Isolation of Bacillus altitudinis 5-DSW with Protease Activity from Deep-Sea Mineral Water and Preparation of Functional Active Peptide Fractions from Chia Seeds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Media
2.2. Isolation, Screening, and Culture Condition
2.3. Identification of the Bacillus Strains from Deep Hot Spring Sea Water
2.4. Protease Activity of B. altitudnis 5-DSW
2.5. Optimization of Protease Activity from B. altitudinis5-DSW
2.6. Preparation of Enzymatic Chia Seed Protein Hydrolysates and Characterization of Hydrolysis Products
2.6.1. Preparation of Enzymatic Chia Seed Protein Hydrolysates
2.6.2. Molecular Weight Analysis by Sodium Dodecyl Sulfate-polyacrylamide Gel Electrophoresis and Size Exclusion Chromatography
2.7. Antioxidant Activity Assays
2.7.1. DPPH Radical Scavenging Activity
2.7.2. ABTS+ Radical Scavenging Activity
2.7.3. Inhibition of Acetylcholinesterase and Butyrylcholinesterase Activities
3. Results and Discussion
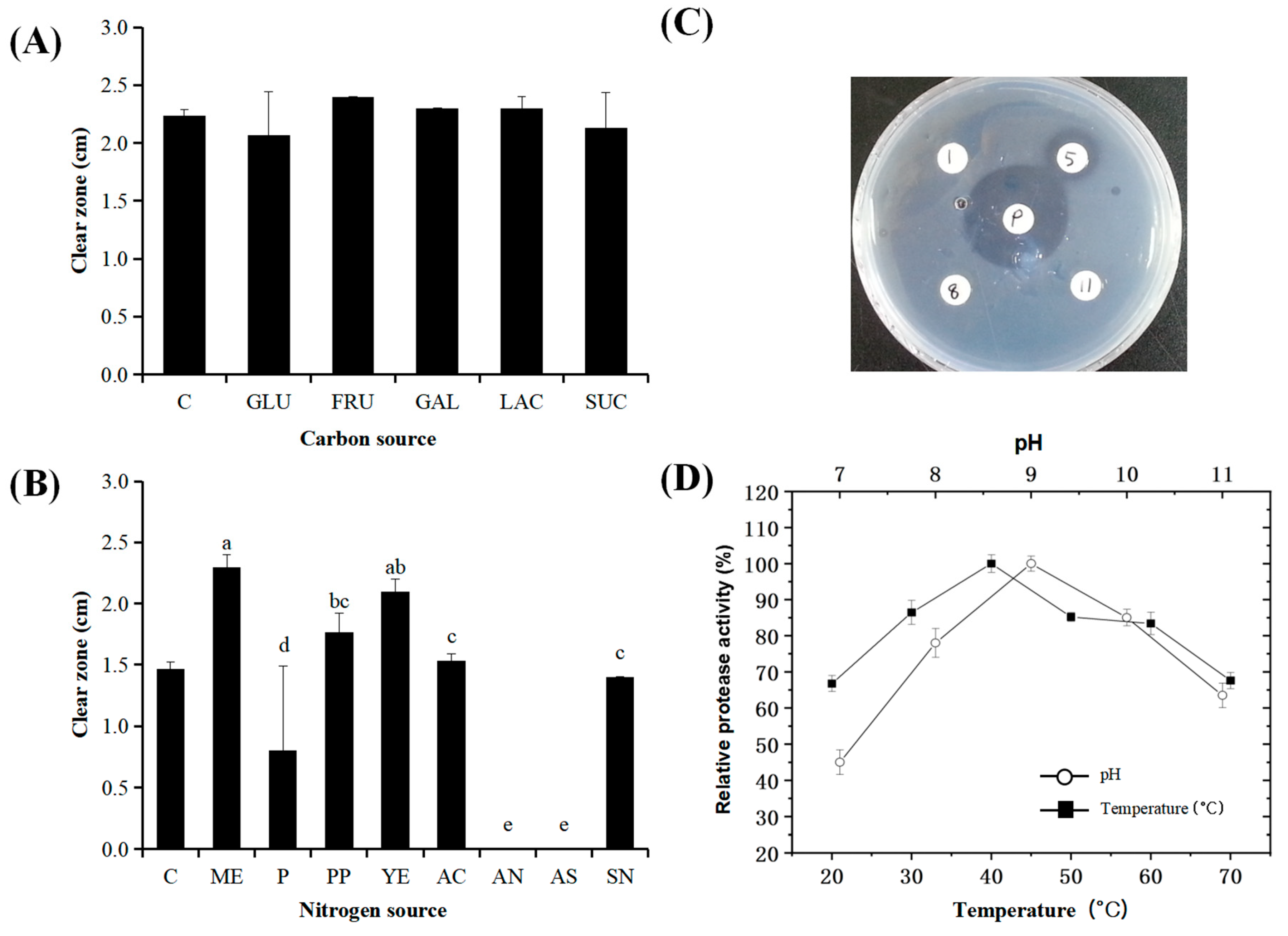
3.1. Isolation of Strains with Protease Activity from Deep Sea Mineral Water
3.2. Effects of Temperature, Carbon, and Nitrogen Sources on Protease Activity
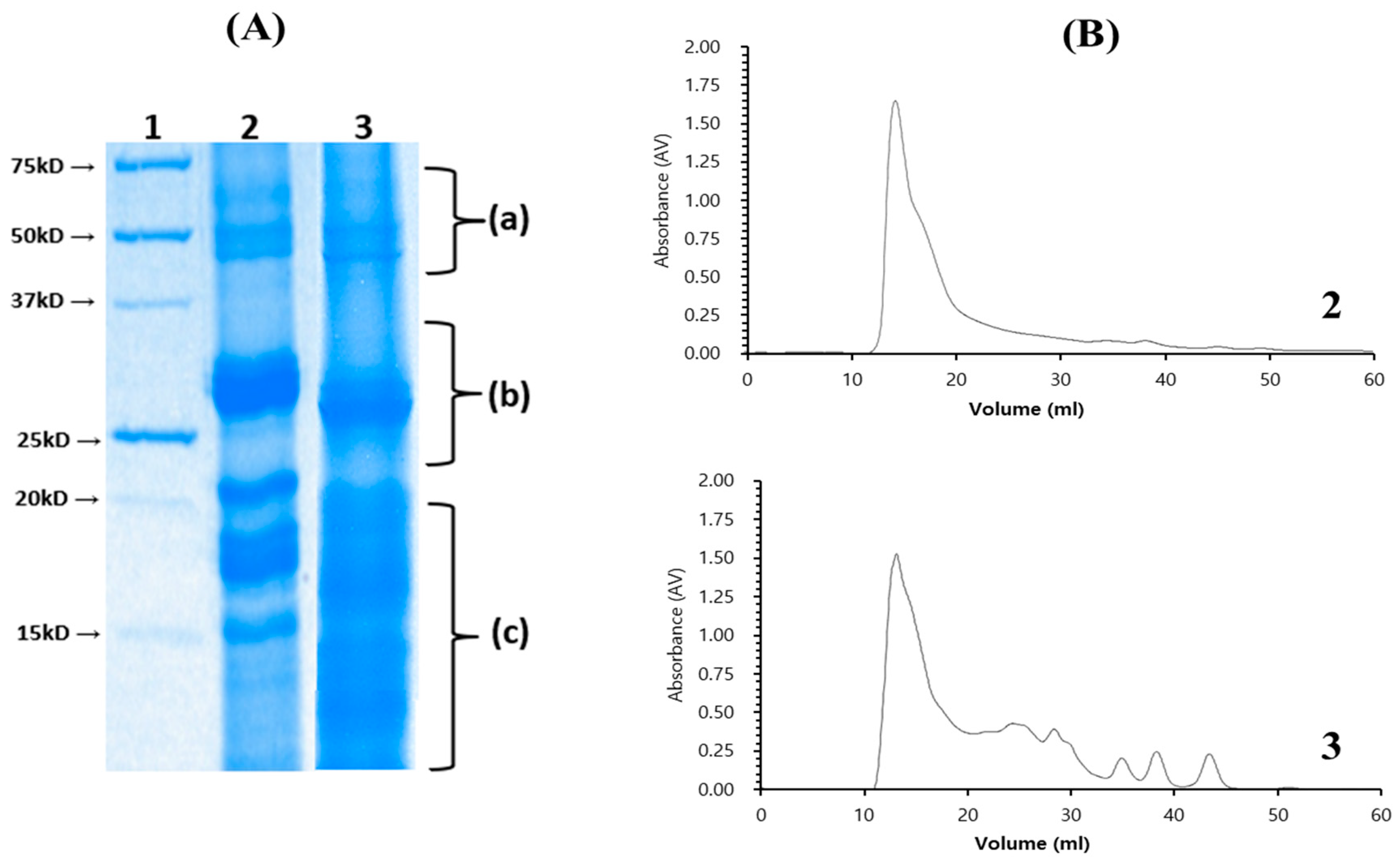
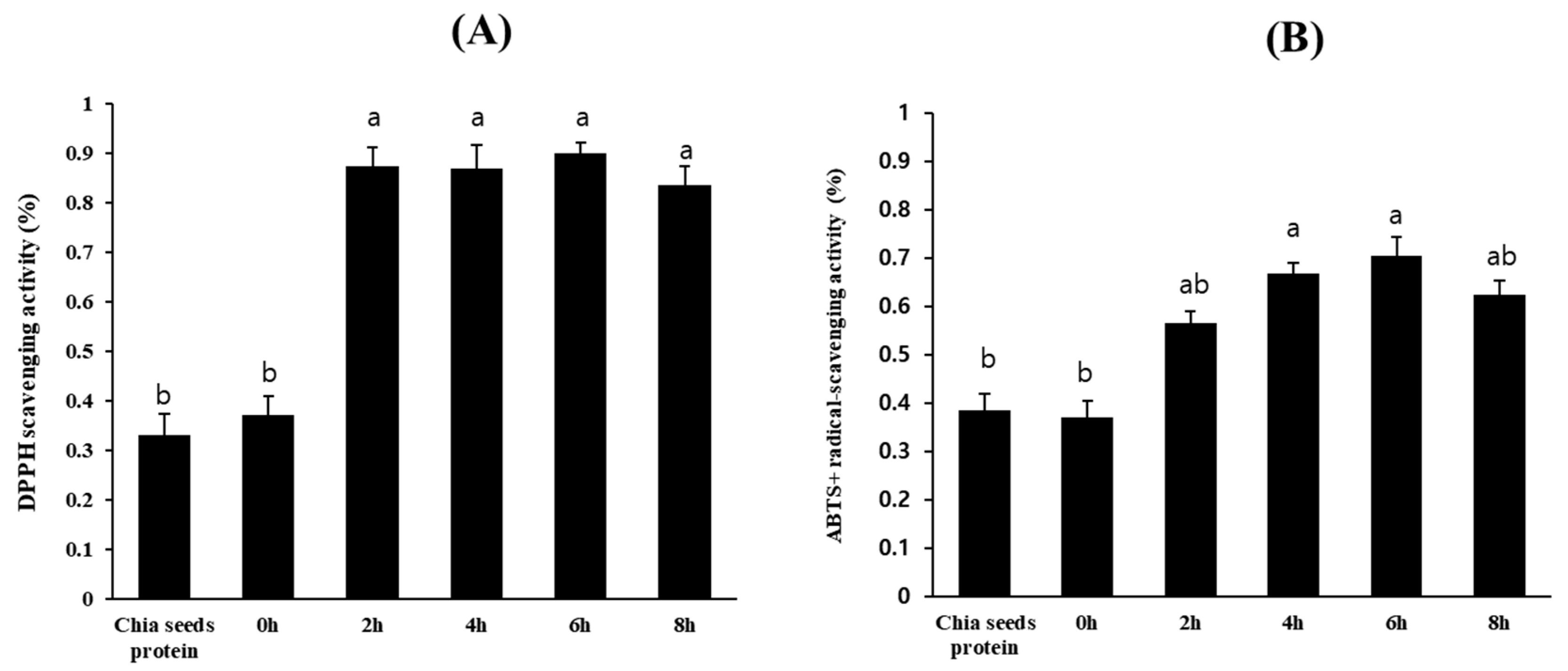
3.3. Isolation of Chia Seed Protein Hydrolysate by Crude Protease from B. altitudinis 5-DSW
3.4. Acetylcholinesterase and butyrylcholinesterase Inhibitory Activities of Chia Seed Protein Hydrolysate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Halder, U.; Banerjee, A.; Chaudhry, V.; Varshney, R.; Mantri, S.; Bandopadhyay, R. Draft genome report of Bacillus altitudinis SORB11, isolated from the Indian sector of the Southern Ocean. Genome Announc. 2017, 5, e00339-17. [Google Scholar] [CrossRef]
- Shafi, S.; Kamili, A.N.; Shah, M.A.; Bandh, S.A.; Dar, R. Dynamics of bacterial class Bacilli in the deepest valley lake of Kashmir-the Manasbal Lake. Microb. Pathog. 2017, 104, 78–83. [Google Scholar] [CrossRef]
- Sunar, K.; Dey, P.; Chakraborty, U.; Chakraborty, B. Biocontrol efficacy and plant growth promoting activity of Bacillus altitudinis isolated from Darjeeling hills, India. J. Basic Microbiol. 2015, 55, 91–104. [Google Scholar] [CrossRef]
- Mao, S.; Lu, Z.; Zhang, C.; Lu, F.; Bie, X. Purification, characterization, and heterologous expression of a thermostable β-1, 3-1, 4-glucanase from Bacillus altitudinis YC-9. Appl. Biochem. Biotechnol. 2013, 169, 960–975. [Google Scholar] [CrossRef]
- Harsonowati, W.; Rahayuningsih, S.; Yuniarti, E.; Susilowati, D.N.; Manohara, D.; Sipriyadi; Widyaningsih, S.; Akhdiya, A.; Suryadi, Y.; Tentrem, T. Bacterial Metal-Scavengers Newly Isolated from Indonesian Gold Mine-Impacted Area: Bacillus altitudinis MIM12 as Novel Tools for Biotransformation of Mercury. Microb. Ecol. 2023, 86, 1646–1660. [Google Scholar] [CrossRef]
- Khan, S.I.; Zarin, A.; Ahmed, S.; Hasan, F.; Belduz, A.O.; Çanakçi, S.; Khan, S.; Badshah, M.; Farman, M.; Shah, A.A. Degradation of lignin by Bacillus altitudinis SL7 isolated from pulp and paper mill effluent. Water Sci. Technol. 2022, 85, 420–432. [Google Scholar] [CrossRef]
- Pranaw, K.; Pidlisnyuk, V.; Trögl, J.; Malinská, H. Bioprospecting of a novel plant growth-promoting bacterium Bacillus altitudinis KP-14 for enhancing Miscanthus× giganteus growth in metals contaminated soil. Biology 2020, 9, 305. [Google Scholar] [CrossRef]
- Jankoski, P.R.; Bach, E.; Bald, D.R.Q.; Passaglia, L.M.P.; Carvalho, J.B.; Oliveira, R.R.; Omori, W.P.; da Motta, A.S. Prospecting the functional potential of Bacillus altitudinis 1.4 isolated from sediment in association with Bradyrhizobium japonicum. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Kuhad, R.C.; Deswal, D.; Sharma, S.; Bhattacharya, A.; Jain, K.K.; Kaur, A.; Pletschke, B.I.; Singh, A.; Karp, M. Revisiting cellulase production and redefining current strategies based on major challenges. Renew. Sustain. Energy Rev. 2016, 55, 249–272. [Google Scholar] [CrossRef]
- Motta, F.; Andrade, C.; Santana, M. A review of xylanase production by the fermentation of xylan: Classification, characterization and applications. Sustain. Degrad. Lignocellul. Biomass-Tech. Appl. Commer. 2013, 1, 251–276. [Google Scholar]
- Zhang, Y.; Li, X.; Xi, R.; Guan, Z.; Cai, Y.; Liao, X. Characterization of an acid-stable catalase KatB isolated from Bacillus altitudinis SYBC hb4. Ann. Microbiol. 2016, 66, 131–141. [Google Scholar] [CrossRef]
- Sarni, S.; Wahab, A.W.; Natsir, H.; La Nafie, N.; Arif, A.R. Production of Crude Uricase Enzyme by Novel Bacillus altitudinis Strain W. IIISRNs_1. 1 from the Hot Spring of Mataumpana, Buton Regency, Southeast Sulawesi. Egypt. J. Chem. 2023, 66, 115–126. [Google Scholar]
- Vijay Kumar, E.; Srijana, M.; Kiran Kumar, K.; Harikrishna, N.; Reddy, G. A novel serine alkaline protease from Bacillus altitudinis GVC11 and its application as a dehairing agent. Bioprocess Biosyst. Eng. 2011, 34, 403–409. [Google Scholar] [CrossRef]
- Kumar, C.G.; Takagi, H. Microbial alkaline proteases: From a bioindustrial viewpoint. Biotechnol. Adv. 1999, 17, 561–594. [Google Scholar] [CrossRef]
- Li-Chan, E.C. Bioactive peptides and protein hydrolysates: Research trends and challenges for application as nutraceuticals and functional food ingredients. Curr. Opin. Food Sci. 2015, 1, 28–37. [Google Scholar] [CrossRef]
- Ganachari, A.; Police Patil, A.; Reddy, M.; Mathad, P.; Khan, H.; Reddy, S.B. Physical properties of Chia (Salvia hispanica L.) seeds required for the design of equipment. Pharma Innov. J. 2022, 11, 2253–2256. [Google Scholar]
- Knez Hrnčič, M.; Ivanovski, M.; Cör, D.; Knez, Ž. Chia Seeds (Salvia hispanica L.): An overview—Phytochemical profile, isolation methods, and application. Molecules 2019, 25, 11. [Google Scholar] [CrossRef]
- Ullah, R.; Nadeem, M.; Khalique, A.; Imran, M.; Mehmood, S.; Javid, A.; Hussain, J. Nutritional and therapeutic perspectives of Chia (Salvia hispanica L.): A review. J. Food Sci. Technol. 2016, 53, 1750–1758. [Google Scholar] [CrossRef]
- Reyes-Caudillo, E.; Tecante, A.; Valdivia-Lopez, M.A. Dietary fibre content and antioxidant activity of phenolic compounds present in Mexican chia (Salvia hispanica L.) seeds. Food Chem. 2008, 107, 656–663. [Google Scholar] [CrossRef]
- Álvarez-Chávez, L.M.; Valdivia-López, M.d.l.A.; Aburto-Juarez, M.d.L.; Tecante, A. Chemical characterization of the lipid fraction of Mexican chia seed (Salvia hispanica L.). Int. J. Food Prop. 2008, 11, 687–697. [Google Scholar] [CrossRef]
- Ixtaina, V.Y.; Martínez, M.L.; Spotorno, V.; Mateo, C.M.; Maestri, D.M.; Diehl, B.W.; Nolasco, S.M.; Tomás, M.C. Characterization of chia seed oils obtained by pressing and solvent extraction. J. Food Compos. Anal. 2011, 24, 166–174. [Google Scholar] [CrossRef]
- Guindani, C.; Podestá, R.; Block, J.M.; Rossi, M.J.; Mezzomo, N.; Ferreira, S.R. Valorization of chia (Salvia hispanica) seed cake by means of supercritical fluid extraction. J. Supercrit. Fluids 2016, 112, 67–75. [Google Scholar] [CrossRef]
- Chai, K.F.; Voo, A.Y.H.; Chen, W.N. Bioactive peptides from food fermentation: A comprehensive review of their sources, bioactivities, applications, and future development. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3825–3885. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Okolie, C.L.; Qian, H.; Ohanenye, I.C.; Agyei, D.; Aluko, R.E. Ribulose-1, 5-bisphosphate carboxylase as a sustainable and promising plant source of bioactive peptides for food applications. Trends Food Sci. Technol. 2017, 69, 74–82. [Google Scholar] [CrossRef]
- Cotabarren, J.; Rosso, A.M.; Tellechea, M.; García-Pardo, J.; Rivera, J.L.; Obregón, W.D.; Parisi, M.G. Adding value to the chia (Salvia hispanica L.) expeller: Production of bioactive peptides with antioxidant properties by enzymatic hydrolysis with Papain. Food Chem. 2019, 274, 848–856. [Google Scholar] [CrossRef]
- Rutherfurd-Markwick, K.J.; Moughan, P.J. Bioactive peptides derived from food. J. AOAC Int. 2005, 88, 955–966. [Google Scholar] [CrossRef]
- Segura-Campos, M.R.; Salazar-Vega, I.M.; Chel-Guerrero, L.A.; Betancur-Ancona, D.A. Biological potential of chia (Salvia hispanica L.) protein hydrolysates and their incorporation into functional foods. LWT-Food Sci. Technol. 2013, 50, 723–731. [Google Scholar] [CrossRef]
- Orona-Tamayo, D.; Valverde, M.E.; Nieto-Rendón, B.; Paredes-López, O. Inhibitory activity of chia (Salvia hispanica L.) protein fractions against angiotensin I-converting enzyme and antioxidant capacity. LWT-Food Sci. Technol. 2015, 64, 236–242. [Google Scholar] [CrossRef]
- Chim-Chi, Y.; Gallegos-Tintoré, S.; Jiménez-Martínez, C.; Dávila-Ortiz, G.; Chel-Guerrero, L. Antioxidant capacity of Mexican chia (Salvia hispanica L.) protein hydrolyzates. J. Food Meas. Charact. 2018, 12, 323–331. [Google Scholar] [CrossRef]
- Grancieri, M.; Martino, H.S.D.; de Mejia, E.G. Digested total protein and protein fractions from chia seed (Salvia hispanica L.) had high scavenging capacity and inhibited 5-LOX, COX-1-2, and iNOS enzymes. Food Chem. 2019, 289, 204–214. [Google Scholar] [CrossRef]
- Coelho, M.S.; Soares-Freitas, R.A.M.; Arêas, J.A.G.; Gandra, E.A.; Salas-Mellado, M.d.l.M. Peptides from chia present antibacterial activity and inhibit cholesterol synthesis. Plant Foods Hum. Nutr. 2018, 73, 101–107. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.; Deering, A.; Liceaga, A. New insights into the antimicrobial properties of hydrolysates and peptide fractions derived from chia seed (Salvia hispanica L.). Probiotics Antimicrob. Proteins 2020, 12, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Schreyer, S.; Klein, C.; Pfeffer, A.; Rasińska, J.; Stahn, L.; Knuth, K.; Abuelnor, B.; Panzel, A.E.C.; Rex, A.; Koch, S. Chia seeds as a potential cognitive booster in the APP23 Alzheimer’s disease model. Sci. Rep. 2020, 10, 18215. [Google Scholar] [CrossRef]
- Kobus-Cisowska, J.; Szymanowska, D.; Maciejewska, P.; Kmiecik, D.; Gramza-Michałowska, A.; Kulczyński, B.; Cielecka-Piontek, J. In vitro screening for acetylcholinesterase and butyrylcholinesterase inhibition and antimicrobial activity of chia seeds (Salvia hispanica). Electron. J. Biotechnol. 2019, 37, 1–10. [Google Scholar] [CrossRef]
- Logan, N.; Berkeley, R. Identification of Bacillus strains using the API system. Microbiology 1984, 130, 1871–1882. [Google Scholar] [CrossRef]
- Capitani, M.I.; Spotorno, V.; Nolasco, S.M.; Tomás, M.C. Physicochemical and functional characterization of by-products from chia (Salvia hispanica L.) seeds of Argentina. LWT-Food Sci. Technol. 2012, 45, 94–102. [Google Scholar] [CrossRef]
- Kim, J.-Y. Isolation and characterization of an alkaline protease produced by Bacillus subtilis JK-1. Korean J. Microbiol. 2007, 43, 331–336. [Google Scholar]
- Sokol, P.A.; Ohman, D.E.; Iglewski, B.H. A more sensitive plate assay for detection of protease production by Pseudomonas aeruginosa. J. Clin. Microbiol. 1979, 9, 538–540. [Google Scholar] [CrossRef]
- Anson, M.L. The estimation of pepsin, trypsin, papain, and cathepsin with hemoglobin. J. Gen. Physiol. 1938, 22, 79. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Pukalskas, A.; van Beek, T.A.; Venskutonis, R.P.; Linssen, J.P.; van Veldhuizen, A.; de Groot, Æ. Identification of radical scavengers in sweet grass (Hierochloe odorata). J. Agric. Food Chem. 2002, 50, 2914–2919. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Payne, G.W.; Vandamme, P.; Morgan, S.H.; LiPuma, J.J.; Coenye, T.; Weightman, A.J.; Jones, T.H.; Mahenthiralingam, E. Development of a recA gene-based identification approach for the entire Burkholderia genus. Appl. Environ. Microbiol. 2005, 71, 3917–3927. [Google Scholar] [CrossRef]
- Kaluniants, K.; Strel'nikova, L.; Shteĭn, I. Effect of sources of carbon, nitrogen, and phosphorus on the synthesis of proteases from Bacillus subtilis cultures. Prikl. Biokhimiia I Mikrobiol. 1979, 15, 57–62. [Google Scholar]
- Patel, R.; Dodia, M.; Singh, S.P. Extracellular alkaline protease from a newly isolated haloalkaliphilic Bacillus sp.: Production and optimization. Process Biochem. 2005, 40, 3569–3575. [Google Scholar] [CrossRef]
- Pedrolli, D.B.; Monteiro, A.C.; Gomes, E.; Carmona, E.C. Pectin and pectinases: Production, characterization and industrial application of microbial pectinolytic enzymes. Open Biotechnol. J. 2009, 3, 9–18. [Google Scholar] [CrossRef]
- Qian, X.-l.; Ning, W.-w.; Tao, N.p. Functional Properties of Protein from Chia (Salvia hispanica L.) Seed. Nat. Prod. Res. Dev. 2018, 30, 20. [Google Scholar]
- Chandran, A.S.; Suri, S.; Choudhary, P. Sustainable plant protein: A recent overview of sources, extraction techniques and utilization ways. Sustain. Food Technol. 2023, 1, 466–483. [Google Scholar] [CrossRef]
- Sangsukiam, T. Encapsulation of Adzuki Bean Vigna Angularis Hydrolysate by Alginate-Based Beads. Master’s Thesis, Chulalongkorn University, Bangkok, Thailand, 2019. [Google Scholar]
- Malomo, S.A.; Aluko, R.E. In vitro acetylcholinesterase-inhibitory properties of enzymatic hemp seed protein hydrolysates. J. Am. Oil Chem. Soc. 2016, 93, 411–420. [Google Scholar] [CrossRef]
- Zare-Zardini, H.; Tolueinia, B.; Hashemi, A.; Ebrahimi, L.; Fesahat, F. Antioxidant and cholinesterase inhibitory activity of a new peptide from Ziziphus jujuba fruits. Am. J. Alzheimer's Dis. Other Dement. 2013, 28, 702–709. [Google Scholar] [CrossRef]
- Ishida, Y.; Shibata, Y.; Fukuhara, I.; Yano, Y.; Takehara, I.; Kaneko, K. Effect of an excess intake of casein hydrolysate containing Val-Pro-Pro and Ile-Pro-Pro in subjects with normal blood pressure, high-normal blood pressure, or mild hypertension. Biosci. Biotechnol. Biochem. 2011, 75, 427–433. [Google Scholar] [CrossRef]
- Mohkam, M.; Nezafat, N.; Berenjian, A.; Mobasher, M.A.; Ghasemi, Y. Identification of Bacillus probiotics isolated from soil rhizosphere using 16S rRNA, recA, rpoB gene sequencing and RAPD-PCR. Probiotics Antimicrob. Proteins 2016, 8, 8–18. [Google Scholar] [CrossRef]
- Keis, S.; Kaim, G.; Dimroth, P.; Cook, G.M. Cloning and molecular characterization of the atp operon encoding for the F1F0–ATPsynthase from a thermoalkaliphilic Bacillus sp. strain TA2. A1. Biochim. Biophys. Acta (BBA)-Gene Struct. Expr. 2004, 1676, 112–117. [Google Scholar] [CrossRef]

| Chia Seed | Crude Protein (g) | Crude Fat (g) | Protein Recovery Yield (%) | |
|---|---|---|---|---|
| Step 1 | Source | 15.4 ± 0.48 | 29.1 ± 0.63 | 100 |
| Step 2 | Defatted process | 11.3 ± 0.75 | 0.93 ± 0.3 | 73.37 |
| Step 3 | Freeze-dried process | 10.9 ± 0.9 | 0.90 ± 0.3 | 70.77 |
| Chia seeds Hydrolysis | AChE IC50 (μg/mL) | BChE IC50 (μg/mL) |
|---|---|---|
| No hydrolysis | 59.06 ± 0.25 | 45.48 ± 0.88 |
| 0 h | 58.67 ± 0.30 | 43.27 ± 0.51 |
| 2 h | 26.17 ± 0.35 | 24.57 ± 0.59 |
| 4 h | 20.69 ± 0.98 | 12.30 ± 0.91 |
| 6 h | 14.48 ± 0.88 | 10.90 ± 0.80 |
| 8 h | 14.57 ± 0.59 | 11.20 ± 0.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, H.; Lee, E.-G.; Khalid, F.; Jo, S.-W.; Baik, S.-H. Isolation of Bacillus altitudinis 5-DSW with Protease Activity from Deep-Sea Mineral Water and Preparation of Functional Active Peptide Fractions from Chia Seeds. Microorganisms 2024, 12, 2048. https://doi.org/10.3390/microorganisms12102048
Jin H, Lee E-G, Khalid F, Jo S-W, Baik S-H. Isolation of Bacillus altitudinis 5-DSW with Protease Activity from Deep-Sea Mineral Water and Preparation of Functional Active Peptide Fractions from Chia Seeds. Microorganisms. 2024; 12(10):2048. https://doi.org/10.3390/microorganisms12102048
Chicago/Turabian StyleJin, Hao, Eun-Gyo Lee, Faiza Khalid, Seung-Wha Jo, and Sang-Ho Baik. 2024. "Isolation of Bacillus altitudinis 5-DSW with Protease Activity from Deep-Sea Mineral Water and Preparation of Functional Active Peptide Fractions from Chia Seeds" Microorganisms 12, no. 10: 2048. https://doi.org/10.3390/microorganisms12102048





