Sex-Dependent Rhizosphere Microbial Dynamics and Function in Idesia polycarpa through Floral and Fruit Development
Abstract
:1. Introduction
2. Results
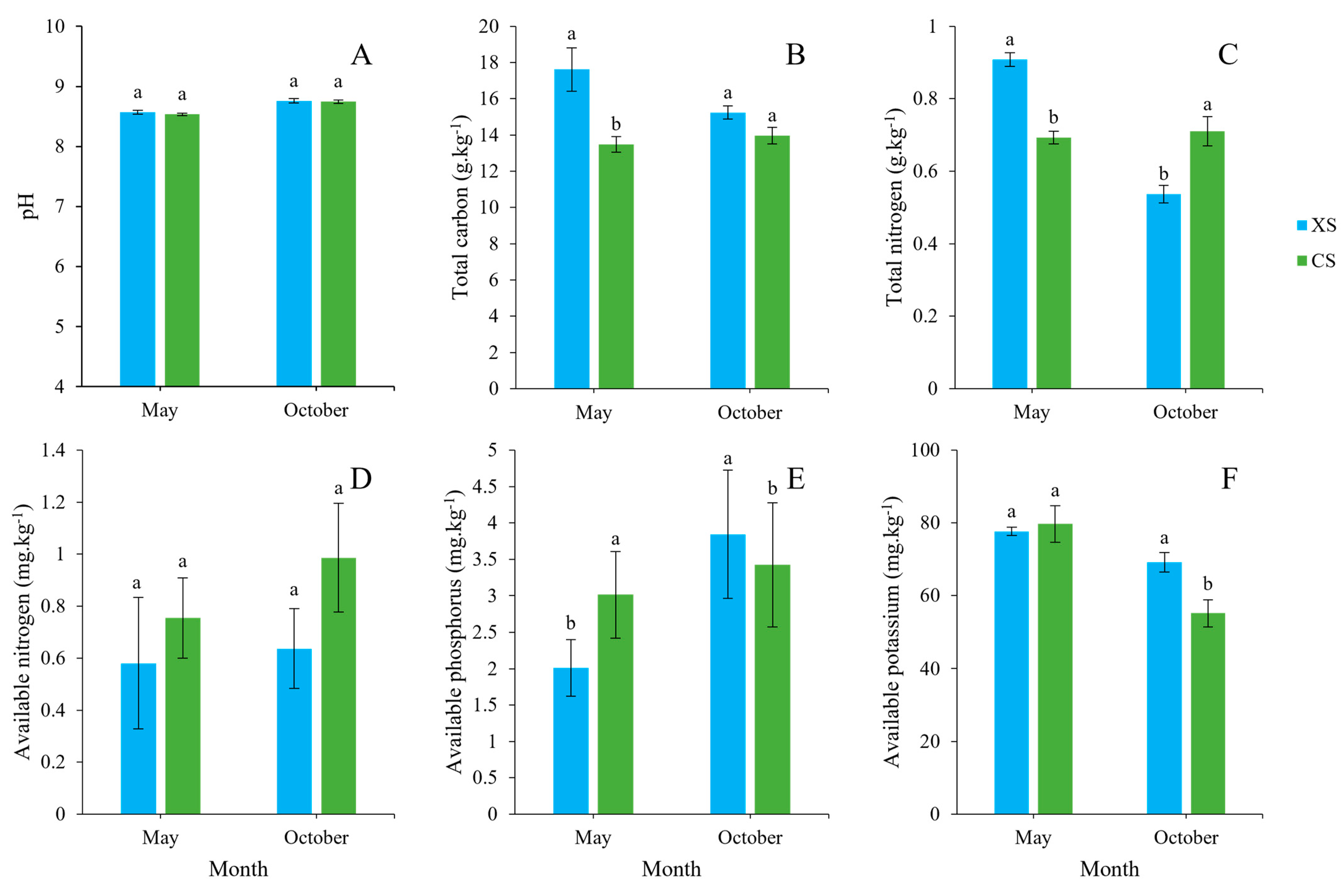
2.1. Variations in Soil Factors over Time
2.2. OTU Distribution of Rhizosphere Bacteria in Different Periods
2.3. Diversity of Bacterial Communities in Soil Environment at Different Stages
2.4. Comparison of Bacterial Community Structure in Soil Environment at Different Times
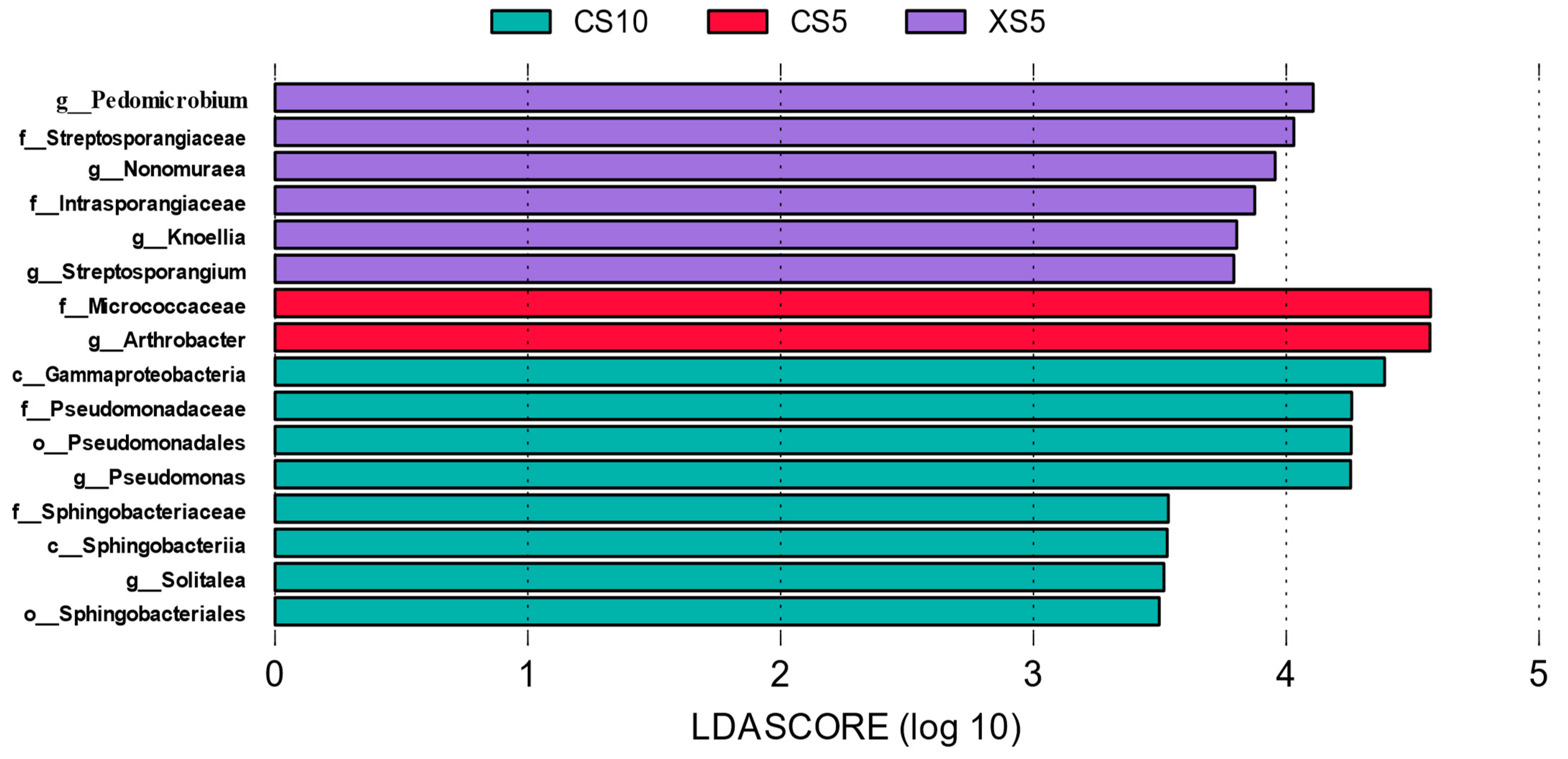
2.5. Difference Analysis of Rhizosphere Bacteria between Plants of Idesia Polycarpa at Different Periods
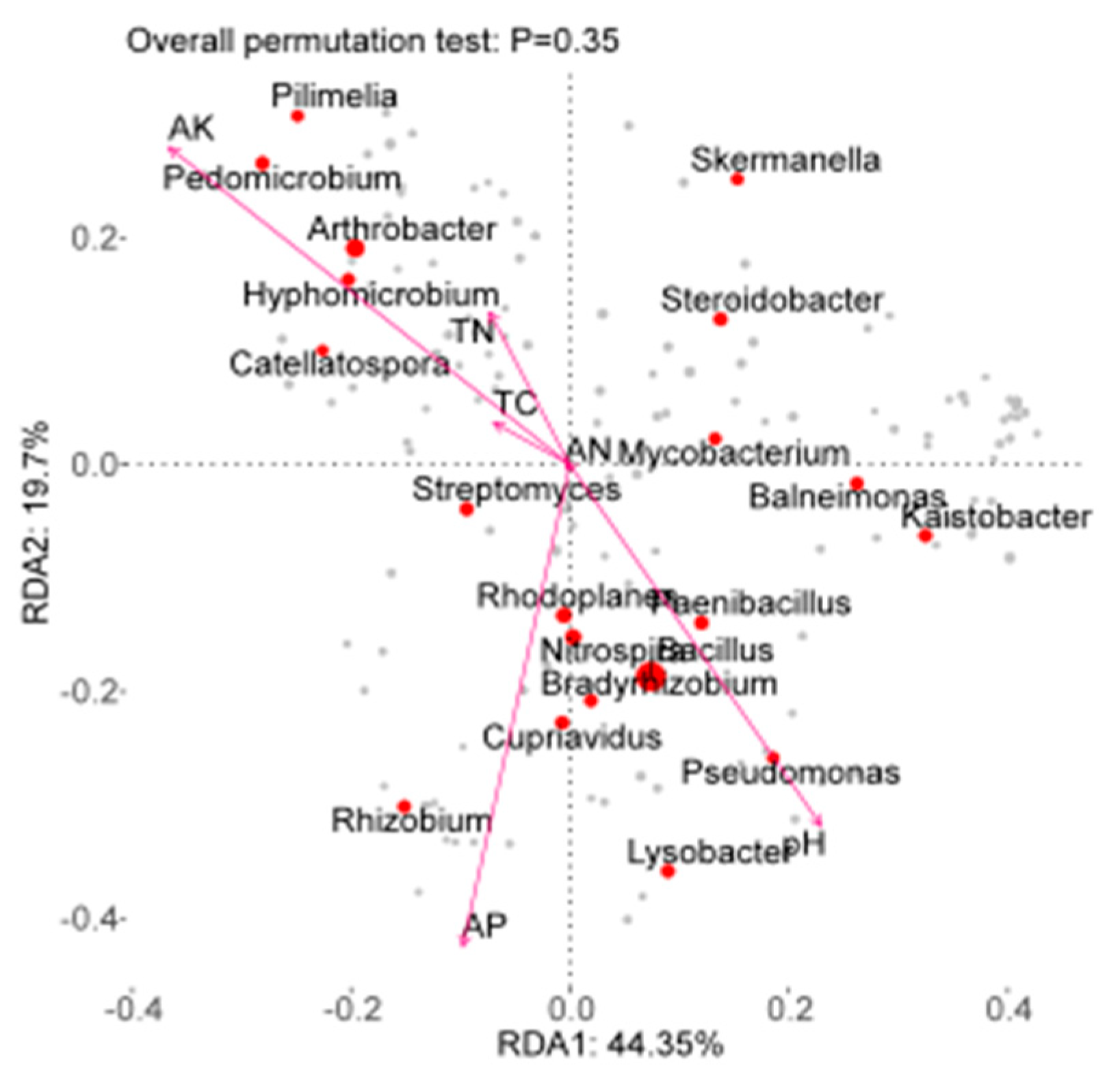
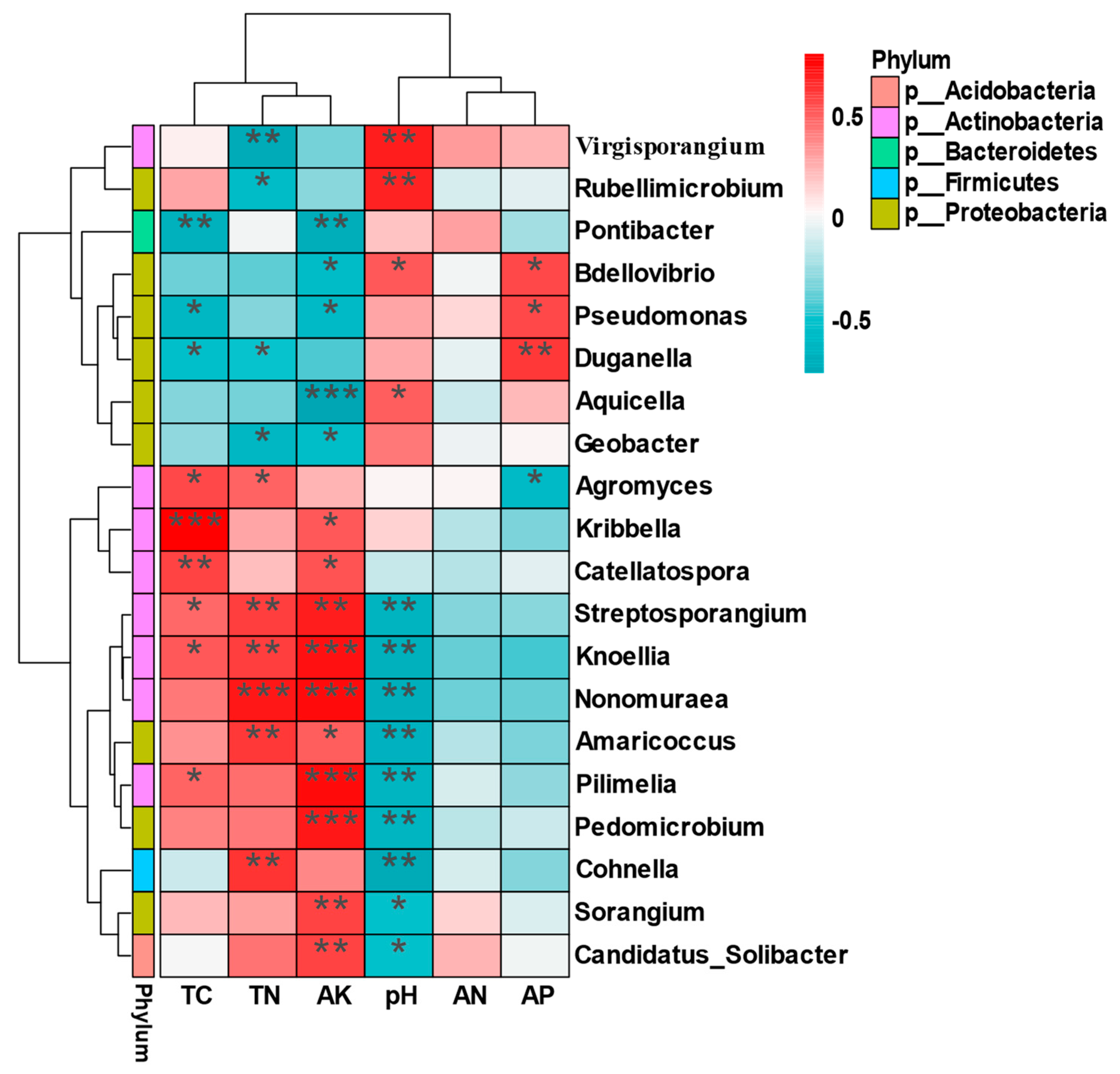
2.6. Correlation Analysis between Soil Factors and Bacterial Community Structure
2.7. Functional Differences in Rhizosphere Bacteria between Male and Female Plants of Idesia Polycarpa in Different Periods
3. Discussion
3.1. Structure and Diversity of the Bacterial Community in Idesia Polycarpa Soil in Different Periods
3.2. Effects of Soil Environmental Factors on the Composition and Structure of Soil Fungal Communities
3.3. Functional Differences in Rhizosphere Bacteria between Male and Female Idesia Polycarpa in Different Periods
4. Materials and Methods
4.1. Test Site
4.2. Experimental Materials and Design
4.3. DNA Extraction and PCR Amplification
4.4. Sequencing Information Statistics and Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Susanne, S.R.; Robert, E.R. Dioecy and its correlates in the flowering plant. Am. J. Bot. 1995, 82, 596–606. [Google Scholar] [CrossRef]
- Chen, X.M.; Wei, H.; Lin, M.Z. Responses of dioecious plants to climate change: A review on the potential mechanisms. Chin. J. Ecol. 2014, 33, 3144–3149. [Google Scholar]
- Xian, T.; Liu, J.Y.; Wen, X.M.; Xu, X.; Dong, Y.F. Effects of soil microbial legacy of female and male Populus cathayana on morphology, growth and photosynthesis of Salix rehderiana. Plant Physiol. J. 2021, 51, 129–138. [Google Scholar]
- Yan, K.; Dong, Y.F.; Zhu, Q.L. Rhizosphere bacterial communities and soil nutrient conditions reveal sexual dimorphism of Populus deltoides. J. For. Res. 2023, 34, 761–771. [Google Scholar]
- Zhang, Y.Z.; Xu, J.; Riera, N.; Jin, T.; Li, J.Y.; Wang, N. Huanglongbing impairs the rhizosphere-to-rhizoplane enrichment process of the citrus root-associated microbiome. Microbiome 2017, 5, 97. [Google Scholar] [CrossRef]
- Liu, L.L.; Li, H.L.; Meng, Z.S.; Peng, J.Y.; Li, X.L.; Peng, C.; Lu, L.; Xu, X. Differences in phyllosphere microbial community diversity and structure between male and female Populus plants. Acta Microbiol. Sin. 2022, 60, 556–569. [Google Scholar] [CrossRef]
- Gao, Y.H. Monoculture and Organic Amendment Change Soil Properties and Rhizosphere Microflora in Cucumber and Tomato Greenhouses. Ph.D. Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2019. [Google Scholar]
- Max, J.J.S.; Shu, H.H.; Corné, M.J.P.; Ioannis, A.S. Coumarin Communication Along the Microbiome-Root-Shoot Axis. Trends Plant Sci. 2021, 26, 169–183. [Google Scholar] [CrossRef]
- Pi, J.; Zhou, X.Y.; Teng, K.; Tian, M.H.; Li, S.L. Plant-microbial interaction mediated by root exudates. Plant Health Med. 2022, 1, 11–17. [Google Scholar] [CrossRef]
- Lugtenberg, B.J.; Dekkers, L.; Bloemberg, G.V. Molecular determinants of rhizosphere colonization by Pseudomonas. Annu. Rev. Phytopathol. 2001, 39, 461–490. [Google Scholar] [CrossRef]
- Farooq, T.H.; Chen, X.Y.; Shakoor, A.; Li, Y.; Wang, J.; Rashid, M.H.U.; Kumar, U.; Yan, W.D. Unraveling the Influence of Land-Use Change on δ13C, δ15N, and Soil Nutritional Status in Coniferous, Broadleaved, and Mixed Forests in Southern China: A Field Investigation. Plants 2021, 10, 1499. [Google Scholar] [CrossRef]
- Fan, Z.Y. The Response of Leaf Traits, Soil Nutrients, and Fruit Quality of Lingwu jujube to Nitrogen Application. Master’s Thesis, Ningxia University, Ningxia, China, 2021. [Google Scholar] [CrossRef]
- Rana, S.; Liu, Z. Study on the pattern of vegetative growth in young dioecious trees of Idesia polycarpa maxim. Trees 2021, 35, 69–80. [Google Scholar] [CrossRef]
- Wang, H.; Rana, S.; Li, Z.; Geng, X.; Wang, Y.; Cai, Q.; Li, S.; Sun, J.; Liu, Z. Morphological and anatomical changes during floral bud development of the trioecious Idesia polycarpa Maxim. Braz. J. Bot. 2022, 45, 679–688. [Google Scholar] [CrossRef]
- Rana, S.; Jemim, R.S.; Li, Z.; Geng, X.; Wang, Y.; Cai, Q.; Liu, Z. Study of the pattern of reproductive allocation and fruit development in young dioecious trees of Idesia polycarpa Maxim. South Afr. J. Bot. 2022, 146, 472–480. [Google Scholar] [CrossRef]
- Hong, Q.; Zhao, Y.; Chen, M.J.; Huang, J.C.; Feng, Z.Y.; Chen, H.; Zhang, J.J. Effents of in situ Stropharia rugosoannulata cultivation residue return on soil organic matter, enzyme activity and bacterial diversity. Acta Edulis Fungi 2022, 29, 27–35. [Google Scholar] [CrossRef]
- Liu, H.W.; Brettell, L.E.; Qiu, Z.G.; Singh, B.K. Microbiome-Mediated Stress Resistance in Plants. Trends Plant Sci. 2020, 25, 733–743. [Google Scholar] [CrossRef]
- Sun, Y.H.; Zhao, Y.H.; Dong, L.Z.L.; Wu, Q.Y.; Wei, Q.G.; Lin, Y.L.; Cui, G.W. Effects of Suaeda salsa on bacterial community structure in rhizosphere soil at different growth stages. Chin. J. Grassl. 2022, 44, 78–86. [Google Scholar] [CrossRef]
- Jia, S.H. Study on Diversity, Antibacterial Activity and Secondary Metabolites of Actinomycetes from Mangrove in Guangxi. Master’s Thesis, Guilin Medical College, Guilin, China, 2021. [Google Scholar] [CrossRef]
- Deng, J.J.; Zhou, Y.B.; Yin, Y.; Wei, Y.W.; Qin, S.J.; Zhu, W.X. Soil bacterial community structure characteristics in coniferous forests of Montane Regions of eastern Liaoning Province, China. Acta Ecol. Sin. 2019, 39, 997–1008. [Google Scholar] [CrossRef]
- Wang, W.X. Correlations between the composition and diversity of bacterial communities and ecological factors in the rhizosphere of Ammopiptanthus mongolicus. Li Xiaowei; HuangWenguang; Yang Junlong. Acta Ecol. Sin. 2020, 40, 8660–8671. [Google Scholar]
- Ren, M. Microbial Communities in the Tarim Basin Soil: Diversity and Their Roles in Carbon and Nitrogen Cycle. PhD Thesis, Huazhong Agricul tural University, Wuhan, China, 2018. [Google Scholar]
- Lauber, C.L.; Strickland, M.S.; Bradford, M.A.; Fierer, N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol. Biochem. 2008, 40, 2407–2415. [Google Scholar] [CrossRef]
- Li Destri Nicosia, M.G.; Mosca, S.; Mercurio, R.; Schena, L. Dieback of Pinus nigra seedlings caused by a strain of Trichoderma viride. Plant Dis. 2015, 99, 44–49. [Google Scholar] [CrossRef]
- Hou, J.W.; Xing, C.F.; Deng, X.M.; Chen, F. Effect of pH on soil bacterial commumity structure in root zone of prickiy ash. J. Northwest A F Univ. (Nat. Sci. Ed.) 2020, 48, 115–122. [Google Scholar] [CrossRef]
- Zhou, W.J.; Yao, P.; Huang, G.H.; Wang, D.L.; Ju, F.C. Effects of sulfur addition on bacterial community diversity in root zone and plant growth and development of blueberry. J. Jilin Agric. Univ. 2023, 46, 1–9. [Google Scholar]
- Wang, Y.; Xu, J.; Shen, J.; Luo, Y.; Scheu, S.; Ke, X. Tillage residue burning and crop rotation alter soil fungal community and water-stable aggregation in arable fields. Soil Tillage Res. 2010, 107, 71–79. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Ruan, Y.N.; Wang, W.; Chen, J.F.; Zhao, W.J.; Chen, H.; Fu, L.B. Effects of different gradient application amounts of nitrogen, phosphorus and potassium on bacterial community structure and diversity in tobacco-growing soil of Yunnan province. Southwest China J. Agric. Sci. 2022, 35, 764–771. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Jiang, A.X.; Guo, D.F. Response of soil fungal community structure to halophytic vegetation succession in the Yellow River Delta. Acta Sci. Circumstantiae 2016, 36, 4146–4152. [Google Scholar] [CrossRef]
- Wagg, C.; Schlaeppi, K.; Banerjee, S.; Kuramae Eiko, E.; van der Heijden Marcel, G.A. Fungal-bacterial diversity and microbiome complexity predict ecosystem functioning. Nat. Commun. 2019, 10, 4841–4850. [Google Scholar] [CrossRef]
- Xu, F.; Cai, T.J.; Yang, X.; Sui, W.Z. Soil fungal community variation by large-scale reclamation in Sanjiang Plain, China. Ann. Microbiol. 2017, 67, 679–689. [Google Scholar] [CrossRef]
- Zhang, X.X.; Liu, Z.; Wang, X.J.; Li, Z.; Cai, Q.F.; Geng, X.D.; Wang, Y.M.; Wu, H.Y. Comparison on the growth performance of 2-year-old Idesia polycarpa seedling from different provenances. J. Northwest For. Univ. 2021, 36, 118–123. [Google Scholar] [CrossRef]
- Kan, W.; Xu, S.S.; Yang, T.Y.; Liu, Y.; Wang, L.C.; Li, H.X.; Tan, Y.H.; Wu, D.; Kong, B.H.; Li, C.Y. Plant Phyllosphere Bacteria Community in Xishuangbanna Tropic Rainforest in China. Southwest China J. Agric. Sci. 2020, 33, 631–636. [Google Scholar] [CrossRef]
- Jiang, S.Y.; Wang, W.X.; Xue, X.X.; Yang, S.H. Analysis of bacterial diversity in oil shale by PCR-DGGE with different 16S rDNA target sequences. J. Northeast. Univ. (Nat. Sci.) 2014, 35, 1033–1038. [Google Scholar] [CrossRef]
- Fu, D.G.; Zhou, Y.; Wu, X.M.; Duan, C.Q.; Zhao, L.Q.; Huang, N. Responses of net photosynthetic rate of cyclobalanopsis glaucoides Seedlings to soil nutrient and water contents in evergreen broad-leaved forest of central Yunnan. J. West China For. Sci. 2019, 48, 75–81. [Google Scholar] [CrossRef]
- Chen, H.X.; Liu, X.R.; Sun, T.Y.; Wang, R.L.; Zhang, S.X. Variation in leaf C: N:P stoichiometry of quercus species along the altitudinal gradient in Taibao muntain, China. Acta Ecol. Sin. 2021, 41, 4503–4512. [Google Scholar]
- Li, Y.; Yang, X.D.; Qin, L.; Lv, G.H.; He, X.M.; Zhang, X.N. The bacterial diversity and community structures in rhizosphere soil of two halophytes, Lycium ruthenicum and Kalidium capsicum. Acta Ecol. Sin. 2018, 38, 3118–3131. [Google Scholar] [CrossRef]



| Period | Treatments | Chao1 Index | Shannon Index | Simpson Index |
|---|---|---|---|---|
| May bacteria | CS5 | 1129.49 ± 48.28 a | 9.57 ± 0.05 a | 0.998 ± 0.0001 a |
| XS5 | 1050.96 ± 16.78 ab | 9.46 ± 0.05 a | 0.9978 ± 0.0003 a | |
| October bacteria | CS10 | 1636.21 ± 29.22 b | 9.84 ± 0.12 b | 0.9975 ± 0.0006 a |
| XS10 | 1808.26 ± 7.9 a | 10.08 ± 0.07 a | 0.9982 ± 0.0002 a |
| Parameter | r2 | p-Value |
|---|---|---|
| TC | 0.131 | 0.535 |
| TN | 0.255 | 0.293 |
| AN | 0.008 | 0.951 |
| AP | 0.724 | 0.103 |
| AK | 0.766 | 0.001 |
| pH | 0.653 | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Yuan, Q.; Wang, S.; Zhang, T.; Wang, Y.; Cai, Q.; Geng, X.; Yang, Y.; Miao, C.; Dai, L.; et al. Sex-Dependent Rhizosphere Microbial Dynamics and Function in Idesia polycarpa through Floral and Fruit Development. Microorganisms 2024, 12, 2022. https://doi.org/10.3390/microorganisms12102022
Li Z, Yuan Q, Wang S, Zhang T, Wang Y, Cai Q, Geng X, Yang Y, Miao C, Dai L, et al. Sex-Dependent Rhizosphere Microbial Dynamics and Function in Idesia polycarpa through Floral and Fruit Development. Microorganisms. 2024; 12(10):2022. https://doi.org/10.3390/microorganisms12102022
Chicago/Turabian StyleLi, Zhi, Qiupeng Yuan, Shasha Wang, Tao Zhang, Yanmei Wang, Qifei Cai, Xiaodong Geng, Yi Yang, Chao Miao, Li Dai, and et al. 2024. "Sex-Dependent Rhizosphere Microbial Dynamics and Function in Idesia polycarpa through Floral and Fruit Development" Microorganisms 12, no. 10: 2022. https://doi.org/10.3390/microorganisms12102022







