Carbohydrate Metabolism Differentiates Pectinatus and Megasphaera Species Growing in Beer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Cultivation
2.2. Beer Spoilage Assay
2.3. Ion Chromatography
2.3.1. Chemicals
2.3.2. Carbohydrate Analysis
2.4. Headspace SPME-GCMS Measurements
2.5. pH Measurements
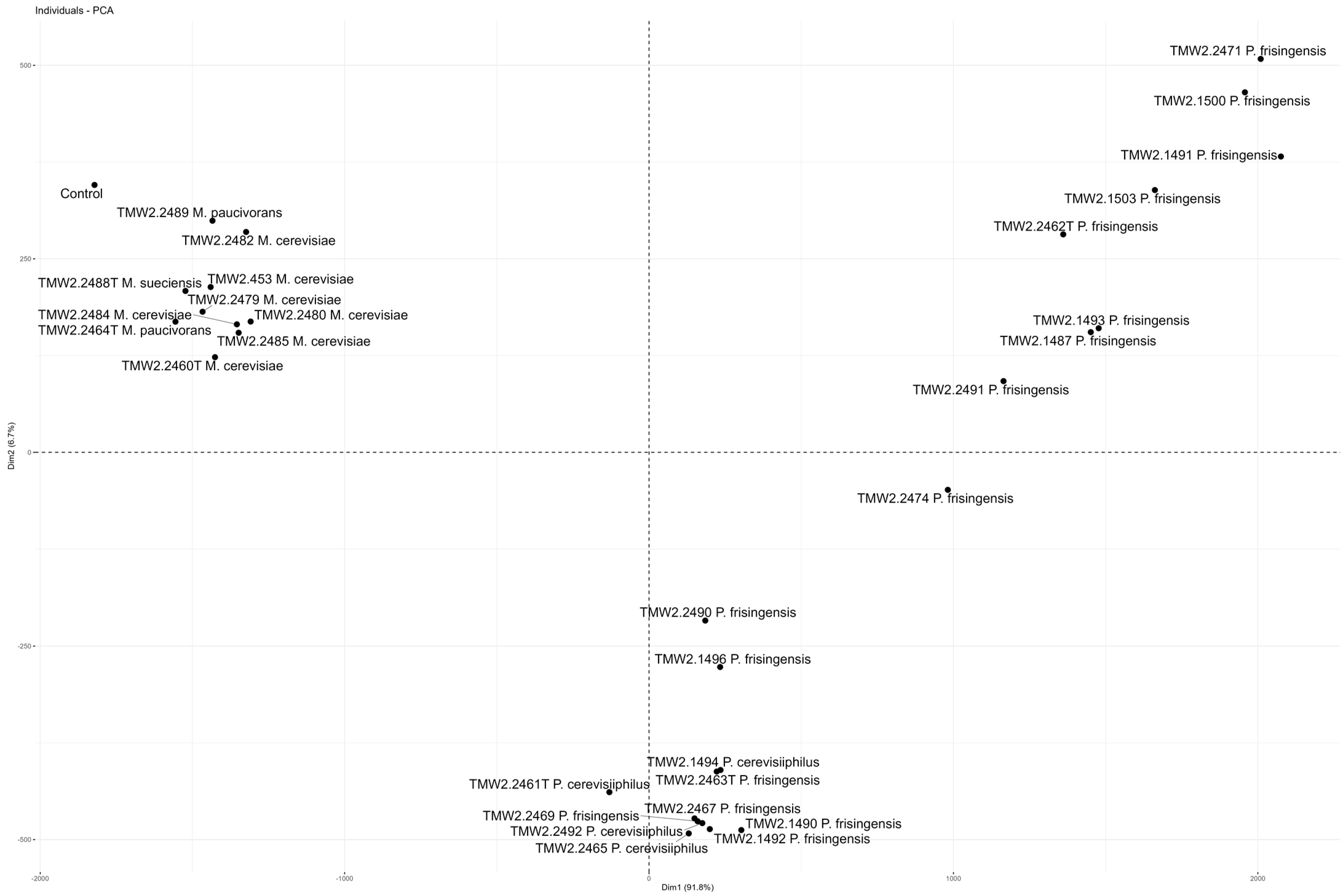
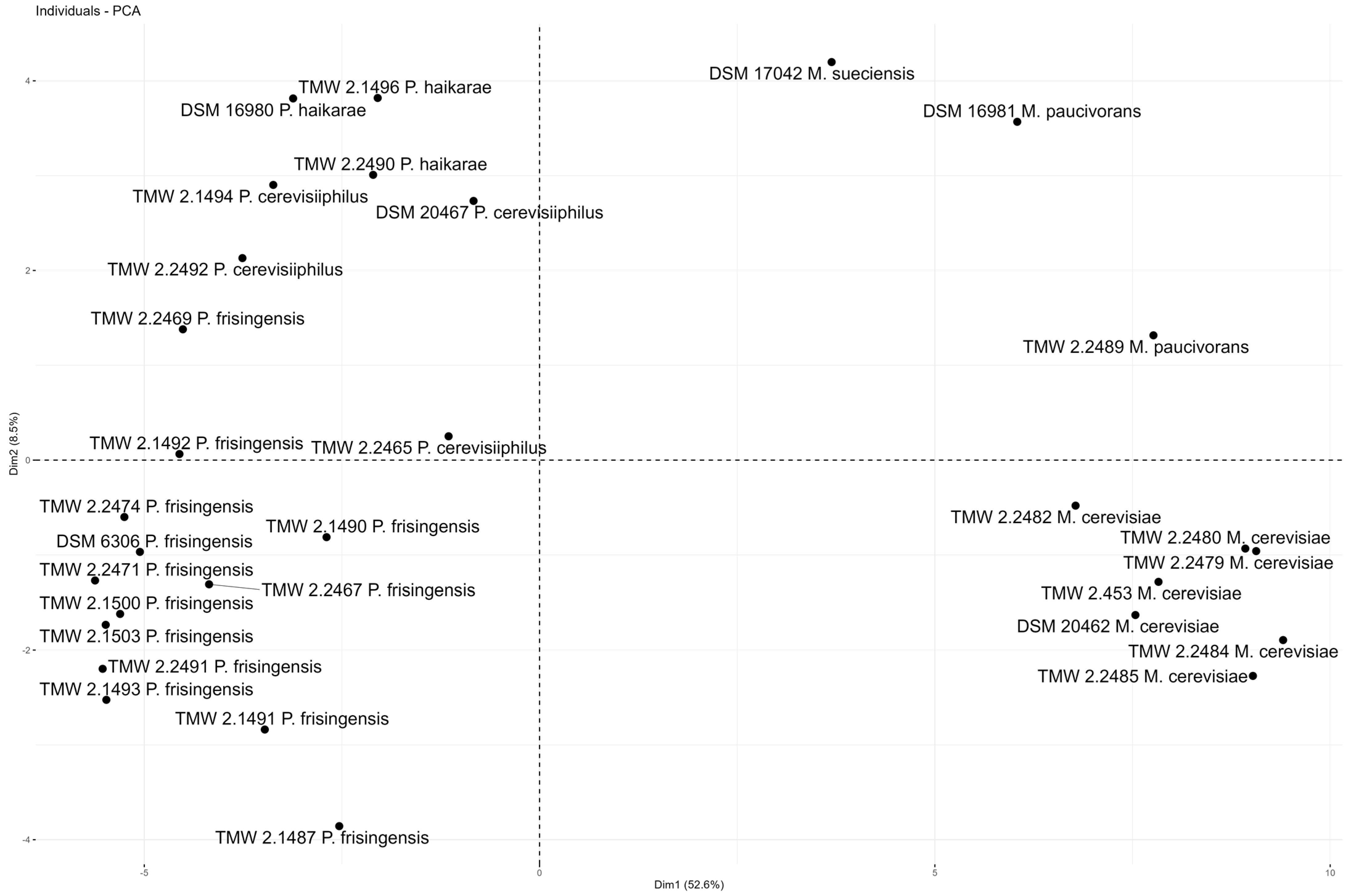
2.6. Statistics and Figures
2.7. Availability of Data and Materials
3. Results
3.1. Residual Carbohydrates in Beer
3.2. Carbohydrate and Glycerol Utilization and Odor Compound Production by Megasphaera spp. Strains during Lager Beer Spoilage
3.3. Carbohydrate and Glycerol Utilization and Odor Compound Production by Pectinatus spp. Strains during Lager Beer Spoilage
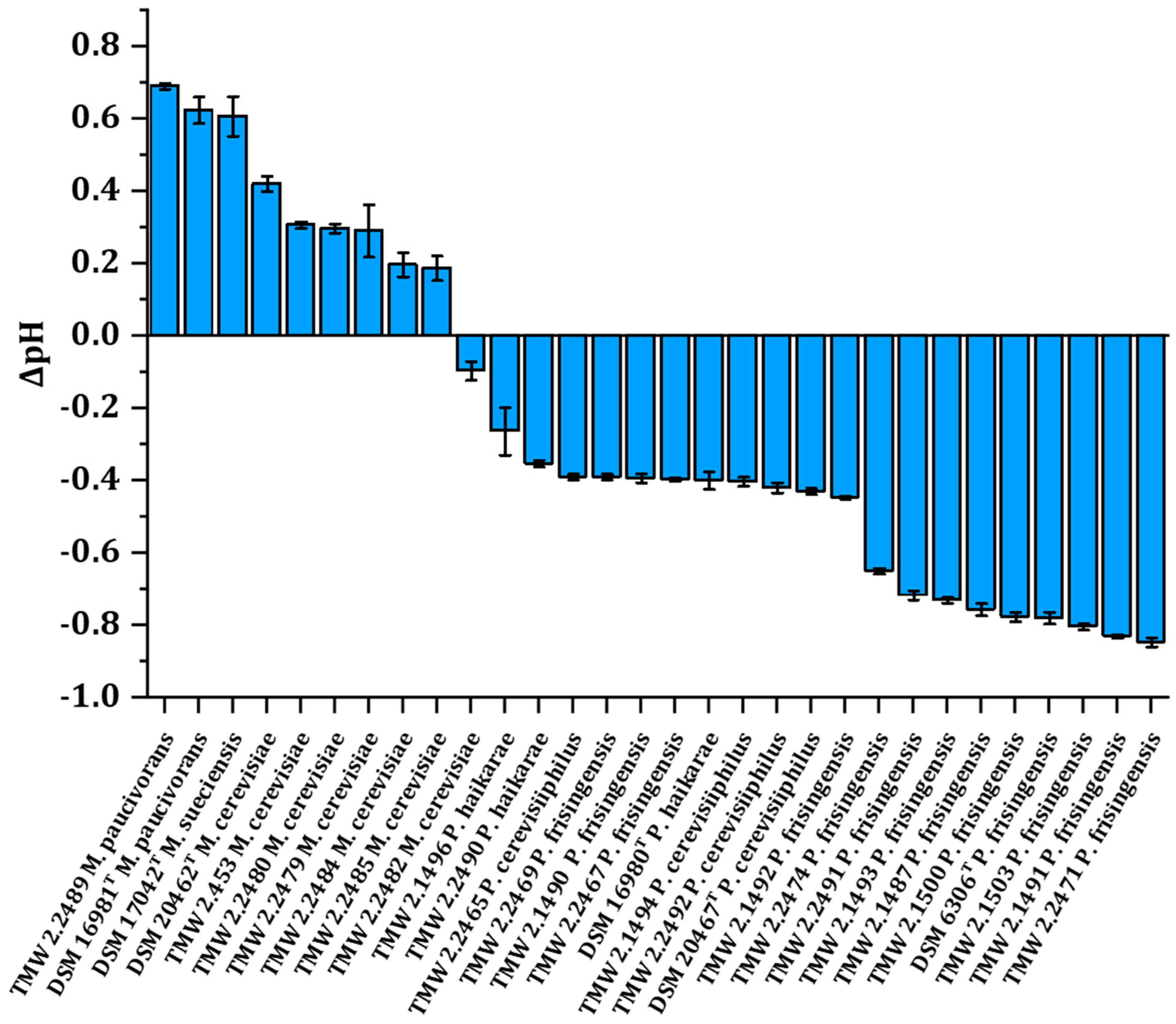
3.4. Change in pH after Spoilage
4. Discussion
4.1. Residual Carbohydrates in Beer
4.2. Carbohydrate and Glycerol Metabolism by Megasphaera spp.
4.3. Carbohydrate and Glycerol Metabolism by Pectinatus spp.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paradh, A.D.; Mitchell, W.J.; Hill, A.E. Occurrence of Pectinatus and Megasphaera in the major UK breweries. J. Inst. Brew. 2011, 117, 498–506. [Google Scholar] [CrossRef]
- Matoulkova, D.; Kosar, K.; Slaby, M.; Sigler, K. Occurrence and species distribution of strictly anaerobic bacterium Pectinatus in brewery bottling halls. Cerevisia 2013, 38, 59. [Google Scholar] [CrossRef]
- Schneiderbanger, J.; Grammer, M.; Jacob, F.; Hutzler, M. Statistical evaluation of beer spoilage bacteria by real-time PCR analyses from 2010 to 2016. J. Inst. Brew. 2018, 124, 173–181. [Google Scholar] [CrossRef]
- Goodman, M.; Neal, J.A.; Corsi, A.; Sirsat, S.A. Isolation of beer-spoiling bacteria from Texas craft breweries. J. Culinary Sci. Tech. 2020, 18, 116–123. [Google Scholar] [CrossRef]
- Schleifer, K.H.; Leuteritz, M.; Weiss, N.; Ludwig, W.; Kirchhof, G.; Seidel-Rüfer, H. Taxonomic study of anaerobic, Gram-negative, rod-shaped bacteria from breweries—Emended description of P. cerevisiiphilus and description of P. frisingensis sp. nov., Selenomonas lacticifex sp. nov., Zymophilus raffinosivorans gen. nov., sp. nov., and Zymophilus paucivorans sp. nov. Int. J. Syst. Bacteriol. 1990, 40, 19–27. [Google Scholar]
- Tholozan, J.L.; Membre, J.M.; Kubaczka, M. Effects of culture conditions on Pectinatus cerevisiiphilus and Pectinatus frisingensis metabolism: A physiological and statistical approach. J. Appl. Bacteriol. 1996, 80, 418–424. [Google Scholar] [CrossRef]
- Tholozan, J.-L.; Membré, J.-M.; Grivet, J.-P. Physiology and development of Pectinatus cerevisiiphilus and Pectinatus frisingensis, two strict anaerobic beer spoilage bacteria. Int. J. Food Microbiol. 1997, 35, 29–39. [Google Scholar] [CrossRef]
- Simpson, W.J.; Smith, A.R.W. Factors affecting antibacterial activity of hop compounds and their derivatives. J. Appl. Bacteriol. 1992, 72, 327–334. [Google Scholar] [CrossRef]
- Osman, Y.A.; Ingram, L.O. Mechanism of ethanol inhibition of fermentation in Zymomonas mobilis CP4. J. Bacteriol. 1985, 164, 173–180. [Google Scholar] [CrossRef]
- Ingram, L.O. Ethanol tolerance in bacteria. Crit. Rev. Biotechnol. 1990, 9, 305–319. [Google Scholar] [CrossRef]
- Hayashi, N.; Ito, M.; Horiike, S.; Taguchi, H. Molecular cloning of a putative divalent-cation transporter gene as a new genetic marker for the identification of Lactobacillus brevis strains capable of growing in beer. Appl. Microbiol. Biotechnol. 2001, 55, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Margolles, A.; van Veen, H.W.; Konings, W.N. Hop resistance in the beer spoilage bacterium Lactobacillus brevis is mediated by the ATP-binding cassette multidrug transporter HorA. J. Bacteriol. 2001, 183, 5371–5375. [Google Scholar] [CrossRef] [PubMed]
- Booth, I.R.; Cash, P.; O’Byrne, C. Sensing and adapting to acid stress. Antonie Van Leeuwenhoek 2002, 81, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Iijima, K.; Suzuki, K.; Ozaki, K.; Yamashita, H. horC confers beer-spoilage ability on hop-sensitive Lactobacillus brevis ABBC45cc. J. Appl. Microbiol. 2006, 100, 1282–1288. [Google Scholar] [CrossRef]
- Bergsveinson, J.; Goerzen, S.; Redekop, A.; Zoerb, S.; Ziola, B. Genetic variability in the hop-tolerance horC gene of beer-spoiling lactic acid bacteria. J. Am. Soc. Brew. Chem. 2016, 74, 173–182. [Google Scholar] [CrossRef]
- Narziß, L.; Back, W. Die Technologie der Würzebereitung, 8. überarb. und erg. Aufl.; WILEY-VCH: Weinheim, Germany, 2009. [Google Scholar]
- Chowdhury, I.; Watier, D.; Hornez, J.-P. Variability in survival of Pectinatus cerevisiiphilus, strictly anaerobic bacteria, under different oxygen conditions. Anaerobe 1995, 1, 151–156. [Google Scholar] [CrossRef]
- Watier, D.; Leguerinel, I.; Hornez, J.P.; Chowdhury, I.; Dubourguier, H.C. Heat resistance of Pectinatus sp., a beer spoilage anaerobic bacterium. J. Appl. Bacteriol. 1995, 78, 164–168. [Google Scholar] [CrossRef]
- Engelmann, U.; Weiss, N. Megasphaera cerevisiae sp. nov.: A new Gram-negative obligately anaerobic coccus isolated from spoiled beer. Syst. Appl. Microbiol. 1985, 6, 287–290. [Google Scholar] [CrossRef]
- Marounek, M.; Fliegrova, K.; Bartos, S. Metabolism and some characteristics of ruminal strains of Megasphaera elsdenii. Appl. Environ. Microb. 1989, 55, 1570–1573. [Google Scholar] [CrossRef]
- Juvonen, R.; Suihko, M.-L. Megasphaera paucivorans sp. nov., Megasphaera sueciensis sp. nov. and Pectinatus haikarae sp. nov., isolated from brewery samples, and emended description of the genus Pectinatus. Int. J. Syst. Evol. Microbiol. 2006, 56, 695–702. [Google Scholar] [CrossRef]
- Marchandin, H.; Juvonen, R.; Haikara, A. Megasphaera; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 1–16. [Google Scholar] [CrossRef]
- Lee, S.Y.; Mabee, M.S.; Jangaard, N.O. Pectinatus, a New Genus of the Family Bacteroidaceae. Int. J. Syst. Bacteriol. 1978, 28, 582–594. [Google Scholar] [CrossRef]
- Zhang, W.-W.; Fang, M.-X.; Tan, H.-Q.; Zhang, X.-Q.; Wu, M.; Zhu, X.-F. Pectinatus brassicae sp. nov., a Gram-negative, anaerobic bacterium isolated from salty wastewater. Int. J. Syst. Evol. Microbiol. 2012, 62, 2145–2149. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, J.M.; Juvonen, R.; Brown, J.; Breidt, F. Pectinatus sottacetonis sp. nov., isolated from a commercial pickle spoilage tank. Int. J. Syst. Evol. Microbiol. 2013, 63, 3609–3616. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K. Emergence of new spoilage microorganisms in the brewing industry and development of microbiological quality control methods to cope with this phenomenon: A review. J. Am. Soc. Brew. Chem. 2020, 78, 245–259. [Google Scholar] [CrossRef]
- Ritter, S.; Nobis, A.; Gastl, M.; Becker, T. Evaluating raffinose family oligosaccharides and their decomposition products by ion chromatography—A method development and advanced repeatability study. Talanta Open 2022, 5, 100086. [Google Scholar] [CrossRef]
- Schnaitter, M.; Wimmer, A.; Kollmannsberger, H.; Gastl, M.; Becker, T. Influence of hop harvest date of the ‘Mandarina Bavaria’ hop variety on the sensory evaluation of dry-hopped top-fermented beer. J. Inst. Brew. 2016, 122, 661–669. [Google Scholar] [CrossRef]
- Signorell, A. DescTools: Tools for Descriptive Statistics 2023. Available online: https://cran.r-project.org/package=DescTools (accessed on 10 August 2024).
- Makowski, D.; Lüdecke, D.; Patil, I.; Thériault, R.; Ben-Shachar, M.S.; Wiernik, B.M. Automated Results Reporting as a Practical Tool to Improve Reproducibility and Methodological Best Practices Adoption. CRAN. 2023. Available online: https://easystats.github.io/report/ (accessed on 10 August 2024).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef]
- Galili, T.; O’Callaghan, A.; Sidi, J.; Sievert, C. heatmaply: An R package for creating interactive cluster heatmaps for online publishing. Bioinformatics 2018, 34, 1600–1602. [Google Scholar] [CrossRef]
- Lemenkova, P. K-means clustering in R libraries cluster and factoextra for grouping oceanographic data. Int. J. Info. Appl. Math. 2019, 2, 1–26. [Google Scholar]
- Otter, G.E.; Popplewell, J.A.; Taylor, L. Quantitative chromatography of the oligosaccharides in wort, beer and brewing syrups. J. Chromatogr. A 1970, 49, 462–468. [Google Scholar] [CrossRef]
- Preedy, V.R. (Ed.) Beer in Health and Disease Prevention; 1. Aufl.; Elsevier: Amsterdam, The Netherlands, 2008; ISBN 0123738911. [Google Scholar]
- Li, M.; Du, J.; Zhang, K. Profiling of carbohydrates in commercial beers and their influence on beer quality. J. Sci. Food Agric. 2020, 100, 3062–3070. [Google Scholar] [CrossRef]
- Powell, C.D.; Quain, D.E.; Smart, K.A. The impact of brewing yeast cell age on fermentation performance, attenuation and flocculation. FEMS Yeast Res. 2003, 3, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.D.; Diacetis, A.N. Long term serial repitching and the genetic and phenotypic stability of brewer’s yeast. J. Inst. Brew. 2007, 113, 67–74. [Google Scholar] [CrossRef]
- Gabriel, P.; Dienstbier, M.; Matoulková, D.; Kosař, K.; Sigler, K. Optimised acidification power test of yeast vitality and its use in brewing practice. J. Inst. Brew. 2008, 114, 270–276. [Google Scholar] [CrossRef]
- Sigler, K.; Matoulková, D.; Dienstbier, M.; Gabriel, P. Net effect of wort osmotic pressure on fermentation course, yeast vitality, beer flavor, and haze. Appl. Microbiol. Biotechnol. 2009, 82, 1027–1035. [Google Scholar] [CrossRef]
- Parker, W.E.; Richardson, P.J. The quantitative determination of glycerol in beer by gas-liquid chromatography. J. Inst. Brew. 1970, 76, 191–198. [Google Scholar] [CrossRef]
- Briggs, D.E. (Ed.) Brewing: Science and Practice; CRC Press: Boca Raton, FL, USA; Woodhead Pub. Ltd.: Cambridge, UK, 2004. [Google Scholar]
- Langstaff, S.A.; Guinard, J.-X.; Lewis, M.J. Instrumental evaluation of the mouthfeel of beer and correlation with sensory evaluation. J. Inst. Brew. 1991, 97, 427–433. [Google Scholar] [CrossRef]
- Sohrabvandi, S.; Mousavi, S.M.; Razavi, S.H.; Mortazavian, A.M.; Rezaei, K. Alcohol-free beer: Methods of production, sensorial defects, and healthful effects. Food Rev. Int. 2010, 26, 335–352. [Google Scholar] [CrossRef]
- Albertyn, J.; Hohmann, S.; Thevelein, J.M.; Prior, B.A. GPD1, which encodes glycerol-3-phosphate dehydrogenase, is essential for growth under osmotic stress in Saccharomyces cerevisiae, and its expression is regulated by the high-osmolarity glycerol response pathway. Mol. Cell. Biol. 1994, 14, 4135–4144. [Google Scholar] [CrossRef]
- Ansell, R.; Granath, K.; Hohmann, S.; Thevelein, J.M.; Adler, L. The two isoenzymes for yeast NAD+-dependent glycerol 3-phosphate dehydrogenase encoded by GDP1 and GDP2 have distinct roles in osmoadaption and redox regulation. EMBO J. 1997, 16, 2179–2187. [Google Scholar] [CrossRef]
- Albers, E.; Larsson, C.; Lidén, G.; Niklasson, C.; Gustafsson, L. Influence of the nitrogen source on Saccharomyces cerevisiae anaerobic growth and product formation. Appl. Environ. Microb. 1996, 62, 3187–3195. [Google Scholar] [CrossRef]
- Peat, S.; Roberts, P.J.P.; Whelan, W.J. The occurence of fructose in rabbit-liver glycogen. Biochem. J. 1952, 51, xvii–xviii. [Google Scholar]
- Radomski, M.W.; Smith, M.D. Isolation of maltulose from alpha-amylase hydrolysates of waxy corn starch. Cereal Chem. 1962, 39, 30–34. [Google Scholar]
- Dias, F.F.; Panchal, D.C. Maltulose formation during saccharification of starch. Starch/Stärke 1987, 39, 64–66. [Google Scholar] [CrossRef]
- Adachi, S.; Hkuwijitjaru, P.; Kobayashi, T. Continuous production of maltulose from maltose in a pressurized hot phosphate buffer. Jpn. J. Food Eng. 2022, 23, 63–69. [Google Scholar] [CrossRef]
- García-Baños, J.L.; Villamiel, M.; Olano, A.; Rada-Mendoza, M. Study on nonenzymatic browning in cookies, crackers and breakfast cereals by maltulose and furosine determination. J. Cereal Sci. 2004, 39, 167–173. [Google Scholar] [CrossRef]
- García-Baños, J.L.; Corzo, N.; Sanz, M.L.; Olano, A. Maltulose and furosine as indicators of quality of pasta products. Food Chem. 2004, 88, 35–38. [Google Scholar] [CrossRef]
- Morales, V.; Olano, A.; Corzo, N. Ratio of maltose to maltulose and furosine as quality parameters for infant formula. J. Agric. Food Chem. 2004, 52, 6732–6736. [Google Scholar] [CrossRef]
- Clapperton, J.F.; MacWilliam, I.C. Fermentation of minor wort carbohydrates by brewing yeasts. J. Inst. Brew. 1971, 77, 519–522. [Google Scholar] [CrossRef]
- Sugawara, S.; Nakamura, Y.; Shimomura, T. Substrate specificity and some properties of crystalline mold maltase. Agric. Biol. Chem. 1961, 25, 358–361. [Google Scholar] [CrossRef]
- Pokusaeva, K.; O’Connell-Motherway, M.; Zomer, A.; Fitzgerald, G.F.; van Sinderen, D. Characterization of two novel alpha-glucosidases from Bifidobacterium breve UCC2003. Appl. Environ. Microb. 2009, 75, 1135–1143. [Google Scholar] [CrossRef]
- Zastrow, C.R.; Hollatz, C.; de Araujo, P.S.; Stambuk, B.U. Maltotriose fermentation by Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 2001, 27, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Dietvorst, J.; Londesborough, J.; Steensma, H.Y. Maltotriose utilization in lager yeast strains: MTT1 encodes a maltotriose transporter. Yeast 2005, 22, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Alves-Jr, S.L.; Herberts, R.A.; Hollatz, C.; Miletti, L.C.; Stambuk, B.U. Maltose and maltotriose active transport and fermentation by Saccharomyces cerevisiae. J. Am. Soc. Brew. Chem. 2007, 65, 99–104. [Google Scholar] [CrossRef]
- Bearson, S.; Bearson, B.; Foster, J.W. Acid stress responses in enterobacteria. FEMS Microbiol. Lett. 1997, 147, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Lund, P.A.; Tramonti, A.; Biase, D. de. Coping with low pH: Molecular strategies in neutralophilic bacteria. FEMS Microbiol. Rev. 2014, 38, 1091–1125. [Google Scholar] [CrossRef]
- Guan, N.; Liu, L. Microbial response to acid stress: Mechanisms and applications. Appl. Microbiol. Biotechnol. 2020, 104, 51–65. [Google Scholar] [CrossRef]
- Sandegren, E.; Enebo, L.; Guthenberg, H.; Ljungdahl, L. Studies on amino acids in brewing. ASBCJ 1954, 12, 63–74. [Google Scholar] [CrossRef]
- Gómez-Alonso, S.; Hermosín-Gutiérrez, I.; García-Romero, E. Simultaneous HPLC analysis of biogenic amines, amino acids, and ammonium ion as aminoenone derivatives in wine and beer samples. J. Agric. Food Chem. 2007, 55, 608–613. [Google Scholar] [CrossRef]
- Kramer, T.; Kelleher, P.; van der Meer, J.; O’Sullivan, T.; Geertman, J.-M.A.; Duncan, S.H.; Flint, H.J.; Louis, P. Comparative genetic and physiological characterisation of Pectinatus species reveals shared tolerance to beer-associated stressors but halotolerance specific to pickle-associated strains. Food Microbiol. 2020, 90, 103462. [Google Scholar] [CrossRef]
- Schwarz, P.B.; Han, J.-Y. Arabinoxylan content of commercial beers. ASBCJ 1995, 53, 157–159. [Google Scholar] [CrossRef]
- Steiner, J.; Kupetz, M.; Becker, T. Advancing quantification of water-extractable arabinoxylan in beer: A high-throughput approach. Polymers 2023, 15, 3959. [Google Scholar] [CrossRef] [PubMed]
- de Wulf, P.; Soetaert, W.; Schwengers, D.; Vandamme, E.J. D-glucose does not catabolite repress a transketolase-deficient D-ribose-producing Bacillus subtilis mutant strain. J. Ind. Microbiol. 1996, 17, 104–109. [Google Scholar] [CrossRef]
- Park, Y.-C.; Choi, J.-H.; Bennett, G.N.; Seo, J.-H. Characterization of D-ribose biosynthesis in Bacillus subtilis JY200 deficient in transketolase gene. J. Biotechnol. 2006, 121, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-C.; Kim, Y.-J.; Lee, C.-W.; Rho, Y.-T.; Kang, J.; Lee, D.-H.; Seong, Y.-J.; Park, Y.-C.; Lee, D.; Kim, S.-G. Production of D-ribose by metabolically engineered Escherichia coli. Process Biochem. 2017, 52, 73–77. [Google Scholar] [CrossRef]
- Haslbeck, K.; Bub, S.; von Kamp, K.; Michel, M.; Zarnkow, M.; Hutzler, M.; Coelhan, M. The influence of brewing yeast strains on monoterpene alcohols and esters contributing to the citrus flavour of beer. J. Inst. Brew. 2018, 124, 403–415. [Google Scholar] [CrossRef]
- Sales, A.; Felipe, L.; Bicas, J.L. Production, properties, and applications of α-Terpineol. Food Bioprocess Technol. 2020, 13, 1261–1279. [Google Scholar] [CrossRef]
- Da Soares Costa, M.; Gonçalves, C.; Ferreira, A.; Ibsen, C.; Guedes de Pinho, P.; Silva Ferreira, A.C. Further insights into the role of methional and phenylacetaldehyde in lager beer flavor stability. J. Agric. Food Chem. 2004, 52, 7911–7917. [Google Scholar] [CrossRef]

| Strain Reference | Genus | Species | Strain | Discovery of Isolate | Date of Sampling |
|---|---|---|---|---|---|
| DSM 20462T | Megasphaera | cerevisiae | PAT 1 | Spoiled beer, Germany | before 7 June 1979 |
| DSM 20467T | Pectinatus | cerevisiiphilus | CCC B-1022 | Spoiled beer, USA | before 2 August 1979 |
| DSM 6306T | Pectinatus | frisingensis | V1 | Turbid beer, Finland | 1978 |
| DSM 16980T | Pectinatus | haikarae | VTT E-88329 | Air of bottling hall in a brewery, Finland | 1989 |
| DSM 16981T | Megasphaera | paucivorans | VTT E-032341 | Spoiled beer, Italy | 2002 |
| DSM 17042T | Megasphaera | sueciensis | VTT E-97791 | Spoiled beer, Sweden | 1997 |
| TMW 2.2465 | Pectinatus | cerevisiiphilus | 33-1 | Brewery environment | Unknown |
| TMW 2.2467 | Pectinatus | frisingensis | HBS 2 | Brewery environment | Unknown |
| TMW 2.2469 | Pectinatus | frisingensis | Mu 2 | Brewery environment | Unknown |
| TMW 2.2471 | Pectinatus | frisingensis | 247-2 | Brewery conveyor belt | Unknown |
| TMW 2.2474 | Pectinatus | frisingensis | 245-1 | Brewery facility table | Unknown |
| TMW 2.2479 | Megasphaera | cerevisiae | SR V 4 | Brewery environment | Unknown |
| TMW 2.2480 | Megasphaera | cerevisiae | Sp. II 9/4 | Brewery environment | Unknown |
| TMW 2.2482 | Megasphaera | cerevisiae | SR V 5 | Brewery environment | Unknown |
| TMW 2.2484 | Megasphaera | cerevisiae | PAT 2a | Brewery environment | Unknown |
| TMW 2.2485 | Megasphaera | cerevisiae | PAT 2b | Brewery environment | Unknown |
| TMW 2.453 | Megasphaera | cerevisiae | M2 | Brewery environment | Unknown |
| TMW 2.1487 | Pectinatus | frisingensis | 140 | Brewery environment | Unknown |
| TMW 2.1490 | Pectinatus | frisingensis | 173 | Brewery environment | Unknown |
| TMW 2.1491 | Pectinatus | frisingensis | 175 | Brewery environment | Unknown |
| TMW 2.1492 | Pectinatus | frisingensis | 225 | Brewery environment | Unknown |
| TMW 2.1493 | Pectinatus | frisingensis | 227 | Brewery environment | Unknown |
| TMW 2.1494 | Pectinatus | cerevisiiphilus | 228 | Brewery environment | Unknown |
| TMW 2.1496 | Pectinatus | haikarae | 233 | Brewery environment | Unknown |
| TMW 2.1500 | Pectinatus | frisingensis | 240 | Brewery environment | Unknown |
| TMW 2.1503 | Pectinatus | frisingensis | 260 | Brewery environment | Unknown |
| TMW 2.2489 | Megasphaera | paucivorans | BEL B | Spoiled beer, Italy | 2010 |
| TMW 2.2490 | Pectinatus | haikarae | BIO Y21 | Spoiled beer, Finland | 2010 |
| TMW 2.2491 | Pectinatus | frisingensis | ABBC437 | Brewery environment | Unknown |
| TMW 2.2492 | Pectinatus | cerevisiiphilus | ABBC474 | Brewery environment | Unknown |
| Species | Glycerol | Arabinose | Xylose | Fructose | Ribose | Maltulose | Maltose | Maltotriose | |
|---|---|---|---|---|---|---|---|---|---|
| Control | - | 1550 ± 45.8 | 78.8 ± 3.0 | 82.9 ± 1.6 | 71.3 ± 2.1 | 39.8 ± 4.7 | 1120 ± 12.2 | 3670 ± 605.6 | 2130 ± 35.0 |
| DSM 20462T | M. cerevisiae | 1190 | 0.0 | 90.4 | 0.0 | 33.4 | 1040 | 3430 | 2010 |
| TMW 2.453 | M. cerevisiae | 1280 | 64.4 | 89.6 | 0.0 | 38.6 | 1040 | 3400 | 2030 |
| TMW 2.2479 | M. cerevisiae | 1250 | 69.0 | 92.6 | 0.0 | 39.6 | 1080 | 3430 | 2080 |
| TMW 2.2480 | M. cerevisiae | 1210 | 71.5 | 87.5 | 0.0 | 42.1 | 1070 | 3280 | 1930 |
| TMW 2.2482 | M. cerevisiae | 1310 | 71.0 | 91.6 | 77.0 | 32.6 | 1050 | 3250 | 2010 |
| TMW 2.2484 | M. cerevisiae | 1210 | 18.4 | 89.6 | 0.0 | 41.0 | 1040 | 3340 | 1983 |
| TMW 2.2485 | M. cerevisiae | 1190 | 16.6 | 89.1 | 0.0 | 40.0 | 1030 | 3340 | 1990 |
| Lit.+ | a | a | |||||||
| Lit.− | a | a | a | a | |||||
| DSM 16981T | M. paucivorans | 1250 | 72.0 | 94.2 | 0.0 | 39.2 | 1080 | 3530 | 2160 |
| TMW 2.2489 | M. paucivorans | 1360 | 72.6 | 87.0 | 0.0 | 39.9 | 1030 | 3350 | 2010 |
| Lit.+ | |||||||||
| Lit.− | b | b | b | b | b | b | |||
| DSM 17042T | M. sueciensis | 1290 | 68.6 | 87.0 | 53.3 | 33.3 | 1080 | 3470 | 2120 |
| Lit.+ | |||||||||
| Lit.− | b | b | b | b | b | b |
| DSM 20462T M. cerevisiae | TMW 2.2479 M. cerevisiae | TMW 2.2480 M. cerevisiae | TMW 2.2482 M. cerevisiae | TMW 2.2484 M. cerevisiae | TMW 2.2485 M. cerevisiae | TMW 2.453 M. cerevisiae | Lit. | DSM 16981T M. paucivorans | TMW 2.2489 M. paucivorans | Lit. | DSM 17042T M. sueciensis | Lit. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimethyl sulfide | X | X | X | X | X | X | |||||||
| Propanol | X | X | X | X | X | X | X | ||||||
| Ethyl acetate | X | ||||||||||||
| Isobutanol | X | X | |||||||||||
| n-Butanol | X | X | X | X | X | X | X | X | X | X | |||
| 3-Methylbutanal | X | ||||||||||||
| 2-Methylbutanal | X | X | X | ||||||||||
| S-Methyl thioacetate | X | X | X | ||||||||||
| Isobutyric acid | X | X | X | X | X | X | X | a | X | b | X | b | |
| Ethyl-Isobutanoate | X | X | X | X | X | X | X | ||||||
| Butyric acid | X | X | X | X | X | X | X | a | X | X | b | X | b |
| Ethyl butanoate | X | X | X | X | X | X | X | X | X | ||||
| 3-Methylbutanoic acid | X | X | X | X | X | X | X | X | X | X | |||
| 2-Methylbutanoic acid | X | X | X | X | X | X | X | ||||||
| Ethyl-2-Methyl butanoate | X | X | X | X | X | X | X | ||||||
| Ethyl-3-Methyl butanoate | X | X | X | X | X | X | |||||||
| Furfural | X | X | X | X | X | ||||||||
| Hexanol | X | X | X | X | X | X | X | X | |||||
| Styrene | X | X | |||||||||||
| Ethyl pentanoate | X | X | X | X | X | ||||||||
| Ethyl furfuryl ether | X | X | X | X | X | ||||||||
| Methional | X | ||||||||||||
| Dimethyl trisulfide | X | X | X | ||||||||||
| Methionol | X | X | X | X | X | X | X | X | |||||
| Caproic acid | X | X | X | X | X | X | X | a | X | X | b | X | b |
| Ethyl hexanoate | X | X | X | X | X | X | X | X | |||||
| Hexyl acetate | X | X | X | X | X | ||||||||
| Limonene | X | ||||||||||||
| Ethyl-5-Methylhexanoate | X | X | |||||||||||
| 2-Acetyl1H-Pyrrol | X | X | |||||||||||
| Octanol | X | X | X | X | X | X | X | X | |||||
| Ethyl heptanoate | X | X | X | X | X | X | |||||||
| 2-Nonanol | X | ||||||||||||
| Heptyl acetate | X | ||||||||||||
| Methionyl acetate | X | ||||||||||||
| a-Terpineol | X | ||||||||||||
| 4-Vinylphenol | X | X | |||||||||||
| Ethyl nicotinoate | X | X | X | X | X | X | X | X | X | X | |||
| Citronellol | X | X | |||||||||||
| Ethyl-2-Phenylacetat | X | X | X | X | X | X | |||||||
| Decanol | X | X | X | ||||||||||
| Geranyl acetate | X |
| Species | Glycerol | Arabinose | Xylose | Fructose | Ribose | Maltulose | Maltose | Maltotriose | |
|---|---|---|---|---|---|---|---|---|---|
| Control | - | 1550 ± 45.8 | 78.8 ± 3.0 | 82.9 ± 1.6 | 71.3 ± 2.1 | 39.8 ± 4.7 | 1120 ± 12.2 | 3670 ± 605.6 | 2130 ± 35.0 |
| DSM 20467T | P. cerevisiiphilus | 69.8 | 0.0 | 0.0 | 0.0 | 29.2 | 1020 | 2510 | 2140 |
| TMW 2.2465 | P. cerevisiiphilus | 0.0 | 0.0 | 0.0 | 0.0 | 41.7 | 968 | 2280 | 1790 |
| TMW 2.1494 | P. cerevisiiphilus | 83.1 | 64.8 | 0.0 | 0.0 | 36.4 | 965 | 2130 | 1620 |
| TMW 2.2492 | P. cerevisiiphilus | 0.0 | 58.3 | 0.0 | 0.0 | 32.9 | 925 | 2240 | 1730 |
| Lit.+ | a | a | |||||||
| Lit.− | a | ||||||||
| DSM 6306T | P. frisingensis | 62.9 | 0.0 | 103 | 0.0 | 0.0 | 637 | 895 | 2160 |
| TMW 2.2471 | P. frisingensis | 0.0 | 0.0 | 107 | 0.0 | 38.3 | 356 | 254 | 2020 |
| TMW 2.1487 | P. frisingensis | 0.0 | 18.6 | 95.9 | 0.0 | 32.4 | 685 | 835 | 1850 |
| TMW 2.1491 | P. frisingensis | 0.0 | 18.5 | 93.9 | 0.0 | 34.3 | 387 | 207 | 1520 |
| TMW 2.1493 | P. frisingensis | 0.0 | 7.63 | 98.5 | 0.0 | 46.2 | 657 | 814 | 1810 |
| TMW 2.1500 | P. frisingensis | 0.0 | 3.07 | 105 | 0.0 | 29.5 | 379 | 312 | 1960 |
| TMW 2.1503 | P. frisingensis | 0.0 | 3.66 | 108 | 0.0 | 43.8 | 525 | 613 | 2060 |
| TMW 2.2491 | P. frisingensis | 81.1 | 0.0 | 88.8 | 0.0 | 37.6 | 704 | 1120 | 1790 |
| TMW 2.2467 | P. frisingensis | 0.0 | 0.0 | 94.6 | 0.0 | 60.8 | 934 | 2260 | 1780 |
| TMW 2.2469 | P. frisingensis | 0.0 | 0.0 | 96.0 | 0.0 | 61.1 | 1050 | 2230 | 1830 |
| TMW 2.2474 | P. frisingensis | 0.0 | 0.0 | 0.0 * | 0.0 | 46.0 | 792 | 1340 | 1930 |
| TMW 2.1490 | P. frisingensis | 0.0 | 8.72 | 90.7 | 0.0 | 57.5 | 1020 | 2090 | 1560 |
| TMW 2.1492 | P. frisingensis | 0.0 | 7.33 | 95.3 | 0.0 | 56.5 | 766 | 2250 | 1550 |
| Lit.+ | b | a | a | ||||||
| Lit.− | a | ||||||||
| DSM 16980T | P. haikarae | 0.0 | 0.0 | 6.37 | 19.5 | 38.3 | 1000 | 2160 | 1950 |
| TMW 2.1496 | P. haikarae | 136 | 81.1 | 0.0 * | 14.4 | 33.6 | 968 | 2090 | 1870 |
| TMW 2.2490 | P. haikarae | 189 | 71.9 | 137 * | 42.7 | 35.5 | 925 | 2120 | 1920 |
| Lit.+ | c | c | c | c | c | ||||
| Lit.− | c |
| DSM 20467T P. cerevisiiphilus | TMW 2.2465 P. cerevisiiphilus | TMW 2.1494 P. cerevisiiphilus | TMW 2.2492 P. cerevisiiphilus | Lit. | DSM 6306T P. frisingensis | TMW 2.1487 P. frisingensis | TMW 2.1491 P. frisingensis | TMW 2.1493 P. frisingensis | TMW 2.1500 P. frisingensis | TMW 2.1503 P. frisingensis | TMW 2.2471 P. frisingensis | TMW 2.2474 P. frisingensis | TMW 2.2491 P. frisingensis | TMW 2.2467 P. frisingensis | TMW 2.2469 P. frisingensis | TMW 2.1490 P. frisingensis | TMW 2.1492 P. frisingensis | Lit. | DSM 16980T P. haikarae | TMW 2.1496 P. haikarae | TMW 2.2490 P. haikarae | Lit. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Methyl mercaptan | X | ||||||||||||||||||||||
| Dimethyl sulfide | X | X | X | X | X | X | X | X | X | X | |||||||||||||
| Propanol | X | X | |||||||||||||||||||||
| Acetic acid | X | X | a, b | X | X | X | X | X | X | X | X | X | X | X | X | X | a, b | X | X | c | |||
| Ethyl acetate | X | X | |||||||||||||||||||||
| Isobutanol | X | X | X | X | |||||||||||||||||||
| 3-Methylbutanal | X | X | X | ||||||||||||||||||||
| 2-Methylbutanal | X | ||||||||||||||||||||||
| Propanoic acid | X | X | X | X | a, b | X | X | X | X | X | X | X | X | X | X | X | X | X | a, b | X | X | X | c |
| Ethyl propanoate | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||
| Propyl acetate | X | X | X | X | X | X | X | X | X | ||||||||||||||
| Isobutyric acid | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| Isobutyl acetate | X | X | X | X | X | ||||||||||||||||||
| 2,3-Butandiol | X | X | X | X | X | X | X | ||||||||||||||||
| Butyric acid | X | X | X | X | X | X | X | X | X | X | |||||||||||||
| 3-Methyl-2-Buten-1-thiol | X | X | X | X | |||||||||||||||||||
| 3-Methyl butyric acid | X | X | X | X | X | X | X | ||||||||||||||||
| Furfural | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||
| Ethyl furfuryl ether | X | X | X | X | X | X | X | X | X | X | |||||||||||||
| Methional | X | X | |||||||||||||||||||||
| Ethyl-4-Methyl pentanoate | X | ||||||||||||||||||||||
| 1-Octen-3-ol | X | X | X | X | X | X | X | X | X | X | X | ||||||||||||
| Methionol | X | X | X | ||||||||||||||||||||
| Caproic acid | X | ||||||||||||||||||||||
| Myrcen | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||
| Octanol | X | ||||||||||||||||||||||
| Ethyl heptanoate | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||
| Linalool | X | ||||||||||||||||||||||
| Ethyl-6-Methyl heptanoate | X | X | |||||||||||||||||||||
| a-Terpineol | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||
| Nerol | X | X | X | X | X | X | X | X | X | ||||||||||||||
| Geraniol | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| 4-Vinyl guajacol | X | ||||||||||||||||||||||
| Methyl-E-geranate | X | ||||||||||||||||||||||
| Citronellyl acetate | X | ||||||||||||||||||||||
| Ethyl dihydrocinnamate | X | ||||||||||||||||||||||
| 3-Methylbutyl octanoate | X | ||||||||||||||||||||||
| 2-Methylbutyl octanoate | X |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arnold, M.J.; Ritter, S.W.; Ehrmann, M.A.; Kurniawan, Y.N.; Suzuki, K.; Becker, T.M.; Liebl, W. Carbohydrate Metabolism Differentiates Pectinatus and Megasphaera Species Growing in Beer. Microorganisms 2024, 12, 2045. https://doi.org/10.3390/microorganisms12102045
Arnold MJ, Ritter SW, Ehrmann MA, Kurniawan YN, Suzuki K, Becker TM, Liebl W. Carbohydrate Metabolism Differentiates Pectinatus and Megasphaera Species Growing in Beer. Microorganisms. 2024; 12(10):2045. https://doi.org/10.3390/microorganisms12102045
Chicago/Turabian StyleArnold, Manuel J., Stefan W. Ritter, Matthias A. Ehrmann, Yohanes N. Kurniawan, Koji Suzuki, Thomas M. Becker, and Wolfgang Liebl. 2024. "Carbohydrate Metabolism Differentiates Pectinatus and Megasphaera Species Growing in Beer" Microorganisms 12, no. 10: 2045. https://doi.org/10.3390/microorganisms12102045
APA StyleArnold, M. J., Ritter, S. W., Ehrmann, M. A., Kurniawan, Y. N., Suzuki, K., Becker, T. M., & Liebl, W. (2024). Carbohydrate Metabolism Differentiates Pectinatus and Megasphaera Species Growing in Beer. Microorganisms, 12(10), 2045. https://doi.org/10.3390/microorganisms12102045






