The Insecticidal Activity of Secondary Metabolites Produced by Streptomyces sp. SA61 against Trialeurodes vaporariorum (Hemiptera: Aleyrodidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Actinomycete Material
2.3. Insect Cultures
2.4. Fermentation and Extraction
2.5. Isolation and Purification
2.6. Effect of Compounds on T. vaporariorum Mortality Rates
3. Results
3.1. Structure Elucidation of Compounds
3.2. Insecticidal Activity Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choi, W.I.; Lee, E.H.; Choi, B.R.; Park, H.M.; Ahn, Y.J. Toxicity of plant essential oils to Trialeurodes vaporariorum (Homoptera: Aleyrodidae). J. Econ. Entomol. 2003, 96, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-García, E.E.; Ortega-Arenas, L.D.; Pérez-Pacheco, R.; Rodríguez-Hernández, C. Repellency, toxicity, and oviposition inhibition of vegetable extracts against greenhouse whitefly trialeurodes vaporariorum (westwood) (hemiptera: Aleyrodidae). Chil. J. Agric. Res. 2014, 74, 41–48. [Google Scholar] [CrossRef]
- CABI. Invasive Species Compendium. Trialeurodes vaporariorum. (Whitefly, Greenhouse). 2021. Available online: http://www.cabi.org/isc/datasheet/54660 (accessed on 16 August 2024).
- Moodley, V.; Gubba, A.; Mafongoya, P.L. A survey of whitefly-transmitted viruses on tomato crops in South Africa. Crop Prot. 2019, 123, 21–29. [Google Scholar] [CrossRef]
- Jones, D.R. Plant viruses transmitted by whiteflies. Eur. J. Plant Pathol. 2003, 109, 195–219. [Google Scholar] [CrossRef]
- Hanssen, I.M.; Lapidot, M. Major tomato viruses in the Mediterranean basin. Adv. Virus Res. 2012, 84, 31–66. [Google Scholar] [PubMed]
- Perring, T.M.; Stansly, P.A.; Liu, T.X.; Smith, H.A.; Andreason, S.A. Whiteflies: Biology, Ecology, and Management. In Sustainable Management of Arthropod Pests of Tomato; Wakil, W., Brust, G.E., Perring, T.M., Eds.; Elsevier Academic Press: San Diego, CA, USA, 2018; pp. 73–110. [Google Scholar]
- Gorman, K.; Devine, G.; Bennison, J.; Coussons, P.; Punchard, N.; Denholm, I. Report of resistance to the neonicotinoid insecticide imidacloprid in Trialeurodes vaporariorum (Hemiptera: Aleyrodidae). Pest. Manag. Sci. 2007, 63, 555–558. [Google Scholar] [CrossRef]
- Millar, N.S.; Denholm, I. Nicotinic acetylcholine receptors: Targets for commercially important insecticides. Invert. Neurosci. 2007, 7, 53–66. [Google Scholar] [CrossRef]
- Soderlund, D.M.; Bloomquist, J.R. Neurotoxic actions of pyrethroid insecticides. Annu. Rev. Entomol. 1989, 34, 77–96. [Google Scholar] [CrossRef]
- Bi, J.L.; Toscano, N.C. Current status of the greenhouse whitefly, Trialeurodes vaporariorum, susceptibility to neonicotinoid and conventional insecticides on strawberries in southern California. Pest. Manag. Sci. 2007, 63, 747–752. [Google Scholar] [CrossRef]
- Kapantaidaki, D.E.; Sadikoglou, E.; Tsakireli, D.; Kampanis, V.; Stavrakaki, M.; Schorn, C.; Ilias, A.; Riga, M.; Tsiamis, G.; Nauen, R.; et al. Insecticide resistance in Trialeurodes vaporariorum populations and novel diagnostics for kdr mutations. Pest. Manag. Sci. 2018, 74, 59–69. [Google Scholar] [CrossRef]
- Gorman, K.; Hewitt, F.; Denholm, I.; Devine, G.J. New developments in insecticide resistance in the glasshouse whitefly (Trialeurodes vaporariorum) and the two-spotted spider mite (Tetranychus urticae) in the UK. Pest. Manag. Sci. 2002, 58, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Katz, L.; Baltz, R.H. Natural product discovery: Past, present, and future. J. Ind. Microbiol. Biotechnol. 2016, 43, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Lorsbach, B.A.; Sparks, T.C.; Cicchillo, R.M.; Garizi, N.V.; Hahn, D.R.; Meyer, K.G. Natural products: A strategic lead generation approach in crop protection discovery. Pest. Manag. Sci. 2019, 75, 2301–2309. [Google Scholar] [CrossRef] [PubMed]
- Sparks, T.C.; Sparks, J.M.; Duke, S.O. Natural Product-Based Crop Protection Compounds—Origins and Future Prospects. J. Agric. Food Chem. 2023, 71, 2259–2269. [Google Scholar] [CrossRef] [PubMed]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Klenk, H.P.; Clément, C.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, Physiology, and Natural Products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2015, 80, 1–43. [Google Scholar] [CrossRef]
- Wang, N.; Zhu, K.; Bi, Y.; Liu, F.; Yu, Z. Anti-Aphid Polyketides from Streptomyces sp. SA61. J. Nat. Prod. 2023, 86, 791–796. [Google Scholar] [CrossRef]
- Waksman, S.A. Classification, identification and descriptions of genera and species. In The Actinomycetes; The Williams & Wilkins Co.: Baltimore, MD, USA, 1961; Volume II, pp. 327–334. [Google Scholar]
- Shirling, E.T.; Gottlieb, D. Methods for characterization of Streptomyces specie. Int. J. Syst. Evol. Microbiol. 1966, 16, 313–340. [Google Scholar]
- Cao, C.W.; Zhang, J.; Gao, X.W.; Liang, P.; Guo, H.L. Overexpression of carboxylesterase gene associated with organophosphorous insecticide resistance in cotton aphids, Aphis gossypii (Glover). Pestic. Biochem. Physiol. 2008, 90, 175–180. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Am. Mosq. Control Assoc. 1987, 3, 302–303. [Google Scholar] [CrossRef]
- Dürr, C.; Schnell, H.J.; Luzhetskyy, A.; Murillo, R.; Weber, M.; Welzel, K.; Vente, A.; Bechthold, A. Biosynthesis of the terpene phenalinolactone in Streptomyces sp. Tü6071: Analysis of the gene cluster and generation of derivatives. Chem. Biol. 2006, 13, 365–377. [Google Scholar] [CrossRef]
- Shimizu, Y.; Ogata, H.; Goto, S. Type III Polyketide Synthases: Functional Classification and Phylogenomics. Chembiochem 2017, 18, 1048–1049. [Google Scholar] [CrossRef] [PubMed]
- Hertweck, C. The biosynthetic logic of polyketide diversity. Angew. Chem. Int. Ed. Engl. 2009, 48, 4688–4716. [Google Scholar] [CrossRef] [PubMed]
- Chooi, Y.H.; Tang, Y. Navigating the fungal polyketide chemical space: From genes to molecules. J. Org. Chem. 2012, 77, 9933–9953. [Google Scholar] [CrossRef] [PubMed]
- Toopaang, W.; Bunnak, W.; Srisuksam, C.; Wattananukit, W.; Tanticharoen, M.; Yang, Y.L.; Amnuaykanjanasin, A. Microbial polyketides and their roles in insect virulence: From genomics to biological functions. Nat. Prod. Rep. 2022, 39, 2008–2029. [Google Scholar] [CrossRef]
- Montesinos, E. Development, registration and commercialization of microbial pesticides for plant protection. Int. Microbiol. 2003, 6, 245–252. [Google Scholar] [CrossRef]
- Wang, X.J.; Zhang, J.; Wang, J.D.; Huang, S.X.; Chen, Y.H.; Liu, C.X.; Xiang, W.S. Four new doramectin congeners with acaricidal and insecticidal activity from Streptomyces avermitilis NEAU1069. Chem. Biodivers. 2011, 8, 2117–2125. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, A.; Zhang, N.; Li, S.; Yuan, T.; Ding, N.; Zhang, S.; Bao, S.; Wang, C.; Zhnag, Y.; et al. Insecticidal Endostemonines A-J Produced by Endophytic Streptomyces from Stemona sessilifolia. J. Agric. Food Chem. 2020, 68, 1588–1595. [Google Scholar] [CrossRef]
- Lewer, P.; Chapin, E.L.; Graupner, P.R.; Gilbert, J.R.; Peacock, C. Tartrolone C: A novel insecticidal macrodiolide produced by Streptomyces sp. CP1130. J. Nat. Prod. 2003, 66, 143–145. [Google Scholar] [CrossRef]
- Xiang, W.S.; Wang, J.D.; Wang, M.; Wang, X.J. New nemadectin congener from Streptomyces microflavus neau3: Fermentation, separation, structure elucidation and biological activities. J. Antibiot. 2010, 63, 171–175. [Google Scholar] [CrossRef]
- Sparks, T.C.; Nauen, R. IRAC: Mode of action classification and insecticide resistance management. Pestic. Biochem. Physiol. 2015, 121, 122–128. [Google Scholar] [CrossRef]
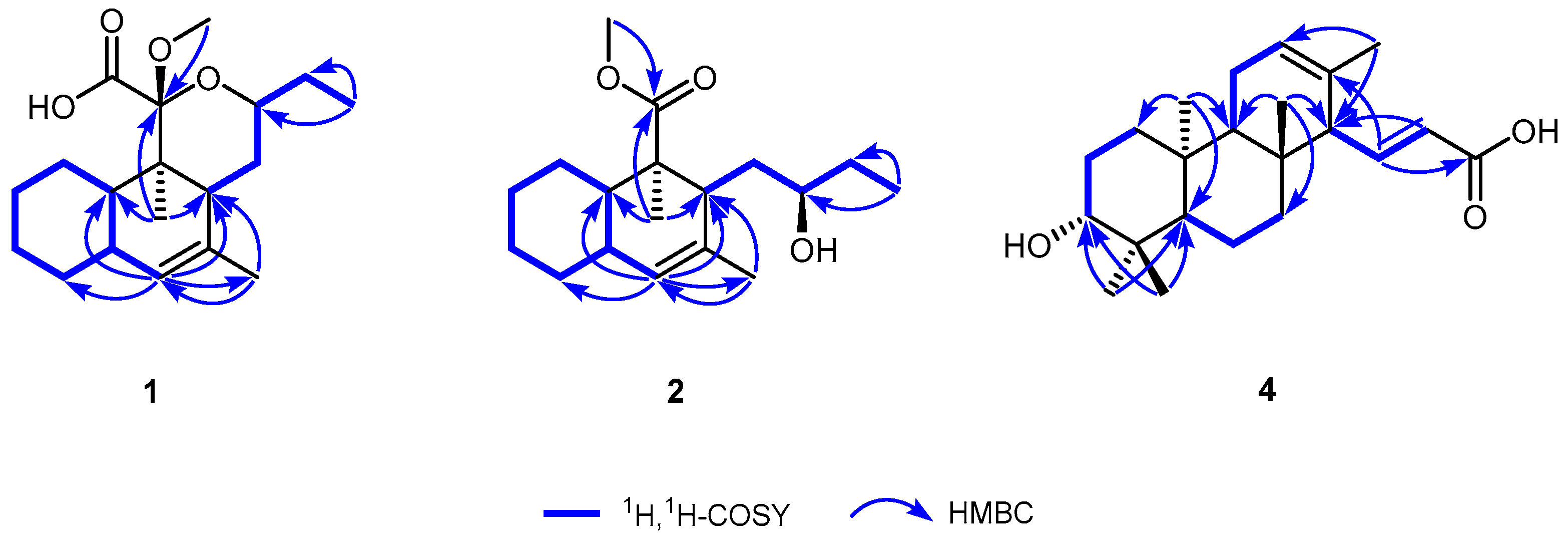

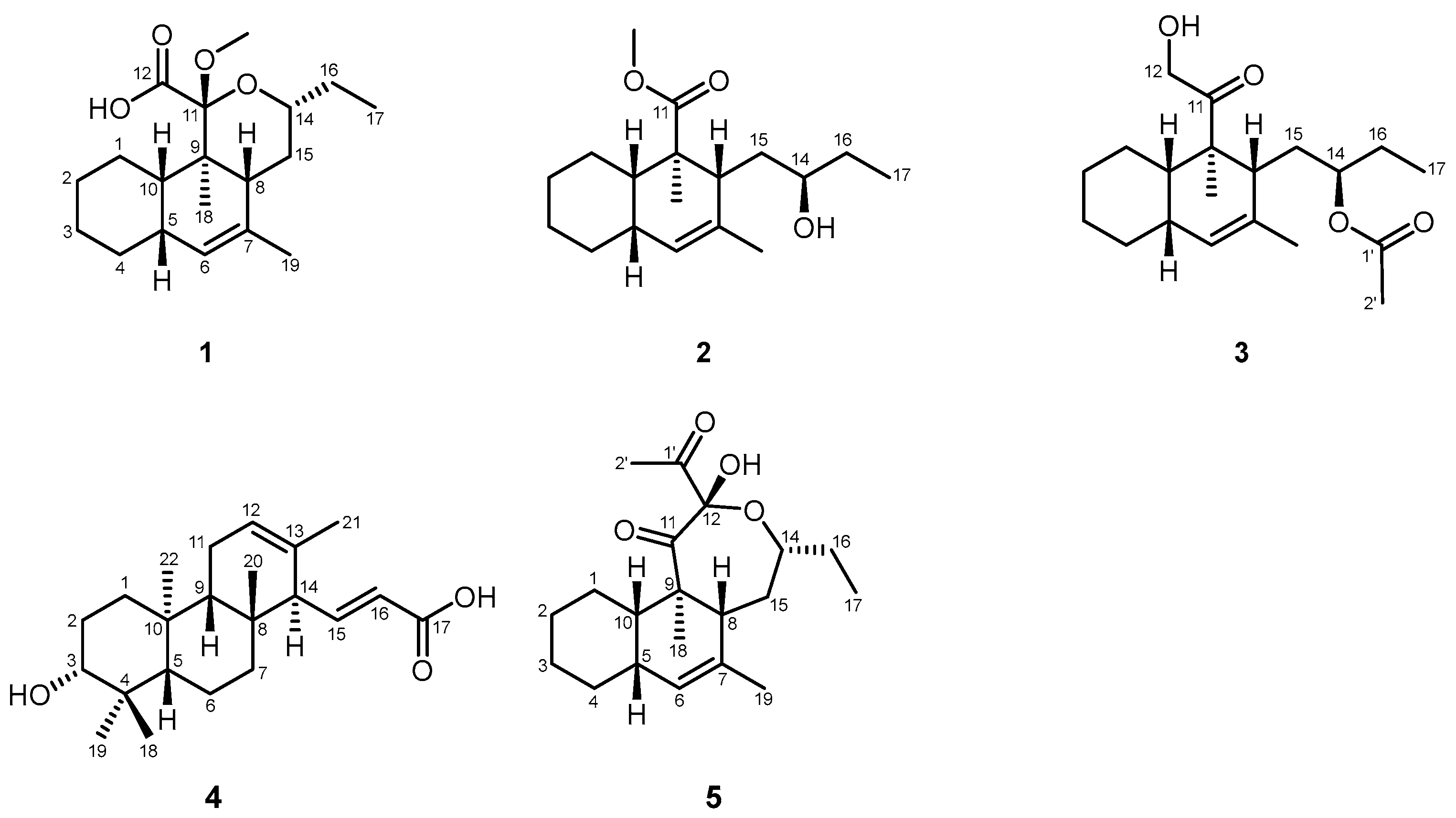
| No. | 1 | 2 | 3 |
|---|---|---|---|
| 1 | 0.76 (1Hα, qd-like, 12.6, 3.3) 1.38 (1Hβ, br d, 12.6) | 1.14 (1Hα, m) 1.55 (1Hβ, m) | 1.14 (1Hα, m) 1.58 (1Hβ, m) |
| 2 | 1.25 (1Hβ, m) 1.60 (1Hα, m) | 1.14 (1Hβ, m) 1.65 (1Hα, m) | 1.15 (1Hβ, m) 1.69 (1Hα, m) |
| 3 | 1.20 (1Hα, m) 1.49 (1Hβ, br d, 12.3) | 1.21 (1Hα, m) 1.39 (1Hβ, m) | 1.17 (1Hα, m) 1.43 (1Hβ, m) |
| 4 | 1.62 (1Hβ, m) 1.79 (1Hα, br d, 13.3) | 1.49 (1Hβ, m) 1.57 (1Hα, m) | 1.47 (1Hβ, m) 1.58 (1Hα, m) |
| 5 | 2.27 (1H, br s) | 2.18 (1H, br s) | 2.09 (1H, br s) |
| 6 | 5.55 (1H, br s) | 5.01 (1H, br s) | 5.02 (1H, br s) |
| 8 | 2.77 (1H, br d, 13.0) | 2.63 (1H, br s) | 2.29 (1H, br s) |
| 10 | 2.62 (1H, dt-like, 13.0, 5.1) | 1.99 (1H, m) | 1.95 (1H, m) |
| 12 | — | — | 4.28, 4.37 (each 1H, d, 18.3) |
| 14 | 3.70 (1H, m) | 3.52 (1H, m) | 4.85 (1H, m) |
| 15 | 1.63 (1Hβ, m) 1.73 (1Hα, q-like, 12.8) | 1.43, 1.61 (each 1H, m) | 1.48 (1H, ddd, 15.3, 5.3, 4.0) 1.86 (1H, ddd, 15.3, 9.5, 2.9) |
| 16 | 1.60–1.69 (2H, m) | 1.42, 1.56 (each 1H, m) | 1.53–1.64 (2H, m) |
| 17 | 1.02 (3H, t, 7.4) | 0.96 (3H, t, 7.4) | 0.91 (3H, t, 7.4) |
| 18 | 0.72 (3H, s) | 1.26 (3H, s) | 1.19 (3H, s) |
| 19 | 1.68 (3H, br s) | 1.74 (3H, br s) | 1.79 (3H, br s) |
| OCH3 | 3.27 (3H, s) | 3.65 (3H, s) | — |
| CH3CO | — | — | 2.04 (3H, s) |
| No. | 1 | 2 | 3 |
|---|---|---|---|
| 1 | 27.9 (t) | 23.7 (t) | 23.6 (t) |
| 2 | 26.7 (t) | 26.3 (t) | 26.4 (t) |
| 3 | 22.6 (t) | 22.7 (t) | 22.5 (t) |
| 4 | 30.3 (t) | 31.5 (t) | 31.4 (t) |
| 5 | 35.4 (d) | 35.0 (d) | 34.5 (d) |
| 6 | 128.3 (d) | 124.5 (d) | 125.2 (d) |
| 7 | 136.2 (s) | 137.5 (s) | 137.1 (s) |
| 8 | 38.3 (d) | 40.4 (d) | 38.5 (d) |
| 9 | 45.1 (s) | 50.6 (s) | 53.8 (s) |
| 10 | 38.5 (d) | 40.9 (d) | 41.1 (d) |
| 11 | 104.4 (s) | 179.1 (s) | 215.2 (s) |
| 12 | 169.1 (s) | — | 64.2 (t) |
| 14 | 74.0 (d) | 74.9 (d) | 77.0 (d) |
| 15 | 28.3 (t) | 38.1 (t) | 34.9 (t) |
| 16 | 28.6 (t) | 30.5 (t) | 27.5 (t) |
| 17 | 10.0 (q) | 10.2 (q) | 9.8 (q) |
| 18 | 14.0 (q) | 20.3 (q) | 20.4 (q) |
| 19 | 19.0 (q) | 22.7 (q) | 22.6 (q) |
| OCH3 | 51.3 (q) | 51.9 (q) | — |
| CH3CO | — | — | 171.0 (s) |
| CH3CO | — | — | 21.2 (q) |
| No. | 1H NMR | 13C NMR |
|---|---|---|
| 1 | 0.95 (1Hβ, m) 1.81 (1Hα, br d, 13.0) | 40.3 (t) |
| 2 | 1.57, 1.63 (each 1H, m) | 28.0 (t) |
| 3 | 3.12 (1H, dd, 11.6, 4.6) | 79.6 (d) |
| 4 | — | 39.9 (s) |
| 5 | 0.85 (1H, m) | 57.0 (d) |
| 6 | 1.46–1.54 (2H, m) | 19.0 (t) |
| 7 | 1.07 (1Hβ, m) 1.81 (1Hα, br d, 13.0) | 39.9 (t) |
| 8 | — | 37.4 (s) |
| 9 | 1.05 (1H, m) | 56.0 (d) |
| 10 | — | 39.3 (s) |
| 11 | 2.09 (1Hα, br d, 19.0) 2.22 (1Hβ, br d, 19.0) | 23.5 (t) |
| 12 | 5.49 (1H, br s) | 123.6 (d) |
| 13 | — | 133.3 (s) |
| 14 | 3.05 (1H, br d, 10.3) | 48.7 (d) |
| 15 | 6.68 (1H, dd, 15.6, 10.3) | 150.8 (d) |
| 16 | 5.89 (1H, d, 15.6) | 127.5 (d) |
| 17 | — | 171.0 (s) |
| 18 | 0.96 (3H, s) | 28.8 (q) |
| 19 | 0.78 (3H, s) | 16.3 (q) |
| 20 | 0.89 (3H, s) | 27.8 (q) |
| 21 | 1.53 (3H, br s) | 23.2 (q) |
| 22 | 0.93 (3H, s) | 15.1 (q) |
| Compound | Toxicity Regression Equation (y) | LC50 (95% CL) (mg/L) | χ2 | p Value |
|---|---|---|---|---|
| Strekingmycin G, 1 | Y = −1.915 + 1.410x | 22.817 (19.090–27.144) | 9.990 | 0.695 |
| Strekingmycin H, 2 | Y = −1.982 + 1.546x | 19.150 (16.132–22.459) | 17.927 | 0.160 |
| Strekingmycin F, 3 | Y = −2.248 + 1.827x | 16.981 (14.587–19.521) | 16.235 | 0.237 |
| Phenalinolactone CD8, 4 | Y = −2.089 + 1.291x | 41.501 (34.149–52.257) | 7.976 | 0.845 |
| Strekingmycin A, 5 | Y = −1.521 + 1.805x | 6.949 (5.415–8.456) | 10.116 | 0.684 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Wang, N.; Wang, Y.; Yu, Z. The Insecticidal Activity of Secondary Metabolites Produced by Streptomyces sp. SA61 against Trialeurodes vaporariorum (Hemiptera: Aleyrodidae). Microorganisms 2024, 12, 2031. https://doi.org/10.3390/microorganisms12102031
Liu F, Wang N, Wang Y, Yu Z. The Insecticidal Activity of Secondary Metabolites Produced by Streptomyces sp. SA61 against Trialeurodes vaporariorum (Hemiptera: Aleyrodidae). Microorganisms. 2024; 12(10):2031. https://doi.org/10.3390/microorganisms12102031
Chicago/Turabian StyleLiu, Fei, Ning Wang, Yinan Wang, and Zhiguo Yu. 2024. "The Insecticidal Activity of Secondary Metabolites Produced by Streptomyces sp. SA61 against Trialeurodes vaporariorum (Hemiptera: Aleyrodidae)" Microorganisms 12, no. 10: 2031. https://doi.org/10.3390/microorganisms12102031





