The Marine-Origin Exopolysaccharide-Producing Bacteria Micrococcus Antarcticus HZ Inhibits Pb Uptake in Pakchoi (Brassica chinensis L.) and Affects Rhizosphere Microbial Communities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacteria, Soil, and Plants
2.2. Determination of Pb Concentration and EPS Yield in Soil Filtrate after Inoculation with Strain HZ
2.3. Potting Experiments
2.4. Plant and Soil Sample Analysis
2.5. Detection of Bacterial Strains Colonizing Inter-Root Soil
2.6. Analysis of Soil Flora
2.7. Statistical Analysis
3. Results
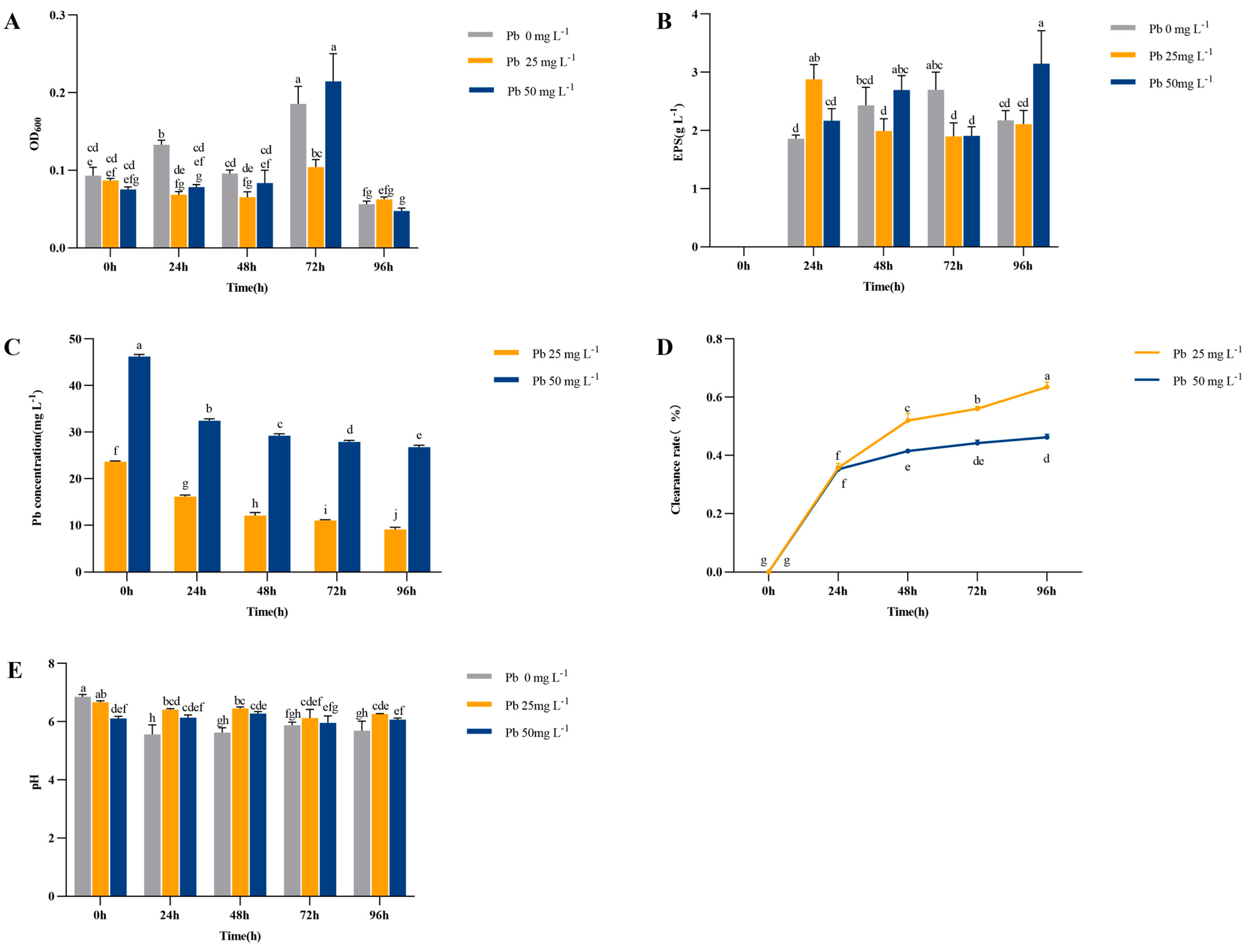
3.1. Determination of Concentrations of Water-Soluble Pb and EPS in Soil Filtrate after Inoculation with Strain HZ
3.2. Colonization of Soil by Strain HZ
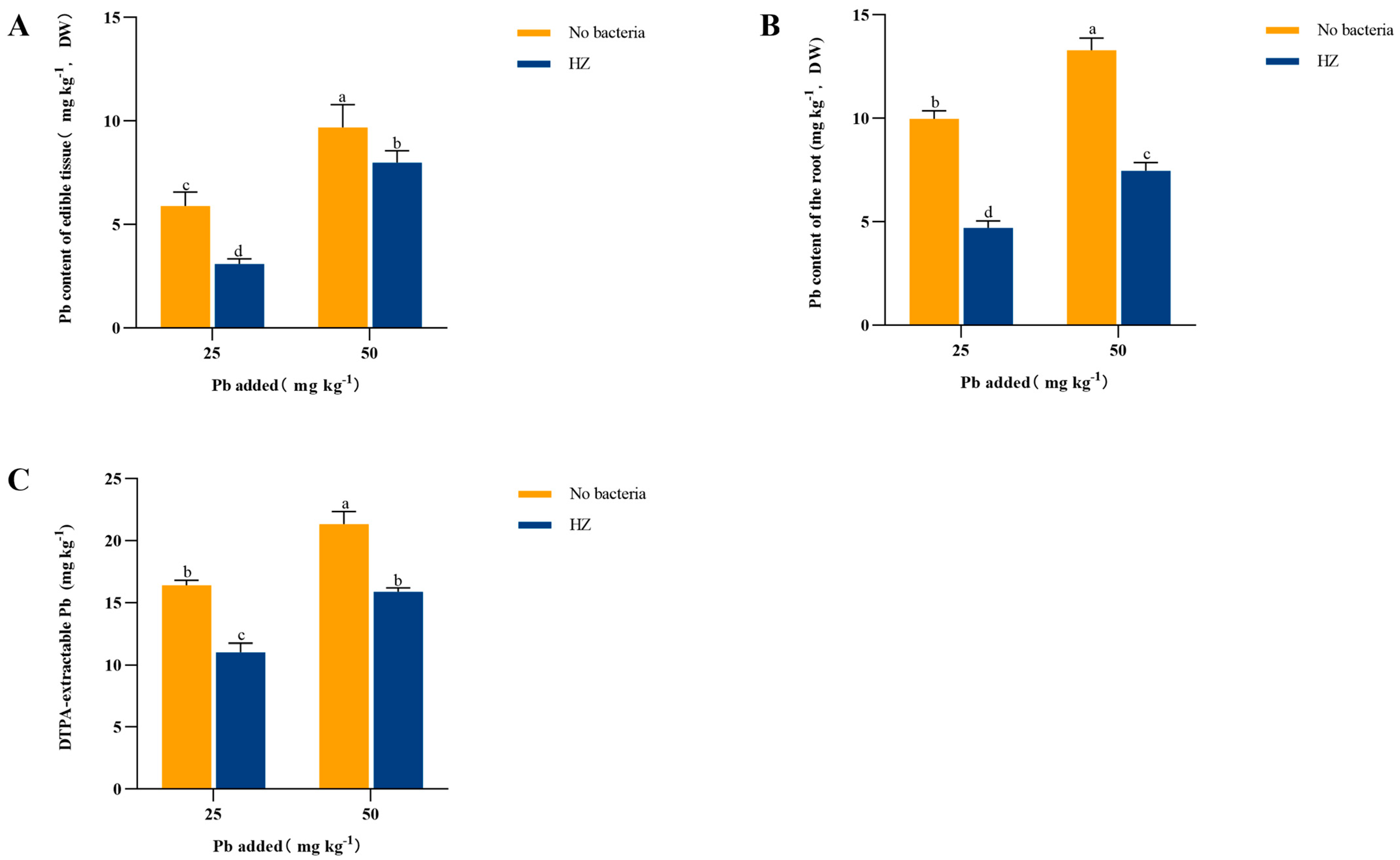
3.3. Effect of Strain HZ on Available Pb in Rhizosphere Soil
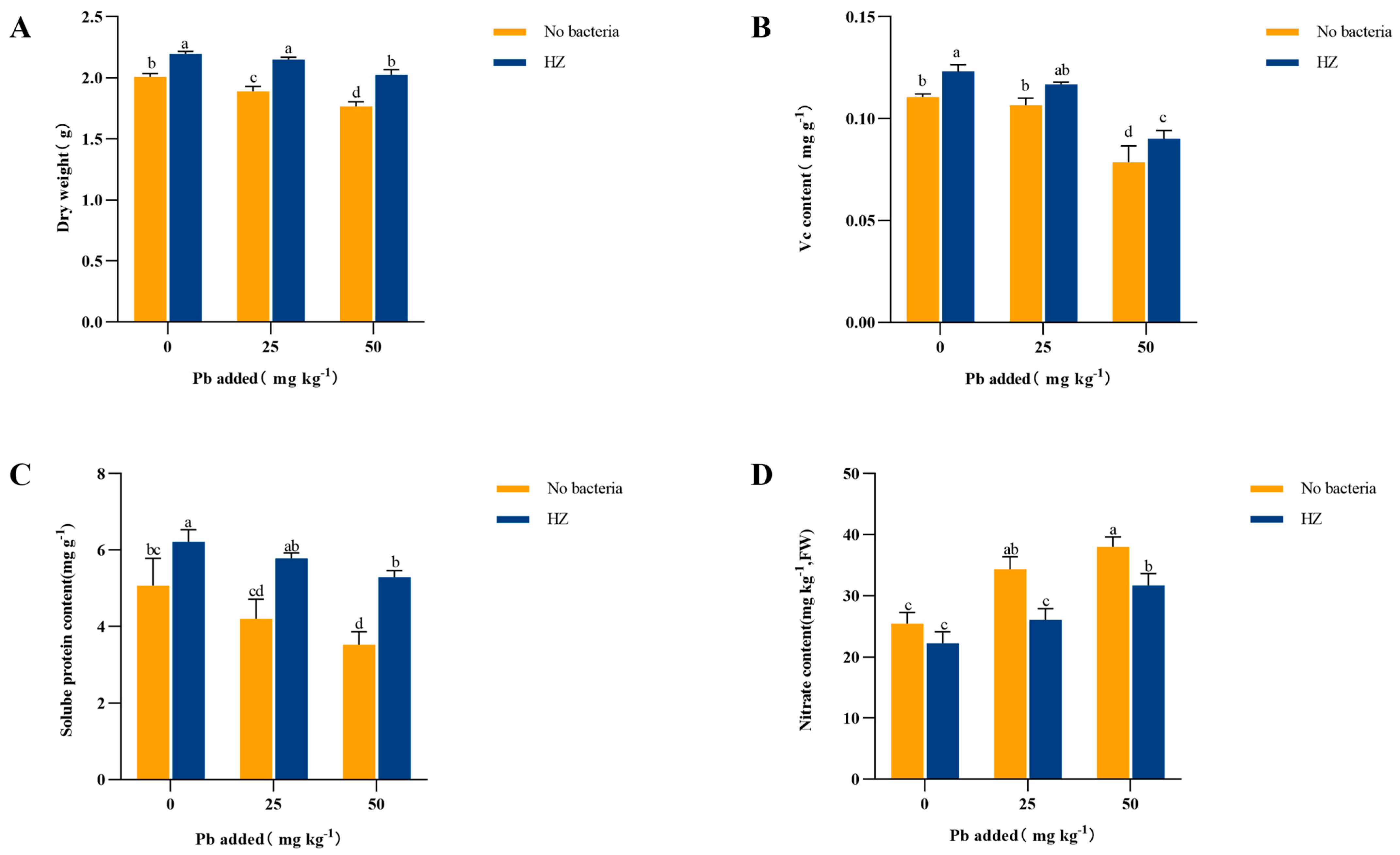
3.4. Effect of Strain HZ on Cabbage Biomass and Edible Tissue Vitamin C, Soluble Protein, and Nitrite Content
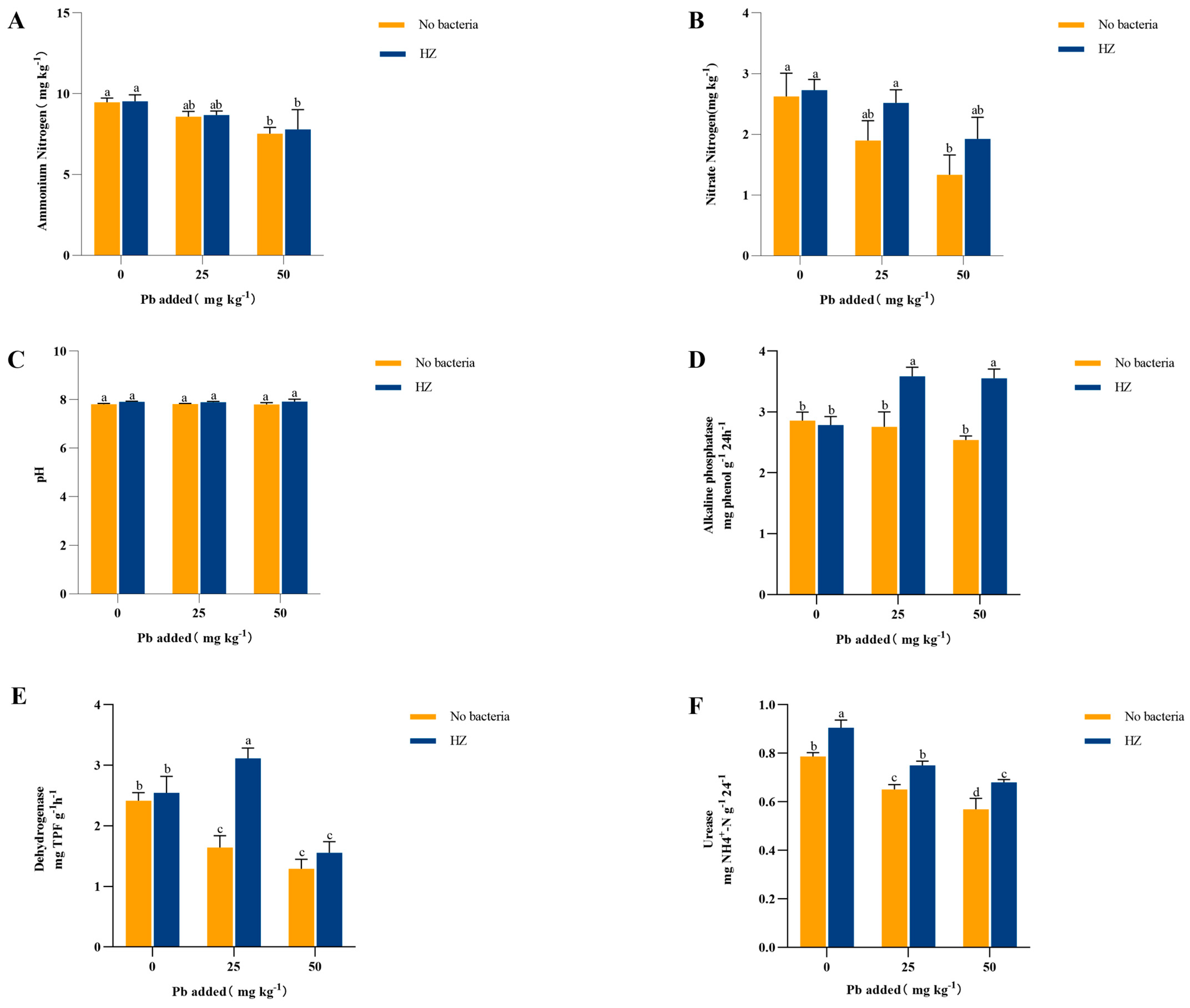
3.5. Effect of Strain HZ on Soil Nitrogen Content and Soil Enzyme Activity
3.6. Bacterial Community Diversity and Structure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, J.; Li, H.; Liu, J. Heavy metal pollution in the soil around municipal solid waste incinerators and its health risks in China. Environ. Res. 2022, 203, 111871. [Google Scholar] [CrossRef]
- Angon, P.B.; Islam, M.S.; Kc, S.; Das, A.; Anjum, N.; Poudel, A.; Suchi, S.A. Sources, effects and present perspectives of heavy metals contamination: Soil, plants and human food chain. Heliyon 2024, 10, e28357. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, D.; Ren, F.; Huang, L. Spatiotemporal variation of soil heavy metals in China: The pollution status and risk assessment. Sci. Total Environ. 2023, 871, 161768. [Google Scholar] [CrossRef]
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A. Effects of exogenously applied salicylic acid and putrescine alone and in combination with rhizobacteria on the phytoremediation of heavy metals and chickpea growth in sandy soil. Int. J. Phytoremed. 2018, 20, 405–414. [Google Scholar] [CrossRef]
- Azhar, U.; Ahmad, H.; Shafqat, H.; Babar, M.; Shahzad Munir, H.M.; Sagir, M.; Arif, M.; Hassan, A.; Rachmadona, N.; Rajendran, S.; et al. Remediation techniques for elimination of heavy metal pollutants from soil: A review. Environ. Res. 2022, 214, 113918. [Google Scholar] [CrossRef]
- Choudhury, S.; Chatterjee, A. Microbial application in remediation of heavy metals: An overview. Arch. Microbiol. 2022, 204, 268. [Google Scholar] [CrossRef]
- Liu, S.; Liu, H.; Chen, R.; Ma, Y.; Yang, B.; Chen, Z.; Liang, Y.; Fang, J.; Xiao, Y. Role of Two Plant Growth-Promoting Bacteria in Remediating Cadmium-Contaminated Soil Combined with Miscanthus floridulus (Lab.). Plants 2021, 10, 912. [Google Scholar] [CrossRef]
- Ruan, Z.; Chen, K.; Cao, W.; Meng, L.; Yang, B.; Xu, M.; Xing, Y.; Li, P.; Freilich, S.; Chen, C.; et al. Engineering natural microbiomes toward enhanced bioremediation by microbiome modeling. Nat. Commun. 2024, 15, 4694. [Google Scholar] [CrossRef]
- Gomes, M.A.; Hauser-Davis, R.A.; de Souza, A.N.; Vitória, A.P. Metal phytoremediation: General strategies, genetically modified plants and applications in metal nanoparticle contamination. Ecotoxicol. Environ. Saf. 2016, 134, 133–147. [Google Scholar] [CrossRef]
- Alami, Y.; Achouak, W.; Marol, C.; Heulin, T. Rhizosphere Soil Aggregation and Plant Growth Promotion of Sunflowers by an Exopolysaccharide-Producing Rhizobium sp. Strain Isolated from Sunflower Roots. Appl. Environ. Microbiol. 2000, 66, 3393–3398. [Google Scholar] [CrossRef]
- de Vries, F.T.; Griffiths, R.I.; Knight, C.G.; Nicolitch, O.; Williams, A. Harnessing rhizosphere microbiomes for drought-resilient crop production. Science 2020, 368, 270–274. [Google Scholar] [CrossRef]
- Li, Z. Screening and Identification of Cadmium-Resistant Bacterium Burkholderia sp. 3-1 and Preliminary Investigation of the Adsorption Characteristics and Mechanism of Cd2+ in Water Bodies. Master’s Thesis, Hainan University, Haikou, China, 2023. [Google Scholar]
- Wang, Z.; Wang, M.; Cai, K.Z.; Cai, Y.X.; Huang, F. Research progress on the mechanism of heavy metal adsorption and detoxification by bacteria. Biotechnol. Bull. 2016, 32, 13–18. [Google Scholar]
- Shi, Y. Research progress of heavy metal ion removal by biosorption. Biotechnol. World 2014, 9, 23. [Google Scholar]
- Raj, K.K.; Sardar, U.R.; Bhargavi, E.; Devi, I.; Bhunia, B.; Tiwari, O.N. Advances in exopolysaccharides based bioremediation of heavy metals in soil and water: A critical review. Carbohydr. Polym. 2018, 199, 353–364. [Google Scholar]
- Abd-El-Haleem, D. Alpha-glucan: A novel bacterial polysaccharide and its application as a biosorbent for heavy metals. J. Genet. Eng. Biotechnol. 2023, 21, 133. [Google Scholar] [CrossRef]
- Hao, L.; Liu, W.; Liu, K.; Shan, K.; Wang, C.; Xi, C.; Liu, J.; Fan, Q.; Zhang, X.; Lu, X.; et al. Isolation, Optimization of Fermentation Conditions, and Characterization of an Exopolysaccharide from Pseudoalteromonas agarivorans Hao 2018. Mar. Drugs 2019, 17, 703. [Google Scholar] [CrossRef]
- Chakraborty, J.; Das, S. Characterization and cadmium-resistant gene expression of biofilm-forming marine bacterium Pseudomonas aeruginosa JP-11. Environ. Sci. Pollut. Res. 2014, 21, 14188–14201. [Google Scholar] [CrossRef]
- Ni, B.J.; Fang, F.; Xie, W.M.; Sun, M.; Sheng, G.P.; Li, W.H.; Yu, H.Q. Characterization of extracellular polymeric substances produced by mixed microorganisms in activated sludge with gel-permeating chromatography, excitation-emission matrix fluorescence spectroscopy measurement and kinetic modeling. Water Res. 2009, 43, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Mohite, B.V.; Koli, S.H.; Narkhede, C.P.; Patil, S.N.; Patil, S.V. Prospective of Microbial Exopolysaccharide for Heavy Metal Exclusion. Appl. Biochem. Biotechnol. 2017, 183, 582–600. [Google Scholar] [CrossRef] [PubMed]
- Raj, R.; Dalei, K.; Chakraborty, J.; Das, S. Extracellular polymeric substances of a marine bacterium mediated synthesis of CdS nanoparticles for removal of cadmium from aqueous solution. J. Colloid Interface Sci. 2016, 462, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Karthik, C.; Elangovan, N.; Kumar, T.S.; Govindharaju, S.; Barathi, S.; Oves, M.; Arulselvi, P.I. Characterization of multifarious plant growth promoting traits of rhizobacterial strain AR6 under Chromium (VI) stress. Microbiol. Res. 2017, 204, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Zainab, N.; Amna; Din, B.U.; Javed, M.T.; Afridi, M.S.; Mukhtar, T.; Kamran, M.A.; Ul Ain, Q.; Khan, A.A.; Ali, J.; et al. Deciphering metal toxicity responses of flax (Linum usitatissimum L.) with exopolysaccharide and ACC-deaminase producing bacteria in industrially contaminated soils. Plant Physiol. Biochem. 2020, 152, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Caruso, C.; Rizzo, C.; Mangano, S.; Poli, A.; Di Donato, P.; Finore, I.; Nicolaus, B.; Di Marco, G.; Michaud, L.; Lo Giudice, A. Production and Biotechnological Potential of Extracellular Polymeric Substances from Sponge-Associated Antarctic Bacteria. Appl. Environ. Microbiol. 2018, 84, e01624-17. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhang, N.; Yang, X.; Song, L.; Yang, S. Toxic metal biosorption by macrocolonies of cyanobacterium Nostoc sphaeroides Kützing. J. Appl. Phycol. 2016, 28, 2265–2277. [Google Scholar] [CrossRef]
- Huët, M.A.L.; Puchooa, D. Bioremediation of heavy metals from aquatic environment through microbial processes: A potential role for probiotics? J. Appl. Biol. Biotech. 2017, 5, 14–23. [Google Scholar]
- Casillo, A.; Lanzetta, R.; Parrilli, M.; Corsaro, M.M. Exopolysaccharides from Marine and Marine Extremophilic Bacteria: Structures, Properties, Ecological Roles and Applications. Mar. Drugs 2018, 16, 69. [Google Scholar] [CrossRef]
- Wang, W.; Ju, Y.; Liu, N.; Shi, S.; Hao, L. Structural characteristics of microbial exopolysaccharides in association with their biological activities: A review. Chem. Biol. Technol. Agric. 2023, 10, 137. [Google Scholar] [CrossRef]
- Ju, Y.; Shan, K.; Liu, W.; Xi, C.; Zhang, Y.; Wang, W.; Wang, C.; Cao, R.; Zhu, W.; Wang, H.; et al. Effect of Different Initial Fermentation pH on Exopolysaccharides Produced by Pseudoalteromonas agarivorans Hao 2018 and Identification of Key Genes Involved in Exopolysaccharide Synthesis via Transcriptome Analysis. Mar. Drugs 2022, 20, 89. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, Q.; An, J.; Sun, Y.; Liu, R. Variations in cadmium accumulation among Chinese cabbage cultivars and screening for Cd-safe cultivars. J. Hazard. Mater. 2010, 173, 737–743. [Google Scholar] [CrossRef]
- Lu, X.P.; Xu, Y.R.; Fan, Q.P.; Wang, C.L.; Hao, L.J. Isolation and identification of bacteria on seedling plates of Pseudosylvestris pseudo spp. Oceanogr. Res. 2017, 35, 85–90. [Google Scholar]
- Christensen, B.E.; Kjosbakken, J.; Smidsrød, O. Partial Chemical and Physical Characterization of Two Extracellular Polysaccharides Produced by Marine, Periphytic Pseudomonas sp. Strain NCMB 2021. Appl. Environ. Microbiol. 1985, 50, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q. The Research of Exopolysaccharide Produced by Pesudoalteromonas issachenkonii HZ. Master’s Thesis, Qilu University of Technology, Jinan, China, 2016. [Google Scholar]
- Soler-Jofra, A.; Pérez, J.; Van Loosdrecht, M.C. Hydroxylamine and the nitrogen cycle: A review. Water Res. 2021, 190, 116723. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.Y.; Tian, W.H.; Jin, C.W. Nitrogen in plants: From nutrition to the modulation of abiotic stress adaptation. Stress Biol. 2022, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, N.; Saifulla; Malhi, S.S.; Zia, M.H.; Naeem, A.; Bibi, S.; Farid, G. Role of mineral nutrition in minimizing cadmium accumulation by plants. J. Sci. Food Agric. 2010, 90, 925–937. [Google Scholar] [CrossRef]
- Mei, P.P.; Wang, P.; Li, L.; Zhang, X.; Gui, G.G.; Huang, J.C. Construction of an efficient nitrogen fixation model of corn/fava bean-rhizobium on newly reclaimed soil. Chin. J. Ecol. Agric. 2018, 26, 62–74. [Google Scholar]
- Zhu, J.; Fang, X.Z.; Dai, Y.J.; Zhu, Y.X.; Chen, H.S.; Lin, X.Y.; Jin, C.W. Nitrate transporter 1.1 alleviates lead toxicity in Arabidopsis by preventing rhizosphere acidification. J. Exp. Bot. 2019, 70, 6363–6374. [Google Scholar] [CrossRef]
- LI, Y.Y.; Yang, R.; Gao, R.; Wei, H.A.; Chen, A.L.; Yong, L.I. Effects of long-term phosphorus fertilization and straw incorporation on phosphorus fractions in subtropical paddy soil. J. Integr. Agric. 2015, 14, 365–373. [Google Scholar] [CrossRef]
- Madhaiyan, M.; Poonguzhali, S.; Kang, B.G.; Lee, Y.-J.; Chung, J.-B.; Sa, T.-M. Effect of co-inoculation of methylotrophic Methylobacterium oryzae with Azospirillum brasilense and Burkholderia pyrrocinia on the growth and nutrient uptake of tomato, red pepper and rice. Plant Soil 2010, 328, 71–82. [Google Scholar] [CrossRef]
- Mahbub, K.R.; Krishnan, K.; Megharaj, M.; Naidu, R. Bioremediation potential of a highly mercury resistant bacterial strain Sphingobium SA2 isolated from contaminated soil. Chemosphere 2016, 144, 330–337. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, W.J.; He, L.Y.; Sheng, X.F. Increased biomass and quality and reduced heavy metal accumulation of edible tissues of vegetables in the presence of Cd-tolerant and immobilizing Bacillus megaterium H3. Ecotoxicol. Environ. Saf. 2018, 148, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Dong, G.; Liu, X.; Zhang, S.; Li, C.; Lu, X.; Xia, T. Poly-γ-glutamic acid-producing bacteria reduced Cd uptake and effected the rhizosphere microbial communities of lettuce. J. Hazard. Mater. 2020, 398, 123146. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Cai, H.; Wang, X.; Hu, X.; Chen, Z.; Yao, L. Heavy metal-immobilizing bacteria increase the biomass and reduce the Cd and Pb uptake by pakchoi (Brassica chinensis L.) in heavy metal-contaminated soil. Ecotoxicol. Environ. Saf. 2020, 195, 110375. [Google Scholar] [CrossRef] [PubMed]
- Jansson, J.K.; Hofmockel, K.S. Soil microbiomes and climate change. Nat. Rev. Microbiol. 2019, 18, 35–46. [Google Scholar] [CrossRef]
- Naylor, D.; Coleman-Derr, D. Drought Stress and Root-Associated Bacterial Communities. Front. Plant Sci. 2018, 8, 2223. [Google Scholar] [CrossRef]
- Li, Y.; Gao, Y.; Chen, W.; Zhang, W.; Lu, X. Shifts in bacterial diversity, interactions and microbial elemental cycling genes under cadmium contamination in paddy soil: Implications for altered ecological function. J. Hazard. Mater. 2024, 461, 132544. [Google Scholar] [CrossRef]
- Ma, H.; Li, P.; Liu, X.; Li, C.; Zhang, S.; Wang, X.; Tao, X. Poly-γ-glutamic acid enhanced the drought resistance of maize by improving photosynthesis and affecting the rhizosphere microbial community. BMC Plant Biol. 2022, 22, 11. [Google Scholar] [CrossRef]
- Naz, M.; Dai, Z.; Hussain, S.; Tariq, M.; Danish, S.; Khan, I.U.; Qi, S.; Du, D. The soil pH and heavy metals revealed their impact on soil microbial community. J. Environ. Manag. 2022, 321, 115770. [Google Scholar] [CrossRef]
- Sazykin, I.; Khmelevtsova, L.; Azhogina, T.; Sazykina, M. Heavy Metals Influence on the Bacterial Community of Soils: A Review. Agriculture 2023, 13, 653. [Google Scholar] [CrossRef]
- Luo, J.; Gu, S.; Guo, X.; Liu, Y.; Tao, Q.; Zhao, H.P.; Liang, Y.; Banerjee, S.; Li, T. Core Microbiota in the Rhizosphere of Heavy Metal Accumulators and Its Contribution to Plant Performance. Environ. Sci. Technol. 2022, 56, 12975–12987. [Google Scholar] [CrossRef]
- Kamal, S.; Prasad, R.; Varma, A. Soil Microbial Diversity in Relation to Heavy Metals. In Soil Heavy Metals; Springer: Berlin/Heidelberg, Germany, 2010; pp. 31–63. [Google Scholar]
- Berg, J.; Brandt, K.K.; Al-Soud, W.A.; Holm, P.E.; Hansen, L.H.; Sørensen, S.J.; Nybroe, O. Selection for Cu-tolerant bacterial communities with altered composition, but unaltered richness, via long-term Cu exposure. Appl. Environ. Microbiol. 2012, 78, 7438–7446. [Google Scholar] [CrossRef] [PubMed]
- Borgulat, J.; Łukasik, W.; Borgulat, A.; Nadgórska-Socha, A.; Kandziora-Ciupa, M. Influence of lead on the activity of soil microorganisms in two Beskidy landscape parks. Environ. Monit. Assess. 2021, 193, 839. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Song, M.; Yang, L.; Zhang, D.; Sun, Y.; Shen, Z.; Luo, C.; Zhang, G. Exploring the Influence of Environmental Factors on Bacterial Communities within the Rhizosphere of the Cu-tolerant plant, Elsholtzia splendens. Sci. Rep. 2016, 6, 36302. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Wang, P.; Liu, H.; Jin, D.; Chen, X.; Zhu, L. Effect of chlorantraniliprole on soil bacterial and fungal diversity and community structure. Heliyon 2023, 9, e13668. [Google Scholar] [CrossRef]
- Narendrula-Kotha, R.; Nkongolo, K.K. Bacterial and fungal community structure and diversity in a mining region under long-term metal exposure revealed by metagenomics sequencing. Ecol. Genet. Genom. 2017, 2, 13–24. [Google Scholar] [CrossRef]
- Chen, C.; Wang, X.; Wang, J. Phytoremediation of cadmium-contaminated soil by Sorghum bicolor and the variation of microbial community. Chemosphere 2019, 235, 985–994. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Sun, Y.B.; Zhang, R.F.; Wang, C.; Jia, H.C. Effects of mercury pollution on soil organic carbon stability and functional microbial communities for carbon sequestration. Environ. Sci. 2024, 1–14. [Google Scholar] [CrossRef]
- Bian, F.; Zhong, Z.; Li, C.; Zhang, X.; Gu, L.; Huang, Z.; Gai, X.; Huang, Z. Intercropping improves heavy metal phytoremediation efficiency through changing properties of rhizosphere soil in bamboo plantation. J. Hazard. Mater. 2021, 416, 125898. [Google Scholar] [CrossRef]
- Tan, L.T.; Chan, K.G.; Khan, T.M.; Bukhari, S.I.; Saokaew, S.; Duangjai, A.; Pusparajah, P.; Lee, L.H.; Goh, B.H. Streptomyces sp. MUM212 as a Source of Antioxidants with Radical Scavenging and Metal Chelating Properties. Front. Pharmacol. 2017, 8, 276. [Google Scholar] [CrossRef]
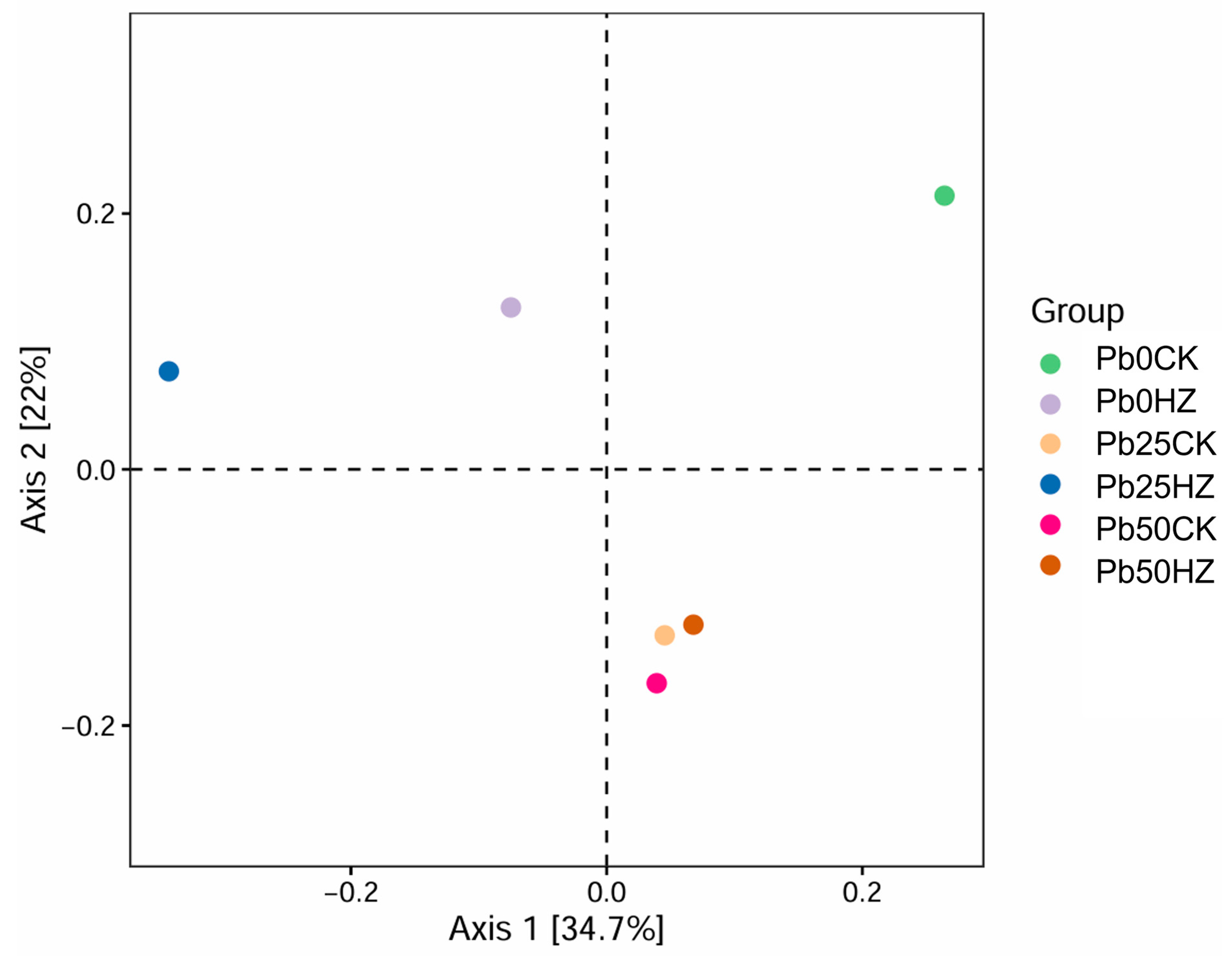
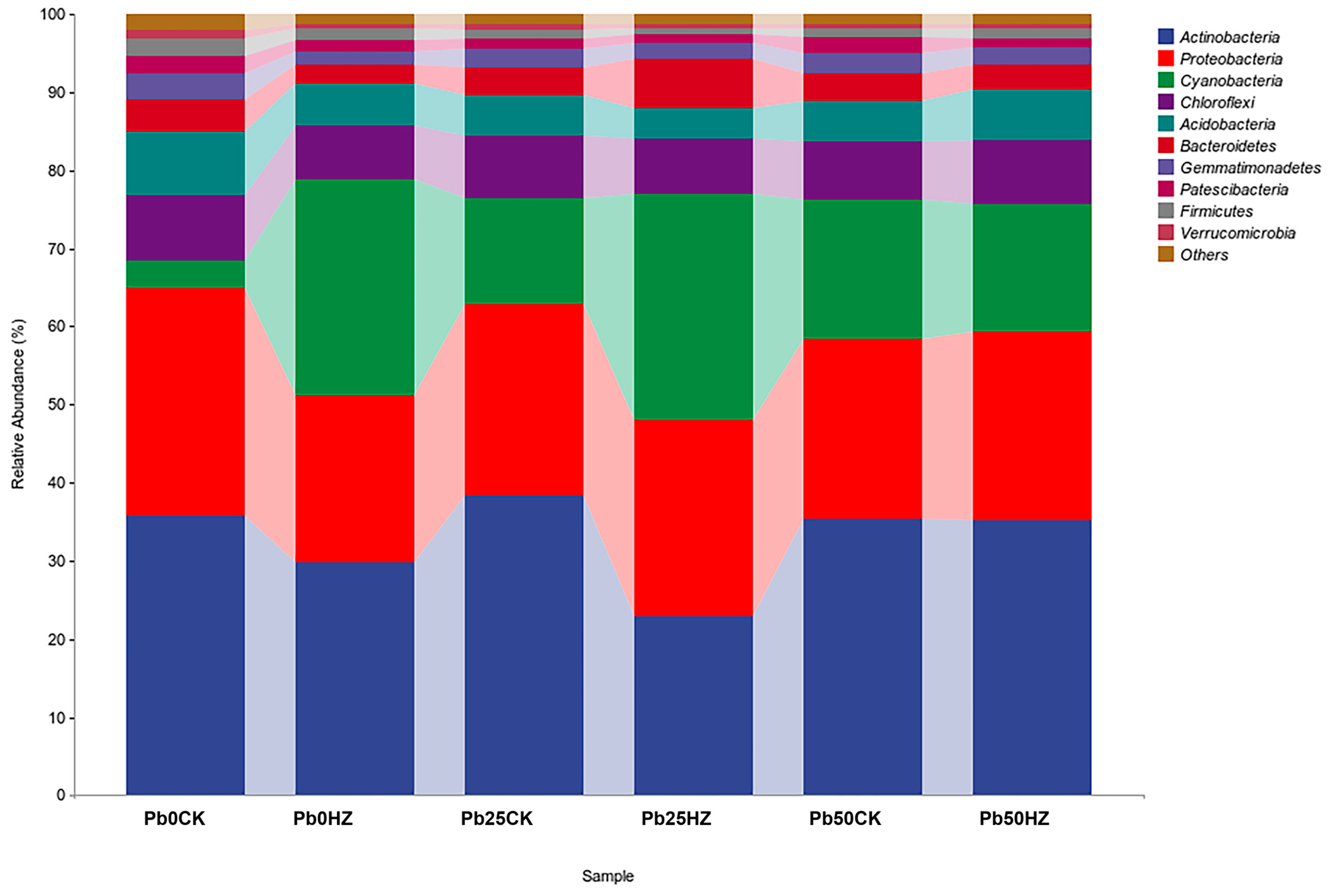
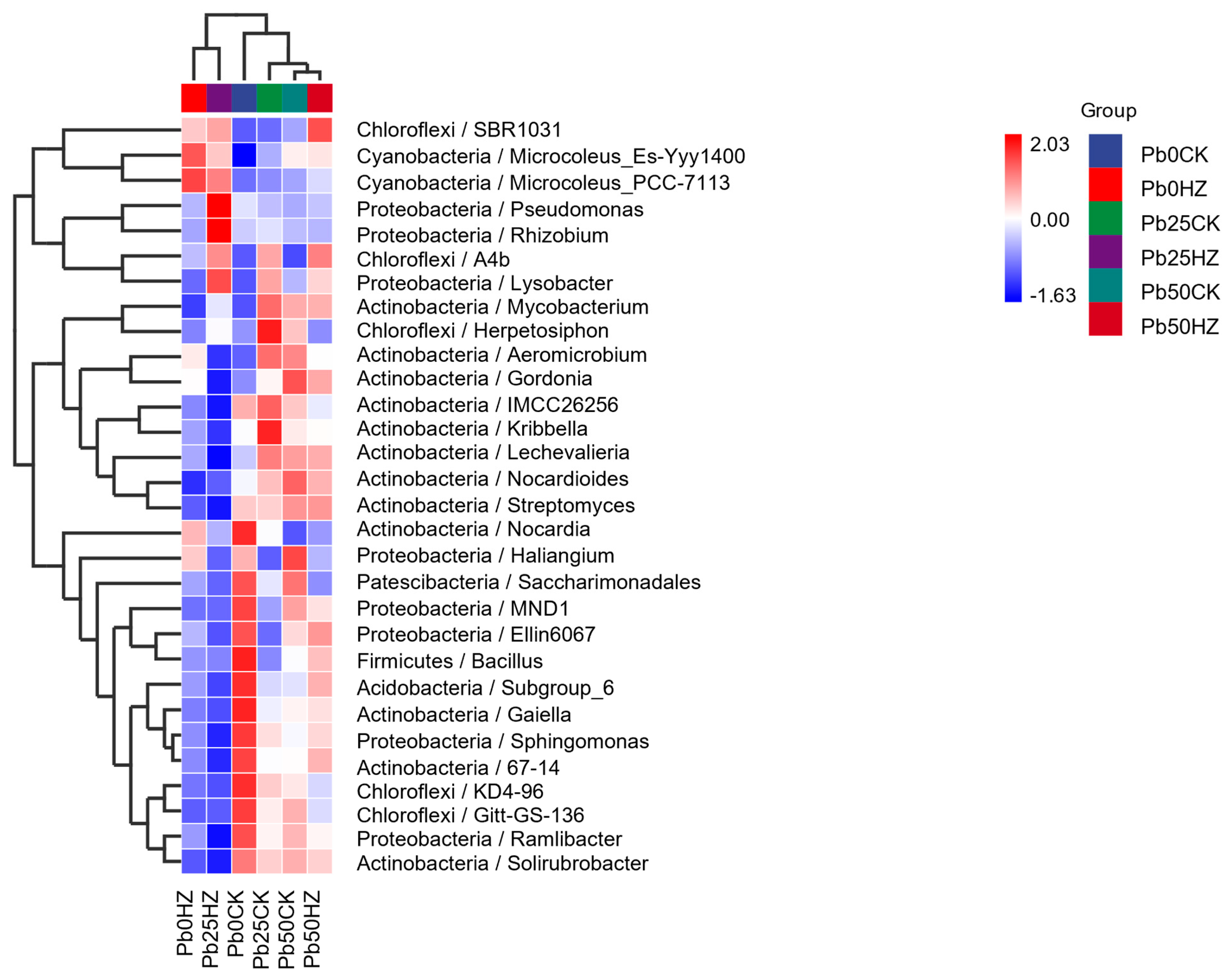

| Treatment | Raw Sequences | Qualified (No.) |
|---|---|---|
| Pb0CK_1 | 139073 | 82,793 |
| Pb0CK_2 | 151032 | 80,818 |
| Pb0CK_3 | 126493 | 69,484 |
| Pb0CK_4 | 129797 | 87,466 |
| Pb0HZ_1 | 131850 | 77,391 |
| Pb0HZ_2 | 130246 | 79,238 |
| Pb0HZ_3 | 128867 | 68,490 |
| Pb0HZ_4 | 148993 | 67,085 |
| Pb25CK_1 | 136723 | 87,648 |
| Pb25CK_2 | 129863 | 74,599 |
| Pb25CK_3 | 126683 | 75,559 |
| Pb25CK_4 | 146233 | 57,391 |
| Pb25HZ_1 | 123545 | 69,729 |
| Pb25HZ_2 | 117928 | 65,192 |
| Pb25HZ_3 | 119091 | 63,747 |
| Pb25HZ_4 | 153962 | 62,999 |
| Pb50CK_1 | 133478 | 72,788 |
| Pb50CK_2 | 126592 | 74,589 |
| Pb50CK_3 | 122184 | 58,901 |
| Pb50CK_4 | 148932 | 66,457 |
| Pb50HZ_1 | 122266 | 74,712 |
| Pb50HZ_2 | 136385 | 84,387 |
| Pb50HZ_3 | 130485 | 78,091 |
| Pb50HZ_4 | 140886 | 53,173 |
| Pb Added (mg kg−1) | Chao1 | Observed Species | Shannon | Simpson |
|---|---|---|---|---|
| 0 * | ||||
| CK | 7619 ± 724 a | 6456 ± 489 a | 11.1 ± 0.3 a | 0.998 ± 0.0009 a |
| HZ | 6399 ± 1088 a | 5383 ± 839 a | 10.1 ± 0.9 ab | 0.9928 ± 0.0051 ab |
| 25 * | ||||
| CK | 6979 ± 590 a | 5827 ± 638 a | 10.7 ± 0.3 ab | 0.9975 ± 0.0007 a |
| HZ | 5586 ± 1539 a | 4802 ± 1200 a | 9.4 ± 1.1 b | 0.9838 ± 0.0104 b |
| 50 * | ||||
| CK | 7088 ± 472 a | 5903 ± 225 a | 10.6 ± 0.2 ab | 0.9961 ± 0.002 ab |
| HZ | 7144 ± 289 a | 6078 ± 269 a | 10.8 ± 0.4 ab | 0.9965 ± 0.0018 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, N.; Zhang, G.; Fang, L.; Geng, R.; Shi, S.; Li, J.; Wang, W.; Lin, M.; Chen, J.; Si, Y.; et al. The Marine-Origin Exopolysaccharide-Producing Bacteria Micrococcus Antarcticus HZ Inhibits Pb Uptake in Pakchoi (Brassica chinensis L.) and Affects Rhizosphere Microbial Communities. Microorganisms 2024, 12, 2002. https://doi.org/10.3390/microorganisms12102002
Liu N, Zhang G, Fang L, Geng R, Shi S, Li J, Wang W, Lin M, Chen J, Si Y, et al. The Marine-Origin Exopolysaccharide-Producing Bacteria Micrococcus Antarcticus HZ Inhibits Pb Uptake in Pakchoi (Brassica chinensis L.) and Affects Rhizosphere Microbial Communities. Microorganisms. 2024; 12(10):2002. https://doi.org/10.3390/microorganisms12102002
Chicago/Turabian StyleLiu, Nan, Gangrui Zhang, Longyu Fang, Rui Geng, Shengbo Shi, Jinghua Li, Wei Wang, Mingchun Lin, Junfeng Chen, Yanru Si, and et al. 2024. "The Marine-Origin Exopolysaccharide-Producing Bacteria Micrococcus Antarcticus HZ Inhibits Pb Uptake in Pakchoi (Brassica chinensis L.) and Affects Rhizosphere Microbial Communities" Microorganisms 12, no. 10: 2002. https://doi.org/10.3390/microorganisms12102002






