (30 mg) c | - | - | - | 20 | I | |||
| Treatment | TSP | HP | ABTS | DPPH | FRAP |
|---|---|---|---|---|---|
| T1 | 3.25 ± 0.15 a | 4.61 ± 0.14 b | 12.59 ± 0.58 a | 23.53 ± 1.12 a | 16.06 ± 0.64 a |
| T2 | 18.21 ± 0.56 c | 6.35 ± 0.01 a | 99.38 ± 3.82 b | 62.37 ± 2.98 b | 74.62 ± 2.74 b |
| T3 | 2.55 ± 0.35 a | 5.17 ± 0.11 c | 19.25 ± 0.55 c | 32.41 ± 0.76 c | 11.65 ± 0.16 c |
| T4 | 11.66 ± 0.36 b | 6.41 ± 0.12 a | 42.14 ± 1.67 d | 45.59 ± 1.38 d | 57.65 ± 1.03 d |
| Treatment | Vacuum Impregnation | Dehydrated |
|---|---|---|
| T1 | 0 | 0 |
| T2 | 0 | 0 |
| T3 | 1.60 × 106 ± 2.80 × 102 a | 3.70 × 106 ± 2.10 × 103 a |
| T4 | 1.90 × 106 ± 1.70 × 102 b | 1.75 × 106 ± 4.29 × 102 b |
| Treatment | BA (%) | NBP (mg GAE/g DW) | PBPA (mg GAE/g DW) |
|---|---|---|---|
| T1 | 20.77 ± 0.65 a | 14.96 ± 0.15 a | 0.96 ± 0.03 a |
| T2 | 30.65 ± 1.45 b | 18.78 ± 0.06 b | 2.55 ± 0.02 b |
| T3 | 17.13 ± 0.46 c | 13.11 ± 0.42 c | 0.39 ± 0.01 c |
| T4 | 11.41 ± 1.05 d | 19.29 ± 0.55 d | n.d. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durán-Castañeda, A.C.; Bueno-Durán, A.Y.; Girón-Pérez, M.I.; Ragazzo-Sánchez, J.A.; Sánchez-Burgos, J.A.; Sáyago-Ayerdi, S.G.; Zamora-Gasga, V.M. In Vitro Digestion of Vacuum-Impregnated Yam Bean Snacks: Pediococcus acidilactici Viability and Mango Seed Polyphenol Bioaccessibility. Microorganisms 2024, 12, 1993. https://doi.org/10.3390/microorganisms12101993
Durán-Castañeda AC, Bueno-Durán AY, Girón-Pérez MI, Ragazzo-Sánchez JA, Sánchez-Burgos JA, Sáyago-Ayerdi SG, Zamora-Gasga VM. In Vitro Digestion of Vacuum-Impregnated Yam Bean Snacks: Pediococcus acidilactici Viability and Mango Seed Polyphenol Bioaccessibility. Microorganisms. 2024; 12(10):1993. https://doi.org/10.3390/microorganisms12101993
Chicago/Turabian StyleDurán-Castañeda, Alba Cecilia, Adela Yolanda Bueno-Durán, Manuel Iván Girón-Pérez, Juan Arturo Ragazzo-Sánchez, Jorge Alberto Sánchez-Burgos, Sonia Guadalupe Sáyago-Ayerdi, and Victor Manuel Zamora-Gasga. 2024. "In Vitro Digestion of Vacuum-Impregnated Yam Bean Snacks: Pediococcus acidilactici Viability and Mango Seed Polyphenol Bioaccessibility" Microorganisms 12, no. 10: 1993. https://doi.org/10.3390/microorganisms12101993






